Abstract
Free full text

The versatile heparin in COVID‐19
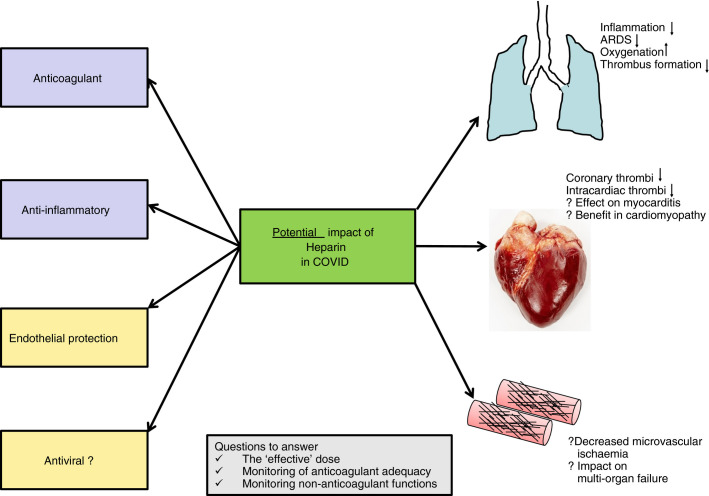
Coagulopathy in coronavirus infection has been shown to be associated with high mortality with high D‐dimers being a particularly important marker for the coagulopathy.1 In the latest paper from the same group, the use of anticoagulant therapy with heparin was shown to decrease mortality as well.2 This is especially so in patients (a) who have met the sepsis induced coagulopathy (SIC) criteria ≥ 4 (40.0% versus 64.2%, P = 0.029) compared to those with SIC score < 4 (29.0% versus 22.6%, P = 0.419) or (b) with markedly elevated D‐dimer (greater than six‐fold at the upper limit of normal). In those with such marked elevation of D‐dimer, approximately 20% reduction in mortality with heparin was found (32.8% versus 52.4%, P = 0.017).2., 3. In this paper, however, only 99/449 patients had received the prophylactic heparin. The authors explain this small number by the fact that consideration of anticoagulant therapy was only made after pulmonary micro‐thrombi were noted at lung dissection from a critically ill patient, although this did assist the authors to retrospectively analyze the difference in outcomes between patients with and without receiving anticoagulant. So, how does this paper impact the current management of COVID‐19 patients? Low molecular weight heparin (LMWH) at prophylactic dose should be considered in patients with markedly elevated D‐dimers or high SIC score. Anecdotal reports from Italy suggest an increased risk of venous thromboembolism in patients admitted to hospitals with COVID‐19. Clearly, prophylactic LMWH would benefit these patients. But may there be other benefits with LMWH in patients with COVID‐19?
Severe lung inflammation and impaired pulmonary gas exchange in COVID‐19 has been suggested to be due to upregulation of pro‐inflammatory cytokines.4 It may be argued that the marked elevation in D‐dimers may also be due to this intense inflammation stimulating intrinsic fibrinolysis in the lungs and spilling into the blood.5 Based on the immuno‐thrombosis model, which highlights a bidirectional relationship between the immune system and thrombin generation, blocking thrombin by heparin may dampen the inflammatory response.6 However, one of the better known non‐anticoagulant properties of heparin, its anti‐inflammatory function, may also be relevant in this setting. Several publications have demonstrated this non‐anticoagulant property and some of the described mechanisms include binding to inflammatory cytokines, inhibiting neutrophil chemotaxis and leukocyte migration, neutralizing the positively charged peptide complement factor C5a, and sequestering acute phase proteins.7., 8., 9., 10. A systematic review concluded that in the clinical environment, heparin can decrease the level of inflammatory biomarkers but urged the need for more data from larger studies.11
Acute respiratory distress syndrome (ARDS) is one of the commonest complications of COVID‐19 infection. Activation of the coagulation system has been shown to be relevant in the pathogenesis of ARDS. Ozoline et al demonstrated that median plasma concentrations of tissue factor and plasminogen activator inhibitor‐1 were significantly higher at day seven in patients with ARDS, as compared to non‐ARDS.12 The mechanisms contributing to this lung coagulopathy are localized tissue factor‐mediated thrombin generation, and depression of bronchoalveolar plasminogen activator‐mediated fibrinolysis, mediated by the PAI‐1 increase. 12., 13. Treatment with heparin may thus be helpful in mitigating this pulmonary coagulopathy. A meta‐analysis noted that adjunctive treatment with LMWH within the initial 7‐day onset of ARDS reduces the risk of 7‐day mortality by 48% and the risk of 28‐day mortality by 37% in addition to significantly improving PaO2/FiO2 ratio (the improvement is particularly important in the subgroup receiving high‐dose LMWH of ≥ 5000 units/d).14 The possible need for a higher dose was also noted in a study of critically ill patients with sepsis who “failed” thromboprophylaxis.15 Of the 42/355 (12.5%) patients who developed a venous thromboembolism, ARDS was the significant risk factor.15 In this context, because the coagulopathy in ARDS may have commenced in the lungs (and in many cases, is limited to it), nebulized anticoagulation would seem a reasonable strategy. However, despite animal trials, this approach has not yet found clinical utility.16
The ubiquitous cell, the vascular endothelium, is often affected by pathogenic invasion with the resulting endothelial dysfunction leading to organ failure. In addition to the pathogen, histones released from damaged cells can also cause endothelial injury.17 Heparin can antagonize histones and thus “protect” the endothelium.18., 19. This protective function can be extended to the endothelial tight junctions as demonstrated in a sepsis model, in which unfractionated heparin could decrease lung edema and vascular leakage following lipopolysaccharide‐induced injury.20 Another mechanism is through its effects on histone methylation and the mitogen‐activated protein kinase (MAPK) and nuclear factor kappa‐light‐chain enhancer of activated B cells (NF‐κB) signal pathways.21 Thus, heparin can impact the microcirculatory dysfunction and possibly decrease organ damage.
Endothelial dysfunction is well known to contribute to cardiac effects, another increasingly recognized complication of COVID.4 Heparin may also be helpful in microvascular dysfunction in cardiac failure because it has been postulated that ischemic hypoxia in the subendocardial layer can make it lose its natural anticoagulant properties.22 A paper published several years ago has also shown that heparin can reduce myocardial inflammation and decrease collagen deposition in an animal model of (chronic) myocarditis.23 These cardiac benefits of heparin are worth noting (and investigating) while treating COVID patients.
Another intriguing concept is the antiviral role of heparin, which has been studied in experimental models. The polyanionic nature of heparin allows it to bind to several proteins and thus act as an effective inhibitor of viral attachment.24 For example, in the case of herpes simplex virus infections, heparin competes with the virus for host cell surface glycoproteins to limit infection and in Zika virus infection, it prevents virus‐induced cell death of human neural progenitor cells.24., 25. In an Italian study, the use of heparin at a concentration of 100 µg/mL halved the infection in experimental vero cells injected with sputum from a patient with SARS‐associated CoV strain pneumonia.26 A very recent online paper has used surface plasmon resonance and circular dichroism and showed that the SARS‐CoV‐2 Spike S1 protein receptor binding domain interacts with heparin.27 But the clinical benefits in any of these viral infections are yet to be determined.
In summary, there are several ways heparin can prove beneficial in patients with COVID‐19 (Figure 1 ). In this respect, the correct dose of LMWH is a matter of immediate interest as a drug can only be effective if administered in an effective dose. Although a prophylactic dose may be adequate in most patients, it would be important to consider a higher dose in individuals with high body mass index. Other matters worthy of investigation are
- • Should we consider a higher than prophylactic dose of LMWH in those with extremely high D‐dimers (eg, six to eight fold) at admission?
- • Should an increase in dose of LMWH be considered in patients who may need higher levels of ventilation (oxygen requirements) or develop ARDS?
- • Should in vitro studies of anti‐inflammatory functions, endothelial protection, and viral inhibition with heparin be considered independently of anticoagulant properties in the COVID scenario?
We are still learning how to adequately manage COVID but the increasing experience shared by extremely dedicated and self‐less health‐care professionals is sure to make us triumph over this pandemic.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/jth.14821
Read article for free, from open access legal sources, via Unpaywall:
http://www.jthjournal.org/article/S1538783622003294/pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/jth.14821
Article citations
Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy.
Pharmaceuticals (Basel), 17(10):1362, 12 Oct 2024
Cited by: 0 articles | PMID: 39459002 | PMCID: PMC11510354
Review Free full text in Europe PMC
Time in Therapeutic Range of Unfractionated Heparin-Based Therapy in Critically Ill Patients with COVID-19 Pneumonia.
Ther Clin Risk Manag, 20:611-618, 10 Sep 2024
Cited by: 0 articles | PMID: 39280635 | PMCID: PMC11401533
Management of Therapeutic-intensity Unfractionated Heparin: A Narrative Review on Critical Points.
TH Open, 8(3):e297-e307, 01 Jul 2024
Cited by: 0 articles | PMID: 39420916 | PMCID: PMC11486528
Review Free full text in Europe PMC
Venous Thromboembolism Management throughout the COVID-19 Era: Addressing Acute and Long-Term Challenges.
J Clin Med, 13(6):1825, 21 Mar 2024
Cited by: 1 article | PMID: 38542049 | PMCID: PMC10971529
The laboratory parameters in predicting the severity and death of COVID-19 patients: Future pandemic readiness strategies.
Biomol Biomed, 24(2):238-255, 11 Mar 2024
Cited by: 0 articles | PMID: 37712883 | PMCID: PMC10950347
Review Free full text in Europe PMC
Go to all (246) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Anticoagulant and antiarrhythmic effects of heparin in the treatment of COVID-19 patients.
J Thromb Haemost, 18(8):2073-2075, 01 Aug 2020
Cited by: 16 articles | PMID: 32408391 | PMCID: PMC7272830
Potential of heparin and nafamostat combination therapy for COVID-19.
J Thromb Haemost, 18(6):1521-1522, 06 May 2020
Cited by: 61 articles | PMID: 32302456 | PMCID: PMC9906352
Differentiating biochemical from clinical heparin resistance in COVID-19.
J Thromb Thrombolysis, 50(4):1015-1016, 01 Nov 2020
Cited by: 7 articles | PMID: 32880796 | PMCID: PMC7471526
Heparin failure and COVID-19: Should we explore other anticoagulants? An observational report regarding in-vitro recovery of anticoagulant action in COVID-19 patients in intensive care.
Thromb Res, 195:226-227, 09 Aug 2020
Cited by: 10 articles | PMID: 32795720 | PMCID: PMC7415167