Abstract
Background:
Multidisciplinary guidelines recommend parathyroidectomy to slow the progression of chronic kidney disease (CKD) in patients with primary hyperparathyroidism (PHPT) and an estimated glomerular filtration rate (eGFR) less than 60mL/min/1.73m2. Limited data address the effect of parathyroidectomy on long-term kidney function.
Objective:
To compare the incidence of a sustained decline in eGFR of at least 50% among PHPT patients treated with parathyroidectomy vs. non-operative management.
Design:
We performed target trial emulation using observational data from adults with PHPT, using an extended Cox model with time-varying inverse probability censoring weighting.
Setting:
Veterans Health Administration.
Patients:
Patients with new biochemical diagnosis of PHPT 2000–2019.
Main Measures:
Sustained ≥50% decline from pre-treatment eGFR.
Results:
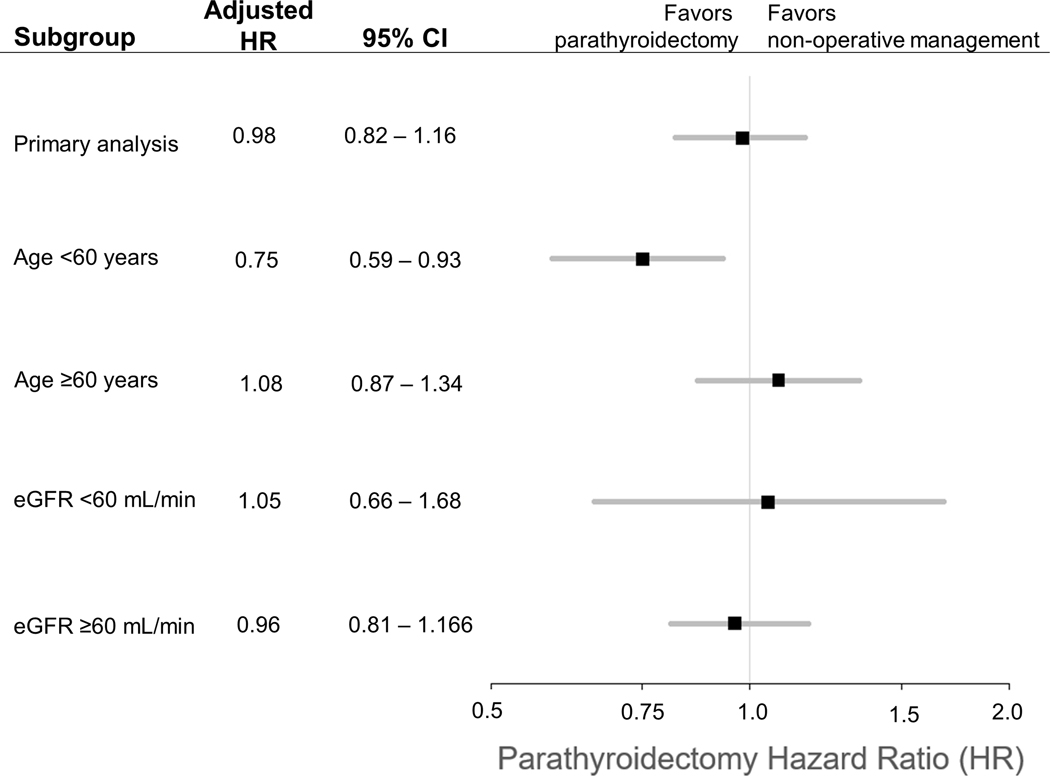
Among 43,697 patients with PHPT (mean age 66.8 years), 2928 (6.7%) developed ≥50% decline in eGFR over a median follow-up of 4.9 years. The weighted cumulative incidence of eGFR decline was 5.1% and 10.8% at 5 and 10 years in patients managed with parathyroidectomy, compared with 5.1% and 12.0% in those managed non-operatively. There was no difference in the adjusted hazard of eGFR decline with parathyroidectomy compared to non-operative management (HR 0.98, 95%CI 0.82–1.16). On subgroup analyses, there was no heterogeneity of treatment effect based on pre-treatment kidney function. Parathyroidectomy was associated with a reduced hazard of the primary outcome among patients age <60 years (HR 0.75, 95%CI 0.59–0.93) that was not evident among those age ≥60 years (HR 1.08, 95%CI 0.87–1.34).
Limitation:
Analyses were performed in a predominantly male cohort using observational data.
Conclusions:
Parathyroidectomy had no effect on long-term kidney function in older adults with PHPT. Potential benefits related to kidney function should not be the primary consideration for PHPT treatment decisions.
INTRODUCTION
Primary hyperparathyroidism (PHPT) is a common endocrine disorder associated with a higher risk of chronic kidney disease (CKD).(1, 2) Definitive treatment for PHPT is parathyroidectomy to remove one or more abnormal parathyroid glands; however, observation for disease progression is an alternative that may be considered in patients without obvious end-organ damage related to PHPT. Multidisciplinary guidelines recommend parathyroidectomy, at least in part, to mitigate the risk of, and effects related to, CKD progression among patients with PHPT and an estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2.(3, 4) However, there are limited data documenting the association of parathyroidectomy with long-term kidney function to support this recommendation. The majority of patients with PHPT are not treated with parathyroidectomy, including those who meet operative guideline criteria based on CKD stage,(5, 6) highlighting clinical uncertainty regarding the effectiveness of parathyroidectomy as a management strategy to preserve kidney function.
The exact pathogenesis of kidney injury in patients with PHPT is unknown. Proposed mechanisms for the development of impaired kidney function in PHPT include kidney stone disease, nephrocalcinosis, hypercalcemia-induced diuresis and volume depletion, or direct toxicity from calcium or parathyroid hormone (PTH). A large population-based, matched cohort study found a 13-fold higher adjusted rate of kidney failure based solely on claim codes among patients with PHPT when compared with age- and sex-matched controls, with 18.9% of PHPT cases developing kidney failure compared with 1.2% of patients without PHPT over a median follow up of 3 years.(2) Whether treatment with parathyroidectomy slows the loss of kidney function in adults with PHPT remains unknown.
To address this evidence gap, we used target trial emulation to estimate the effect of parathyroidectomy on kidney function among adults with a new biochemical diagnosis of PHPT. We hypothesized that parathyroidectomy would be associated with a lower rate of a sustained decline in eGFR of 50% or more after accounting for differences in baseline severity of PHPT and CKD risk factors between operatively and non-operatively managed groups.
METHODS
To estimate the effect of parathyroidectomy vs. non-operative management on the incidence of a sustained decline in eGFR of at least 50%, we emulated a hypothetical randomized trial (the target trial) comparing patients who were treated with parathyroidectomy within one year of PHPT diagnosis with those who were managed non-operatively. The specifications of our target trial and emulated trial are listed in Table 1.
Table 1:
Specification and emulation of a target trial of parathyroidectomy versus non-operative management for PHPT in adults within the VHA 2000–2019.
| Component | Target trial | Emulated trial using real-world data |
|---|---|---|
| Eligibility | Adults with a new biochemical diagnosis of PHPT between 2000–2019 within VHA | Same |
| Exclusions | Adults in nursing home, hospice, or assisted living, Charlson score >=5, prior parathyroidectomy | Same |
| Treatment strategies | 1. Early parathyroidectomy (within one year of diagnosis) 2. Early observation (observation/no surgery within one year of diagnosis) |
1. Early parathyroidectomy (within one year of diagnosis) 2. Early observation (observation/no surgery within one year of diagnosis) |
| Treatment assignment | Patients are randomly assigned to either strategy | Patients are non-randomly assigned to the treatment strategy their observational data are compatible with. Randomization is emulated via cloning of patients in both arms |
| Treatment implementation | None | 1-year grace period |
| Primary outcome | >=50% sustained decline in eGFR | Same |
| Follow up | Follow up starts at PHPT diagnosis (equivalent to time of treatment assignment) and ends at >=50% sustained decline in eGFR, last eGFR measurement, or December 31, 2019 | Same |
| Causal contrast | Intention-to-treat effect | Observational analog of the per-protocol effect |
| Statistical analysis | Cox regression analysis comparing time to >=50% sustained decline in eGFR between treatment arms. | Extended Cox model with time-varying inverse probability weights comparing time to >=50% sustained decline in eGFR between treatment arms. Cloning was used to avoid immortal time bias, censoring to compare individuals with data compatible with their assigned strategy, and inverse-probability weighting was used to account for the selection bias introduced by censoring. |
Data and Study Population
We used data from patients with a new biochemical diagnosis of PHPT within the Veterans Health Administration (VHA) from January 1, 2000 to December 31, 2019. We accessed national VHA data stored in the Corporate Data Warehouse (CDW) using the VA Informatics and Computing Infrastructure (VINCI) platform. All patient data was retrieved from tables mapped to the Observational Medical Outcomes Partnership (OMOP) Common Data Model. This study was approved by the Stanford University Institutional Review Board (IRB) and the Veterans Affairs Palo Alto Research and Development Committee. This study was performed and reported in accordance with STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines for observational research.(7)
Target Trial Eligibility Criteria and Exclusions
Patients were considered eligible for the target trial if they had a new biochemical diagnosis of PHPT during the study period. We defined biochemical diagnosis of PHPT as having an elevated parathyroid hormone (PTH) level (>65 ng/mL) within 6 months after an elevated serum calcium (>10.2 mg/dL). To exclude patients with secondary or tertiary hyperparathyroidism, we required subjects to have an eGFR above 30 mL/min/1.73 m2 for 12 months prior to PHPT diagnosis and excluded patients with claim codes or lab results indicating stage 5 CKD, receipt of any hemodialysis, or kidney transplant prior to PHPT diagnosis. To establish a baseline eGFR, we excluded patients without a serum creatinine measurement within one year prior to PHPT diagnosis. We calculated eGFR using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) creatinine equation refit without the race variable.(8, 9) To ensure that our emulated target trial would only include patients who were suitable for both operative or non-operative management of PHPT, we excluded patients in hospice, residing in nursing homes or assisted living facilities, who had undergone prior parathyroidectomy, and with Charlson-Deyo Comorbidity Index scores ≥5. We also excluded patients with missing demographic or body mass index (BMI) data.
Treatment Strategies
Our target trial was focused on comparing kidney outcomes in patients treated with early parathyroidectomy (within one year of PHPT diagnosis) compared to those who underwent early observation (no surgery within one year of PHPT diagnosis). Patients treated with parathyroidectomy were identified based on International Classification of Diseases, Ninth Revision (ICD-9) procedure codes 06.81, 06.89, 06.99; Tenth Revision (ICD-10) procedure codes 0GBLx, 0GBMx, 0GBNx, 0GBPx, 0GBQx, 0GBRx; and Current Procedural Terminology (CPT) codes 60500, 60502, 60505.
Covariates
We abstracted patient demographics including age at diagnosis, sex (male, female), designated race (white, Black, other, unknown), and ethnicity (Hispanic or Latino, not Hispanic or Latino, unknown). We included the following covariates and comorbid conditions: baseline Charlson-Deyo Comorbidity Index (categorized to 0, 1, or ≥ 2), serum calcium ≥11.3 mg/dL (1 mg/dL greater than the upper limit of normal), and risk factors for CKD, including history of coronary artery disease, stroke, kidney stones, hypertension, diabetes, use of an angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers, and BMI. All clinical covariates were assessed in the two years before PHPT diagnosis, except for history of kidney stones, which was assessed any time before PHPT diagnosis, and baseline eGFR and ACE inhibitor or ARB use, which were assessed in the year before PHPT diagnosis to more accurately represent pre-treatment kidney function.
Outcome and Follow Up
The primary outcome was the development of a sustained decline in eGFR of at least 50% from baseline. To avoid identifying patients with transient acute kidney injury, we required that the 50% or greater decline from baseline eGFR be sustained on at least two occasions that were at least 90 days apart. Time zero for our analysis was the date of PHPT diagnosis, when all patients met trial eligibility criteria. The grace period of one year for treatment assignment was chosen to reflect typical practice patterns for the management of PHPT. We reasoned that a one-year grace period would encompass the time from which a decision about operative or non-operative management is made after a biochemical diagnosis to the time needed for evaluation by a surgeon and scheduling of parathyroidectomy. We followed patients up to 24 months after the date of their last eGFR measurement within the study period, requiring patients to have an eGFR measurement every 24 months to remain under follow-up in the target trial. Therefore, patients who did not experience the primary outcome were censored at end of the first 24-month gap in eGFR measurements or on December 31st, 2019.
Statistical Analysis
The statistical analysis for our target trial emulation follows the procedure of Maringe et al.(10) To account for confounding and immortal-time bias that may result from the delay between time zero and receipt of treatment within the grace period, we used a technique in which each patient contributes two clones to the dataset, with one clone assigned to each treatment arm, doubling the size of our dataset at study initiation.(10–12) In each treatment arm, we censored clones within or at the end of the grace period when the received treatment was no longer compatible with the assigned treatment arm. For patients who underwent surgery during the grace period, we censored clones assigned to the non-operative arm at the time of surgery, whereas we censored non-operative patient clones assigned to the surgery arm at the end of the grace period. We adjusted for informative censoring of clones using inverse probability weighting. We computed weights from an individual’s predicted probability of parathyroidectomy during the grace period, accounting for patient demographics and baseline characteristics associated with treatment and the outcome of interest.(10) After weighting, we compared covariate balance between patients treated with parathyroidectomy and patients managed non-operatively using standardized mean difference (SMD).(13)
We estimated the effect of parathyroidectomy on time to a sustained 50% decline in eGFR using an extended Cox model with time-varying inverse probability weights. Weighted cumulative incidence curves were plotted for both treatment groups. Because guidelines suggest parathyroidectomy for patients with an eGFR <60 ml/min/1.73m2, we used the methods described above to perform subgroup analyses according to pre-treatment eGFR (<60 mL/min/1.73 m2 and ≥60 mL/min/1.73 m2) and age (<60 years, ≥60 years) via stratification to generate hazard ratios for parathyroidectomy vs. non-operative management within each subgroup. We also performed a sensitivity analysis, censoring patients at the time of delayed parathyroidectomy (after the one-year grace period) to determine whether delayed parathyroidectomy may have affected our results.
We queried the VA OMOP tables using MS SQL Server. We conducted all data analyses using the R statistical programming language and its various packages. We assessed two-sided statistical significance and derived confidence intervals at the α=0.05 level.
Role of the Funding Source
The National Institute on Aging and other funders of this work had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
RESULTS
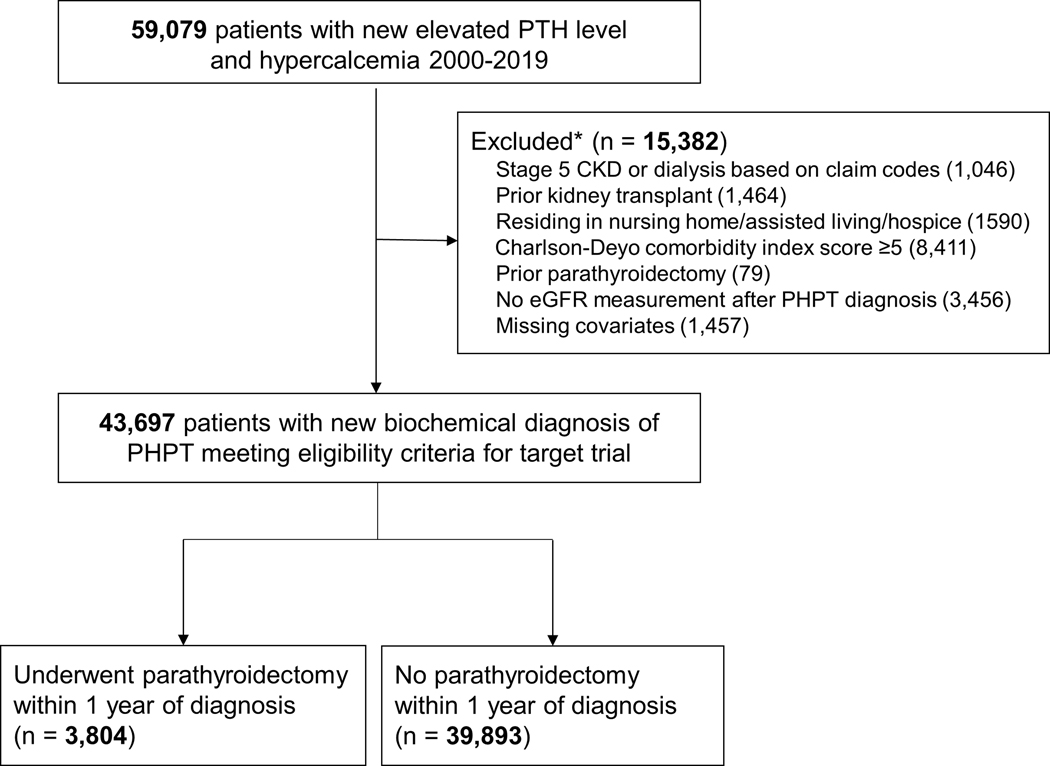
We identified 43,697 patients with a biochemical diagnosis of PHPT who met eligibility criteria for our target trial between January 1, 2000 to December 31, 2019 (Figure 1). A total of 3804 (8.7%) patients were treated with parathyroidectomy and 39,893 (91.3%) were managed non-operatively within one year. Patients treated with parathyroidectomy were younger and more likely to be white and have a lower Charlson-Deyo Comorbidity Index score compared with patients managed non-operatively (Table 2). The mean pre-treatment eGFR was 71.8 mL/min/1.73 m2 in the overall population, 80.2 mL/min/1.73 m2 in the parathyroidectomy group, and 71.0 mL/min/1.73 m2 in the non-operatively managed group. Patients who underwent parathyroidectomy were more likely to have a serum calcium level ≥1 mg/dL above the upper limit of normal and less likely to have risk factors for CKD or to be on ACE inhibitors or ARBs. Baseline characteristics were similar and balanced between treatment groups after inverse probability weighting based on SMD (eFigure 1).
Figure 1.
Flowchart of eligible and included individuals for the emulation of a target trial of parathyroidectomy vs. non-operative management for adults with PHPT.
Table 2.
Baseline characteristics of patients with a new biochemical diagnosis of PHPT (2000–2019).
| Overall cohort | Parathyroidectomy | Non-operatively managed | ||||
|---|---|---|---|---|---|---|
| N | %/SD | N | %/SD | N | %/SD | |
| N | 43697 | 100 | 3804 | 8.7 | 39893 | 91.3 |
| Sex | ||||||
| Female | 5449 | 12.5 | 606 | 15.9 | 4843 | 12.1 |
| Male | 38248 | 87.5 | 3198 | 84.1 | 35050 | 87.9 |
| Age (years - mean, SD) | 66.8 | SD 12.1 | 60.8 | SD 11.3 | 67.4 | SD12.0 |
| Race | ||||||
| White | 29492 | 67.5 | 2785 | 73.2 | 26707 | 66.9 |
| Black | 9677 | 22.1 | 689 | 18.1 | 8988 | 22.5 |
| Other | 603 | 1.4 | 66 | 1.7 | 537 | 1.3 |
| Unknown | 3925 | 9.0 | 264 | 6.9 | 3661 | 9.2 |
| Ethnicity | ||||||
| Not Hispanic or Latino | 39645 | 90.7 | 3488 | 91.7 | 36157 | 90.6 |
| Hispanic or Latino | 1763 | 4.0 | 169 | 4.4 | 1594 | 4.0 |
| Unknown | 2289 | 5.2 | 147 | 3.9 | 2142 | 5.4 |
| Charlson Comorbidity Index | ||||||
| 0 | 13935 | 31.9 | 1807 | 47.5 | 12128 | 30.4 |
| 1 | 9554 | 21.9 | 792 | 20.8 | 8762 | 22.0 |
| 2+ | 20208 | 46.2 | 1205 | 31.7 | 19003 | 47.6 |
| Calcium ≥ 11.3 mg/dL | ||||||
| No | 37838 | 86.6 | 2499 | 65.7 | 35339 | 88.6 |
| Yes | 5859 | 13.4 | 1305 | 34.3 | 4554 | 11.4 |
| eGFR (Mean, SD) | 71.8 | SD 20.6 | 80.2 | SD 19.5 | 71.0 | SD 20.5 |
| Serum Calcium (mg/dL - mean, SD) | 10.8 | SD 0.5 | 11.2 | SD 0.8 | 10.8 | SD 0.5 |
| Parathyroid Hormone (ng/mL, mean, SD) | 115.1 | SD 73.8 | 162.6 | SD 123.3 | 110.5 | SD 65.5 |
| CKD Risk Factors | ||||||
| Diabetes | ||||||
| No | 40500 | 92.7 | 3627 | 95.3 | 36873 | 92.4 |
| Yes | 3197 | 7.3 | 177 | 4.7 | 3020 | 7.6 |
| Hypertension | ||||||
| No | 12476 | 28.6 | 1451 | 38.1 | 11025 | 27.6 |
| Yes | 31221 | 71.4 | 2353 | 61.9 | 28868 | 72.4 |
| BMI | ||||||
| Underweight or normal weight | 8871 | 20.3 | 683 | 18.0 | 8188 | 20.5 |
| Overweight | 16708 | 38.2 | 1522 | 40.0 | 15186 | 38.1 |
| Obese | 15582 | 35.7 | 1344 | 35.3 | 14238 | 35.7 |
| Severely obese | 2536 | 5.8 | 255 | 6.7 | 2281 | 5.7 |
| History of kidney stones | ||||||
| No | 39209 | 89.7 | 3126 | 82.2 | 36083 | 90.4 |
| Yes | 4488 | 10.3 | 678 | 17.8 | 3810 | 9.6 |
| Coronary artery disease | ||||||
| No | 35824 | 82.0 | 3371 | 88.6 | 32453 | 81.4 |
| Yes | 7873 | 18.0 | 433 | 11.4 | 7440 | 18.6 |
| Stroke | ||||||
| No | 42853 | 98.1 | 3758 | 98.8 | 39095 | 98.0 |
| Yes | 844 | 1.9 | 46 | 1.2 | 798 | 2.0 |
| ACE inhibitor or ARB use | ||||||
| No | 26937 | 61.6 | 2660 | 69.9 | 24277 | 60.9 |
| Yes | 16760 | 38.4 | 1144 | 30.1 | 15616 | 39.1 |
Abbreviations: eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; BMI, body mass index; ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker.
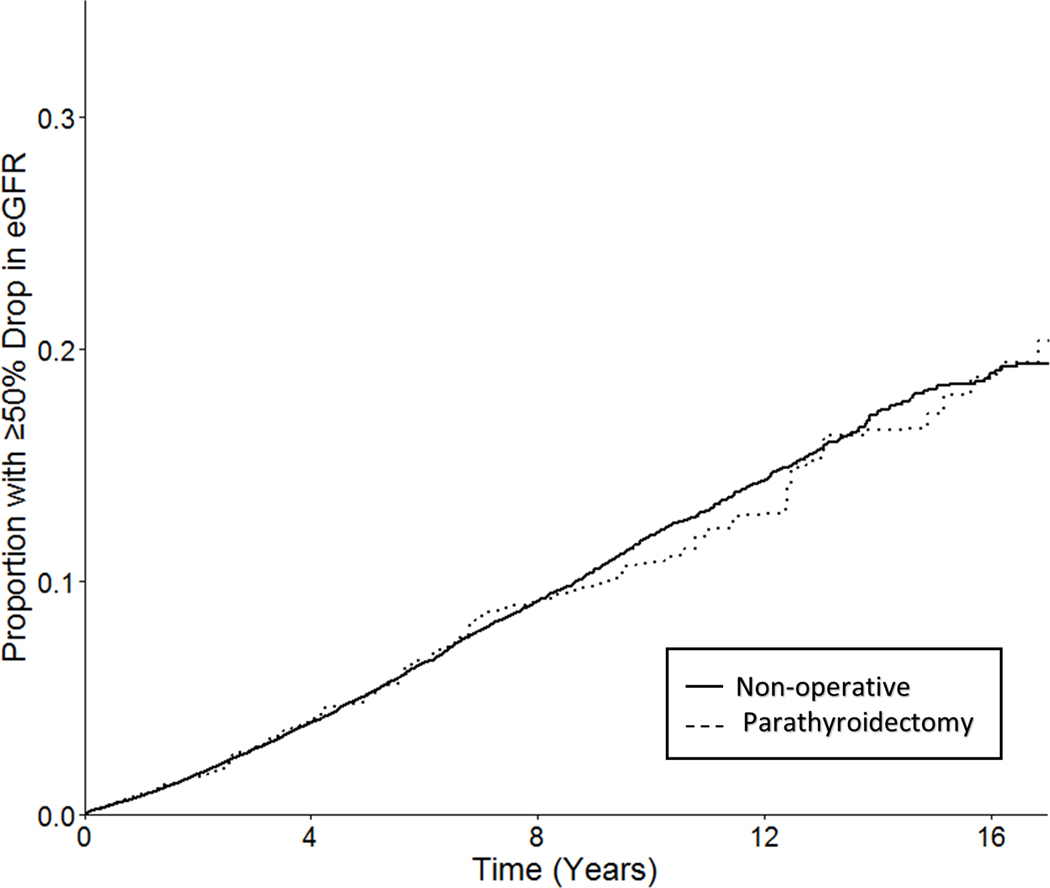
During the study period, serum creatinine measurements were obtained at a similar frequency in patients managed with parathyroidectomy and those managed non-operatively (median 4.5 measurements per year vs. 4.6 per year, respectively). A total of 2928 (6.7%) patients developed a sustained decline in eGFR of 50% or more over a median follow up of 4.9 years, including 263 (6.9%) of 3804 patients managed with parathyroidectomy and 2665 (6.7%) of 39,893 patients managed non-operatively. In the primary analysis, the weighted cumulative incidence of a 50% decline in eGFR was 5.1% at 5 years and 10.8% at 10 years in patients managed with parathyroidectomy, compared with 5.1% at 5 years and 12.0% at 10 years in those managed non-operatively (Figure 2).
Figure 2.
Weighted cumulative incidence curve for target trial emulation.
Weighted cumulative incidence curves demonstrating the probability of remaining free of the primary outcome, a sustained decline in eGFR of at least 50%, among patients managed with parathyroidectomy (green) and non-operative management (red).
Patients with PHPT treated with parathyroidectomy experienced no difference in the adjusted rate of a sustained decline in eGFR of at least 50% (HR 0.98, 95%CI 0.82–1.16). In analyses stratified by pre-treatment kidney function or age, there was no effect of parathyroidectomy on the adjusted rate of a sustained decline in eGFR in patients with eGFR <60 mL/min/1.73 m2 (HR 1.05, 95% CI 0.66–1.68), in patients with eGFR ≥60 mL/min/1.73 m2 (HR 0.96, 95% CI 0.81–1.16), or in patients 60 years or older (HR 1.08, 95% CI 0.87–1.34). Among patients younger than age 60, those treated with parathyroidectomy experienced a lower adjusted rate of a sustained decline in eGFR (HR 0.75, 95%CI 0.59–0.93) compared with those treated with non-operative management.
A total of 2939 (6.7%) patients underwent delayed parathyroidectomy (>1 year after PHPT diagnosis), which occurred a median of 2.4 years after PHPT diagnosis. There was no change in the estimated effect of parathyroidectomy when we censored patients at the time of delayed parathyroidectomy (HR 0.99 [95%CI 0.83–1.18]).
DISCUSSION
In this emulated target trial using observational data, we found that treatment of PHPT with parathyroidectomy resulted in no difference in the development of a sustained decline in eGFR compared with non-operative management. This finding remained consistent in subgroups with varying pre-treatment eGFR and in the subgroup of adults older than age 60. Patients younger than 60 treated with parathyroidectomy had a reduced hazard of a sustained decline in eGFR. Taken together, these findings suggest that parathyroidectomy does not help to preserve kidney function in older adults with PHPT and should not be a primary consideration when making treatment decisions for these individuals.
Few studies have addressed the comparative effectiveness of parathyroidectomy vs. non-operative management in preventing long-term loss of kidney function in patients with PHPT. Small, single-center studies have shown no change or a small decrease in eGFR with short-term follow up after parathyroidectomy, but these studies lacked a non-operative comparator group.(14–18) Other small, prospective studies of PHPT management did not report kidney function as an outcome.(19–21)
The findings of our study add to our understanding of the risks and benefits of parathyroidectomy for PHPT, advance our knowledge of appropriate indications for operative management, and complement the data recently provided in this journal by Pretorius et al. These investigators reported the 10-year morbidity and mortality from the 191-patient Scandinavian Investigation of Primary Hyperparathyroidism (SIPH) randomized controlled trial.(22) Among patients with mild PHPT (serum calcium between 10.4 and 11.2 mg/dL), they found no difference between those treated with parathyroidectomy or observation in the primary endpoint of mortality (HR 1.17, 95%CI 0.43–3.24) or secondary morbidity endpoints, including fractures, cardiovascular events, and kidney stones. A published interim analysis showed no difference in eGFR between treatment groups at 2-year follow up.(23) These results led the authors to conclude that parathyroidectomy does not reduce morbidity or mortality in patients with mild PHPT. However, due to a higher than expected dropout rate (24 out of 95 [25%] in the parathyroidectomy group, 30 out of 96 [31.3%] in the observation group), this study was underpowered for both the primary outcome and secondary outcomes. As a result, the reported hazard ratios had wide confidence intervals that ranged from a significant protective effect to a significant harmful effect of parathyroidectomy. Consequently, we believe the findings of the SIPH trial neither support nor refute the benefits of parathyroidectomy among patients with mild PHPT. Our target trial emulation includes a large number of patients with PHPT, allowing us to compare long-term outcomes and to examine the effects in key subgroups. While a randomized clinical trial can inform treatment decisions for patients with PHPT, a large number of patients and a long time period for follow-up would be needed to power a trial for relevant morbidity outcomes. An additional logistical challenge includes the need to recruit a diverse group of older adults, who are a key demographic in PHPT that are typically not well represented in randomized trials.(24, 25) Until such barriers can be overcome, we believe that target trial emulation using observational data can inform guidelines and patient care.
Although large epidemiological studies are limited, current data suggest that the majority of adults diagnosed with PHPT are older, typically in their 60s and 70s.(26) The findings of our study suggest that older adults with PHPT do not realize the potential benefit of parathyroidectomy for long-term preservation of kidney function. Given parathyroidectomy is associated with higher quality of life and lower risk of fracture among patients of all age groups and frailty categories(27), patients and clinicians should weigh these factors more heavily when deciding whether to proceed with operative management of PHPT in older adults. Current guidelines recommend parathyroidectomy for those with an eGFR less than 60 mL/min/1.73m2,(1) based on a study suggesting eGFR stabilizes in this group following curative parathyroidectomy(27) and the concern that lower kidney function is associated with lower bone mineral density in patients with PHPT.(28) Our results do not support recommendations for parathyroidectomy in patients with eGFR <60 mL/min/1.73m2 for the purpose of preserving kidney function. However, we agree that parathyroidectomy should be considered for potential skeletal benefits among groups at higher risk of fractures, such as those with lower eGFR, based on evidence that parathyroidectomy reduces fracture risk.(27, 29)
Multidisciplinary guidelines recommend parathyroidectomy based on age only for those younger than 50 years, a threshold that has not changed since the original consensus guidelines were published in 1990.(1, 4, 5) Our findings suggest that there may be long-term benefits for preservation of kidney function after parathyroidectomy for younger patients (<60 years of age) with PHPT. This is clinically significant for the following reasons. First, incident CKD is a risk factor for future cardiovascular events and death.(30) Second, kidney function affects drug dosing and contrast administration. Loss of kidney function to an eGFR less than 30 mL/min/1.73m2 can restrict the use of medications for primary cardiovascular prevention, as well as certain antibiotics, chemotherapeutic agents, pain medications, and the use of contrast agents for diagnostic testing. Thus, for younger patients, we propose that a fundamental shift, from a treatment strategy centered on operative intervention following documented and clinically apparent metabolic complications of PHPT to one focused on timely surgical intervention to prevent end-organ damage, could reduce morbidity and adverse health outcomes in patients with PHPT. Given parathyroidectomy is a generally low-risk operation with a high rate of successful PHPT cure that has been shown to be more cost effective than non-operative management,(8, 9) the benefits of preventing adverse effects of PHPT in the long-term, such as loss of kidney function, fractures, and cardiovascular disease,(27, 31, 32) should be weighed against the time-limited perioperative risks associated with an invasive intervention like parathyroidectomy in younger patients with good life expectancy.
Strengths of our study include its long-term follow up of a large and diverse cohort of patients with a proven biochemical diagnosis of PHPT, longitudinal measurements of kidney function, and focus on clinically meaningful kidney outcomes. However, our study has limitations related to the patient population and the use of administrative claims for risk adjustment. The VA patient population was 88% male and likely had higher rates of comorbidity than non-Veteran populations.(33) Patients with PHPT are predominantly female. Given our power to detect an interaction based on sex was limited in this study, we should use caution when generalizing the results of our study to the general PHPT population. In addition, we did not access Medicare or private insurance data to identify patients treated with parathyroidectomy outside of the VHA, but we believe that this did not affect treatment assignment because previous publications have shown little difference in the rate of operative management for PHPT between Veterans with primary VA and dual insurance coverage.(34) Lastly, the observational nature of this study leaves open the possibility of residual confounding; while these data can be used to inform clinical practice, the results should be interpreted with due caution.
CONCLUSION
Emulating a target trial using data from a large cohort of adults with PHPT, we found that early parathyroidectomy had no estimated effect on the development of a sustained decline in eGFR when compared to early non-operative management. Subgroup analyses suggest patients younger than 60 treated with early parathyroidectomy are more likely to preserve long-term kidney function. When participating in shared decision-making for older adults with PHPT, clinicians should not consider parathyroidectomy for potential benefits of preservation of kidney function. For younger patients, clinicians should discuss the potential benefit of parathyroidectomy to reduce the risk of chronic kidney disease and associated complications in adults with PHPT.
Supplementary Material
eFigure 1. Standardized mean differences before and after applying inverse probability weighting. This figure shows the absolute standardized mean differences for each of the 23 baseline covariates in the overall cohort before and after inverse probability weighting. The vertical line indicates a standardized mean difference of 0.1, which corresponds an effect size considered to be very small.(13, 35)
Figure 3.
Forest plot illustrating the relative hazard of a sustained drop in eGFR of at least 50% for parathyroidectomy versus non-operative management in the overall analysis and subgroups according to age at diagnosis and pre-treatment eGFR, with 95% confidence intervals shown as dashed lines.
Acknowledgements:
Dr. Seib and Mr. Furst had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding:
The authors acknowledge funding support from the National Institutes of Health, National Institute on Aging by awards R03AG060097, K76AG068526 (Dr. Seib) and K24AG073615 (Dr. Kurella Tamura), the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases by award K24DK085446 (Dr. Chertow), and a Veterans Affairs Merit Grant I01HX003091 (Drs. Pao, Leppert, Ganesan). Funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Primary Funding Source:
National Institute on Aging
Footnotes
Conflict of Interest Disclosures: The authors have no conflicts of interest relevant to this project. Dr. Seib reported prior consulting for Virtual Incision Corporation. Dr. Suh is a consultant for Medtronic, Prescient Surgical, and RPWB. No other disclosures were reported.
REFERENCES
- 1.Assadipour Y, Zhou H, Kuo EJ, et al. End-organ effects of primary hyperparathyroidism: A population-based study. Surgery. 2019;165(1):99–104. [DOI] [PubMed] [Google Scholar]
- 2.Yu N, Donnan PT, Leese GP. A record linkage study of outcomes in patients with mild primary hyperparathyroidism: The Parathyroid Epidemiology and Audit Research Study (PEARS). Clinical Endocrinology. 2011;75(2):169–76. [DOI] [PubMed] [Google Scholar]
- 3.Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the Management of Asymptomatic Primary Hyperparathyroidism: Summary Statement from the Fourth International Workshop. The Journal of Clinical Endocrinology & Metabolism. 2014;99(10):3561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilezikian JP, Khan AA, Silverberg SJ, et al. Evaluation and management of primary hyperparathyroidism: summary statement and guidelines from the Fifth International Workshop. Journal of Bone and Mineral Research. 2022;37(11):2293–314. [DOI] [PubMed] [Google Scholar]
- 5.Seib CD, Meng T, Suh I, et al. Undertreatment of primary hyperparathyroidism in a privately insured US population: Decreasing utilization of parathyroidectomy despite expanding surgical guidelines. Surgery. 2021;169(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seib C, Suh I, Meng T, et al. Patient Factors Associated with Parathyroidectomy in Older Adults with Primary Hyperparathyroidism. JAMA Surgery. 2021;156(4):334–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ev Elm, Altman DG, Egger M, et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inker LA, Eneanya ND, Coresh J, et al. New creatinine-and cystatin C–based equations to estimate GFR without race. New England Journal of Medicine. 2021;385(19):1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgado C, Baweja M, Crews DC, et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. American Journal of Kidney Diseases. 2022;79(2):268–88.e1. [DOI] [PubMed] [Google Scholar]
- 10.Maringe C, Benitez Majano S, Exarchakou A, et al. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. International journal of epidemiology. 2020;49(5):1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernán MA, Sauer BC, Hernández-Díaz S, et al. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. Journal of clinical epidemiology. 2016;79:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emilsson L, García-Albéniz X, Logan RW, et al. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA oncology. 2018;4(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical power analysis for the behavioral sciences: Routledge; 2013. [Google Scholar]
- 14.Tassone F, Guarnieri A, Castellano E, et al. Parathyroidectomy halts the deterioration of renal function in primary hyperparathyroidism. The Journal of Clinical Endocrinology & Metabolism. 2015;100(8):3069–73. [DOI] [PubMed] [Google Scholar]
- 15.Egan RJ, Dewi F, Arkell R, et al. Does elective parathyroidectomy for primary hyperparathyroidism affect renal function? A prospective cohort study. International Journal of Surgery. 2016;27:138–41. [DOI] [PubMed] [Google Scholar]
- 16.García-Martín F, Guadalix S, García-Boyano F, et al. Does renal function improve after parathyroidectomy in primary hyperparathyroidism? Nefrología (English Edition). 2019;39(2):160–7. [DOI] [PubMed] [Google Scholar]
- 17.ÇALIŞKAN M, KIZILGÜL M, Beysel S, et al. Factors associated with glomerular filtration rate variation in primary hyperparathyroidism after parathyroidectomy. Turkish Journal of Medical Sciences. 2019;49(1):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belli M, Martin RM, Brescia MDEG, et al. Acute and long-term kidney function after parathyroidectomy for primary hyperparathyroidism. PLoS One. 2020;15(12):e0244162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverberg SJ, Shane E, Jacobs TP, et al. A 10-year prospective study of primary hyperparathyroidism with or without parathyroid surgery. New England Journal of Medicine. 1999;341(17):1249–55. [DOI] [PubMed] [Google Scholar]
- 20.Ambrogini E, Cetani F, Cianferotti L, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. The Journal of Clinical Endocrinology & Metabolism. 2007;92(8):3114–21. [DOI] [PubMed] [Google Scholar]
- 21.Rao DS, Phillips ER, Divine GW, et al. Randomized controlled clinical trial of surgery versus no surgery in patients with mild asymptomatic primary hyperparathyroidism. The Journal of Clinical Endocrinology & Metabolism. 2004;89(11):5415–22. [DOI] [PubMed] [Google Scholar]
- 22.Pretorius M, Lundstam K, Heck A, et al. Mortality and Morbidity in Mild Primary Hyperparathyroidism: Results from A 10 Year Prospective Randomised Controlled Trial Of Parathyroidectomy vs Observation. Annals of Internal Medicine. 2022;175(6):812–9. [DOI] [PubMed] [Google Scholar]
- 23.Bollerslev J, Jansson S, Mollerup CL, et al. Medical Observation, Compared with Parathyroidectomy, for Asymptomatic Primary Hyperparathyroidism: A Prospective, Randomized Trial. The Journal of Clinical Endocrinology & Metabolism. 2007;92(5):1687–92. [DOI] [PubMed] [Google Scholar]
- 24.Sedrak MS, Freedman RA, Cohen HJ, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA: a cancer journal for clinicians. 2021;71(1):78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cherubini A, Oristrell J, Pla X, et al. The Persistent Exclusion of Older Patients From Ongoing Clinical Trials Regarding Heart Failure. Archives of Internal Medicine. 2011;171(6):550–6. [DOI] [PubMed] [Google Scholar]
- 26.Yeh MW, Ituarte PHG, Zhou HC, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. The Journal of Clinical Endocrinology & Metabolism. 2013;98(3):1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seib CD, Meng T, Suh I, et al. Risk of Fracture Among Older Adults With Primary Hyperparathyroidism Receiving Parathyroidectomy vs Nonoperative Management. JAMA Internal Medicine. 2022;182(1):10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gianotti L, Tassone F, Cesario F, et al. A slight decrease in renal function further impairs bone mineral density in primary hyperparathyroidism. The Journal of Clinical Endocrinology & Metabolism. 2006;91(8):3011–6. [DOI] [PubMed] [Google Scholar]
- 29.Yeh MW, Zhou H, Adams AL, et al. The Relationship of Parathyroidectomy and Bisphosphonates With Fracture Risk in Primary Hyperparathyroidism: An Observational Study. Annals of Internal Medicine. 2016;164(11):715–23. [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Chertow GM, Fan D, et al. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. New England Journal of Medicine. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 31.Seib CD, Meng T, Cisco RM, et al. Adverse Cardiovascular Outcomes Among Older Adults with Primary Hyperparathyroidism Treated with Parathyroidectomy vs. non-operative Management. Annals of Surgery. 2022: 10.1097/SLA.0000000000005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Axelsson KF, Wallander M, Johansson H, et al. Analysis of Comorbidities, Clinical Outcomes, and Parathyroidectomy in Adults With Primary Hyperparathyroidism. JAMA Network Open. 2022;5(6):e2215396-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazis LE, Ren XS, Lee A, et al. Health status in VA patients: results from the Veterans Health Study. American Journal of Medical Quality. 1999;14(1):28–38. [DOI] [PubMed] [Google Scholar]
- 34.Alore EA, Suliburk JW, Ramsey DJ, et al. Diagnosis and Management of Primary Hyperparathyroidism Across the Veterans Affairs Health Care System. JAMA Internal Medicine. 2019;179(9):1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28(25):3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Standardized mean differences before and after applying inverse probability weighting. This figure shows the absolute standardized mean differences for each of the 23 baseline covariates in the overall cohort before and after inverse probability weighting. The vertical line indicates a standardized mean difference of 0.1, which corresponds an effect size considered to be very small.(13, 35)