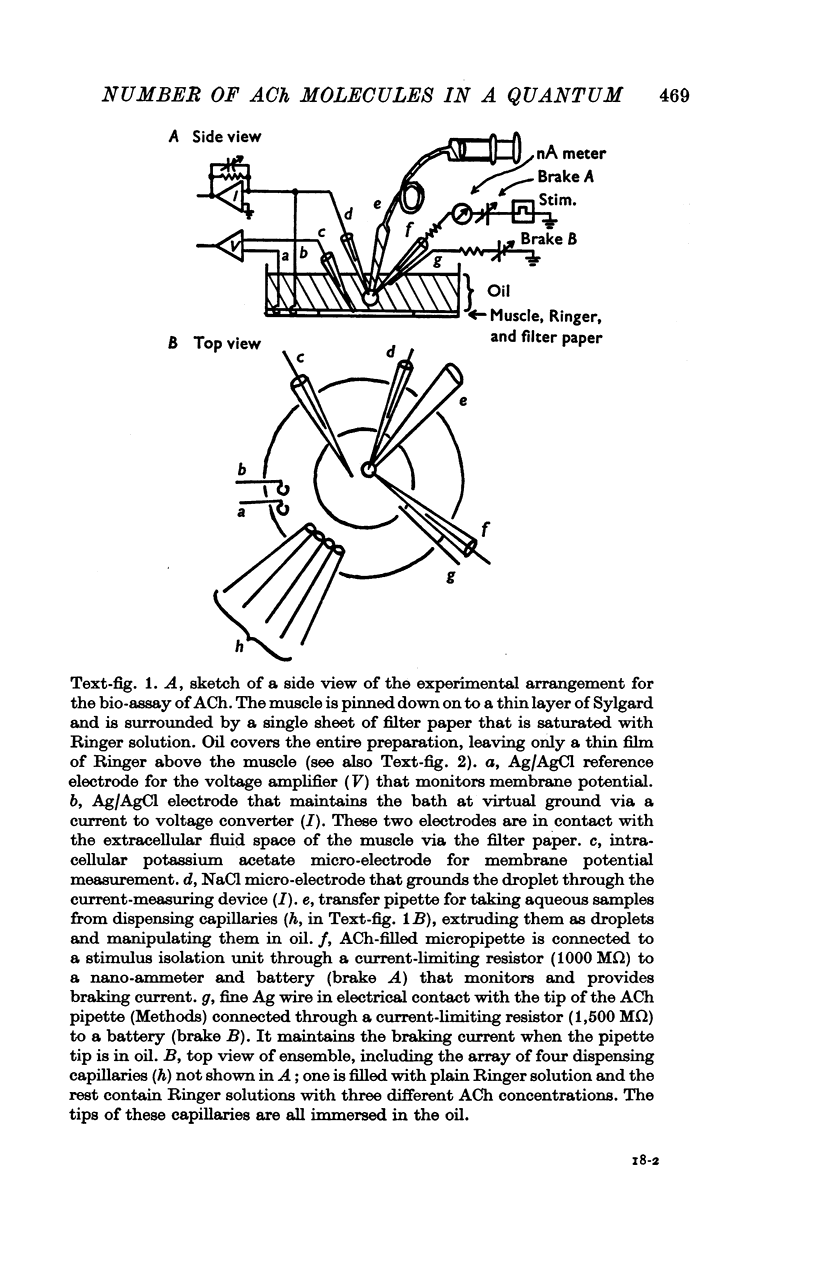
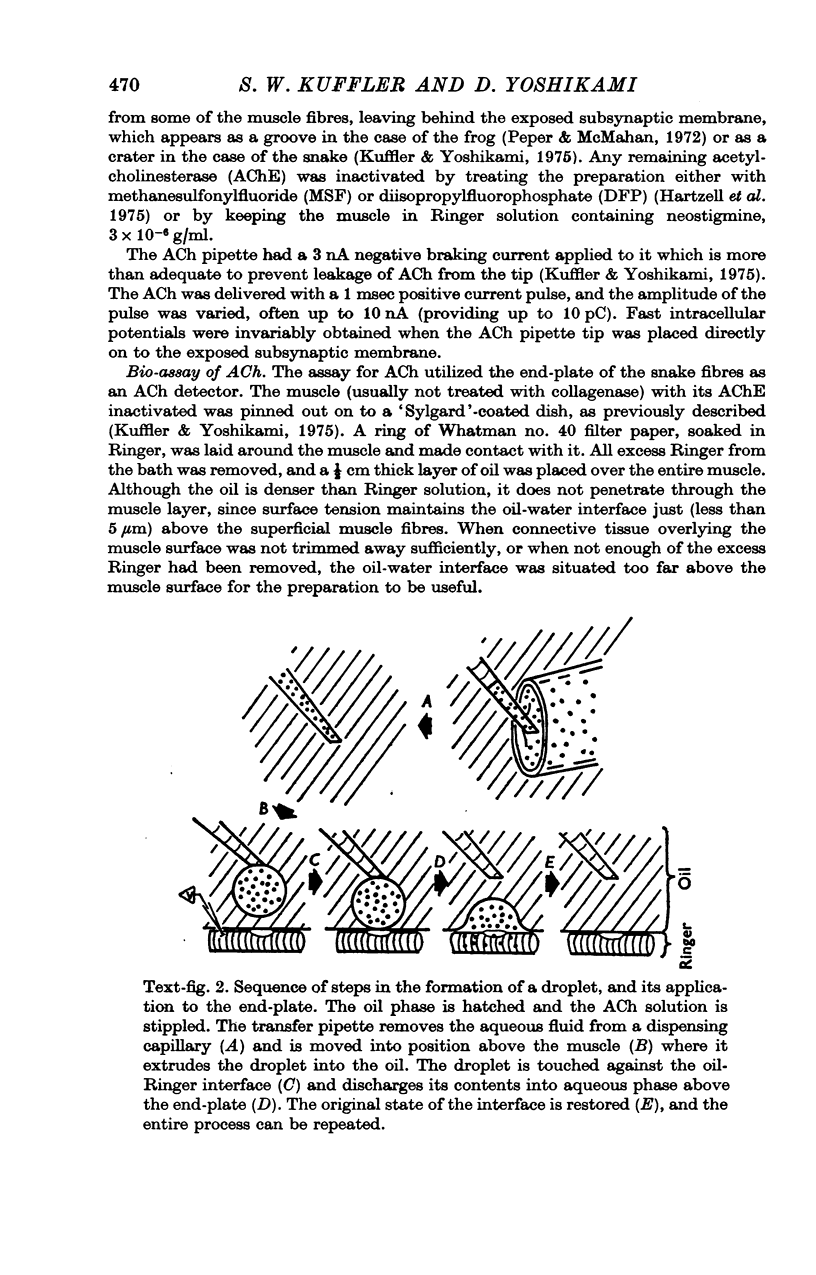
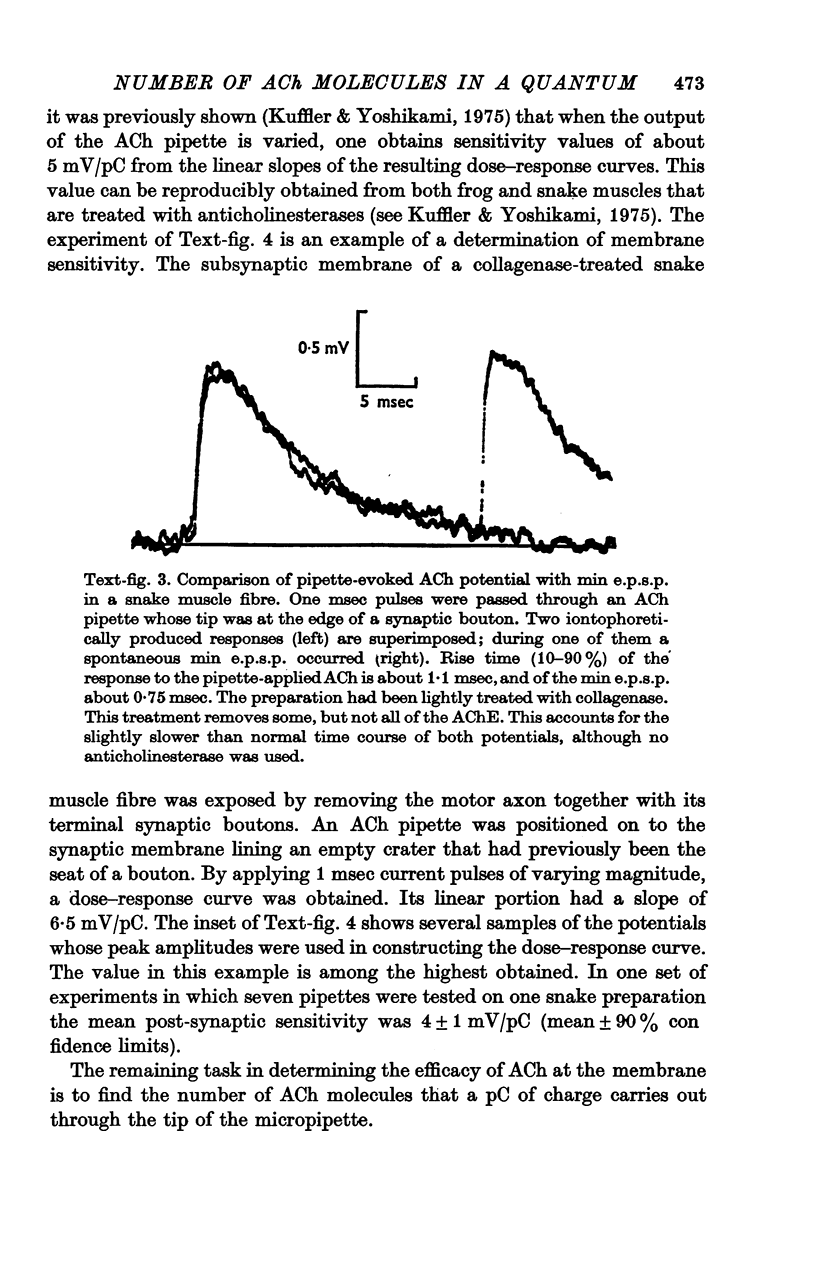
Abstract
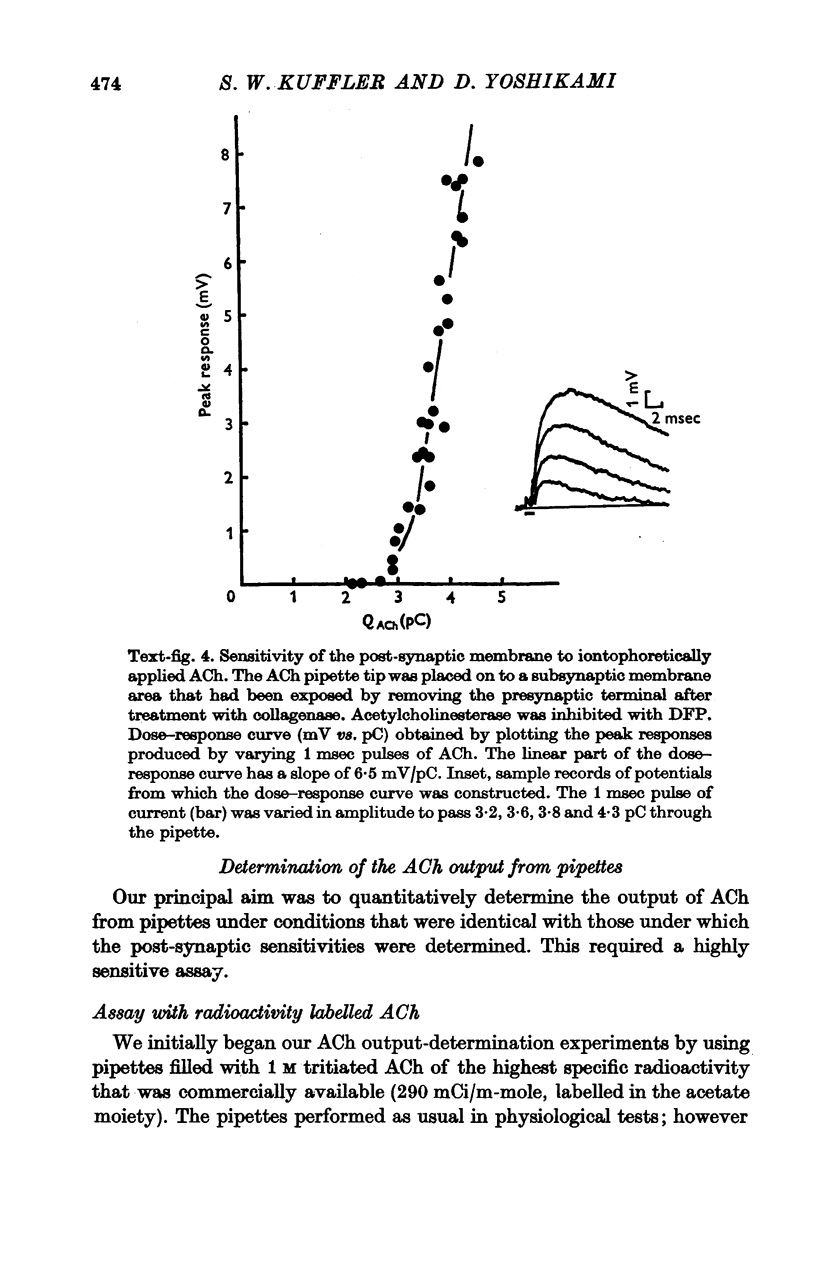
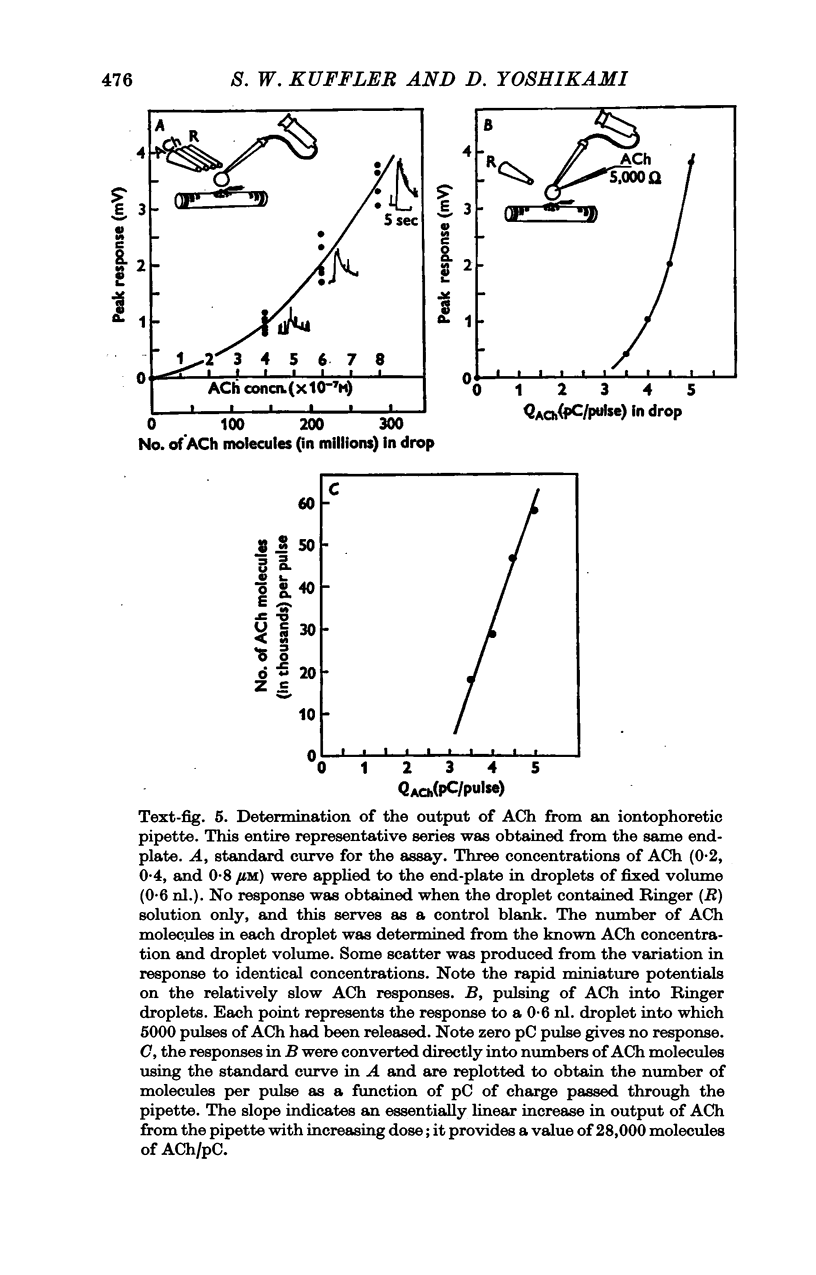
1. The sensitivity of the subsynaptic membrane of twitch muscles of the frog and snake to iontophoretically applied acetylcholine (ACh) was determined. Optimal placement of ACh micropipettes on to the postsynaptic membrane resulted in potentials that were similar, though not identical, to the miniature excitatory post-synaptic potentials (min e.p.s.p.s). A sensitive bio-assay was developed to measure the output of ACh from micropipettes; this allowed an estimate to be made of the upper limit of the number of ACh molecules in a quantum of transmitter that is released from the nerve to produce a min e.p.s.p. 2. The assay to calibrate the output of ACh from micropipettes used the end-plate of the snake muscle as an ACh concentration detector. The end-plate was situated within a few mum of an oil-water interface, and a 0-6 nl. droplet of Ringer solution containing a known concentration of ACh (1 muM or less) was formed in the oil phase. The droplet was brought to the interface and, upon touching it, discharged its contents into the Ringer phase immediately above the end-plate. This resulted in a membrane depolarization that was recorded with an intracellular microelectrode. By applying droplets containing various known ACh concentrations a standard curve was constructed. To measure the ACh output of micropipettes a 0-6 nl. droplet of Ringer solution was suspended in the oil. The ACh pipette tip was inserted into the droplet and several thousand pulses of ACh were then delivered. The ACh content of the test droplet was measured by comparing its effectiveness in depolarizing the end-plate with the standard curve. In this manner the number of ACh molecules released in a single pulse was determined as a function of charge passed through the pipette. The output of ACh was linear and an average of 30,000 molecules of ACh were released per pC. 3. The sensitivity of the subsynaptic membrane to iontophoretically applied ACh, using the linear slopes of dose-response curves, in preparations from frog and snake treated with anticholinesterases was usually about 5 mV/pC. It follows that 6000 molecules of ACh are sufficient to produce a depolarization of 1 mV in the subsynaptic membrane. 4. The mean min e.p.s.p.s of muscle fibres treated with anticholinesterase range from 1 to 3 mV. Since the ACh released from an iontophoretic pipette is less effective than the same amount released from the nerve, it is concluded that a quantum of transmitter consists of less than 10,000 molecules of ACh. 5. It is calculated that for each molecule of ACh released in a quantum there results a minimum net flow of 3000 univalent ions across the synaptic membrane.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A., Wieckowski J., Chiu T. H. Cholinergic receptor molecules and cholinesterase molecules at mouse skeletal muscle junctions. Nature. 1971 Nov 26;234(5326):207–209. doi: 10.1038/234207a0. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Harris A. J., Kuffler S. W. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- Fertuck H. C., Salpeter M. M. Localization of acetylcholine receptor by 125I-labeled alpha-bungarotoxin binding at mouse motor endplates. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1376–1378. doi: 10.1073/pnas.71.4.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I. Microphysiology of vertebrate neuromuscular transmission. Physiol Rev. 1973 Jul;53(3):674–723. doi: 10.1152/physrev.1973.53.3.674. [DOI] [PubMed] [Google Scholar]
- Hubbard J. I., Wilson D. F. Neuromuscular transmission in a mammalian preparation in the absence of blocking drugs and the effect of D-tubocurarine. J Physiol. 1973 Jan;228(2):307–325. doi: 10.1113/jphysiol.1973.sp010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., MILEDI R. Acetylcholine in mammalian neuromuscular transmission. Nature. 1958 Sep 20;182(4638):805–806. doi: 10.1038/182805b0. [DOI] [PubMed] [Google Scholar]
- McMahan U. J., Kuffler S. W. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc R Soc Lond B Biol Sci. 1971 Apr 27;177(1049):485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- Sheridan M. N., Whittaker V. P., Israël M. The subcellular fractionation of the electric organ of Torpedo. Z Zellforsch Mikrosk Anat. 1966;74(3):293–307. [PubMed] [Google Scholar]