Abstract
Background
Serum cystatin C (Scys) has been proposed as a potential replacement for serum creatinine (Scr) in glomerular filtration rate (GFR) estimation. We report development and evaluation of GFR estimating equations using Scys alone and Scys, Scr or both with demographic variables.
Study Design
Test of diagnostic accuracy.
Setting and Participants
Participants screened for three chronic kidney disease (CKD) studies in the US (n=2980) and a clinical population in Paris, France (n=438)
Reference Test
Measured GFR (mGFR).
Index Test
Estimated GFR using the four new equations based on Scys alone, Scys, Scr or both with age, sex and race. New equations were developed using regression with log GFR as the outcome in 2/3 data from US studies. Internal validation was performed in remaining 1/3 of data from US CKD studies; external validation was performed in the Paris study.
Measurements
GFR was measured using urinary clearance of 125I-iothalamate in the US studies and chromium-ethylenediaminetetraacetate (51Cr-EDTA) in the Paris study. Scys was measured by Dade Behring assay, standardized Scr.
Results
Mean mGFR, Scr and Scys were 48 (5th–95th percentile 15–95) ml/min/1.73m2 2.1 mg/dL and 1.8 mg/L respectively. For the new equations, the coefficients for age, sex and race were significant in the equation with Scys but 2 to 4 fold smaller than in the equation with Scr. Measures of performance among new equations were consistent across development, internal and external validation datasets. Percent of eGFR within 30% of mGFR for equations based on Scys alone, Scys, Scr or both with age, sex and race were 81, 83, 85, and 89%, respectively. The equation using Scys alone yields estimates with small biases in age, sex and race subgroups, which are improved in equations including these variables.
Limitations
Study population composed mainly of patients with CKD.
Conclusions
Scys alone provides GFR estimates that are nearly as accurate as Scr adjusted for age, sex and race thus providing an alternative GFR estimate that is not linked to muscle mass. An equation including Scys in combination with Scr, age, sex and race provide most accurate estimates.
Keywords: creatinine, cystatin, diagnostic tests, accuracy, bias, precision, GFR estimating equations
Glomerular filtration rate (GFR) is an important indicator of kidney function, critical for detection, evaluation and management of chronic kidney disease (CKD). GFR cannot be practically measured for routine clinical or research purposes and therefore, serum creatinine is often used to estimate GFR. Several factors affect the level of serum creatinine other than GFR, including its generation from muscle metabolism. GFR estimating equations, such as the Modification of Diet in Renal Disease (MDRD) Study equation, include age, sex, and race to account for the average differences in muscle mass among subgroups; however, the magnitude of the association of muscle mass with age, sex, and race vary among populations, compromising the generalizability of the equations. Furthermore, incorporation of age, sex and race in the estimating equation does not account for variation in creatinine generation due to diet or other clinical conditions, such as illnesses complicated by malnutrition, inflammation or deconditioning, that also affect muscle mass. These other causes of creatinine generation lead to imprecision in the estimates.1, 2
Cystatin C is being considered as a potential replacement for serum creatinine as a filtration marker. Most studies show that serum levels of cystatin C (Scys) are more closely correlated with GFR than serum creatinine (Scr); however, the few studies that have compared serum cystatin C to estimates based on serum creatinine, age, sex and race have shown them to be comparable.3–5 No studies have examined whether the addition of age, sex, race and creatinine to an equation based on cystatin C would improve GFR estimates based on cystatin alone.
The aim of this study was to develop and compare the accuracy of a GFR estimating equation that includes only cystatin C to equations that include cystatin C, creatinine or both adjusted for age, sex and race.
Methods
Sources of Data
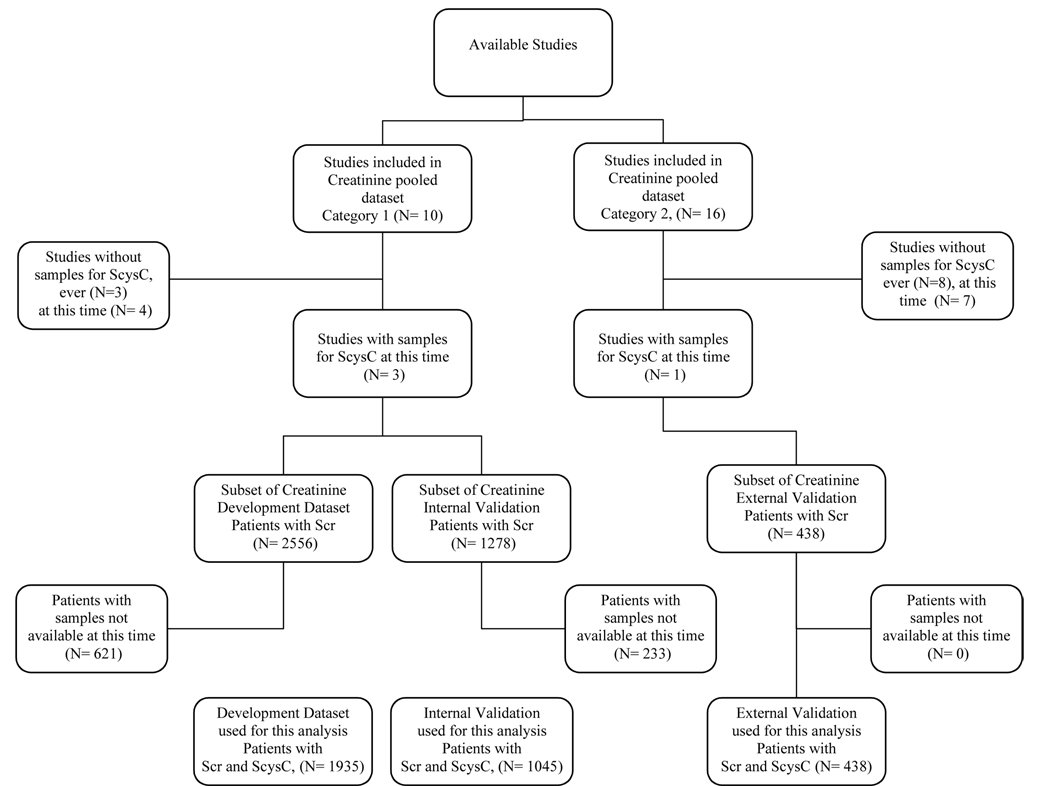
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) is a research group formed to develop and validate improved estimating equations for GFR by pooling data from research studies and clinical populations (hereafter referred to as “studies”). (See appendix text and appendix figure for description of datasets included in CKD-EPI). The current analysis is based on a pooled individual-level patient data from the Modification of Diet in Renal Disease (MDRD) Study, African American Study of Kidney Disease (AASK), Collaborative Study Group (CSG), and a clinical population in Paris, France. Each study has been previously described.1, 6–10 The first three were used for model development and internal validation, and the fourth study was used for external validation.
Appendix Figure. Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) Datasets.
Figure shows allocation of studies to category 1 (development and interval validation) and category 2 (external validation) datasets, and availability of serum samples for cystatin C at the time of this analysis. ScysC, serum cystatin C; Scr, serum creatinine.
Measurements
GFR was measured as four period urinary clearance of 125I-iothalamate in the MDRD Study, AASK and CSG and as five periods of urinary clearance of chromium ethylenediaminetetraacetate (51Cr-EDTA) in Paris. Comparisons of 125I-iothalamate and 51Cr-EDTA clearances to urinary clearance of inulin, the reference standard for GFR measurements, demonstrate high correlation.10–12 Serum creatinine assays were calibrated to standardized serum creatinine values at the Cleveland Clinic Research Laboratory (CCRL). The results of the calibration procedure for the MDRD Study, AASK and CSG have been previously described.13, 14 Calibration of the Paris dataset was performed similarly using 215 frozen specimens measured at CCRL. Samples for all four studies had been frozen at −70°C until 2005 to 2006, when serum cystatin C was measured at the CCRL using a particle-enhanced immuno-nephelometric assay (N Latex Cystatin C, Dade Behring, IL). With a range of 0.23–7.25 mg/l (17.2–543.0 nmol/L), this assay is currently the most precise automated assay across the clinical concentration range.15 The inter-assay coefficients of variation (CV) for the assay were 5.05% and 4.87% at mean concentrations of 0.97 and 1.90 mg/L (72.7 and 142.3 nmol/L), respectively. Serum cystatin C has been reported as robust to multiple freeze-thaw cycles.16
Model Development and Evaluation
We developed new models in the development dataset (n=1935), assessed stability of new models in the internal validation dataset (n=1045), and compared model performance in the external validation dataset (n= 438) (appendix figure). To improve the precision and generalizability of the final estimating equations, we used data from the total study population (n=3134) to revise the regression coefficients in new models.
Models were developed using least squares linear regression. We restricted variables in model development to serum cystatin C, serum creatinine, age, sex, and race. As in the MDRD Study equation, serum creatinine, serum cystatin C and GFR were log transformed to capture the multiplicative relationship between GFR and the level of the filtration marker and to equalize the variance across the range of GFR. Age was also log transformed to be consistent with the form used in the MDRD Study equation. Race was defined as “Black” or “other” and was assigned as in the individual studies. GFR was adjusted for body surface area (BSA) as ml/min/1.73 m2.17 In equations that included age, sex and race, all variables were initially included but were maintained only for a P value of less than 0.001. For optimal comparisons between equations based on serum creatinine and cystatin C, a model with serum creatinine, age, sex and race was developed (equivalent to “refitting” the coefficients from the MDRD Study equation). GFR was also estimated using the MDRD Study equation and as the average of the estimates from model that used cystatin C alone and the MDRD Study equation. For these computations, we used the MDRD Study equation re-expressed for use with the serum creatinine values standardized to isotope dilution mass spectroscopy (IDMS) (GFR = 175 × standardized Scr −1.154 × age−0.203 × 1.212 [if black] × 0.742 [if female]).1
Statistical analyses
Measured (mGFR) and estimated (eGFR) were compared for each patient graphically by plotting mGFR and the difference (mGFR-eGFR) against eGFR. Bias was expressed as the difference (mGFR-eGFR) and percent difference [(mGFR-eGFR) / mGFR*100]), with positive values indicating lower eGFR than mGFR (underestimation). Precision was expressed as inter-quartile range (IQR) for the differences and root mean square error (RMSE) calculated on the logarithmic scale. Accuracy was expressed as the percent of eGFR within 30% of mGFR (P30). A difference in the P30 between equations reflects a difference in the magnitude of outliers. Accuracy of GFR estimates to within 30% of measured GFR has been used as a benchmark for evaluation of GFR estimates for use in clinical practice.18, 19 Percent difference, RMSE and P30 account for the expectation that the absolute magnitudes of errors increase proportionately to the level of GFR level; however, these measures overemphasize errors at the lower GFR range. Bias was not shown for the development dataset since it is close to zero for equations evaluated in the population in which they were developed. Confidence intervals were computed by bootstrap methods (2000 bootstraps) for difference and percent difference, interquartile ranges and RMSE, and by using the binomial approximation to estimate standard errors for the method for accuracy (P30).20 We selected RMSE as the primary measure of model fit in the development phase and as the primary measure of model validation in the internal and external validation phases. We evaluated performance for all patients and for subgroups defined by age, sex and race. Differences among equations in their performance were determined by examination of non-overlapping confidence intervals
Analyses were computed using R (Version 2, Free Software Foundation, Inc., Boston, MA) and SAS software (version, 9.1, Cary, NC). Smoothed estimates of the mean in the figures were created using the lowess function in R.
Role of the Funding Source
CKD-EPI is funded by grants from the National Institute of Diabetes, Digestive and Kidney Disease (NIDDK) as part of a cooperative agreement in which the NIDDK has substantial involvement in the design of the study and the collection, analysis, and interpretation of the data. The NIDDK was not required to approve publication of the finished manuscript. The institutional review boards of all participating institutions approved the study.
Results
Clinical Characteristics
Table 1 summarizes clinical characteristics by study, for the development, internal and external validation datasets, as well as for the overall population. All patients were considered to have chronic kidney disease. Mean GFR (5th –95th percentile) was 48 (15–95) ml/min/1.73m2 [0.8(0.3–1.6) ml/s/1.73m2]. The mean (SD) serum concentrations of creatinine and cystatin C were 2.1 (1.1) mg/dL [186(97) umol/L] and 1.8 (0.8) mg/L [135(60) nmol/L], respectively, with a correlation between them of 0.85.
Table 1.
Study and clinical characteristics
| Development & Internal Validation Studies |
External Validation (Paris) | Total Sample | |||||
|---|---|---|---|---|---|---|---|
| MDRD | AASK | CSG | Development | Internal Validation | |||
| Study Characteristics | |||||||
| Type | RCT | RCT | RCT | - | - | CP | - |
| Year | 1989–1992 | 1995–1998 | 1987–1992 | - | - | 2004–2005 | - |
| Filtration marker | iothalamate | iothalamate | iothalamate | EDTA | |||
| Clearance method | Urinary | Urinary | Urinary | Urinary | |||
| Population characteristics | |||||||
| Sample size | 1047 | 1647 | 286 | 1935 | 1045 | 438 | 3418 |
| Age, years | 52 (13) | 54 (10) | 34 (8) | 51 (13) | 51 (12) | 59 (15) | 52 (13) |
| Female | 39% | 36% | 47% | 37% | 40% | 29% | 37% |
| Black | 10% | 100% | 8% | 61% | 57% | 8% | 53% |
| White | 83% | 0% | 91% | 37% | 40% | 79% | 43% |
| Other | 7% | 0% | 1% | 2% | 3% | 13% | 4% |
| Diabetes | 6% | 0% | 100% | 12% | 11% | 22% | 13% |
| Height, m | 171 (10) | 171 (10) | 169 (9) | 171 (10) | 171 (10) | 168 (9) | 171 (10) |
| Weight, Kg | 79(16) | 90(21) | 73(14) | 85(20) | 83(20) | 74(16) | 83(20) |
| Body Mass Index, kg/m2 | 27(4) | 31(7) | 25(5) | 29(6) | 29(6) | 26(5) | 29(6) |
| Body Surface Area, m2 | 1.9(0.2) | 2.0 (0.2) | 1.8(0.2) | 2.0 (0.2) | 1.9(0.2) | 1.8(0.2) | 1.9 (0.2) |
| Albumin, g/dL | 4(0.4) | 4.3(0.4) | 3.7(0.5) | 4.1(0.4) | 4.1(0.4) | 4.1(0.5) | 4.1(0.4) |
| GFR, ml/min/1.73m2 | 33 (14) | 57 (23) | 75 (33) | 51 (26) | 50 (26) | 34 (17) | 48 (25) |
| (5th–95th %ile) | 13–57 | 22–98 | 24–134 | 15–98 | 16–95 | 11–66 | 15–95 |
| Standardized Serum Creatinine, mg/dL | 2.5 (1.1) | 1.9(0.9) | 1.4(0.6) | 2.1(1.0) | 2(1.0) | 2.5(1.2) | 2.1(1.1) |
| Serum Cystatin, mg/L | 2.2 (0.8) | 1.5 (0.7) | 1.4 (0.7) | 1.8 (0.8) | 1.8 (0.8) | 2.2 (0.8) | 1.8 (0.8) |
MDRD Study, Modification of Diet in Renal Disease Study; AASK, African American Study of Kidney Diseases and Hypertension; CSG, Collaborative Study Group: Captopril in Diabetic Nephropathy Study; RCT, randomized clinical trial; Scr, serum creatinine; SD, standard deviation; GFR is measured in ml/min per 1.73 m2; Mean (SD) reported for continuous data ; Percents for dichotomous data. To convert albumin from g/dL to g/L, multiply by 10. To convert GFR from mL/min/1.73 m2 to mL/sec per 1.73 m2, multiply by 0.01666. To convert serum creatinine from mg/dL to umol/L, multiply by 88.4. To convert cystatin C from mg/L to nmol/L, multiply by 74.9‥
Equation Development
Table 2 shows the coefficients and performance of the six equations in the development dataset. Exploration of a quadratic form of log (cystatin C) did not yield better results than the simple logarithmic transformation and is not reported here. The coefficients for the variables in the equation including creatinine, age, sex and race are similar to those in the MDRD Study equation. When included in the equation with cystatin C, the age, sex and race variables were significant at a p-value of <0.001; however, their estimated coefficients had magnitudes were estimated at 2- to 4-fold smaller than for the equation with serum creatinine. The coefficients for serum cystatin C and creatinine were approximately 2-fold smaller and comparable to each other when included in the same equation than when included in separate equations.
Table 2.
Coefficients (standard errors) and performance of the models developed in the development dataset
| Model | Variables |
Model Fit |
||||||
|---|---|---|---|---|---|---|---|---|
| intercept | logCys | logScr | logAge | Female | Black | RMSE | R2 | |
| (95% CI)* | (95% CI) | |||||||
| 1. Cystatin | 4.34 | −1.18 | 0.237 | 0.817 | ||||
| (0.01) | (0.01) | (0.227, 0.248) | (0.800,0.833) | |||||
| 2. Cystatin, age, sex, race | 4.78 | −1.15 | −0.12 | −0.07 | 0.06 | 0.232 | 0.824 | |
| (0.08) | (0.01) | (0.02) | (0.01) | (0.01) | (0.222,0.243) | (0.808,0.840) | ||
| 3. Creatinine | 4.38 | −1.10 | 0.282 | 0.742 | ||||
| (0.01) | (0.01) | (0.271,0.292) | (0.720,0.764) | |||||
| 4. Creatinine, age, sex, race | 5.40 | −1.12 | −0.27 | −0.26 | 0.20 | 0.228 | 0.831 | |
| (0.07) | (0.01) | (0.02) | (0.01) | (0.01) | (0.217,0.239) | (0.813,0.848) | ||
| 5. Creatinine, Cystatin | 4.39 | −0.82 | −0.41 | 0.220 | 0.843 | |||
| (0.01) | (0.02) | (0.02) | (0.210,0.230) | (0.827,0.857) | ||||
| 6. Creatinine, Cystatin, age, sex, race | 5.13 | −0.60 | −0.62 | −0.19 | −0.17 | 0.11 | 0.199 | 0.872 |
| (0.07) | (0.02) | (0.02) | (0.02) | (0.01) | (0.01) | (0.188,0.209) | (0.857,0.886) | |
| 7. MDRD Study equation | 5.16 | −1.15 | −0.203 | −0.298 | 0.19 | 0.231 | 0.829 | |
| (0.220,0.242) | (0.811,0.846) | |||||||
Scr, serum creatinine; Cys, serum Cystatin C; RMSE, root mean square error; CI, confidence interval; MDRD Study, Modification of Diet in Renal Disease Study.
The 95% confidence interval around the estimates of bias, IQR, P30 and RMSE provides the range of values which is likely to include the parameter in 95% of circumstances. Comparison of confidence intervals around any metric for two equations provides information as to difference between them. If the two metrics were the same, the chance that the upper and lower bounds of the confidence intervals would not overlap is less than 5%. Therefore, it can be concluded that they are statistically significantly different
The model fit was better in the equation that included only cystatin C than in the equation using only creatinine. Addition of age, sex, and race as predictor variables substantially improved the fit of the model for creatinine (p-value < 0.001) and slightly improved the model for cystatin C (p-value < 0.001). The best-fitting equation included both creatinine and cystatin with age, sex and race. We elected to carry forward the equations that used cystatin C alone; cystatin C with age, sex and race; creatinine, age, sex and race; and finally cystatin C and creatinine with age, sex and race, for further evaluation in the internal and external validation datasets.
Performance in validation datasets
Table 3 shows the performance in the internal and external validation datasets of the four new equations, the MDRD Study equation, and the average of the cystatin C alone and MDRD Study equations. In the internal validation dataset, RMSE of all equations was similar to that in the development dataset, with the serum creatinine based equations showing more stability between the development and interval validation datasets than the cystatin C based equations.
Table 3.
Bias, Precision and Accuracy in Validation for Different Models for Estimating GFR
| Model | Difference | Percent Difference | ||||
|---|---|---|---|---|---|---|
| Median | IQR** | Median | IQR* | P30 | RMSE | |
| (95% CI)* | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Internal Validation (n=1045) | ||||||
| 1. Cystatin C | 0 (−1,1) | 13 (12,14) | 0 (−2,2) | 29 (27,31) | 82 (81,83) | 0.257 (0.238, 0.275) |
| 2. Cystatin C, age, sex and race | 0 (0,1) | 12 (11,13) | 1(−1,3) | 28 (26,29) | 84 (83,85) | 0.250 (0.231, 0.267) |
| 3. Creatinine, age, sex, and race | 0 (−1,1) | 10 (9,11) | 1 (−1,2) | 26 (24,28) | 85 (84,86) | 0.226 (0.209, 0.244) |
| 4. Cysatin C and creatinine, age, sex and race | 0 (0,1) | 10 (9,11) | 0 (−1,2) | 23 (21,24) | 89 (88,90) | 0.200 (0.184, 0.215) |
| 5. MDRD Study equation | 1 (0,1) | 10 (9,11) | 2 (1,3) | 26 (24,28) | 85 (84,86) | 0.226 (0.210, 0.244) |
| 6. Average #1 & # 5 eGFR Scys & MDRD | 1 (0,1) | 10 (9,11) | 2 (0,3) | 23 (21,24) | 88 (87,89) | 0.204 (0.190, 0.220) |
| External Validation (n=438) | ||||||
| 1. Cystatin C | −3 (−3,−2) | 8 (7, 9) | −10 (−13,−7) | 31 (28, 36) | 73 (72,74) | 0.264 (0.239, 0.289) |
| 2. Cystatin C, age, sex and race | −2 (−2,−1) | 8 (7, 9) | −6 (−8,−4) | 30 (26, 32) | 79 (78,80) | 0.248 (0.223, 0.271) |
| 3. Creatinine, age, sex, and race | 2 (1,3) | 8 (7, 9) | 7 (4,9) | 25 (22, 29) | 84 (83,85) | 0.229 (0.210, 0.247) |
| 4. Cysatin C and creatinine, age, sex and race | 0 (0,1) | 7 (6, 8) | 1 (−1,3) | 22 (20, 25) | 90 (89,91) | 0.193 (0.174, 0.211) |
| 5. MDRD Study equation | 2 (2,3) | 8 (7, 9) | 8 (6,11) | 24 (22, 27) | 85 (84,86) | 0.231 (0.213, 0.249) |
| 6. Average #1 & # 5 eGFR Scys & MDRD | 0 (−1,1) | 7 (6, 8) | 1 (−2,3) | 24 (22, 27) | 90 (89,91) | 0.196 (0.176, 0.217) |
Difference is calculated as measured GFR-estimated GFR. Units are in mL/min per 1.73 m2. To convert GFR from mL/min/1.73 m2 to mL/sec per 1.73 m2, multiply by 0.01666. Percent difference is calculated as (measured GFR-estimated GFR)/measured GFR, and units are in percent. Median values measure bias and the inter-quartile ranges measure precision. P30 is calculated as the percentage of estimated GFR within 30% of measured GFR. IQR, inter-quartile range; RMSE, root mean square error; CI, confidence interval; MDRD Study, Modification of Diet in Renal Disease Study; eGFR, estimated glomerular filtration rate; Scys, serum cystatin C.
The 95% confidence interval around the estimates of bias, IQR, P30 and RMSE provides the range of values which is likely to include the parameter in 95% of circumstances. Comparison of confidence intervals around any metric for two equations provides information as to difference between them. If the two metrics were the same, the chance that the upper and lower bounds of the confidence intervals would not overlap is less than 5%. Therefore, it can be concluded that they are statistically significantly different.
IQR is the width of the 25th to 75th percentile. For example, the 25th to 75th of the difference is −5.4 and 7.2, for an IQR of 13. For the percent difference, 25th to 75th is −13.8% and 14.8% which leads to the calculated IQR of 29%.
In the external validation dataset, equations based on cystatin C over-estimated mGFR while equations based on serum creatinine under-estimated mGFR. The equation incorporating both cystatin C and creatinine was unbiased, as was the average of GFR estimates from the equation based on cystatin C alone and the MDRD Study equation. Precision (IQR) was better in models using creatinine compared to models not using creatinine when expressed on the percent scale but similar across all models when expressed on the raw scale. P30 was higher for the equation based on creatinine, age, sex and race as well as for the equation based on cystatin C, age, sex and race compared to the equation based on cystatin C alone. Accuracy was highest for the equation based on all variables and for the average of the cystatin C only equation and the MDRD Study equation. Given the similarity in performance of equations across the three datasets, the data were combined and final coefficients were determined. Table 4 provides the three cystatin C-based equations fit using the combined datasets.
Table 4.
GFR estimating equations to predict GFR from serum cystatin C. Refit coefficients from entire dataset
| Equation 1: eGFR | = 76.7*CysC−1.19 |
| Equation 2: eGFR | = 127.7* CysC−1.17 * age−0.13 * (0.91 if female) * (1.06 if black) |
| Equation 3: eGFR | = 177.6* SCr−0.65 * CysC−0.57 * age−0.20 * (0.82 if female) * (1.11 if black) |
Performance by level of eGFR and age, sex and race
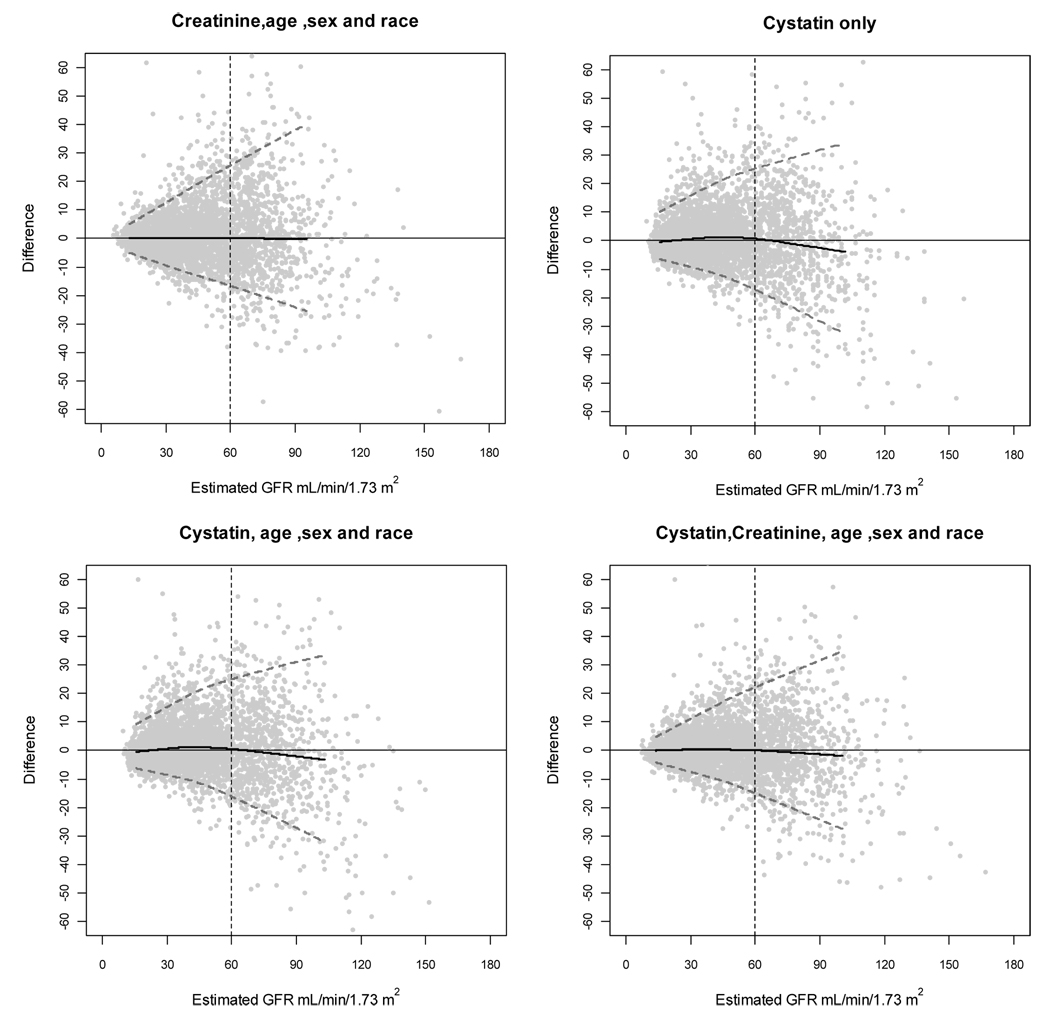
Figure 1 shows the performance of the four main equations by level of eGFR in the combined dataset. All equations show minimal bias and similar precision up to levels of eGFR of 90 ml/min/1.73 m2 (1.5 ml/s/1.73m2). There is greater overestimation at the higher range of eGFR using equations based on cystatin C compared to equations based on creatinine.
Figure 1. Performance in the pooled dataset by level of estimated GFR.
Difference is calculated as (measured GFR-estimated GFR). Solid horizontal line indicates no difference. Solid black curve is a non-linear regression of the mean difference, which measures bias. Dashed grey lines are quantile regressions of the 5th and 90th percentiles of the differences, which measures precision. The grey dotted vertical line indicates 60 ml/min per 1.73 m2. To convert GFR from mL/min/1.73 m2 to mL/sec per 1.73 m2, multiply by 0.01666. GFR, glomerular filtration rate.
Table 5 shows performance of the equations within subgroups. There are larger differences in bias, precision and accuracy among young, middle and old age subgroups; between men and women; and between blacks and non-blacks in the equations that use cystatin C without adjustment for demographic variables. The addition of the age, sex and race reduces bias in some subgroups, particularly individuals of older age, females and blacks.
Table 5.
Performance by subgroups defined by age, sex, race and eGFR in the entire dataset
| Group | N | Difference | Percent Difference | P30 | RMSE | |||
|---|---|---|---|---|---|---|---|---|
| Median (95% CI)* | IQR | Median | IQR | |||||
| Cystatin alone | ||||||||
| Overall | 3418 | 0.2 (−0.1, 0.6) | 11.7 | 0.7(−0.2,1.4) | 29.8 | 81 | 0.246 | |
| <40 | 666 | 0.9 (0.2, 1.9) | 13.9 | 2.7 (0.8,4.3) | 29.6 | 80 | 0.266 | |
| 40–65 | 2152 | 0.5 (0.1, 1.0) | 11.5 | 1.3(0.3,2.5) | 28.8 | 82 | 0.238 | |
| Age | >65 | 600 | −1.4 (−1.9, −0.6) | 11.0 | −3.7(−6.5,−1.8) | 32.7 | 79 | 0.252 |
| Y | 1261 | −2.1 (−2.9, −1.6) | 11.6 | −5.8(−7.1,−4.0) | 33.2 | 76 | 0.263 | |
| Female | N | 2157 | 1.5 (1.0, 1.9) | 11.2 | 3.5(2.7, 4.5) | 26.2 | 84 | 0.236 |
| White and other | 1610 | −0.8 (−1.2, −0.4) | 9.7 | −2.5(−3.7,−1.3) | 31.2 | 79 | 0.251 | |
| Race | African American | 1808 | 1.5 (1.0, 2.0) | 13.7 | 3.3(2.0,4.4) | 27.2 | 83 | 0.242 |
| Cystatin +age +race +sex | ||||||||
| Overall | 3418 | 0 (−0.2, 0.4) | 11.2 | 0.1(−0.6,1.0) | 28.2 | 83 | 0.239 | |
| <40 | 666 | −0.7 (−1.7, 0.1) | 12.5 | −2(−4.1,0.2) | 30.3 | 80 | 0.263 | |
| 40–65 | 2152 | 0.5 (−0.1, 0.9) | 10.8 | 1(−0.2,2.2) | 27.0 | 84 | 0.229 | |
| Age | >65 | 600 | −0.1 (−0.5, 0.6) | 11.1 | −0.3(−1.9,1.7) | 30.4 | 82 | 0.245 |
| Y | 1261 | 0.0 (−0.6, 0.6) | 11.7 | −0.1(−1.5,1.3) | 31.3 | 81 | 0.253 | |
| Female | N | 2157 | 0.1 (−0.3, 0.5) | 10.9 | 0.3(−0.7,1.6) | 26.7 | 84 | 0.230 |
| White and other | 1610 | −0.2 (−0.6, 0.3) | 9.7 | −0.6(−1.7,0.9) | 30 | 83 | 0.243 | |
| Race | African American | 1808 | 0.3 (−0.2, 0.9) | 13.5 | 0.6(−0.4,2.0) | 27.1 | 83 | 0.235 |
| Cystatin +creatinine + age + sex+ race | ||||||||
| Overall | 3418 | 0.1 (−0.3, 0.3) | 9.2 | 0.1(−0.7,0.9) | 23 | 89 | 0.197 | |
| <40 | 666 | −0.9(−1.5, −0.3) | 9.3 | −2.2(−4.0,−0.7) | 23.1 | 88 | 0.207 | |
| 40–65 | 2152 | 0.4(0.1, 0.7) | 9.2 | 1.0(0.2,1.9) | 22.3 | 90 | 0.194 | |
| Age | >65 | 600 | −0.3(−0.6, 0.4) | 8.7 | −0.9(−2.2,1.5) | 22.9 | 89 | 0.197 |
| Y | 1261 | −0.2(−0.5, 0.2) | 9.5 | −0.6(−1.6,0.8) | 25 | 87 | 0.210 | |
| Female | N | 2157 | 0.2(−0.2, 0.5) | 8.9 | 0.6(−0.6,1.1) | 21.7 | 91 | 0.189 |
| White and other | 1610 | −0.1(−0.4, 0.2) | 7.4 | −0.6(−1.4,0.7) | 22.2 | 90 | 0.192 | |
| Race | African American | 1808 | 0.4(−0.3, 0.9) | 11.3 | 0.7(−0.6,1.9) | 23.3 | 89 | 0.202 |
| Creatinine +age +race +sex | ||||||||
| Overall | 3418 | 0.1(−0.1, 0.3) | 10.8 | 0.3(−0.5, 0.9) | 26.9 | 85 | 0.226 | |
| <40 | 666 | −0.3(−1.0, 0.3) | 11.3 | −0.8(−2.5, 0.7) | 27.1 | 83 | 0.235 | |
| 40–65 | 2152 | 0.2(−0.1, 0.5) | 10.9 | 0.6(−0.3, 1.4) | 26.4 | 85 | 0.226 | |
| Age | >65 | 600 | 0(−0.6, 0.6) | 10.1 | 0.1(−2.2, 1.5) | 28.2 | 87 | 0.217 |
| Y | 1261 | −0.1(−0.5, 0.2) | 10.3 | −0.4(−1.7, 0.7) | 28.3 | 83 | 0.239 | |
| Female | N | 2157 | 0.2(−0.1, 0.5) | 11.1 | 0.7(−0.3, 1.3) | 26 | 86 | 0.219 |
| White and other | 1610 | 0(−0.3, 0.3) | 8.2 | 0.1(−0.9, 1.0) | 26.1 | 85 | 0.220 | |
| Race | African American | 1808 | 0.1(−0.3, 0.7) | 13.7 | 0.4(−0.8, 1.3) | 27.6 | 84 | 0.232 |
Difference is calculated as measured GFR-estimated GFR. Units are in mL/min per 1.73 m2. To convert GFR from mL/min/1.73 m2 to mL/sec per 1.73 m2, multiply by 0.01666. Percent difference is calculated as (measured GFR-estimated GFR)/measured GFR, and units are in percent. Median values measure bias and the inter-quartile ranges (IQR) measure precision. P30 is calculated as the percentage of estimated GFR within 30% of measured GFR. GFR, glomerular filtration rate; RMSE, root mean square error; CI, confidence interval.
The 95% confidence interval around the estimates of bias, IQR, P30 and RMSE provides the range of values which is likely to include the parameter in 95% of circumstances. Comparison of confidence intervals around any metric for two equations provides information as to difference between them. If the two metrics were the same, the chance that the upper and lower bounds of the confidence intervals would not overlap is less than 5%. Therefore, it can be concluded that they are statistically significantly different.
Using the equation based on cystatin C alone, the serum level corresponding to eGFR levels of 45, 60, 75 and 90 ml/min/1.73 m2 (0.75, 1.0, 1.3, and 1.5 ml/s/1.73m2) are 1.57, 1.23, 1.02 and 0.88 mg/L (118, 92.1, 76.4, and 65.9 nmol/L), respectively. In the equation that includes cystatin C, age, sex and race, the serum cystatin C levels for an eGFR of 60 ml/min/1.73 m2 (1.0 ml/s/1.73m2) was 1.21 mg/L (89.9 nmol/L) for a 60 year old white man; 1.12 mg/L (83.9 nmol/L) for a 60 year old white woman; 1.27 mg/L (95.1 nmol/L) for a 60 year old black man; and 1.17 mg/L (87.6 nmol/L) for a 60 year old black woman.
Discussion
In this study, we pooled data from 3418 patients with CKD in three research studies and one clinical population to develop and compare GFR estimating equations using serum creatinine, cystatin C or both. Strengths of the study include the large study population; calibration of the creatinine assays in each study to standardized values; measurement of cystatin C in a single laboratory; multiple period urinary clearances of validated filtration markers for measurement of GFR; and the use of a separate external validation dataset. The key findings are that in populations with CKD, cystatin C alone provides GFR estimates that are more accurate than serum creatinine alone and nearly as accurate as serum creatinine, age, sex and race, thus providing an alternative estimate of GFR which is not linked to serum creatinine and muscle mass. Nevertheless, the addition of age, sex and race to cystatin C reduced bias in some subgroups defined by these variables, and an equation that uses both serum creatinine and cystatin C with age, sex and race was better than equations that use only one of these markers.
Cystatin C is an endogenous, 13 kilodalton protein filtered by the glomeruli and reabsorbed and catabolized by the tubular epithelial cells with only small amounts excreted in the urine, and reported to be generated at a relatively constant rate irrespective of muscle mass. Thus, it was anticipated that cystatin C would provide a better estimate of GFR than estimating equations based on serum creatinine. We found a weaker association of age, sex and race with cystatin C than with serum creatinine, which is consistent with this a priori hypothesis as well as with most published cystatin C based estimating equations that do not include terms for age, sex or race.4, 5, 21–23
Both markers provide independent information for the estimation of GFR. The combination of both markers in an equation with age, sex and race provided the most accurate estimate in our dataset, and was equivalent to the average of the estimates from the equation that used cystatin C alone and the MDRD Study equation. More work is needed to determine optimal use of the combination or sequential use of filtration markers, to provide even more accurate GFR estimates.
Several findings in our study suggest that cystatin C levels are affected by factors other than GFR. First, variation in cystatin C alone among subgroups defined by age, sex and race was observed. For the same level of eGFR, serum cystatin levels were 9% lower for women than men, 6% higher for blacks than for whites, and 9% lower for 40 year olds compared to 20 year olds. These differences may reflect differences in the genreration of cystatin among these groups. Accordingly, there was a small improvement in performance of the equation with the addition of these variables. Second, cystatin C based equations slightly overestimated measured GFR at eGFR greater than 90 ml/min/1.73 m2 (1.5 ml/s/1.73m2), whereas the creatinine based equations remained unbiased. Finally, precision was lower for the cystatin C based estimating equations that did not include creatinine. Previous studies have also showed preliminary evidence for non-GFR determinants of cystatin C, including non-renal elimination as well differences in generation among individuals as related to such factors as inflammation, steroid use, thyroid disease, and some studies have shown an effect of body composition.4, 22, 24–28 Variation in these factors among individuals likely account for the lower precision of the cystatin based estimates. The absence of urinary excretion has made it difficult to rigorously evaluate cystatin C as a filtration marker and to examine its non-GFR determinants. The specific nature and magnitude of these factors is not known and requires further study.
The performance of equations using serum creatinine and cystatin C is known to vary among populations. For example, the MDRD Study equation performs well in patients with CKD but is less accurate in potential kidney donors, young people with type 1 diabetes and patients with substantially reduced muscle mass.2, 13, 29 Rule et al showed that a cystatin C based estimating equation performed differently among patients with native kidney disease, kidney transplant recipients and potential kidney donors; therefore, we cannot draw conclusions about the relative performance of serum cystatin C or creatinine in other populations.4 This has important implications for use of these equations in screening populations for CKD, as the equations have not been tested in populations without known CKD.
Serum cystatin C has been shown to have a stronger association with mortality and cardiovascular disease than serum creatinine, particularly in studies of older adults.30–33 In the Cardiovascular Health Study (CHS), participants with eGFR greater than 60 ml/min/1.73 m2 (1.0 ml/s/1.73m2) (based on the MDRD Study equation) and cystatin C greater than 1.0 mg/L (75 nmol/L) had a worse outcome than participants with cystatin C less than 1.0 mg/L (75 nmol/L). A cystatin C of 1.0 mg/L (75 nmol/L) corresponds to an eGFR of 77 ml/min/1.73 m2 (1.3 ml/s/1.73m2), according to the cystatin equation developed here31 The similarity of the performance of equations based on serum creatinine and cystatin C at this level of eGFR in the current study suggests that the stronger association of cystatin C to adverse outcomes in CHS may be due to factors other than GFR that effect the level of serum cystatin C or creatinine.34 An alternative explanation is that the study populations in this report differ from CHS. CHS participants were recruited from Medicare eligibility lists and therefore reflect the general population of older adults, including the frail elderly, whereas the studies included here mostly included patients with CKD who were not frail; therefore, the factors that may confound the relationship between serum creatinine or cystatin C to GFR may not be reflected in the current estimating equations. Studies of GFR measurements in older adults with and without CKD and across the range of health and functional status are required to determine whether cystatin C is a better filtration marker in this population.
Previous studies have shown a wide variability in eGFR for the same level of cystatin C.35 For example, cystatin C levels equivalent to eGFR of 60 ml/min/1.73 m2 (1.0 ml/s/1.73m2) using the equations published by Rule and Grubb would be 1.09 mg/L (81.6 nmol/L) and 1.40 (105 nmol/L) respectively, compared to 1.23 mg/L (92.1 nmol/L) derived from our equation that uses cystatin C alone. These differences may be related to the variation among populations discussed above, or to differences among assays or GFR measurement methods. The high level of variation in the cystatin C assay is beginning to be recognized, and standardization and calibration of clinical laboratories will be important to obtain accurate GFR estimation using cystatin C, as has been shown for creatinine.36
There are several limitations to this analysis. First, as discussed above, the study population was composed mainly of patients with CKD; however, the intent of the current work is to compare estimating equations based on cystatin C to the best available equation based on serum creatinine. Indeed, to maximize this comparison, we did not test all possible transformations of cystatin C or other variables besides age, sex and race (e.g. diabetes), or their interactions. Second, only one external validation dataset was used; therefore, our findings may not be applicable to other populations. In particular, the small bias observed in the external validation dataset could be related to differences between the exogenous filtration markers used for clearance measurements, iothalamate for the development dataset, and EDTA for the validation dataset. Previous studies have shown both filtration markers to provide similar values to inulin clearance; however, a direct comparison cannot be performed as the two radioactive tracers can interfere with one another in the counting, EDTA is not approved for use in the United States, and iothalamate is not available in Europe.10–12 As well, differences between markers are likely to be manifested by bias‥ Since a bias is expected when applying equations in new dataset, the presence of a small bias in the external validation dataset suggests that the difference between filtration markers for clearance measurements is not likely to an important factor in the result. Use of only one external validation dataset also means that we were unable to test if the coefficient for black race differs between blacks in the United States and Europe. Third, the equation coefficients were derived from a pooled analysis of individual studies, rather than from a representative population, with some studies representing a substantial portion of certain demographic groups (e.g. Blacks in AASK). Therefore, it is possible that findings observed within a demographic group may reflect study differences and not characteristics of that group. Indeed, study participants were likely selected on the basis of previous serum creatinine values and given the colinearity between creatinine and cystatin C, this may lead to bias in the coefficients in all equations. However, all studies were of CKD populations and previous studies have suggested that differences among subgroups based on demographic characteristics are minimal for populations with native kidney disease.2, 13 Pooling across studies is probably preferable to using a single study in the absence of data from large representative samples. Fourth, equations were not compared with respect to classification of patients with measured GFR less than versus greater or equal to 60 ml/min/1.73 m2. Our study population has a mean GFR that is well below 60 ml/min per 1.73 m2, thus, these analyses would not be very sensitive to differences in accuracy of the equations. In addition, these comparisons would not take into account error in measured GFR. Finally, these equations were not tested for assessment of change in GFR over time.
GFR estimating equations using cystatin C have the promise to provide more accurate estimates of GFR than equations using serum creatinine. Implementation of these equations in routine clinical practice requires standardization of the cystatin C assay, further investigation of the factors other than GFR that influence the level of cystatin C, and availability of wide-spread and cost-effective assays for additional markers. At the current time, the equations developed here may provide more accurate estimates in people in whom estimates based on serum creatinine are likely to be inaccurate due to conditions affecting muscle mass or diet or to estimate change in GFR over time in people with changing muscle mass or diet.
Acknowledgments
Support:This study was supported by rant numbers UO1 DK 053869, UO1 DK 067651 and UO1 DK 35073.
Appendix
Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) is a research group formed to develop and validate improved estimating equations for glomerular filtration rate (GFR) by pooling data from research studies and clinical populations (hereafter referred to as “studies”). The studies include individuals with diverse clinical characteristics, with and without kidney disease, and across a wide range of GFR. Key inclusion criteria were GFRs measured by investigators with experience and well-documented quality control data; ability to calibrate serum creatinine; and willingness of the investigators to share individual patient data. The creatinine pooled dataset consists of 10 studies (Category 1) for development (random selection of two-thirds of the data) and internal validation (remaining one-third of data) and 16 studies (Category 2) for external validation.13
The cystatin C pooled dataset consists of a subset of these studies in which serum samples were available for assay of cystatin C at the time of these analyses (appendix figure). Four studies had frozen samples available for assay of cystatin C at this time and were therefore included in the current analysis: three from category 1, the Modification of Diet in Renal Disease (MDRD) Study, African American Study of Kidney Disease (AASK), Collaborative Study Group (CSG); and one from category 2, a clinical population in Paris, France.1, 6–10 A subset of patients within each of these studies had samples available for measurement of cystatin C. Consistent with the design of CKD-EPI, the first three studies were used for model development and internal validation, and the fourth study was used for external validation. Study and patient assignments to datasets were maintained, such that the cystatin development, internal validation and external validation datasets are subsets of the creatinine development, internal validation and external validation datasets, respectively.
Investigators and research staff of CKD-EPI include
Tufts-New England Medical Center: Andrew S. Levey, MD; Lesley A. Stevens, MD, MS; Christopher H. Schmid, PhD; Yaping (Lucy) Zhang, MS; Robert D. Bruce III, BA; Cleveland Clinic: Frederick VanLente, PhD, Liang Li, PhD; University of Utah: Tom Greene, PhD; John Hopkins University: Josef Coresh, MD PhD MHS, Jane Manzi, PhD, Brad Astor, PhD, MPH; Elizabeth Selvin, PhD MPH; University of Pennsylvania: Harold I. Feldman, MD, MSCE, J. Richard Landis, PhD; Marshall Joffe MD MPH PhD; National Institute of Diabetes and Digestive and Kidney Diseases: John W. Kusek, PhD, Paul W. Eggers, PhD, Robert Star MD
Collaborators contributing data for this study
Modification of Diet in Renal Disease Study: Gerald Beck, PhD, Cleveland Clinic Foundation; Collaborative Study Group: Captopril in Diabetic Nephropathy Study: Rodger Rodby, MD, Rush University; Richard Rohde, Rush University; African American Study of Kidney Disease and Hypertension: Gabriel Contreras, MD, University of Miami School of Medicine, Julie Lewis, MD, Vanderbilt Univerwsity; Paris: Jerôme Rossert, MD, PhD, Geroges Pompidou European Hospital, Marc Froissart MD, PhD, Georges Pompidou European Hospital.
Footnotes
Presented in abstract form at the Annual Meeting of the American Society of Nephrology in Philadelphia in November, 2005 and in San Diego in November, 2006
Financial disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N section: Because an author of this manuscript is an editor for AJKD, the peer-review and decision-making processes were handled entirely by an Associate Editor (Marcello Tonelli, MD, University of Alberta) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
REFERENCES
- 1.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 2.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function - measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 3.Grubb A, Nyman U, Bjork J, et al. Simple cystatin C-based prediction equations for glomerular filtration rate compared with the Modification of Diet in Renal Disease Prediction Equation for adults and the Schwartz and the Counahan-Barratt Prediction Equations for children. Clin Chem. 2005;51:1420–1431. doi: 10.1373/clinchem.2005.051557. [DOI] [PubMed] [Google Scholar]
- 4.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS. Glomerular filtraton rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006;69:399–405. doi: 10.1038/sj.ki.5000073. [DOI] [PubMed] [Google Scholar]
- 5.Filler G, Lepage N. Should the Schwartz formula for estimation of GFR be replaced by cystatin C formula? Pediatr Nephrol. 2003;18:981–985. doi: 10.1007/s00467-003-1271-5. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK Trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EJ, Kunsicker LG, Bain RP, Rohde RD Collaborative Study Group. The effect of angiotensin-converting enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 9.Klahr S, Levey A, Beck G, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 10.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the MDRD and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 11.Israelit A, Long D, White M, Hull A. Measurement of glomerular filtration rate utilizing a single subcutaneous injection of 125I-iothalamate. Kidney Int. 1973;4:346–349. doi: 10.1038/ki.1973.127. [DOI] [PubMed] [Google Scholar]
- 12.Perrone R, Steinman T, Beck G, et al. Utility of radioisotopic filtration markers in chronic renal insufficiency: simultaneous comparison of 125I-Iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. Am J Kidney Dis. 1990;26:224–235. doi: 10.1016/s0272-6386(12)81022-5. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 2007;50:21–35. doi: 10.1053/j.ajkd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Levey A, Coresh J, Greene T, et al. Expressing the MDRD study equation for estimating GFR with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 15.Newman D, Cystatin C. Ann Clin Biochem. 2002;39:89–104. doi: 10.1258/0004563021901847. [DOI] [PubMed] [Google Scholar]
- 16.Kyhse-Andersen J, Schmidt C, Nordin G, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40:1921–1926. [PubMed] [Google Scholar]
- 17.Mosteller RD. Simplified Calculation of Body Surface Area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. (letter) [DOI] [PubMed] [Google Scholar]
- 18.Myers G, Miller W, Coresh J, et al. Recommendations for improving serum creatinine measurement: A report from the laboratory working group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 20.Dixon PM. The bootstrap and the jackknife: Describing the precision of ecological indices. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. New York: Chapman and Hall; 1993. pp. 290–318. [Google Scholar]
- 21.Hoek F, Kemperman F, Krediet R. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–2031. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 22.Sjostrom P, Tidman M, Jones I. Determination of the production rate and non-renal clearance of cystatin C and estimation of the glomerular filtration rate from the serum concentration of cystatin C in humans. Scand J Clin Lab Invest. 2005;65:111–124. doi: 10.1080/00365510510013523. [DOI] [PubMed] [Google Scholar]
- 23.Larsson A, Malm J, Grubb A, Hansson L. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/min. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 24.Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47:2055–2059. [PubMed] [Google Scholar]
- 25.Grubb AO. Cystatin C-properties and use as diagnostic marker. Adv Clin Chem. 2000;35:63–99. doi: 10.1016/S0065-2423(01)35015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight E, Verhave J, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 27.Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28:346–349. doi: 10.1007/BF03347201. [DOI] [PubMed] [Google Scholar]
- 28.Wasen E, Isoaho R, Mattila K, Vahlberg T, Kivela S, Irjala K. Serum cystatin C in the aged: relationships with health status. Am J Kidney Dis. 2003;42:36–43. doi: 10.1016/s0272-6386(03)00406-2. [DOI] [PubMed] [Google Scholar]
- 29.Greene T. Effect of Source Population on the Relationship of GFR Estimates with "True GFR" (abstract) J Am Soc Nephrol. 2006;17:142A. [Google Scholar]
- 30.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 31.Shlipak M, Katz R, Sarnak M, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 33.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 34.Stevens LA, Levey AS. Chronic kidney disease in the elderly -- How to assess risk? [Editorial] N Engl J Med. 2005;352:2122–2124. doi: 10.1056/NEJMe058035. [DOI] [PubMed] [Google Scholar]
- 35.Madero M, Sarnak MJ, Stevens LA. Serum cystain C as a marker of glomerular filtration rate. Curr Opin Neph Hyper. 2006;15:610–616. doi: 10.1097/01.mnh.0000247505.71915.05. [DOI] [PubMed] [Google Scholar]
- 36.Flodin M, Hansson L, Larsson A. Variations in assay protocol for the Dako cystatin C method may change patient results by 50% without changing the results for controls. Clin Chem Lab Med. 2006;44:1481–1485. doi: 10.1515/CCLM.2006.271. [DOI] [PubMed] [Google Scholar]