Abstract
Study Objectives:
Examine age-adjusted odds and racial/ethnic differences in self-reported difficulties falling and staying asleep and early morning awakening in midlife women to determine whether difficulty sleeping increased with progression through the menopausal transition.
Design:
Longitudinal analysis.
Setting:
Community-based.
Participants:
3,045 Caucasian, African American, Chinese, Japanese, and Hispanic women, aged 42-52 years and pre- or early peri-menopausal at baseline, participating in the Study of Women's Health Across the Nation (SWAN).
Interventions:
None.
Measurements and Results:
Self-reported number of nights of difficulty falling asleep, staying asleep, and early morning awakening during the previous 2 weeks were obtained at baseline and 7 annual assessments. Random effects logistic regression was used to model associations between each of the 3 sleep measures and the menopausal transition, defined by bleeding patterns, vasomotor symptoms (VMS), and estradiol (E2) and follicle stimulating hormone (FSH) serum levels. Adjusted odds ratios (ORs) for difficulty falling asleep and staying asleep increased through the menopausal transition, but decreased for early morning awakening from late perimenopause to postmenopause. Naturally and surgically postmenopausal women using hormones, compared with those who were not, generally had lower ORs for disturbed sleep. More frequent VMS were associated with higher ORs of each sleep difficulty. Decreasing E2 levels were associated with higher ORs of trouble falling and staying asleep, and increasing FSH levels were associated with higher ORs of trouble staying asleep. Racial/ethnic differences were found for staying asleep and early morning awakening.
Conclusions:
Progression through the menopausal transition as indicated by 3 menopausal characteristics—symptoms, bleeding-defined stages, and endogenous hormone levels—is associated with self-reported sleep disturbances.
Citation:
Kravitz HM; Zhao X; Bromberger JT; Gold EB; Hall MH; Matthews KA; Sowers MR. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. SLEEP 2008;31(7):979-990.
Keywords: Estradiol, follicle stimulating hormone, longitudinal study, menopausal transition, race/ethnicity, sleep difficulties, vasomotor symptoms
TWO RELATIVELY CONSISTENT FINDINGS HAVE EMERGED FROM EPIDEMIOLOGIC STUDIES OF SLEEP DISTURBANCES: THAT SUBJECTIVE REPORTS OF difficulty sleeping are more prevalent in women than men and that the prevalence of this difficulty increases with aging.1,2 A female preponderance in the prevalence of self-reported sleep problems is evident by midlife.3–7 Data presented at the NIH State-of-the-Science Conference on Management of Menopause-Related Symptoms8 indicated that sleep problems are reported by 16%-42% of premenopausal women, 39%-47% of perimenopausal women, and 35%-60% of postmenopausal women. In the Study of Women's Health Across the Nation (SWAN) cross-sectional survey of more than sixteen thousand women aged 40–55 years, 38% experienced difficulty sleeping within the 2 weeks preceding the interview.9 Relative to being premenopausal, being perimenopausal was associated with difficulty sleeping even after adjusting for multiple relevant covariates.
Both age and hormonal changes can contribute to disturbed sleep in middle-aged women undergoing the menopausal transition.10–14 Whereas the increase in sleep difficulties that emerge at midlife suggest an aging effect,10,11,14 gender differences at midlife suggest that the role of aging per se must be distinguished from sleep disturbances due to other age-related risk factors.13 In the initial SWAN report,9 we may have found no “age effect” because we only included women in a narrow age range during a period of marked hormonal transition when ovarian age may be more informative than chronological age.
Attributes of the menopausal transition may confer risk for sleep disturbances beyond the effects of age alone, but studies examining these factors have tended to be cross-sectional. Potential precipitating factors during the menopausal transition include onset and exacerbation of vasomotor symptoms (VMS; hot flashes, night sweats, cold sweats)15 and changing reproductive hormone levels (especially follicle stimulating hormone; FSH).16 The etiology of perimenopausal-related sleep changes and whether onset of these changes is associated with hormonal changes and VMS that occur during this transition are not well understood.17,18 VMS are highly prevalent in peri- and postmenopausal women (35%-80%),8,19 and there is considerable overlap between VMS and sleep difficulties.20 Whereas sleep disturbance and VMS are strongly associated, these 2 symptoms are not perfectly correlated, and sleep difficulties may continue long after hot flashes have subsided.21
Menopausal hormonal changes may plausibly be related to acute sleep disturbances, but evidence relating self-reported sleep difficulties to hormonal changes, independent of VMS, during the menopausal transition has been mixed.22 In SWAN,16 FSH concentrations, but not FSH-adjusted estradiol levels, are strongly related to VMS. Others have shown that in women aged 35–49 years, poor sleep quality is associated with lower follicular phase plasma estradiol.23 Data from SWAN's Daily Hormone Study (daily collection of first morning urine for up to 50 days and self-reported sleep difficulties) showed that compared with premenopausal women, early perimenopausal women had 29% higher odds of reporting trouble sleeping.12 This increased reporting was associated with levels of the urinary progesterone metabolite, pregnanediol glucuronide, in perimenopausal women and with FSH levels in premenopausal women, independent of VMS.12
An additional, though largely unexplored issue, is the type of sleep difficulty most prevalent during the menopausal transition. An examination of sleep problems over 12 months in the National Comorbidity Survey Replication, a nationally representative household survey of men and women 18 years and older, showed little variation in types of reported problems: 16.4% had difficulty initiating sleep, 19.9% had difficulty maintaining sleep, and 16.7% had early morning awakenings.24 However, these cross-sectional data were not reported by age or sex. Little is known about the prevalence of these 3 types of disturbed sleep during and after the menopausal transition and how they vary over long periods of time.
We undertook a longitudinal analysis of data from an ethnically diverse cohort of midlife women to determine how each type of sleep continuity difficulty changes as they progressed through the menopausal transition. Specifically, we examined whether three aspects of the menopausal transition, i.e., changes in bleeding patterns, reproductive hormone levels, and VMS, affected sleep symptom reports after accounting for the effects of aging and a variety of health and psychosocial factors. We also examined whether the associations varied among the 5 racial/ethnic groups represented in SWAN.
METHODS
Study Design and Participants
SWAN is a community-based, multisite cohort study of the menopausal transition. A total of 3,302 women were enrolled at 7 sites: Boston, MA, Chicago, IL, Detroit area, MI, Los Angeles and Oakland, CA, Newark, NJ, and Pittsburgh, PA. The study design and cohort recruitment have been described in detail previously.25 Recruitment strategies varied due to differences in resources in each of the seven communities. Each site recruited Caucasian women and a minority group sample. Women were of Caucasian, African American (Boston, Chicago, Detroit, Pittsburgh), Chinese (Oakland), Japanese (Los Angeles), and Hispanic (Newark) origins. SWAN cohort eligibility included the following: age 42–52 years, premenopausal or early perimenopausal, intact uterus and ≥1 ovary, ≥1 menstrual period in the previous 3 months, no sex steroid hormone use in the previous 3 months, and not pregnant. Each site's institutional review board approved the study, and all women gave written informed consent to participate.
Procedures and Measures
From the study cohort of 3,302 participants, 257 women were excluded for having no follow-up data or missing all sleep measures at baseline, leaving a total of 3,045 women. Baseline and annual visit data through the first 7 annual assessments were used. Except for participants at the New Jersey site, for whom no data were available for year 7, each woman could contribute up to 8 observations. The average number of observations was 6.7 (6.9 when excluding the New Jersey site). At the seventh annual visit, the overall SWAN retention rate (including deceased participants) was 72.8% (80.8% when excluding the New Jersey site). Also, at the sixth assessment only 54 observations were available from New Jersey participants. Because these data did not differ statistically from those for the 378 New Jersey participants who dropped out before year 6 in terms of ethnicity, marital status, education, age, body mass index (BMI), difficulty paying for basics, smoking status, or for the menopausal transition variables or all sleep measures at baseline or the fifth annual assessment, these 54 observations were included in the analyses. Data from all observations are reported because the conclusions were the same after removing the New Jersey data. Most model estimates changed less than 10% and only estimates for late perimenopausal women in trouble falling asleep and waking up early changed around 15%. The P-value for the pairwise comparison of late perimenopausal versus premenopausal became more significant (P < 0.001).
Dependent Variables
Trouble falling asleep (sleep initiation difficulty), waking up several times a night (sleep maintenance difficulty), and waking up earlier than planned and unable to fall asleep again (early morning awakening) were self-reported at baseline and at each annual assessment. Participants were asked about the frequency of sleep problems (“your sleep habits”) in the past 2 weeks: (1) none, (2) less than once a week, (3) one or two times a week, (4) three or four times a week, (5) five or more times a week. These 3 sleep measures were dichotomized as “no” (<3 times a week) or “yes” (≥3 times a week), which is consistent with the frequency criterion most commonly used in insomnia research.26,27
Independent Variables
Menopausal Status
Transition status was determined using bleeding criteria, and participants were classified into one of the following 6 categories: premenopausal, early perimenopausal, late perimenopausal, postmenopausal, surgically menopausal, or unknown (pre- or peri-menopausal and using hormone therapy [HT]). HT use since last study visit was assessed annually. Women who were not postmenopausal yet and reported any HT use since their last study visit were categorized as “unknown” menopausal status because HT use affects bleeding patterns. For women who already were naturally or surgically postmenopausal, HT use did not affect their menopausal status classification; however, to assess the impact of HT use on these postmenopausal categories, we split the 2 categories into 4 categories as follows: naturally postmenopausal with current HT use (17%) and without current HT use (83%), and surgically postmenopausal with current HT use (43%) and without current HT use (57%). Hence, we will use this new created menopausal status/HT use variable with 8 levels in the respective models.
Vasomotor Symptoms (VMS)
VMS was a time-varying summary variable that included hot flashes/flushes, cold sweats, and night sweats from the annual self-administered questionnaire. Factor analysis of the items revealed a single factor suggesting that VMS can be treated as a single variable.15 For each vasomotor symptom women were asked to check one of the following frequency categories: none, 1–5 days, 6–8 days, 9–13 days and every day in the past 2 weeks. VMS were coded according to the maximum of the frequency of the 3 components: 0 if none, 1 if all symptoms were reported less than 6 days, and 2 if at least one VMS was reported ≥6 days in the past 2 weeks. These levels were chosen because we considered more frequent symptom reports to indicate a more clinically meaningful symptom frequency compared with no or fewer than 6 days15 and wanted to examine a dose-response effect.
Reproductive Hormones
At baseline and annually, fasting blood specimens were drawn from participants during days 2 to 5 (follicular phase) of their menstrual cycles. If a day 2–5 specimen could not be obtained after 2 attempts, a random fasting specimen was taken within 90 days of the annual assessment. Blood was refrigerated 1–2 h after phlebotomy, centrifuged, and the serum aliquotted, frozen at −80°C, and shipped on dry ice to a central laboratory, the University of Michigan CLASS (Central Ligand Assay Satellite Services) Endocrine Laboratory. Specimens were catalogued and assayed continuously upon arrival, using an ACS-180 automated analyzer (Bayer Diagnostics Corporation). Serum follicle stimulating hormone (FSH) concentrations were measured using a chemiluminometric immunoassay, and serum estradiol (E2) was measured using a modified, off-line ACS-180 (E2-6) immunoassay.16 In modeling the hormone effect, whether the blood draw occurred in the 2 to 5-day window was adjusted for as a 2-level categorical covariate: day 2–5, versus not day 2–5 or unknown.
Covariates
Participants provided extensive health, psychosocial, lifestyle and biologic data at baseline and at annual assessments. Variables selected have been related to trouble sleeping or mediated the association between reproductive hormones and trouble sleeping in previous research.20,28
Sociodemographics
Race/ethnicity was determined by self-identification as African American, Caucasian, Chinese, Japanese, or Hispanic. Other sociodemographic variables included age, marital status, educational level, annual household income, and study site (see Table 1).
Table 1.
Baseline Characteristics, by Race/Ethnicity
| Categorical variable †, n, (%) | Caucasian* | African American* | Hispanic* | Chinese* | Japanese* | Total* |
|---|---|---|---|---|---|---|
| (n = 1445) | (n = 852) | (n = 229) | (n = 244) | (n = 275) | (n = 3045) | |
| Trouble falling asleep | 136 (9.4) | 101 (11.9) | 33 (14.4) | 18 (7.4) | 18 (6.5) | 306 (10.1) |
| Wake up several times | 410 (28.4) | 230 (27.2) | 50 (21.8) | 46 (18.9) | 50 (18.2) | 786 (25.9) |
| Wake up early | 168 (11.6) | 122 (14.4) | 43 (18.8) | 29 (11.9) | 16 (5.8) | 378 (12.4) |
| Menopausal status | ||||||
| Premenopausal | 742 (52.6) | 419 (49.8) | 122 (57.5) | 148 (61.4) | 170 (62.5) | 1601 (53.8) |
| Early Perimenopausal | 668 (47.4) | 422 (50.2) | 90 (42.5) | 93 (38.6) | 102 (37.5) | 1375 (46.2) |
| Vasomotor symptoms | ||||||
| None | 909 (63.1) | 444 (53.0) | 117 (51.1) | 168 (70.0) | 148 (65.8) | 1786 (60.1) |
| <6 days in last 2 weeks | 385 (26.7) | 263 (31.4) | 82 (35.8) | 55 (22.9) | 61 (27.1) | 846 (28.5) |
| ≥6 days in last 2 weeks | 146 (10.1) | 130 (15.5) | 30 (13.1) | 17 (7.1) | 16 (7.1) | 339 (11.4) |
| Blood drawn in cycle day 2–5 window ‡ | 1162 (80.6) | 641 (75.4) | 189 (82.9) | 183 (75.0) | 229 (83.9) | 2404 (79.2) |
| Marital status | ||||||
| Single | 170 (12.0) | 186 (22.0) | 10 (4.6) | 21 (8.7) | 20 (7.3) | 407 (13.6) |
| Married/living with partner | 1030 (72.5) | 399 (47.2) | 163 (75.5) | 194 (80.2) | 222 (81.0) | 2008 (67.0) |
| Separated/Windowed/Divorced | 220 (15.5) | 261 (30.9) | 43 (19.9) | 27 (11.2) | 32 (11.7) | 583 (19.4) |
| Education | ||||||
| ≤High school | 222 (15.5) | 219 (26.0) | 154 (69.4) | 70 (28.7) | 48 (17.5) | 713 (23.6) |
| Some college | 444 (30.9) | 341 (40.5) | 47 (21.2) | 51 (20.9) | 95 (34.5) | 978 (32.4) |
| College | 313 (21.8) | 135 (16.0) | 16 (7.2) | 71 (29.1) | 85 (30.9) | 620 (20.5) |
| More than college | 457 (31.8) | 147 (17.5) | 5 (2.3) | 52 (21.3) | 47 (17.1) | 708 (23.5) |
| Annual household income | ||||||
| <$20,000 | 100 (7.0) | 177 (21.6) | 124 (55.6) | 12 (5.0) | 7 (2.7) | 420 (14.2) |
| $20,000–$49,999 | 450 (31.5) | 333 (40.7) | 77 (34.5) | 81 (33.9) | 57 (22.0) | 998 (33.6) |
| ≥$50,000 | 878 (61.5) | 309 (37.7) | 22 (9.9) | 146 (61.1) | 195 (75.3) | 1550 (52.2) |
| Difficulty paying for basics | ||||||
| Very difficult | 75 (5.2) | 104 (12.3) | 56 (25.1) | 13 (5.3) | 10 (3.6) | 258 (8.5) |
| Somewhat difficult | 372 (25.9) | 278 (32.8) | 125 (56.1) | 55 (22.5) | 71 (25.9) | 901 (29.8) |
| Not very difficult | 992 (68.9) | 465 (54.9) | 42 (18.8) | 176 (72.1) | 193 (70.4) | 1868 (61.7) |
| Current (past month) hormone therapy§ | 103 (7.4) | 38 (5.2) | 4 (1.9) | 8 (3.4) | 5 (1.9) | 158 (5.6) |
| Self-assessed overall health | ||||||
| Excellent | 425 (29.9) | 128 (15.1) | 10 (4.6) | 41 (16.9) | 54 (19.7) | 658 (21.9) |
| Very good/good | 910 (64.0) | 584 (69.1) | 147 (68.1) | 150 (62.0) | 174 (63.5) | 1965 (65.5) |
| Fair/Poor | 87 (6.1) | 133 (15.7) | 59 (27.3) | 51 (21.1) | 46 (16.8) | 376 (12.5) |
| Medical conditions | ||||||
| None | 377 (26.4) | 127 (15.0) | 37 (17.0) | 96 (39.7) | 93 (33.9) | 730 (24.3) |
| 1 | 476 (33.4) | 263 (31.0) | 68 (31.2) | 81 (33.5) | 93 (33.9) | 981 (32.6) |
| 2 or more | 573 (40.2) | 459 (54.1) | 113 (51.8) | 65 (26.9) | 88 (32.1) | 1298 (43.1) |
| Urinate at night | ||||||
| Never/rarely | 604 (41.8) | 292 (34.3) | 80 (34.9) | 113 (46.3) | 149 (54.2) | 1238 (40.7) |
| Once/a few times per week | 442 (30.6) | 195 (22.9) | 26 (11.4) | 67 (27.5) | 77 (28.0) | 807 (26.5) |
| ≥1 per night | 398 (27.6) | 365 (42.8) | 123 (53.7) | 64 (26.2) | 49 (17.8) | 999 (32.8) |
| Physical symptoms (maximum = 5) | ||||||
| None | 785 (54.4) | 495 (58.4) | 113 (49.6) | 168 (68.9) | 171 (62.2) | 1732 (57.0) |
| 1 symptom | 405 (28.0) | 216 (25.5) | 62 (27.2) | 44 (18.0) | 71 (25.8) | 798 (26.3) |
| ≥2 symptoms | 254 (17.6) | 136 (16.1) | 53 (23.2) | 32 (13.1) | 33 (12.0) | 508 (16.7) |
| Taking pain medication | 431 (30.0) | 210 (24.6) | 91 (39.9) | 18 (7.4) | 32 (11.7) | 782 (25.8) |
| Taking nervous/sleep medication | 241 (16.7) | 77 (9.0) | 35 (15.4) | 17 (7.0) | 15 (5.5) | 385 (12.7) |
| Smoking status | ||||||
| Never | 751 (52.1) | 453 (54.0) | 150 (66.1) | 228 (93.4) | 174 (63.7) | 1756 (58.0) |
| Past | 472 (32.7) | 186 (22.2) | 44 (19.4) | 12 (4.9) | 64 (23.4) | 778 (25.7) |
| Current | 219 (15.2) | 200 (23.8) | 33 (14.5) | 4 (1.6) | 35 (12.8) | 491 (16.2) |
| Passive smoking status | ||||||
| None | 569 (39.8) | 292 (34.9) | 141 (61.8) | 187 (77.3) | 163 (61.0) | 1352 (45.0) |
| 1–4 person-hours during past week | 462 (32.3) | 211 (25.2) | 28 (12.3) | 37 (15.3) | 62 (23.2) | 800 (26.6) |
| ≥5 person-hours during past week | 400 (28.0) | 334 (39.9) | 59 (25.9) | 18 (7.4) | 42 (15.7) | 853 (28.4) |
| Stressful life events | ||||||
| None | 680 (47.1) | 371 (44.0) | 122 (53.3) | 177 (72.5) | 177 (64.4) | 1527 (50.3) |
| 1 event | 303 (21.0) | 176 (20.9) | 53 (23.1) | 41 (16.8) | 46 (16.7) | 619 (20.4) |
| ≥2 events | 460 (31.9) | 296 (35.1) | 54 (23.6) | 26 (10.7) | 52 (18.9) | 888 (29.3) |
| Alcohol consumption | ||||||
| None | 568 (39.4) | 485 (56.9) | 111 (48.5) | 191 (78.6) | 153 (56.5) | 1508 (49.7) |
| ≤1 serving per day | 761 (52.8) | 332 (39.0) | 110 (48.0) | 48 (19.8) | 98 (36.2) | 1349 (44.4) |
| >1 serving per day | 112 (7.8) | 35 (4.1) | 8 (3.5) | 4 (1.6) | 20 (7.4) | 179 (5.9) |
| Continuous variable ††, mean, (median) | ||||||
| Age (range: 42.0–53.0) | 46.3 (46.0) | 46.3 (46.2) | 46.3 (46.0) | 46.5 (46.6) | 46.7 (46.7) | 46.4 (46.2) |
| Body mass index (range:15.0–64.8) | 27.7 (26.0) | 31.7 (30.2) | 29.3 (28.2) | 23.2 (22.4) | 22.9 (22.1) | 28.1 (26.5) |
| Depression (summed CES-D score, range: 0–54) | 10 (8.0) | 11 (8.0) | 15.8 (14.5) | 8.4 (6.0) | 8.3 (6.0) | 10.5 (8.0) |
| Anxiety (summed score, range: 0–6) | 2.6 (2.0) | 2.4 (2.0) | 3.2 (3.0) | 2.0 (2.0) | 1.8 (2.0) | 2.5 (2.0) |
| Perceived stress (summed score, range: 4–19) | 8.4 (8.0) | 8.5 (8.0) | 9.8 (11.0) | 8.2 (8.0) | 8.8 (9.0) | 8.5 (8.0) |
| Bodily pain (SF-36 score, range: 0–100) | 71.1 (74.0) | 67.2 (72.0) | 54.4 (51.0) | 74.6 (74.0) | 75.4 (74.0) | 69.4 (74.0) |
| Social support (summed score, range: 0–16) | 12.7 (13.0) | 12.2 (13.0) | 10.8 (12.0) | 11.8 (12.0) | 13.2 (14.0) | 12.4 (13.0) |
| Estradiol (range: 5.4–1493.6) | 80.2 (56.2) | 73.3 (53.7) | 81.1 (56.9) | 65.2 (50.8) | 67.9 (51.8) | 76.0 (54.8) |
| Follicle Stimulating Hormone (range: 1.1–303.2) | 23.4 (15.4) | 25.3 (16.6) | 26.7 (15.7) | 24.8 (16.5) | 23.8 (14.5) | 24.4 (15.9) |
| Total physical activity score without work (range: 3–14) | 8.1 (8.1) | 7.3 (7.3) | 6.7 (6.6) | 7.3 (7.3) | 7.9 (7.8) | 7.7 (1.8) |
| Caffeine intake per day, mg (range: 0–1859.7) | 276.9 (214.5) | 200.4 (141.8) | 209.5 (163.6) | 162.4 (116.3) | 256 (216.6) | 239 (176.1) |
Category Ns for all characteristics do not sum to total for each race/ethnic group and for total due to missing data. Percents are calculated after excluding participants with missing data. Column percentages for categorical variables may not sum to 100% due to rounding.
All P values < 0.001 except for trouble falling asleep (P = 0.007), blood drawn in cycle day 2-5 window (P = 0.002).
This dichotomous variable was included only in the hormone models.
Visit 01 data are reported here. At baseline, no one used hormone therapy.
All P values <0.001 except for age (P = 0.2), estradiol (P = 0.009), follicle stimulating hormone (P = 0.29).
Physical Health and Medication-Related Variables
Self-assessed overall health29 was coded as excellent, very good/good, fair/poor. Comorbid medical conditions were coded 0–2 if a participant reported having been told by a health care provider in the past year that she had or was treated for none, one, or two or more of the following 13 conditions: anemia, diabetes, high blood pressure, hypercholesterolemia, migraines, stroke, arthritis, thyroid disease, heart attack, angina, osteoporosis, fibroids, cancer (other than skin). Physical symptoms were coded 0–2 if a participant reported none, one, or 2 or more of 5 symptoms (stiffness/soreness in joints, neck or shoulder, back aches or pain, vaginal dryness, dizzy spells, headaches) on 6 or more days in the past 2 weeks. Nighttime urinary frequency was ascertained from responses to “how often do you usually get up from bed at night to urinate”: never/rarely, once/a few times per week, once or more per night. Bodily pain score was measured with the SF-36 subscale.29 Body mass index (BMI) was computed as weight in kilograms/height in meters squared). Use of psychotropic medications, such as tranquilizers or sedatives, or medication taken to sleep or for pain, was assessed at baseline and annually, and was included as a categorical time-varying covariate (yes/no). “Current HT use,” defined as having taken an exogenous hormone preparation in the month prior to the follow-up interview, was assessed annually, coded yes/no, and included as a separate categorical time-varying covariate in the data modeling. This concurrent HT use was adjusted for in models estimating the effect of the menopausal transition.
Psychological and Psychosocial Variables
Depression was assessed at baseline and annually with the Center for Epidemiologic Studies Depression (CES-D) Scale30; a summary score of the 20 items was used to reflect the frequency of experiencing depressive symptoms in the previous week (range 0–60). Anxiety was assessed at baseline and annually as a continuous summary measure of the frequency of 4 items (irritability/grouchiness, tense/nervous, heart pounding/racing, fearful) which were scored 0–4 based on the frequency (i.e., number of days) each symptom was experienced in the previous 2 weeks (range 0–16).31 Social support was assessed at baseline and annually as a summed scale of how often 4 types of needed emotional and instrumental supports, each scored 0–4, were available (range 0–16).32 The Perceived Stress Scale (4-item short version33) was administered at baseline and annually (range 4–20); higher scores indicated higher levels of stress. Stressful life events were assessed at baseline and annually with a checklist of 18 life events, which were rated according to how stressful they were; women were categorized as having experienced none, one, or 2 or more “very upsetting” events since their previous annual visit. Difficulty paying for basics, a 3-level categorical measure of financial strain, was assessed at baseline.
Health Behavior Variables (Table 1)
Smoking status was assessed by standard questions34 and passive smoke exposure was assessed by a validated instrument.35 Physical activity was measured by a composite score based on the Kaiser Permanente Activity Score,36 a modification of the Baecke scale37 assessing 3 domains (sports, leisure, and household activities). Daily alcohol consumption and caffeine intake also were assessed.38
For the covariates listed above, race/ethnicity, study site, education, difficulty paying for basics, physical activity, and alcohol and caffeine consumption were assessed at baseline and treated as time-independent covariates in modeling. The remaining covariates were treated as time-dependent variables in modeling, and most were assessed at baseline and annually in 7 years of follow-up. Social support and nighttime urinary frequency were not assessed at visit 7, and bodily pain was not assessed at visits 5 and 7; data for the missing visits were replaced by corresponding values from the prior visit.
Data Analysis
The analytic sample included a total of 20,407 observations from 3,045 women, with women contributing 2–8 data points from baseline through the seventh annual assessment. Baseline summary statistics across race/ethnicity for the dependent (sleep measures) and independent (menopausal transition-related) variables and covariates were computed by using a chi-square analysis or one-way analysis of variance approach. The dependent variables consisted of 3 individual sleep symptoms: trouble falling asleep, waking up several times a night, and early morning awakening. These symptoms were analyzed separately so that we could examine relationships between menopausal transition characteristics and specific types of sleep continuity disturbances.
Longitudinal random effects logistic regression models39,40 were used to evaluate the association between each of the 3 self-reported sleep difficulties and menopausal transition status (approximated by bleeding patterns, frequency of vasomotor symptoms, serum reproductive hormones E2 and FSH) over time. This approach allowed women to contribute different numbers of observations, thus fully using all of the available data. In modeling, both E2 and FSH were log-transformed to reduce the skewness, and the log-transformed hormone values at each visit were separated into 2 components: baseline and change over follow-up after baseline for distinguishing between the cross-sectional effect and the longitudinal effect.39
We first estimated the age-adjusted association by including only concurrent age as a covariate in each model. Then, we constructed multivariable models by adjusting for additional potential confounders, which were identified from the literature, as described above. After checking for collinearity, covariate variables that were suggestive of a possible bivariate association (i.e., P < 0.15) were included in the initial multivariable models. Backward elimination was used to identify suitable multivariable models, with selection criteria set at P < 0.05 for those covariates remaining in the final models. Ethnicity, site, age, and current HT use were forced into all the final multivariable models because of their importance as potential confounders and so that the models could be compared; also, the blood draw in window variable was forced into the hormone models. Due to a specific SWAN study interest in ethnic differences, interactions of race/ethnicity with the independent variables were included to test whether demonstrated associations varied significantly among racial/ethnic groups. Finally, to assess the impact of HT use on models of vasomotor symptoms and hormones (note, the impact of HT use on models of menopausal status was evaluated by using an 8-level menopausal status/HT use variable), we first reran our multivariable analysis by omitting the subgroup (13.3% of the sample) that reported HT use since last study visit. The results (data not shown, all model estimates changed less than 7%) were very similar to results presented here that include HT users. We also tested the interaction terms between HT use and vasomotor symptoms as well as change in hormones since baseline. None of the interaction terms we tested was statistically significant, and therefore the results are not presented.
P-values, odds ratios (ORs), and 95% confidence intervals (CIs) for independent variables were computed and reported across sleep dependent variables. We used Stata Release 8 xtlogit procedure41 to estimate the longitudinal random effects logistic regression models, and the remaining analyses were conducted using SAS Version 8.42
RESULTS
Participant Baseline Characteristics
Of our total sample, 47.5% were Caucasian, 28% were African American, 9% were Japanese, 8% were Chinese, and 7.5% were Hispanic (Table 1). Racial/ethnic groups differed significantly (P < 0.05) on all baseline characteristics except age and FSH. Using menstrual bleeding criteria, 52.6% of the sample were premenopausal and 45.2% were early perimenopausal at baseline; 2.3% (n = 69) were missing bleeding pattern regularity information. Most were married or living with a partner (65.9%; 1.5% missing) and had at least some college, (75.7%; 0.9% had no information).
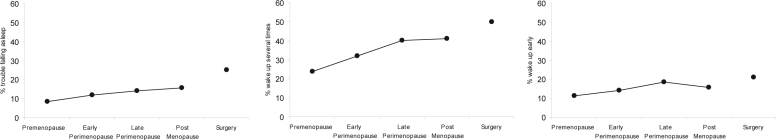
At baseline, 30.8% of the cohort reported at least one of the 3 sleep difficulties (28.0% of premenopausal women and 34.3% of early perimenopausal women). Sleep maintenance, the most prevalent problem, was reported by 25.9% of the baseline cohort. Waking early (12.4%) and trouble falling asleep (10.1%) were notably less prevalent. Over the course of the transition, sleep difficulties increased with the exception that early morning awakening decreased somewhat during the transition from late perimenopause to postmenopause (Figure 1). The highest percentages of each of the 3 sleep problems were reported by those who were surgically menopausal.
Figure 1.
Prevalence (percentage) of women reporting sleep difficulty ≥3 times per week in the past 2 weeks across menopausal transition status (N = 3045). Percentages reporting each sleep difficulty (Y-axis) are plotted as “trouble falling asleep” (left figure), “wake up several times” (center figure), and “wake up early” (right figure).
Note: Percentages were computed as average rates across menopausal status. Women could contribute to more than one menopausal transition state, and within each menopausal transition state a woman could contribute more than one observation. For women with more than one observation within a specific menopausal transition state, the within-woman average sleep score was computed before dichotomizing. Also, “surgery” (i.e., surgically menopausal status) could follow any menopausal status. HT users were excluded from this figure.
Menopausal Transition State and Sleep Difficulties
Age-adjusted results, displayed in Table 2, revealed a progressive and statistically significant increment in the odds of trouble falling asleep as women transitioned to postmenopause over the 7 years. A similar trend was observed for waking up several times and for waking up early, except that the highest ORs were observed in late perimenopause, followed by a small decrement during the transition from late perimenopause to postmenopause.
Table 2.
Age-Adjusted Odds Ratios for Menopausal Status, Vasomotor Symptoms, and Estradiol and Follicle Stimulating Hormone Associated with 3 Types of Sleep Difficulties§
| Trouble falling asleep: 18549∼20245 obs from 3036∼3045 women OR (95% CI) | Wake up several times: 18544∼20239 obs from 3036∼3045 women OR (95% CI) | Wake up early: 18545∼20242 obs from 3036∼3045 women OR (95% CI) | |
|---|---|---|---|
| Menopausal Status | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| Premenopausal (no HT) | 1.00 | 1.00 | 1.00 |
| Early perimenopausal (no HT) | 1.36 (1.11, 1.67)** | 1.41 (1.21, 1.63)*** | 1.20 (1.00, 1.44)* |
| Late perimenopausal (no HT) | 1.95 (1.45, 2.63)*** | 2.39 (1.92, 2.97)*** | 1.93 (1.48, 2.50)*** |
| Postmenopausal (no HT) | 2.35 (1.75, 3.17)*** | 2.24 (1.79, 2.80)*** | 1.54 (1.18, 2.01)** |
| Surgically menopausal (no HT) | 3.34 (2.13, 5.23)*** | 2.52 (1.73, 3.67)*** | 2.05 (1.34, 3.12)** |
| Unknown† | 1.87 (1.39, 2.52)*** | 1.52 (1.22, 1.90)*** | 1.58 (1.22, 2.06)** |
| Postmenopausal (HT) | 1.24 (0.78, 1.97) | 1.44 (1.05, 1.98)* | 1.17 (0.79, 1.72) |
| Surgically menopausal (HT) | 1.44 (0.81, 2.54) | 1.90 (1.23, 2.92)** | 1.93 (1.19, 3.13)** |
| Vasomotor symptoms | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| None | 1.00 | 1.00 | 1.00 |
| < 6 days/2 weeks | 1.86 (1.59, 2.18)*** | 1.68 (1.51, 1.87)*** | 1.70 (1.49, 1.94)*** |
| 6–14 days/2 weeks | 5.28 (4.44, 6.28)*** | 4.89 (4.29, 5.58)*** | 3.89 (3.34, 4.52)*** |
| E2 (log scale) ‡ | |||
| Baseline | 1.14 (0.97, 1.34) | 1.08 (0.95, 1.22) | 1.03 (0.89, 1.18) |
| Change since baseline | 1.15 (1.07, 1.24)*** | 1.12 (1.07, 1.18)*** | 1.06 (0.99, 1.13) |
| FSH (log scale) | |||
| Baseline FSH | 1.02 (0.86, 1.22) | 1.08 (0.95, 1.22) | 1.04 (0.90, 1.21) |
| Change since baseline | 1.10 (1.01, 1.21)* | 1.20 (1.12, 1.28)*** | 1.07 (0.99, 1.16) |
Abbreviations: OR, odds ratio; CI, confidence interval; FSH, follicle stimulating hormone; E2, estradiol; obs, observations (note: numbers of obs and women differ slightly across models due to small differences in missing information between dependent variables and independent variables and covariates).
Estimates were adjusted for time-varying age.
“Unknown” category of menopausal status indicates those women who used hormone therapy but were not postmenopausal yet.
Estimates presented for decreasing E2.
Note: *P < 0.05; **P < 0.01; ***P < 0.001.
After adjusting for all other covariates the general trends described above tended to persist and remained statistically significant, particularly for trouble falling asleep and waking up several times (Table 3). However, some of the sequential pairwise menopausal transition effects (not indicated in the table) were no longer statistically significant. Women transitioning from premenopause to early perimenopause, compared to women who remained premenopausal, had higher odds of reporting waking up several times (OR = 1.26; 95% CI = 1.08, 1.47; P = 0.003); women transitioning from early to late perimenopause had higher ORs of reporting both waking up several times (OR = 1.55; 95% CI = 1.30, 1.86; P < 0.001) and early morning awakening (OR = 1.45; 95% CI = 1.17, 1.79; P = 0.001); and women transitioning from late perimenopause to postmenopause had a lower OR of reporting early morning awakening (OR = 0.77; 95% CI = 0.61, 0.98; P = 0.03). Among the 8 menopausal status/HT use categories, women who were surgically menopausal without current HT use had the highest ORs for trouble falling asleep and waking up several times. In naturally postmenopausal women, compared to HT non-users, women who were HT users had lower ORs for trouble falling asleep (OR = 0.53; 95% CI = 0.34, 0.85; P = 0.007) and waking up several times (OR = 0.64; 95% CI = 0.48, 0.85; P = 0.002). Similarly, surgically postmenopausal women with HT use had lower ORs for trouble falling asleep than did those without HT use (OR = 0.51; 95% CI = 0.26, 0.99; P = 0.047). Because the “unknown” category includes a mix of pre-, early, and late perimenopausal HT users, we could not differentiate the HT effect for any of these 3 groups to permit interpretable “HT–no HT” comparisons within each group.
Table 3.
Multivariable Adjusted Odds Ratios for Menopausal Status, Vasomotor Symptoms, Estradiol and Follicle Stimulating Hormone Associated with 3 Types of Sleep Difficulties§
| Trouble falling asleep: 17692∼18748 obs from 2997∼3006 women OR (95% CI) | Wake up several times: 18070∼19670 obs from 3032∼3043 women OR (95% CI) | Wake up early: 18234∼19809 obs from 3033∼3043 women OR (95% CI) | |
|---|---|---|---|
| Menopausal Status | P = 0.0006 | P < 0.0001 | P = 0.0041 |
| Premenopausal (no HT) | 1.00 | 1.00 | 1.00 |
| Early perimenopausal (no HT) | 1.16 (0.93, 1.45) | 1.26 (1.08, 1.47)** | 1.06 (0.88, 1.28) |
| Late perimenopausal (no HT) | 1.49 (1.08, 2.06)* | 1.96 (1.56, 2.46)*** | 1.53 (1.17, 2.01)** |
| Postmenopausal (no HT) | 1.77 (1.29, 2.44)*** | 1.78 (1.42, 2.23)*** | 1.19 (0.91, 1.56) |
| Surgically menopausal (no HT) | 2.05 (1.21, 3.49)** | 2.04 (1.38, 3.03)*** | 1.50 (0.96, 2.33) |
| Unknown† | 1.55 (1.13, 2.14)** | 1.22 (0.97, 1.54) | 1.39 (1.07, 1.82) * |
| Postmenopausal (HT) | 0.95 (0.57, 1.57) | 1.13 (0.82, 1.57) | 1.03 (0.70, 1.53) |
| Surgically menopausal (HT) | 1.05 (0.58, 1.88) | 1.48 (0.95, 2.30) | 1.60 (0.98, 2.61) |
| Vasomotor symptoms | P < 0.0001 | P < 0.0001 | P < 0.0001 |
| None | 1.00 | 1.00 | 1.00 |
| < 6 days/2 weeks | 1.37 (1.16, 1.62)*** | 1.31 (1.17, 1.47)*** | 1.35 (1.17, 1.55)*** |
| 6–14 days/2 weeks | 2.60 (2.14, 3.14)*** | 2.93 (2.55, 3.37)*** | 2.16 (1.84, 2.54)*** |
| E2 (log scale) ‡ | |||
| Baseline | 1.09 (0.94, 1.27) | 1.04 (0.93, 1.17) | 0.99 (0.87, 1.12) |
| Change since baseline | 1.12 (1.04, 1.21)** | 1.08 (1.02, 1.14)** | 1.03 (0.97, 1.10) |
| FSH (log scale) | |||
| Baseline | 1.01 (0.86, 1.18) | 1.01 (0.90, 1.14) | 0.98 (0.86, 1.12) |
| Change since baseline | 1.05 (0.95, 1.16) | 1.11 (1.03, 1.19)** | 1.02 (0.94, 1.11) |
Abbreviations: OR, odds ratio; CI, confidence interval; FSH, follicle stimulating hormone; E2, estradiol; HT, hormone therapy; CES-D, Center for Epidemiologic Studies Depression Scale; obs, observations (note: numbers of obs and women differ slightly across models due to small differences in missing information between dependent variables and independent variables and covariates).
Ethnicity, site, age, and current HT use were forced into all the final multivariable models. Estimates also were adjusted for the following covariates (P < 0.05) in Menopausal Status models for all 3 sleep difficulties: CES-D score, anxiety symptom score, urinate at night, number of physical symptoms, bodily pain score, very upsetting life events, and psychotropic/sleep medication use. For Trouble Falling Asleep: model also was adjusted for difficulty paying for basics, body mass index, smoking status, and education. Education overall effect was not statistically significant but was included because it was an important confounder for the smoking effect. For Wake Up Several Times: model also was adjusted for pain medication use and self-assessed overall health. In all Vasomotor Symptoms models the sleep difficulties were adjusted for the same set of covariates as in the respective Menopausal Status models. Covariate adjustment in the Hormone (E2 and FSH) models − for Trouble Falling Asleep: same as Menopausal Status model; for Wake Up Several Times: same as Menopausal Status model, plus number of medical conditions; for Wake Up Early: same as Menopausal Status models. Only Hormone models were additionally adjusted for whether the blood level was drawn within the cycle day 2–5 window. In multivariable hormone models, compared with women who had blood drawn in cycle day 2–5 window, women who did not have blood drawn in window were more likely to report sleep difficulties with odds ratio of 1.18–1.38.
“Unknown” category of menopausal status indicates those women who used hormone therapy but were not postmenopausal yet.
Estimates presented for decreasing E2.
Note: *P < 0.05; **P < 0.01; ***P < 0.001.
Vasomotor Symptoms and Sleep Difficulties
For all 3 types of sleep difficulties, a significant dose-response relationship was observed with VMS in the age-adjusted models (Table 2), which persisted, although of lesser magnitude (ORs for ≥ 6 days = 2.16–2.93) after adjusting for the additional covariates (Table 3). More frequent VMS were associated with a significantly higher OR of each sleep difficulty as compared with reports of either no VMS or 1–5 days of VMS. These effects of VMS (and menopausal status) were each independently related to sleep difficulties in multivariable models (Table 3).
E2 and FSH Levels
In modeling the hormone effects, the log-transformed E2 and FSH levels in the follow-up visits were split into baseline (cross-sectional) and change since baseline (longitudinal) values. Age-adjusted results (Table 2) were attenuated modestly in the multivariable-adjusted models, which also were adjusted for blood draw cycle day (Table 3). We observed negative associations between E2 levels and sleep problems and positive associations between FSH and sleep problems. Decreases in annual E2 levels were significantly associated with higher odds of trouble falling asleep and of waking up several times. Increases in annual FSH levels were significantly associated with higher odds of waking up several times.
Race/Ethnicity and Sleep Difficulties
Pairwise comparisons between race/ethnic groups were performed for all multivariable models. For trouble falling asleep, no differences were found between ethnic groups. For waking up several times, the estimates for Caucasian women were significantly higher than for all the other race/ethnic groups, and the estimates for Hispanic women were significantly lower than for all the other race/ethnic groups. For early morning awakening, the estimates for Caucasian women were significantly higher than those for Hispanic women in all models and were marginally significantly lower than those for Chinese women in menopausal status and VMS models. The estimates for Hispanic women were significantly lower than for African American women and for Chinese women in all models. The lower estimates for Japanese women compared with those for Chinese women were of only marginal statistical significance in all models.
In addition to the mean difference between race/ethnic groups, we also examined the potential interaction effect between race/ethnic groups and each of the menopausal transition-related variables in all multivariable models. Statistically significant interactions were found in 2 of 12 models. For waking up several times, we found variation in race/ethnic effects across menopausal status (χ2 = 61.22, df = 28, P = 0.0003); however, because of the many cells (number of cells = 40), some of them with few participants, further interpretation is ambiguous. After removing HT users, surgically menopausal women, and New Jersey participants to reduce model fitting complexity, we observed no statistically significant interaction (χ2 = 14.56, df = 9, P = 0.1038), indicating that the adjusted associations between sleep difficulties and menopausal status did not vary across race/ethnic groups. In the full sample, the VMS-by-race/ethnicity interaction for early morning awakening was significant (χ2 = 24.52, df = 8, P = 0.0019). Within each racial/ethnic group, ORs for early morning awakening increased with increasing VMS frequency compared with no VMS, reaching statistical significance for all groups with VMS ≥ 6 days (P ≤ 0.001). Groups differed in their pattern and magnitude of increase. ORs were highest for Chinese (OR = 3.97) and Japanese (OR = 4.22) women reporting VMS ≥ 6 days, and Hispanic women had similar ORs for 1–5 days (OR = 2.87) and ≥ 6 days (OR = 2.96). Within each of the 3 VMS levels, comparisons between the Caucasian group and other race/ethnic groups revealed only two statistically significant differences – in those without VMS, Hispanic women were less likely to report early morning awakening (OR = 0.37, P = 0.002) and, for VMS ≥ 6 days, Chinese women were more likely (OR = 2.87, P = 0.001) to report early morning awakening. After removing HT users, surgically menopausal women, and New Jersey participants, the interaction remained statistically significant (χ2 = 12.71, df = 6, P = 0.0479).
DISCUSSION
In this prospective longitudinal multi-ethnic, multisite, community-based study of over three thousand women, we have shown that the menopausal transition is fraught with the perception of sleep difficulties. This finding is based on examination of 3 transition-related variables—menopausal status according to bleeding criteria, E2 and FSH levels, and vasomotor symptoms—while simultaneously adjusting for age, HT use, and multiple factors that have been associated with self-reported sleep difficulties.
Our baseline rate of 30.8% of the premenopausal and early perimenopausal women reporting at least one of the 3 sleep difficulties ≥ 3 times a week is very similar to the prevalence of one or more sleep problems “on most nights,” 30.4%, in a UK population-based sample of mixed gender and age (median age 50 years, 55% women).43 Our longitudinal assessments extended such previous findings as those reported by Morphy et al43 by examining in longitudinal models specific types of sleep problems over a longer period of time. Among HT non-users, self-reported sleep maintenance (waking up several times) and early morning awakening worsened significantly through late perimenopause, and the latter improved during postmenopause to a level no longer significantly different from premenopause. One consideration is that less early morning awakening could be due to increasing fatigue from the other sleep difficulties; we did not examine fatigue in these analyses. Another possibility is that change in depression might explain the finding since early morning awakening is a sleep related symptom of depression. Bromberger et al44 examined change in menopausal status and risk of clinically relevant depressive symptoms in SWAN participants, and observed that the risk was nonsignificantly less in postmenopause compared to late perimenopause. We observed a similar pattern, with a nonsignificantly decreased OR for a CES-D score ≥ 16 after age adjustment. Including HT users and after adjusting for relevant covariates, menopausal status and vasomotor symptoms each remained significantly and independently associated with difficulty sleeping (Table 3), despite large contributions from physical and psychological symptoms and nighttime urinary frequency.
Although not all studies of the sleep of middle-aged women show an effect for menopausal status,18 the negative studies tend to be cross-sectional. Our longitudinal results are consistent with those of our previous cross-sectional analysis of a larger sample using the SWAN screening data, in which VMS and mood symptoms were strongly associated with trouble sleeping in premenopausal and perimenopausal women.9 Subjective sleep difficulties observed also were consistent with observations in the Wisconsin Sleep Cohort that transitioning women reported increasing sleep dissatisfaction.45 Young et al45 also observed no difference in polysomnographically measured sleep parameters between menopausal women who did and did not report hot flashes associated with sleep and concluded that hot flashes do not cause sleep disturbances.
Many epidemiologic studies have found associations between sleep continuity disturbance and vasomotor symptoms, particularly hot flashes (the most common perimenopausal symptom46) during the menopausal transition in perimenopausal and postmenopausal women.47,48 Both VMS and the “psychological complaints,” or mood symptoms, tend to occur more during perimenopause, although they may be more elevated during early postmenopause than during premenopause.49,50 Earlier research conducted under controlled laboratory conditions suggested that hot flashes tended to follow rather than precede arousals and awakenings and therefore may not necessarily produce symptomatic sleep continuity disturbance.47,51 More recent data suggest that REM-associated suppression of thermoregulatory effector responses may account for discrepancies between self- and laboratory-reports of hot flash-induced sleep disturbance.52 Thus, the basis for this relationship may be an underlying physiological change that may contribute both to sleep continuity disturbance and VMS. On the other hand, women may associate hot flashes with awakenings if they occur very close in time or if they become more perceptually aware of their occurrence.
Sleep changes in transitioning women have been attributed at least in part to changing sex steroid hormone profiles, although precise relationships remain to be defined. Hormonal effects may be indirect and associated with changes in thermoregulation, or may be direct, due to an evolving hormonal milieu.18 We observed decreases in E2 in association with trouble falling asleep and waking up several times, and increases in FSH in association with waking up several times. Both findings are consistent with known hormonal changes in women progressing through the menopausal transition to menopause, in whom estrogen is decreasing and FSH is increasing.
Longitudinal data in the literature are sparse on relationships between steroid hormone levels and sleep problems in women transitioning from pre- to postmenopause. In a group of regularly menstruating women aged 35–47 years followed for 2 years, Hollander et al23 reported that low follicular phase plasma E2 (but not FSH) was associated with poor sleep. However, this study was of relatively short duration and measurements of “poor quality sleep” were based on ratings for only the previous night. Dennerstein et al53 followed 172 women who were premenopausal at baseline and had transitioned to peri- or postmenopause by the end of 7 years of follow-up. The single symptom checklist item “trouble sleeping” showed a small gradual increment across the transition that was not predicted by annually measured FSH or E2 blood levels. The 9-year follow-up by Dennerstein and coworkers54 confirmed this graded increment, noting a significant difference between late reproductive and early menopausal transition stages combined compared with late menopausal transition or postmenopausal women, as well as those using HT. They also found an association between a decline in E2 and sleep problems, but the effect of changing E2 levels was mediated by hot flashes and night sweats. Woods et al55 also found no significant correlations between sleep disruption (nighttime awakenings or early morning awakening) and urinary FSH, estrone, or testosterone levels, but hot flashes were significantly correlated with sleep symptoms. This report, too, was based on a small subsample—41 women with a final menstrual period who completed symptom ratings on cycle days 5–7 and urine collections on day 6 monthly or quarterly (or on similar consistent days if no longer menstruating).
There is little epidemiologic research on how sleep, and particularly sleep continuity disturbance, varies by race/ethnicity, and the literature is nearly silent on the sleep of Hispanics.56 We observed racial/ethnic differences in staying asleep and early morning awakening but not for trouble falling asleep in women going through the menopausal transition. Compared with our other racial/ethnic groups, Caucasian women had higher rates of difficulty staying asleep and Hispanics had lower rates of difficulty staying asleep and early morning awakenings. Most published studies of race/ethnic sleep have focused on differences between African Americans and Caucasians. For instance, Durrence and Lichstein57 reported that African Americans have lighter (increased stages 1 and 2, decreased stages 3 and 4) and more fragmented sleep (longer sleep latencies, greater wakefulness after sleep onset) than Caucasians. In a study that included Hispanics, Karacan and coworkers58 observed that Hispanic women had less difficulty staying asleep than did African American women, as we did, but that they were similar to Caucasian women. Lower rates of sleep difficulties reported by Hispanic women are particularly intriguing in light of the findings of Hale and Do56 of higher odds of short sleep in Hispanics (excluding Mexican Americans) and other minorities and require further investigation.
One limitation of our study was the lack of objective measures of sleep quality to validate self-reports. Discrepancies between subjective and objective sleep quality have been observed in studies of menopausal women.45,59 One could argue that the 2 measures reflect different aspects of sleep phenomena and that subjective sleep problems are no less valid than objective ones and may be more relevant or important in terms of women's use of health care and their quality of life. Moreover, in a community-based sample, self-selection bias for reporting trouble sleeping should be minimized. Further, our large sample size, multi-ethnic sample and longitudinal follow-up design with good retention, and controlling for multiple factors that have been associated with self-reported sleep difficulties are significant strengths of our study. We recognize that self-report alone is not sufficient for distinguishing causes of sleep difficulties.
Another limitation is that nocturnal hot flashes were not measured. Due to time limitations (i.e., respondent burden), SWAN did not include a number of questions it might have, including nightly hot flashes. In addition to hot flashes, our vasomotor summary variable included night sweats, which may be a prominent nighttime manifestation accompanying hot flashes, and cold sweats. These symptoms had a high single factor loading, indicating they were substantially correlated.15
In the SWAN ancillary sleep study, we are collecting polysomnographic data using ambulatory monitoring on a subsample of this cohort and will be able to compare and report differences between self-report and monitored sleep data; preliminary data from this study have been presented.60 Sleep related skin temperature change and vasomotor symptom data also are being collected.
In summary, we have found that sleep maintenance in particular but also sleep initiation and early morning awakenings are relatively prevalent concerns among women going through the menopausal transition. The Women's Health Initiative61 reported that hormone therapy led to modest improvement in sleep. In SWAN, women with high levels of sleep impairment had more improvement in bodily pain after initiating HT and tended towards improvement in role emotional functioning and vitality.62 However, the negative effects of hormone therapy on other health outcomes preclude routine use of hormone therapy in perimenopausal women. Thus, a better understanding of the trajectory of changes in endogenous reproductive hormones during the transition and their effects on VMS and sleep is particularly important.
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, DHHS, through the National Institute on Aging, the National Institute of Nursing Research and the NIH Office of Research on Women's Health (Grants AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495; NR004061).
Clinical Centers: University of Michigan, Ann Arbor—MaryFran Sowers, PI; Massachusetts General Hospital, Boston, MA—Robert Neer, PI 1994–1999; Joel Finkelstein, PI 1999- present; Rush University Medical Center, Chicago, IL—Lynda Powell, PI; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; Nanette Santoro, PI 2004-present; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Marcia Ory 1994–2001; Sherry Sherman 1994-present; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001; University of Pittsburgh, Pittsburgh, PA—Kim Sutton-Tyrrell, PI 2001-present.
Steering Committee: Chris Gallagher, Chair; Susan Johnson, Chair
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Disclosure Statement
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.Brugge KL, Kripke DF, Ancoli-Israel S, Garfinkel L. The association of menopausal status and age with sleep disorders. [Abstract] Sleep Res. 1989;18:208. [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 3.Cirignotta F, Mondini S, Zucconi M, Lenzi PL, Lugaresi E. Insomnia: an epidemiological survey. Clin Neuropharmacol. 1985;8(Suppl. 1):S49–54. doi: 10.1097/00002826-198508001-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Kales A, Soldatos CR, Kales JD, Healey S. Prevalence of sleep disorders in the Los Angeles metropolitan area. Am J Psychiatry. 1979;136:1257–62. doi: 10.1176/ajp.136.10.1257. [DOI] [PubMed] [Google Scholar]
- 5.Karacan I, Thornby JI, Anch M, et al. Prevalence of sleep disturbance in a primarily urban Florida county. Soc Sci Med. 1976;10:239–44. doi: 10.1016/0037-7856(76)90006-8. [DOI] [PubMed] [Google Scholar]
- 6.Quera-Salva MA, Orluc A, Goldenberg F, Guilleminault C. Insomnia and use of hypnotics: study of a French population. Sleep. 1991;14:386–91. doi: 10.1093/sleep/14.5.386. [DOI] [PubMed] [Google Scholar]
- 7.Weyerer S, Dilling H. Prevalence and treatment of insomnia in the community: results from the Upper Bavarian Field Study. Sleep. 1991;14:392–8. [PubMed] [Google Scholar]
- 8.NIH State-of-the-Science Panel. National Institutes of Health State-of-the-Science conference statement: management of menopause-related symptoms. Ann Intern Med. 2005;142:1003–13. [PubMed] [Google Scholar]
- 9.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Blois R, Feinberg I, Gaillard JM, Kupfer DJ, Webb WB. Sleep in normal and pathological aging. Experientia. 1983;39:551–686. doi: 10.1007/BF01971096. [DOI] [PubMed] [Google Scholar]
- 11.Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the “middle” years of life (20y-59y) J Sleep Res. 1997;6:230–7. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 12.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165:2370–6. doi: 10.1001/archinte.165.20.2370. [DOI] [PubMed] [Google Scholar]
- 13.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 14.Williams RL, Karacan I, Hursch CJ. Electroencephalography (EEG) of human sleep: clinical applications. New York: John Wiley & Sons; 1974. Changes in sleep variables across age groups; pp. 69–95. [Google Scholar]
- 15.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women's Health Across the Nation (SWAN) Am J Public Health. 2006;96:1226–35. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Randolph JF, Sowers MF, Bondarenko I, et al. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–12. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 17.Empson JA, Purdie DW. Effects of sex steroids on sleep. Ann Med. 1999;31:141–5. doi: 10.3109/07853899708998790. [DOI] [PubMed] [Google Scholar]
- 18.Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–55. Errata. Sleep 2000;23:145–9. [PubMed] [Google Scholar]
- 19.Gold EB, Sternfeld B, Kelsey JL, et al. Relation of demographic and lifestyle factors to symptoms in a multi-racial/ethnic population of women 40–55 years of age. Am J Epidemiol. 2000;152:463–73. doi: 10.1093/aje/152.5.463. [DOI] [PubMed] [Google Scholar]
- 20.Dzaja A, Arber S, Hislop J, et al. Women's sleep in health and disease. J Psychiatr Res. 2005;39:55–76. doi: 10.1016/j.jpsychires.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Moe KE. Reproductive hormones, aging, and sleep. Semin Reprod Endocrinol. 1999;17:339–48. doi: 10.1055/s-2007-1016243. [DOI] [PubMed] [Google Scholar]
- 22.Shaver JLF, Zenk SN. Sleep disturbance in menopause. J Womens Health Gend Based Med. 2000;9:109–18. doi: 10.1089/152460900318605. [DOI] [PubMed] [Google Scholar]
- 23.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98:391–7. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 24.Roth T, Jaeger S, Jin R, Kalsekar A, Stang PE, Kessler RC. Sleep problems, comorbid mental disorders, and role functioning in the National Comorbidity Survey Replication. Biol Psychiatry. 2006;60:1364–71. doi: 10.1016/j.biopsych.2006.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Marcus R, Kelsey J, editors. Menopause: biology and pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 26.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 27.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 28.Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Arch Intern Med. 1992;152:1634–7. [PubMed] [Google Scholar]
- 29.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- 31.Neugarten BL, Kraines RJ. “Menopausal symptoms” in women of various ages. Psychosom Med. 1965;27:266–73. doi: 10.1097/00006842-196505000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–14. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 33.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 34.Ferris BG. Epidemiology Standardization Project (American Thoracic Society) Am Rev Respir Dis. 1978;118:1–120. [PubMed] [Google Scholar]
- 35.Coghlin J, Hammond SK, Gann PH. Development of epidemiologic tools for measuring environmental tobacco smoke exposure. Am J Epidemiol. 1989;130:696–704. doi: 10.1093/oxfordjournals.aje.a115391. [DOI] [PubMed] [Google Scholar]
- 36.Sternfeld B, Ainsworth BA, Quesenberry CP., Jr Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–23. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 37.Baecke JAH, Burema J, Fritjers JER. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–42. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 38.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–69. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 39.Diggle PJ, Heagerty PJ, Liang K-Y, Zeger SL. 2nd ed. New York, NY: Oxford University Press Inc; 2002. Analysis of longitudinal data. [Google Scholar]
- 40.Stata. College Station, TX: Stata Press; 2003. Cross-Sectional Time-Series Reference Manual, Release 8. [Google Scholar]
- 41.StataCorp. College Station, TX: Stata Corporation; 2003. Stata Statistical Software: Release 8.0. [Google Scholar]
- 42.Cary, NC: SAS Institute; 2001. SAS/STAT Software changes and enhancements, release 8.2. [Google Scholar]
- 43.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 44.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN) J Affect Disord. 2007;103:267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young T, Rabago D, Zgierska A, Austin D, Finn L. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 46.Avis NE, Crawford SL, McKinlay SM. Psychosocial, behavioral, and health factors related to menopause symptomatology. Womens Health. 1997;3:103–20. [PubMed] [Google Scholar]
- 47.Freedman RR. Hot flashes: behavioral treatments, mechanisms, and relation to sleep. Am J Med. 2005;118(12B):124S–130S. doi: 10.1016/j.amjmed.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 48.Ohayon MM. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166:1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 49.Boulet MJ, Oddens BJ, Lehert P, Vemer HM, Visser A. Climacteric and menopause in seven south-east Asian countries. Maturitas. 1994;19:157–76. doi: 10.1016/0378-5122(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 50.Bromberger JT, Assmann SF, Avis NE, Schocken M, Kravitz HM, Cordal A. Persistent mood symptoms in a multiethnic community cohort of pre- and perimenopausal women. Am J Epidemiol. 2003;158:347–56. doi: 10.1093/aje/kwg155. [DOI] [PubMed] [Google Scholar]
- 51.Freedman RR, Roehrs TA. Lack of sleep disturbance from menopausal hot flashes. Fertil Steril. 2004;82:138–44. doi: 10.1016/j.fertnstert.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 52.Freedman RR, Roehrs TA. Effects of REM sleep and ambient temperature on hot flash-induced sleep disturbance. Menopause. 2006;13:576–83. doi: 10.1097/01.gme.0000227398.53192.bc. [DOI] [PubMed] [Google Scholar]
- 53.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet Gynecol. 2000;96:351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 54.Dennerstein L, Lehert P, Guthrie JR, Burger HG. Modeling women's health during the menopausal transition: a longitudinal analysis. Menopause. 2007;14:53–62. doi: 10.1097/01.gme.0000229574.67376.ba. [DOI] [PubMed] [Google Scholar]
- 55.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women's Health Study. J Womens Health. 2007;16:667–77. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 56.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30:1096–103. doi: 10.1093/sleep/30.9.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durrence HH, Lichstein KL. The sleep of African Americans: a comparative review. Behav Sleep Med. 2006;4:29–44. doi: 10.1207/s15402010bsm0401_3. [DOI] [PubMed] [Google Scholar]
- 58.Karacan I, Thornby JI, Williams RL. Sleep disturbance: a community survey. In: Guilleminault C, Lugaresi E, editors. Sleep/wake disorders: natural history, epidemiology, and long-term evolution. New York: Raven Press; 1983. pp. 37–60. [Google Scholar]
- 59.Polo-Kantola P, Erkkola H, Irjala K, Helenius H, Pullmen S, Polo O. Climacteric symptoms and sleep quality. Obstet Gynecol. 1999;94:219–24. doi: 10.1016/s0029-7844(99)00284-7. [DOI] [PubMed] [Google Scholar]
- 60.Hall M, Kravitz HM, Gold E, et al. Sleep during the menopausal transition in a multi-ethnic cohort: Feasibility and preliminary results. [Abstract 0350] Sleep. 2005;28(Abstract Supplement):A119. [Google Scholar]
- 61.Hays J, Ockene JK, Brunner RL, et al. for the Women's Health Initiative Investigators Effects of estrogen plus progestin on health-related quality of life. N Engl J Med. 2003;348:1839–54. doi: 10.1056/NEJMoa030311. [DOI] [PubMed] [Google Scholar]
- 62.Hess R, Colvin A, Avis NE, et al. The impact of hormone therapy on health-related quality of life: longitudinal results from the Study of Women's Health Across the Nation. Menopause. 2008;15:422–428. doi: 10.1097/gme.0b013e31814faf2b. [DOI] [PubMed] [Google Scholar]