Summary
The epithelial-mesenchymal transition (EMT) is a key developmental program that is often activated during cancer invasion and metastasis. Induction of an EMT in immortalized human mammary epithelial cells (HMLEs) results in the acquisition of mesenchymal traits, but in addition the expression of stem-cell markers, and an increased ability to form mammospheres, a property associated with mammary epithelial stem cells. Independent of this, stem cell-like cells isolated from HMLE cultures form mammospheres, differentiate into duct-like structures, and express markers similar to those of HMLEs that have undergone an EMT. Moreover, stem-like cells isolated either from mouse mammary glands, human reduction mammoplasty tissues, or mammary carcinomas express markers associated with cells that have undergone an EMT. Finally, transformed human mammary epithelial cells that have undergone an EMT form mammospheres, soft agar colonies, and tumors more efficiently. These findings illustrate a direct link between the EMT and the gain of epithelial stem-cell properties.
Introduction
The epithelial-mesenchymal transition (EMT) and the reverse process, termed the mesenchymal-epithelial transition (MET), play central roles in embryogenesis (Hay, 1995; Perez-Pomares and Munoz-Chapuli, 2002; Thiery and Sleeman, 2006). For example, during early embryonic development, the mesoderm generated by EMTs develops into multiple tissue types, and later in development, mesodermal cells generate epithelial organs, such as the kidney and ovary, via METs (Davies, 1996).
Developmental genetics research has revealed a number of pleiotropically acting transcription factors that play critical roles in embryogenesis by orchestrating EMTs (Briegel, 2006). In recent years, these embryonic transcription factors have been found to confer malignant traits, such as motility, invasiveness, and resistance to apoptosis, on neoplastic cells (Cheng et al., 2007; Comijn et al., 2001; Hartwell et al., 2006; Huber et al., 2005; Mani et al., 2007; Oft et al., 2002; Peinado et al., 2007; Savagner et al., 2005; Yang et al., 2004). Some of these transcription factors also appear to play key roles in wound healing (Savagner et al., 2005).
Independent of these findings, a large body of research has described stem cells in normal tissues, which are capable of renewing themselves through asymmetrical cell division while simultaneously generating committed progenitor cells whose descendants may eventually differentiate and carry out tissue-specific functions (Reya et al., 2001). More recently, studies of neoplastic tissues has provided evidence of self-renewing, stem-like cells within tumors, which have been called cancer stem cells (CSCs). CSCs constitute a small minority of neoplastic cells within a tumor and are defined operationally by their ability to seed new tumors. For this reason, they have also been termed “tumor-initiating cells” (Reya et al., 2001).
CSCs were first identified in the hematopoietic system (Bonnet and Dick, 1997); more recently, however, they have also been discovered in solid tumors, including those arising in the breast, colon and brain (Al-Hajj et al., 2003; O'Brien et al., 2007; Ricci-Vitiani et al., 2007; Singh et al., 2004). For example, a small subpopulation of cancer cells is present within some human breast tumors that exhibits a CD44high/CD24low antigenic phenotype; these cells are highly enriched for tumor-initiating cells in comparison to the majority of carcinoma cells of the CD44low/CD24high phenotype found in the same tumors (Al-Hajj et al., 2003).
During the process of tumor metastasis, which is often enabled by EMTs (Thiery, 2003), disseminated cancer cells would seem to require self-renewal capability, similar to that exhibited by stem cells, in order to spawn macroscopic metastases. This raises the possibility that the EMT process, which enables cancer cell dissemination, may also impart a self-renewal capability to disseminating cancer cells. Indeed, the metastatic process is at least superficially similar to the processes that occur during tissue repair and regeneration and enable adult stem cells to exit tissue reservoirs, such as the bone marrow, enter and survive in the circulation, and exit into secondary tissue sites, where they proliferate, differentiate and participate in tissue reconstruction (Kondo et al., 2003).
Together, these diverse lines of evidence suggested a possible link between less differentiated stem cells and the mesenchymal-appearing cells generated by EMTs. In this report, we used a variety of human and murine mammary epithelial cells to address the possible association between the EMT and the formation of stem cells. We show that cells that have undergone an EMT behave in many respects similar to stem cells isolated from normal or neoplastic cell populations.
Results
EMTs generate stem cell-like cells
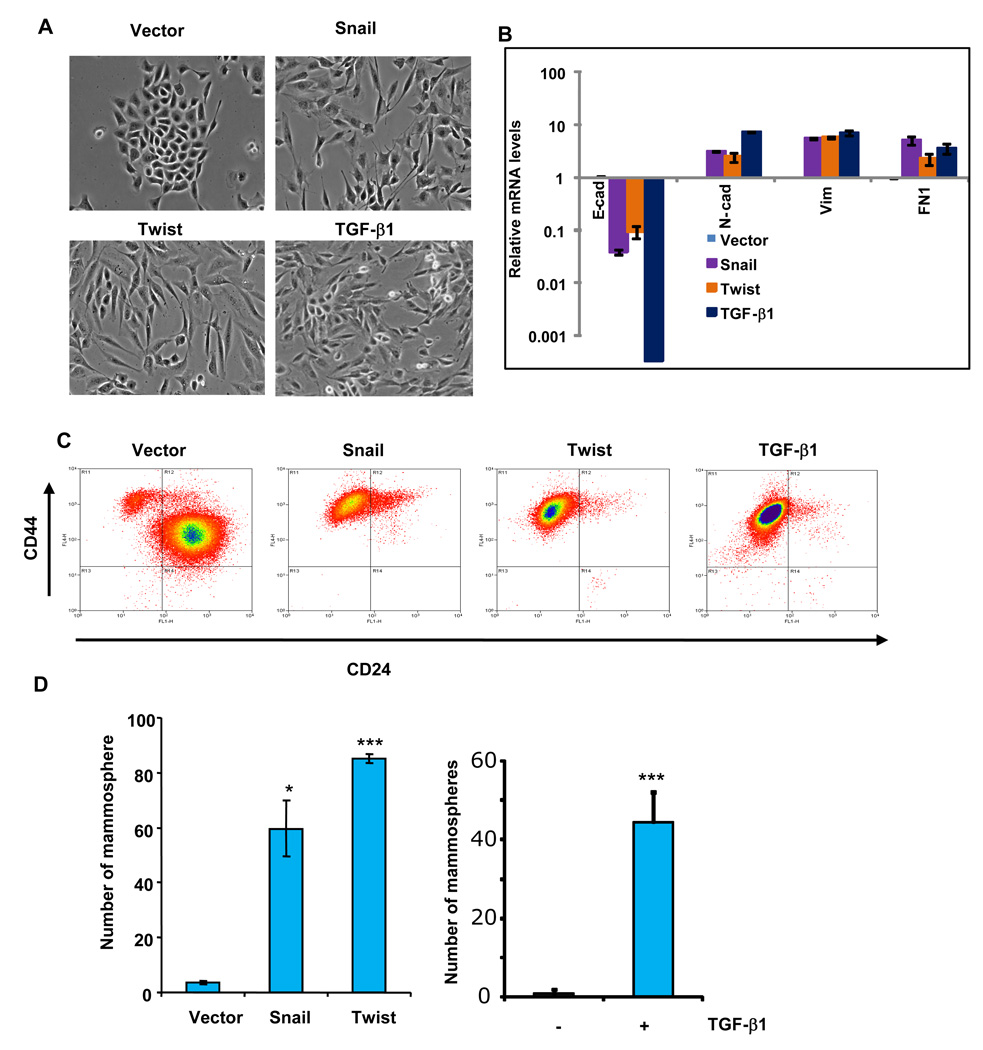
To determine whether adult cells that have undergone an EMT and adult stem cells have similar traits, we induced an EMT in non-tumorigenic, immortalized human mammary epithelial cells (HMLEs) by ectopic expression of either the Twist or Snail transcription factors, both of which are capable of inducing EMTs in epithelial cells (Batlle et al., 2000; Cano et al., 2000; Yang et al., 2004). As anticipated, the resulting cells acquired fibroblast-like, mesenchymal appearances (Figure 1A), down-regulated the expression of mRNAs encoding epithelial markers, such as E-cadherin, and upregulated mRNAs encoding mesenchymal markers, such as N-cadherin, vimentin, and fibronectin (Figure 1B and Supplementary Figure 1). We then used flow cytometry analysis to sort the cells based on the expression CD44 and CD24, two cell-surface markers whose expression in the CD44high/CD24low configuration is associated with both human breast CSCs and normal mammary epithelial stem cells (Al-Hajj et al., 2003; Sleeman et al., 2006).
Figure 1.
The epithelial-mesenchymal transition (EMT) generates cells with properties of stem cells. (A) Phase-contrast images of HMLE cells expressing Snail, Twist or the control vector, as well as HMLE cells treated with recombinant TGF-β1 (2.5 ng/ml) for 12 days (bottom right). (B) Relative expression of the mRNAs encoding E-cadherin, N-cadherin, vimentin, and fibronectin in HMLE cells induced to undergo EMT by the methods outlined in panel A, as determined by Real-time RT-PCR. GAPDH mRNA was used to normalize the variability in template loading. The data are reported as mean +/− SEM). (C) FACS analysis of cell-surface markers, CD44 and CD24, in the cells described in panel A. (D) In vitro quantification of mammospheres formed by cells described in panel A. The data are reported as the number of mammospheres formed/1,000 seeded cells +/− SEM, (* - P < 0.05; *** - P<0.001 comparing to the control).
We found that most, if not all of the mesenchymal-like cells generated by these EMTs acquired a CD44high/CD24low expression pattern — precisely the antigenic phenotype that has been ascribed to neoplastic mammary stem cells (Al-Hajj et al., 2003; Liao et al., 2007; Sleeman et al., 2006); this shift was not observed in cells expressing a control vector (Figure 1C). Similarly, induction of an EMT in HMLEs by exposure to TGF-β1 resulted in the appearance of mesenchymal-looking cells (Figure 1A) and the acquisition of the CD44high/CD24low antigen phenotype (Figure 1C). These various observations showed that the HMLEs that have undergone an EMT develop antigenic markers associated with normal and neoplastic mammary stem cells.
Both human and murine mammary epithelial stem cells can form mammospheres in suspension culture (Dontu et al., 2003; Liao et al., 2007); in addition, following implantation into the mammary stromal fat pad, the murine stem cells can reconstitute an entire mammary ductal tree (Shackleton et al., 2006; Stingl et al., 2006). Our own studies (Liao et al., 2007) and those of others (Liu et al., 2006; Moraes et al., 2007) have demonstrated that the ability to form mammospheres in vitro depends on the presence of self-renewing, gland-reconstituting stem cells within the population. Moreover, the use of mammosphere assay to assess the presence of stem cells in a population of MECs is further validated by the fact that a single murine mammosphere can regenerate an entire mammary ductal tree when implanted into a cleared mouse mammary stromal fat pad (Moraes et al., 2007).
With these findings in mind, we examined the mammosphere-forming ability of the HMLEs forced to undergo an EMT by ectopic expression of Snail or Twist. Significantly, we found that the HMLEs that underwent an EMT formed >30-fold more mammospheres than did HMLEs infected with the corresponding control vector (Figure 1D). In addition, after we induced an EMT in HMLE cells by exposing them to TGF-β1, these cells formed at least >40-fold more mammospheres than did untreated control cells (Figure 1D). Based on this functional assay, we concluded that the cells generated by an EMT acquired yet another attribute of mammary stem cells.
To further assess whether the appearance of the CD44high/CD24low cells and the gain of mammosphere-forming ability are correlated with the EMT process, we activated either Snail or Twist function reversibly in HMLE cells that expressed the respective transcription factors fused, in each case, to a modified estrogen receptor (ER). Following activation of these fusion proteins, achieved by addition of the ER ligand tamoxifen (4-OHT) to these cells, we monitored the expression of the cell-surface markers CD44 and CD24 and the acquisition of mammosphere-forming ability.
Following tamoxifen addition, the HMLE-Snail-ER and HMLE-Twist-ER cells developed a mesenchymal morphology similar to HMLE cells stably expressing Snail or Twist (Supplementary Figure 2A). Moreover, the great majority of HMLE-Snail-ER or Twist-ER cells treated with tamoxifen for 12 days also showed increased expression of vimentin (Supplementary Figure 9). As anticipated, HMLE-Snail-ER cells acquired a mesenchymal mRNA expression profile, as evidenced by increased expression of the mRNAs encoding the N-cadherin, vimentin, and fibronectin proteins and decreased expression of E-cadherin mRNA (Supplementary Figure 2B). Similar results were obtained with HMLE-Twist-ER cells (data not shown). In addition, the tamoxifen-treated cells progressively developed the CD44high/CD24low marker phenotype (Supplementary Figure 2C). In accord with the results cited earlier, following tamoxifen treatment, these cells gradually acquired the ability to form increased numbers of mammospheres when this latter trait was measured subsequently in the absence of further tamoxifen treatment (~10 fold increase in Snail-ER and ~81 fold increase in Twist-ER) (Supplementary Figure 2D). In addition, these mammospheres contained mammosphere-forming stem cells that could be passaged in vitro as mammospheres for at least one additional generation in the absence of tamoxifen (Supplementary Figure 4D). Accordingly, the ability to seed mammospheres persisted in these cells after functional inactivation of the EMT-inducing transcription factors achieved by withdrawal of tamoxifen from their culture media.
Similar to immortalized human mammary epithelial cells, tamoxifen-mediated activation of Snail in primary human mammary epithelial cells resulted in the appearance of mesenchymal-looking cells (Supplementary Figure 3A), decreased expression of E-cadherin (~3 fold), and increased expression of fibronectin (~ 3 fold) and vimentin (~1.5 fold) (Supplementary Figure 3B and C). In addition, these cells exhibited a greatly increased ability (~10 fold) to form mammospheres, as assayed once again in the absence of ongoing tamoxifen treatment (Supplementary Figures 3D and E). Collectively, these data provided further evidence that untransformed human mammary epithelial cells acquire stem-cell characteristics following passage through an EMT.
CD44high/CD24low cells isolated from HMLEs exhibit many properties of stem cells
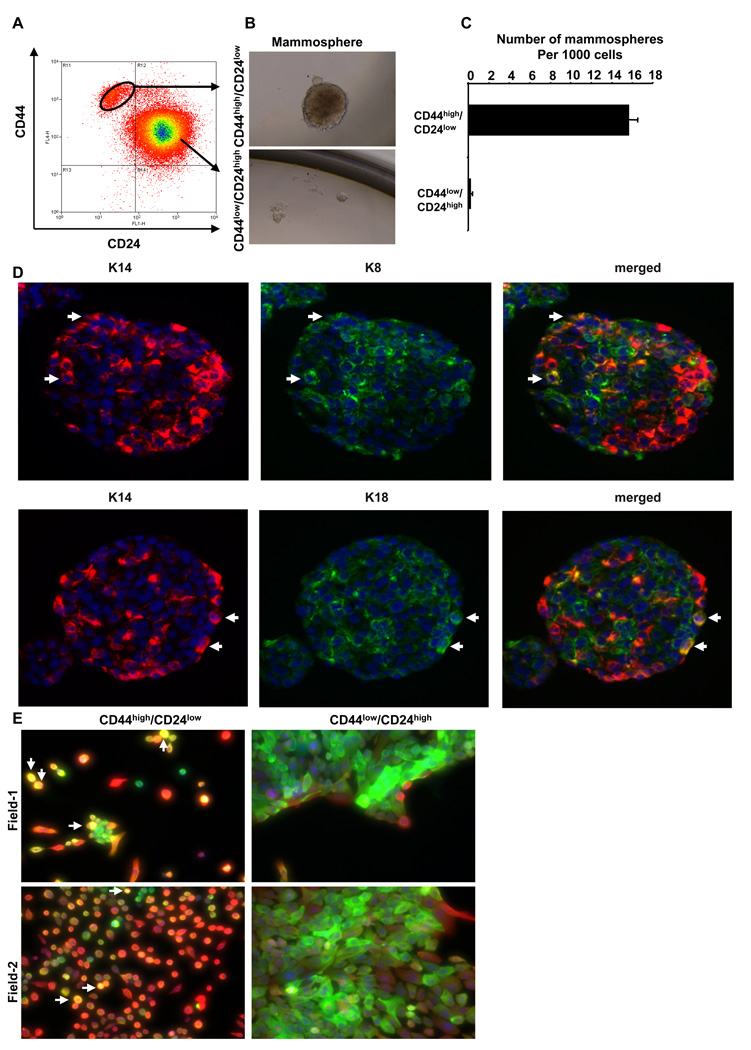
Since the cells that passed through an experimentally induced EMT showed attributes of stem cells, we speculated that naturally arising stem cells might exhibit phenotypes similar to cells that have been caused to undergo an EMT. To test this notion, we first fractionated the HMLE human mammary epithelial cells based on their CD44/CD24 antigen marker profile (Figure 2A) (Al-Hajj et al., 2003; Sleeman et al., 2006). We then subjected these CD44high/CD24low and CD44low/CD24high cells to the mammosphere assay. Indeed, the CD44high/CD24low cells formed mammospheres efficiently (Figure 2B & 2C). In contrast, the CD44low/CD24high cells did not form mammospheres (Figure 2B & 2C).
Figure 2.
CD44high/CD24low cells exhibit many properties of stem cells. (A) CD44high/CD24low cells and CD44low/CD24high cells were separated by FACS. (B) Phase-contrast images of mammospheres seeded by CD44high/CD24low (top) and CD44low/CD24high (bottom) cells (C) Quantification of mammospheres formed by cells from the sorted CD44high/CD24low and CD44low/CD24high populations. The data are reported as mean +/− SEM (P<0.001). (D) Images of mammospheres immunostained with antibodies against basal cytokeratin 14 (K14, red) and luminal cytokeratin 8 (K8, green, top panel) or cytokeratin 18 (K18, green, bottom panel). In the merged images (right), the arrows indicate K8/K14 or K18/K14 double-positive cells (yellow). (E) Images of single-cell clones derived from CD44high/CD24low cells (left) or CD44low/CD24high cells (right) stained using antibodies against K14 (red) and K18 (green). The arrows indicate K14/K18 double-positive cells (yellow).
To test the self-renewal capability of the mammosphere-forming cells, we dissociated the primary mammospheres into single cells and performed secondary mammosphere assays. Interestingly, we found ~ 40 secondary mammospheres formed per 1,000 seeded cells (4.0%). Moreover, the proportion of mammosphere-forming cells isolated from already-formed mammospheres remained the same (~4–6%) through 4 subsequent serial passages (Supplementary Figure 4). Hence, mammosphere-forming cells, indicating that the mammosphere-forming cells are capable of self-renewing and the conditions of mammosphere culture encourage an increase and subsequent stable maintenance of the numbers of self-renewing cells among the larger population of HMLE cells.
To further investigate whether cells within such mammospheres contain bipotential (i.e., luminal and basal/myoepithelial) precursor cells, we introduced HMLEs into mammosphere cultures at a clonal density to ensure that each of the resulting mammospheres arose from a single stem cell and immunostained the resulting mammospheres for the presence of a basal/myoepithelial marker (cytokeratin 14) and the luminal epithelial markers (cytokeratins 8 and 18). This staining revealed that the majority of cells in each mammosphere expressed either the basal epithelial marker cytokeratin 14 or the luminal epithelial markers cytokeratin 8 or 18; however, a minority cell population (~5%) in these mammospheres co-expressed basal and luminal cytokeratin (Figure 2D). Together, these observations suggested that a single mammosphere-forming cell can generate cells of both luminal and basal lineages.
We transferred individual mammospheres into Matrigel culture in order to test whether they could differentiate into secondary structures (Dontu et al., 2003). These conditions of culture revealed that the mammospheres differentiated and formed complex secondary structures (Supplementary Figure 5A). Remarkably, immunostaining of these secondary structures showed Muc1 (luminal marker)-positive cells in the inner luminal layer and CD49f/integrin α6-positive (basal marker) cells in the outer layer, similar to normal mammary ducts (Gudjonsson et al., 2002; Stingl et al., 2006) (Supplementary Figure. 5B &C). These observations indicated that each mammosphere contains a small percentage of bi-potential progenitor cells, as observed previously by others (Dontu et al., 2003).
We also wished to determine whether the CD44high/CD24low cells isolated from monolayer cultures of HMLE cells could generate the CD44low/CD24high non-mammosphere-forming cells in vitro. To do so, we introduced the FACS-purified CD44high/CD24low cells into monolayer culture and assayed for the appearance of a CD44low/CD24high population. Indeed, within 8 days these CD44high/CD24low cells developed into a cell population containing a 71% CD44low/CD24high cells (Supplementary Table 1). In contrast, no CD44high/CD24low cells were generated by culturing the sorted CD44low/CD24high cells in monolayer for the same period of time (Supplementary Table 1). This suggested a lineage hierarchy in which CD44highCD24low cells give rise to CD44lowCD24high cells under these conditions of culture, in which the reverse conversion was not observed. We confirmed and extended these findings by purifying single-cell clones from CD44highCD24low and CD44lowCD24high cell populations and observed similar behavior in vitro (Supplementary Table 2).
We also performed immunostaining to further determine whether the mesenchymal-appearing CD44high/CD24low cells were indeed bi-potential precursor cells that could give rise to populations containing a mixture of luminal cells, myoepithelial cells and a small subpopulation of bipotential cells. We found that culturing of single-cell clones from the CD44high/CD24low population indeed generated heterogeneous populations of cells displaying either the luminal CK18 marker, the basal/myoepithelial CK14 marker or, in the case of a small minority of cells, both luminal and basal/myoepithelial markers (CK14/18) simultaneously (Figure 2E). Conversely, the single-cell clones derived from CD44low/CD24high cells expressed mainly the luminal cytokeratin (CK18), with a few cells at the outer edges of the epithelial cell islands expressing the basal/myoepithelial marker (CK14). We found no bipotential cells in single-cell clones derived from the CD44low/CD24high cells (Figure 2E). These observations further supported our conclusion that CD44high/CD24low cells behave like bipotential precursor cells.
We further addressed the possibility that induction of an EMT forces the conversion of CD44low/CD24high cells to stem-like CD44highCD24low by expressing the Snail or Twist protein in FACS-sorted population of CD44low/CD24high cells or single-cell clones derived from CD44low/CD24high cells. The majority of these CD44low/CD24high cells underwent an EMT, as judged by their morphology, and generated CD44high/CD24low cells (Supplementary Figures 6A and C). In addition, the mesenchymal-appearing cells derived de novo by inducing EMT in both the sorted CD44low/CD24high population (Supplementary Figure 6B) and the clonal CD44low/CD24high population (Supplementary Figure 6D) formed >10-fold more mammospheres than did the sorted CD44low/CD24high population and the clonal CD44low/CD24high population infected with a corresponding control vector.
These observations, together with those cited earlier, indicate that the mammosphere-forming CD44high/CD24low cells can self-renew in vitro to generate more CD44high/CD24low cells as well as give rise to non-mammosphere-forming CD44low/CD24high cells. Conversely, the non-mammosphere-forming CD44low/CD24high cells proliferated well in culture but could not form CD44high/CD24low cells. This provided further evidence that the CD44high/CD24low cells possess certain attributes of stem cells.
Stem-like cells express markers associated with EMT
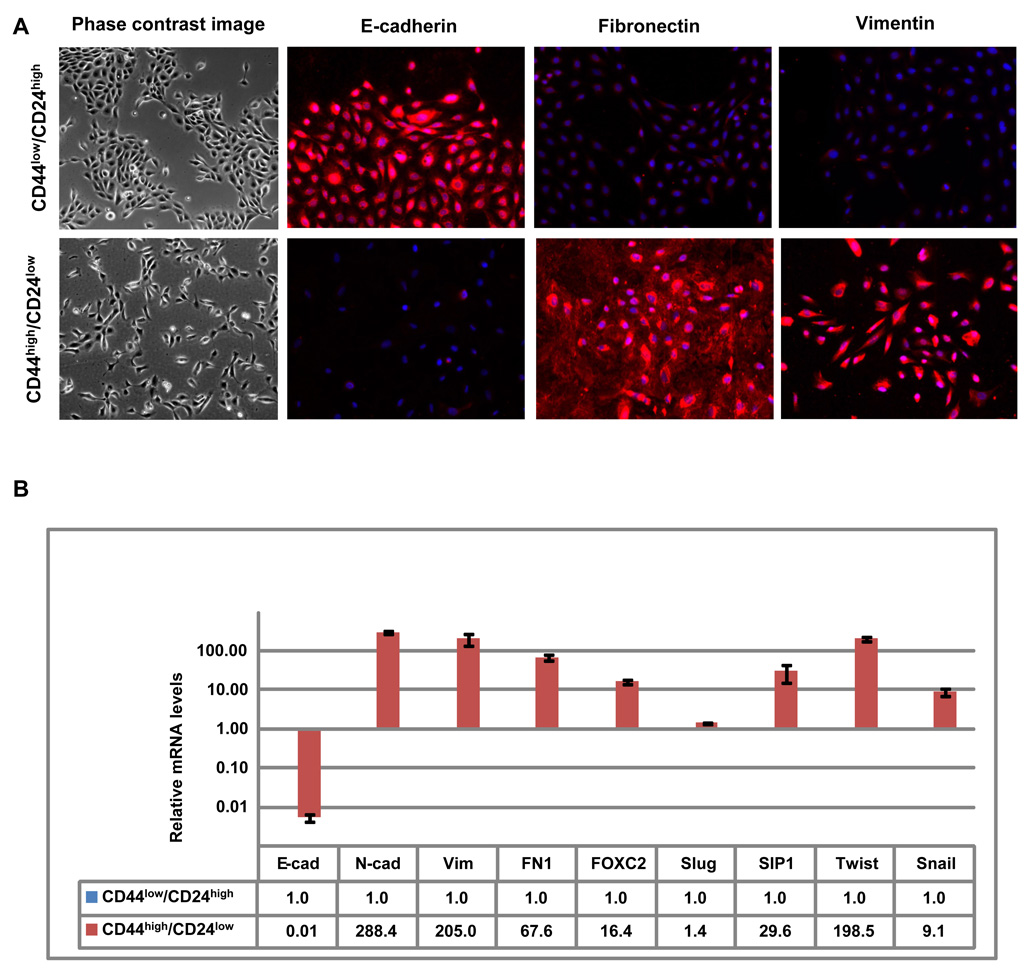
To determine whether naturally present, stem-like CD44high/CD24low cells exhibit phenotypes similar to cells that have undergone an EMT, we first introduced CD44high/CD24low and CD44low/CD24high HMLE cells fractionated by FACS (Figure 2A) into monolayer culture. Of note, the CD44high/CD24low cells introduced into monolayer culture exhibited mesenchymal morphology (Figure 3A) similar to that of cells forced to undergo an EMT by ectopic expression of either Twist or Snail (Figure 1A). Conversely, the CD44low/CD24high HMLE cells formed cobblestone cell islands typical of epithelial cells (Figure 3A).
Figure 3.
Stem-like/CD44high/CD24low cells isolated from HMLE cells exhibit attributes of cells that have undergone an EMT (A) Phase-contrast images (left) and immunofluorescence images of CD44high/CD24low and CD44low/CD24high cells stained using antibodies against E-cadherin, fibronectin or vimentin (right panels). (B) The expression levels of the mRNAs encoding E-cadherin, N-cadherin, vimentin, fibronectin, FOXC2, Slug, SIP1, Twist and Snail in CD44high/CD24low cells relative to CD44low/CD24high as determined by Real-time RT-PCR. GAPDH mRNA was used to normalize the variability in template loading. The data are reported as mean +/− SEM.
To learn whether these naturally present, cultured stem-like CD44high/CD24low HMLE cells have gene expression characteristics observed in epithelial cells that have passed through an EMT, we used both immunofluorescence and real-time RT-PCR to measure the expression of multiple EMT-associated genes. Indeed we found the expression levels of these EMT-associated genes in CD44high/CD24low cells resembled the levels seen in cells that have undergone EMT, while the expression levels of these genes in the CD44low/CD24high cells did not. Specifically, relative to levels in the CD44low/CD24high cells, the CD44high/CD24low cells exhibited a strong reduction in the E-cadherin protein and a significantly increased expression of fibronectin and vimentin (Figure 3A). Moreover, RT-PCR analyses revealed that, relative to the expression in CD44low/CD24high cells, the level of the E-cadherin mRNA in CD44high/CD24low was strongly reduced (~150-fold), while expression of mRNAs encoding mesenchymal markers was markedly increased, specifically N-cadherin (~200-fold), fibronectin (~60-fold), and vimentin (~200-fold). There was also a considerable increase in the expression of EMT-inducing transcription factors, specifically FOXC2 (~16-fold), SIP1 (~30-fold), Snail (~9-fold), and Twist (~198-fold) (Figure 3B), Slug expression was only marginally increased (~1.5 fold) (Figure 3B) in these CD44high/CD24low cells. These measurements provided further indication that the CD44high/CD24low cells exist in a mesenchymal-like cell state that closely resembles that of cells that have undergone an EMT.
Normal mouse mammary stem cells express markers associated with an EMT
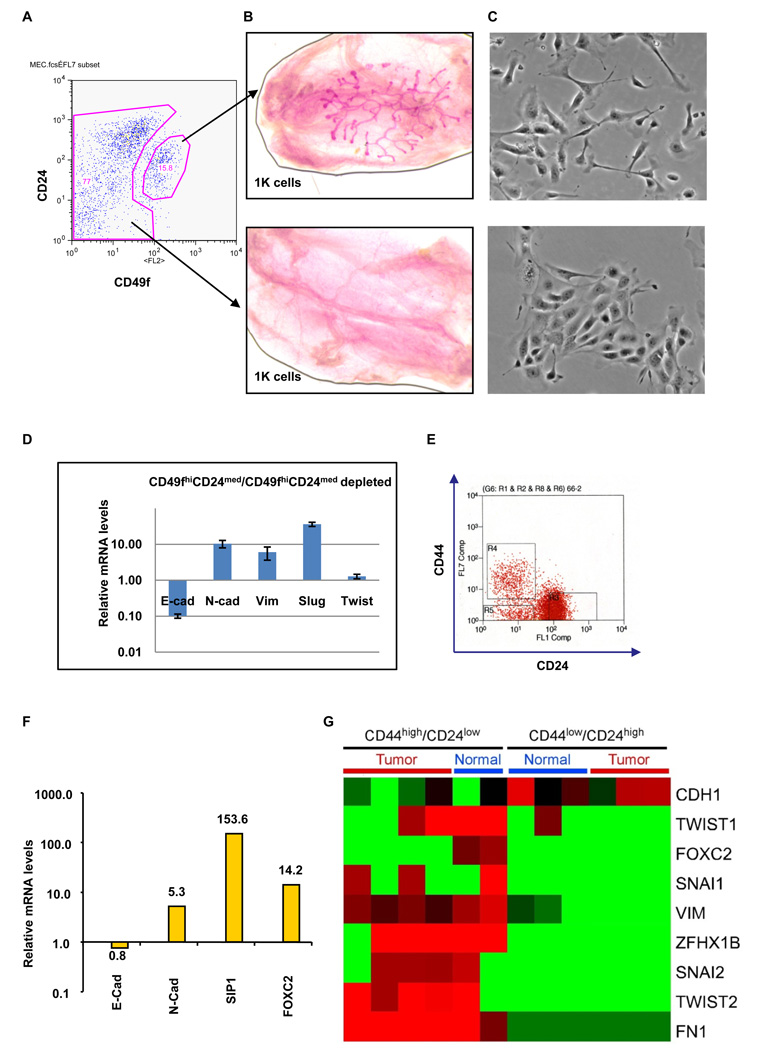
We also used the well-established CD49fhigh and CD24med (Stingl et al., 2006) antigen phenotype to enrich for murine stem cells (Figure 4A) in order to learn whether stem cells isolated directly from mouse mammary tissues also express markers associated with passage through an EMT. Indeed, as reported earlier (Stingl et al., 2006) 1,000 of the FACS-enriched CD49fhigh/CD24med cells reconstituted cleared mouse mammary fat-pads efficiently, while 1,000 cells from the CD49fhigh/CD24med-depleted population failed to do so (Figure 4B). We also found that cells in CD49fhigh/CD24med population appeared mesenchymal in monolayer culture when compared with the cells in the CD49fhigh/CD24med-depleted populations (Figure 4C).
Figure 4.
Primary mouse mammary stem cells, normal human breast stem-like cells, and neoplastic human breast stem-like cells express markers associated with EMT. (A) Primary mouse mammary epithelial cells were separated into CD49fhigh/CD24med and CD49fhigh/CD24med-depleted populations using FACS. (B) A representative image of a cleared mouse mammary fat pad reconstituted using 1000 of CD49fhigh/CD24med cells (top); the same number of CD49fhigh/CD24med-depleted cells failed to reconstitute the fat pad (bottom). (C) Phase-contrast images of CD49fhigh/CD24med and CD49fhigh/CD24med-depleted cells. (D) The expression levels of the mRNAs encoding E-cadherin, N-cadherin, vimentin, Slug and Twist in the CD49fhigh/CD24med relative to CD49fhigh/CD24med-depleted cells, as determined by Real-time RT-PCR. GAPDH mRNA was used to normalize variability in template loading. The data are reported as mean +/− SEM. (E) CD44high/CD24low cells (R4) and CD44low/CD24high cells (R3) were isolated from human reduction mammoplasty tissues using FACS. (F) The expression levels of the mRNAs encoding E-cadherin, N-cadherin, SIP-1 and FOXC2 in CD44high/CD24low cells (R4) relative to CD44low/CD24high cells (R3), as determined by Real-time RT-PCR. GAPDH mRNA was used to normalize the variability in template loading. The data are reported as mean +/− SEM. (G) Heat map depicting the expression levels of mRNAs encoding EMT markers in CD44high/CD24low cells compared to CD44low/CD24high cells, as determined by SAGE analysis. Red and green squares correspond to high and low mRNA levels, respectively.
Similar to observations described above, compared with the CD49fhigh/CD24med-depleted cells, the mesenchymal-appearing CD49fhigh/CD24med cells expressed a number of markers associated with passage through an EMT, notably reduced expression of E-cadherin (~10-fold), an increased expression of N-cadherin (~10-fold) and vimentin (~6-fold), as well as a significant increase in the EMT-inducing transcription factors Twist (~1.6-fold) and Slug (~36-fold) relative to CD49fhigh/CD24med-depleted cells (Figure 4D). Once again, a population of cells enriched for stem-cell activity exhibited a mesenchymal morphology and expressed markers associated with cells that have undergone an EMT.
Normal and neoplastic human breast stem-like cells express markers associated with an EMT
We proceeded to extend these analyses to human mammary epithelial cells isolated directly either from reduction mammoplasties or breast carcinomas. We initially isolated CD44high/CD24low and CD44low/CD24high mammary epithelial cells from normal reduction mammoplasty tissues (Figure 4E) and, as before, gauged their mRNA expression pattern using real-time RT-PCR. The CD44high/CD24low cells expressed low levels of E-cadherin mRNA, high levels of N-cadherin mRNA, and elevated levels of the mRNAs specifying two key EMT-inducing transcription factors – SIP1 and FOXC2 – relative to the expression levels of the respective mRNAs in the CD44low/CD24high population (Figure 4F).
To extend and generalize these results, we isolated CD44high/CD24low cells and CD44low/CD24high cells from three normal reduction mammoplasty tissues and from five neoplastic human breast tissues and carried out serial analysis of gene expression (SAGE) – an alternative method of gauging the spectrum of mRNAs expressed within tissues (Shipitsin et al., 2007). Relative to the CD44low/CD24high cells, the CD44high/CD24low cells expressed high levels of the mRNAs encoding mesenchymal markers, specifically CDH2 (N-cadherin), VIM (Vimentin), FN1 (Fibronectin), ZEB2 (SIP-1), FOXC2, SNAIL1 (Snail), SNAIL2 (Slug), TWIST1 and TWIST2, and a low level of the CDH1I (E-cadherin) mRNA (Figure 4G and Table 1). These data indicate that the CD44high/CD24low cells isolated from cultured immortalized MECs as well as from normal and neoplastic human tissue samples express multiple genes associated with cells that have undergone an EMT.
Table 1.
The sequence of SAGE tags specific for known EMT genes and the numbers of each tags present in CD44high/CD24low and CD44low/CD24high populations isolated from normal and neoplastic human mammary glands. The tumors samples were prepared from two invasive ductal carcinomas, one pleural effusion, and one ascites; the normal samples were prepared from two reduction mammoplasties.
| CD44high/CD24low | CD44low/CD24high | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAGE Tag | Normal | Tumor | Normal | Tumor | Gene Name | ||||||||
| TGTGGGTGCTGATAATT | 18 | 0 | 0 | 8 | 8 | 22 | 115 | 18 | 34 | 12 | 81 | 78 | CDH1(E-cadherin) |
| ATCTTGTTACTGTGATA | 7 | 30 | 46 | 30 | 91 | 26 | 0 | 0 | 0 | 0 | 0 | 0 | FN1 (Fibronectin 1) |
| TCCAAATCGATGTGGAT | 1038 | 642 | 332 | 504 | 472 | 300 | 101 | 71 | 5 | 0 | 0 | 0 | VIM (Vimentin) |
| TGCGTCCCGCCCGCCCT | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | FOXC2 |
| GAATTCCCTCCTGAGTG | 11 | 0 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | SNAI1 (Snail) |
| CTCCATTGTCTTACTAT | 0 | 5 | 4 | 0 | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | SNAI2 (Slug) |
| GTAGATGCAAGGGAAAC | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | TCF-3 (Transcription factor 3) |
| GTAAAATGCAAATAGAT | 18 | 15 | 0 | 0 | 4 | 22 | 0 | 3 | 0 | 0 | 0 | 0 | Twist1 (Twist homolog 1) |
| ATTGTTTCAAGTCAGCC | 0 | 10 | 4 | 15 | 15 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | Twist2 (Twist homolog 2) |
| CTCGAATAAAAATGTAG | 39 | 42 | 57 | 0 | 15 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | ZFH1B (Zinc finger homeobox 1b) |
EMT promotes the generation of cancer stem cells
The above observations suggested mammary epithelial stem-like cells could be generated from more differentiated populations of normal mammary epithelial cells by inducing an EMT. By extension, we speculated that EMT could promote the generation of cancer stem cells from more differentiated neoplastic cells. To address this possibility, we induced an EMT in experimentally immortalized human mammary epithelial cells (Elenbaas et al., 2001; Hahn et al., 1999) that had been transformed by introduction of an activated form of the HER2/neu oncogene (HMLEN cells). These cells were also infected with a vector expressing the tamoxifen-activatable form of either the Snail (Snail-ER) or the Twist (Twist-ER) transcription factors as described earlier.
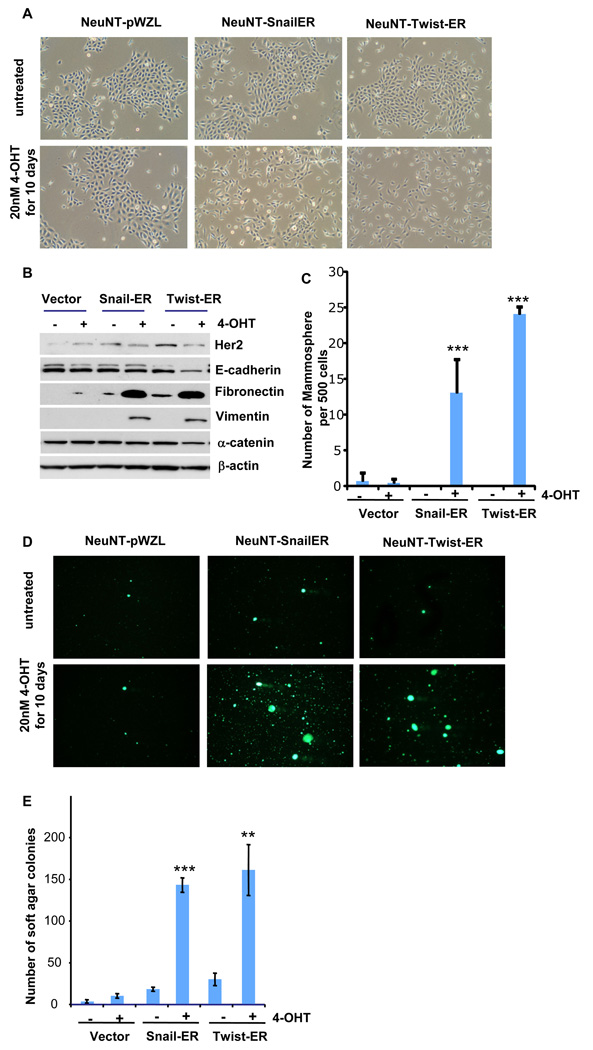
These cells underwent an EMT when treated with tamoxifen for ten days in monolayer culture (Figure 5A and 5B), similar to the behavior of the immortalized, untransformed precursors of these HER2/neu-transformed cells described earlier (Supplementary Figure 2A and B). Following withdrawal of tamoxifen, we subjected these HMLEN cells to both soft agar and tumor sphere assays in the absence of tamoxifen; the first of these assays serves as an in vitro surrogate measure of tumorigenicity (Cifone and Fidler, 1980; Singh et al., 2004), while the second gauges stemness. Interestingly, we observed that the cells that had undergone an EMT following tamoxifen treatment formed at least 10-fold more tumor spheres than did control cells that had not been exposed to tamoxifen (Figure 5C). Equally important, the transformed cells that underwent an EMT formed ~10-fold more colonies in soft agar suspension culture than did the control, untreated cells (Figure 5D).
Figure 5.
EMT induces phenotypes associated with cancer stem cells. (A) Phase-contrast images of NeuNT-Snail-ER, NeuNT-Twist-ER and NeuNT-control vector cells treated with tamoxifen for a period of 10 days as well as images of untreated cells. (B) Western blot analysis of expression of HER2/neu, E-cadherin, fibronectin, and vimentin proteins in the cells shown in panel A. β-actin was used as a loading control. (C) Quantification of the mammospheres seeded by NeuNT-Snail-ER, NeuNT-Twist-ER or NeuNT-control vector cells treated or not treated with tamoxifen for 10 days. The data are reported as mean +/− SD. (D) Images of the colonies formed during soft agar culture of NeuNT-Snail-ER, NeuNT-Twist-ER and NeuNT-control vector cells after being treated with tamoxifen for 10 days. The soft agar assays were performed in the absence of tamoxifen. (E) Quantification of the soft agar colonies shown in panel D. The data are reported as mean +/− SD (** - P<0.01; *** - P<0.001 compared to the control).
In order to gauge the tumorigenicity of the transformed cells that have undergone an EMT, we injected HMLEN cells that carried either Snail-ER or Twist-ER and had been treated with 4-OHT for 12 days prior to implantation in limiting dilutions into subcutaneous sites of mouse hosts. These cells failed to form tumors in vivo, which suggested that long-term maintenance of the EMT/stem-cell state depends on continuous EMT-inducing signals, at least in this experimental model. To test this notion further, we cultured the HMLEN cells that had undergone an EMT in vitro for an additional 15 days in the absence of 4-OHT. These cells reverted completely back to an epithelial phenotype (Supplemental Figure 10), suggesting that maintenance of the stem-cell state by these cells depends, at least in vitro, on continuous EMT-inducing signals.
In order to test whether the constitutive expression of an EMT-inducing transcription factor succeeded in altering the tumor-initiating frequency of transformed cells, we injected HMLER cells [i.e., HMLE cells transformed with a V12H-Ras oncogene to render them tumorigenic; (Elenbaas et al., 2001)] and constitutively expressing either Snail or Twist into immunodeficient hosts. Similar to the previously observed behavior of HMLE cells, both Snail and Twist induced EMTs in these HMLER cells and increased the number of CD44high/CD24low cells and the ability to form mammospheres (Supplementary Figure 7). The majority of the mice that were injected with 103 Twist- or Snail-expressing HMLER cells formed tumors (6 of 9 – Snail; 7 of 9 – Twist), while no tumors arose when an equal number of cells expressing a control vector were injected into mice (Table 2). In fact, 105 of the control cells (lacking either Twist or Snail expression) were required to initiate tumor formation, and even then, tumor formation was inefficient (3 of 9 injected hosts). Hence, expression of either the Twist or Snail EMT-inducing transcription factor significantly increased (by ~2 orders of magnitude) the number of tumor-initiating cells. The histology of the tumors developed from both the control cells and the cells expressing Snail or Twist appeared as squamous metaplasias, similar to that of the tumors formed by the parental cells (Supplementary Figure 8), as reported earlier (Elenbaas et al., 2001).
Table 2.
Tumor incidence of transformed HMLEs induced to undergo EMT by ectopic expression of Snail or Twist and then injected into host mice in limiting dilutions.
| Cells injected |
Tumors incidence/number of injections |
|||
|---|---|---|---|---|
| 1×106 | 1×105 | 1×104 | 1×103 | |
| HMLE-Vector-Ras | 2/6 | 3/9 | 0/9 | 0/9 |
| HMLE-Snail-Ras | 6/6 | 9/9 | 9/9 | 6/9 |
| HMLE-Twist-Ras | 6/6 | 9/9 | 9/9 | 7/9 |
Discussion
The findings presented here describe an unexpected convergence of two lines of recent research; stem cells have been documented in a variety of normal tissues and, more recently, this paradigm was extended to neoplasias (Clarke et al., 2006; Reya et al., 2001). Independent of these discoveries, the epithelial-mesenchymal transition (EMT) has been described over the past decade as a cell-biological program that is required for the remodeling of cells and tissues during embryogenesis, during certain types of wound healing, and during the acquisition of malignant traits by carcinoma cells (Hay, 2005; Thiery, 2003). None of these findings hinted at a link between these two sets of phenomena.
The discovery that the EMT generates cells with many of the properties of self-renewing stem cells holds the promise of resolving a major problem in cancer biology. Many types of cancer cells leaving primary carcinomas appear to rely on the EMT program to facilitate execution of most of the steps of the invasion-metastasis cascade (Thiery, 2003). However, the last step, termed colonization, which involves the growth of micrometastases into macroscopic metastases, has represented a conceptual dilemma: If the vast majority of cells leaving a primary tumor and disseminating to distant sites lack self-renewal capability, their ability to found macroscopic metastases is compromised from the outset because of their limited proliferative potential. This factor would dictate that successful formation of a macroscopic metastasis is an exceedingly rare event. This problem may be addressed, at least in part, by the present findings, since the EMT program that enables cancer cells to disseminate from a primary tumor also promotes their self-renewal capability. Indeed, just such a connection between metastasis and stem-cell state has previously been proposed in a speculative voice as a means of enabling the formation of macroscopic metastases (Brabletz et al., 2005).
While the present work indicates that the EMT confers many of the properties of the normal and neoplastic stem cell state, it does not yet demonstrate all of them. In the case of normal mammary stem cells, the case for the EMT leading to a stem-cell state has been supported, only indirectly: the EMT leads to great increases in the number of self-renewing cells that can initiate the seeding of mammospheres and, as shown by others and ourselves, mammospheres are enriched in stem cells and can seed entire mammary epithelial trees when implanted into cleared mammary fat pads (Supplementary Table 3) (Liao et al., 2007; Liu et al., 2006; Moraes et al., 2007). A more direct demonstration of this point will depend on a transient induction of an EMT in explanted mammary epithelial cells, and the demonstration that, at various limiting dilutions, such an induction leads to substantial increases in mammary gland-repopulating cells upon implantation into cleared mammary fat-pads.
In principle, some of the presently observed behaviors of the HMLE human mammary epithelial cells might be attributable to the introduced genes that were used previously to immortalize these cells, specifically hTERT, which encodes the catalytic subunit of the human telomerase holoenzyme, as well the SV40 early region. For example, these genes might facilitate the reprogramming of the HMLE cells initiated by EMT-inducing transcription factors such as Snail or Twist. However, we observe exactly the same responses to Snail or Twist in non-immortalized primary mammary epithelial cells, which indicates that the hTERT and SV04 T antigens appear to have no effect on the induction of the EMT.
An analogous demonstration of the connection between the EMT and the generation of neoplastic stem cells is also still required. We have shown here that the induction of an EMT in transformed human mammary epithelial cells yields cells with a CD44high/CD24low antigenic phenotype; others have shown, and we have confirmed, that such cells are greatly enriched in tumor-initiating cells. (Al-Hajj et al., 2003); Guo et al, ms. in preparation). We have also demonstrated that the number of tumor-initiating cells is increased by at least two orders of magnitude if transformed cells were forced to constitutively express either a Twist or Snail EMT-inducing transcription factor. However, in the end a more definitive test of the connections between the EMT and tumor-initiating ability will come from inducing an EMT through activation of Twist-ER or Snail-ER in transformed cells ex vivo followed by tests of their tumorigenicity in vivo in the absence of ongoing Twist or Snail activity.
We note that the transient induction in mammary epithelial cells of the EMT yields great increases in their ability to form mammospheres. Since this capability can be transmitted through repeated cycles of serial passage of mammosphere-forming cells from one culture to the next, it seems apparent that the transient induction of an EMT creates a heritable, self-renewing state that continues to be manifest in the distant lineal descendants of a cell that is initially forced to undergo an EMT, indeed long after the EMT-inducing stimulus has been removed.
The presently described connections appear to hold a number of implications for the biology of epithelial cells. Thus, they suggest that the stem cells of certain epithelial organs show many of the attributes of the mesenchymal cell state. Moreover, the transient induction of an EMT in large populations of cancer cells may make possible the generation of relatively unlimited numbers of cancer stem cells, whose biology may then be studied with far greater facility. Perhaps more importantly, the use of genetic or pharmacologic techniques to transiently induce an EMT in large populations of differentiated normal epithelial cells may provide a means of generating at will large numbers of tissue-reconstituting, normal epithelial stem cells.
Materials and Methods
Cell culture
The immortalized human mammary epithelial cells (HMLE) were maintained as previously described (Elenbaas et al., 2001). For 4-hydroxy tamoxifen (4-OHT) treatment, the HMLE-Snail-ER or Twist-ER cells were exposed to 4-OHT at final concentration of 20 nM for the indicated number of days. For TGF-β1 treatment, the HMLE cells were cultured in DME: F12 media (1:1) supplemented with insulin, EGF, hydrocortisone and 5% calf serum and treated with 5 ng/ml of TGF-β1 for 12 days. Freshly sorted primary mouse mammary epithelial cells were cultured in MM+ medium (1:1 ratio of DME/F12, 10 ng/ml of EGF, 10 ug/ml of insulin, 1% bovine serum albumin and 2% calf serum). Cultured cells were photographed on day 2. The plasmids used in this study, procedures used to produce virus, the procedure for the infection of target cells, and the derivation of different cell lines and their culture conditions are provided as Supplementary Methods.
Antibodies, Western Blotting and Immunofluorescence
Cells were lysed in the presence of 50mM Tris PH7.5, 150mM NaCl and 0.5% NP-40 on ice. 50 µg of total protein from each sample was resolved on a 4–12% Bis-Tris Gel with MOPs Running Buffer and transferred to PVDF membranes. The blots were then probed with various antibodies, such as anti-β-actin (Abcam), anti-E-cadherin (BD Transduction), anti-Fibronectin (BD Transduction), anti-vimentin V9 (NeoMarkers), or anti-N-cadherin (BD Transduction). Detailed immunofluorescence methods are also given in the Supplementary Methods.
Mammosphere culture and differentiation
Mammosphere culture was performed as described in Dontu et al 2003 (Dontu et al., 2003) with slight modification, as described in Supplementary Methods. The mammosphere differentiation assay was adopted from Weaver and Bissell (1999) and Dontu et al (2003) (Dontu et al., 2003; Weaver and Bissell, 1999). Essentially, single mammospheres were transferred to 50% Matrigel and cultured in MEGM medium (Clonetics) supplemented with 10ng/ml FGF, 4ng/ml heparin and 2ug/ml prolactin for additional 10–14 days. The structures were then photographed and embedded in OCT for sectioning and immunofluorescence analysis.
Soft agar and Tumorigenesis assays
Soft agar assays were performed as described previously (Cifone and Fidler, 1980). Cultures were photographed, and the colonies with diameters larger than 500 µm were counted using ImageJ software (previously NIH image). 105, 104, and 103 of HMLE-Snail-Ras or HMLE-Twist-Ras or HMLE-Vector-Ras cells were injected subcutaneously into athymic nude mice. The tumor incidence was monitored for 25 days following injection. All mouse procedures were preapproved by the Animal Care and Use Committee of the Massachusetts Institute of Technology and performed according to institutional policies.
Reverse Transcriptase PCR analysis
SYBR-Green Real-time RT-PCR and the corresponding data analysis were described earlier (Yang et al., 2004). For all RT-PCR analysis GAPDH mRNA was used to normalize RNA inputs. Primers sequence used to amplify genes are listed in the Supplemental Methods.
FACS analysis
The anti-CD44 (clone G44-26) and anti-CD24 (clone ML5) antibodies used for FACS analysis were obtained from BD Bioscience. To sort primary mouse mammary epithelial cells, mouse mammary glands were digested with collagenase (1µg/ml), and the organoids were collected by brief centrifugation (800 rpm for 45 seconds). The organoids were digested with 0.025% trypsin for 5 min at 37°C in order to dissociate into single cells. The dissociated cells were stained with antibodies against CD49f (Pharmingen) and CD24 (Pharmingen) and several lineage markers (CD45, CD31, Ter-119 and CD140a (ebioscience), as described in Stingl et al 2006. The Lin− -cells were used for sorting.
Primary human samples and SAGE libraries
CD44high/CD24low and CD44low/CD24high cells were isolated from normal reduction mammoplasty tissues and breast tumors by sequential capturing of cell types with antibody-coupled beads specific for various cell markers (Shipitsin et al., 2007). Fresh tissue samples were collected from Harvard-affiliated hospitals and cell fractions were immediately purified. Three normal tissues, 1 pleural effusion, 2 ascites, and 3 invasive ductal carcinomas were collected in triplicate from different patients. Specimens were collected without patient identifiers following protocols approved by the Dana-Farber Cancer Institute Institutional Review Board.
Heat map
The heat map in Figure 4C was produced using the SAGE tag counts in Table 1, which were visualized using MapleTree (developed by L. Simirenko). Before visualization, tag counts were log2-transformed (treating a count of 0 as if it were 1), median-centered by tag, and subjected to hierarchical complete linkage clustering by tags and SAGE libraries with uncentered correlation similarity metrics using Cluster (Eisen et al., 1998)
Statistical analysis
All data are presented as mean ± SEM except other stated. When two groups were compared, the Student t-test was used (P < 0.05 was considered significant).
Supplementary Material
Note: Supplementary information available
Acknowledgments
We thank Richard Goldsby, Rick Lee, Lynn Waldman, Thijn Brummelkamp, Matthew Saelzler, Kurt Evans and members of the Weinberg laboratory for critical review of the manuscript; Christine Hickey for administrative help. This research was supported by grants from the Breast Cancer Research Foundation, National Cancer Institute Program PO-CA080111 and National Institute of Health RO1- CA078461. R.A.W. is an American Cancer Society and Ludwig Foundation professor. S.A.M. received U.S. Army Breast Cancer Research Program Postdoctoral Fellowship DAMD17-01-1-0457. W.G is a Susan G. Komen Breast Cancer Foundation Postdoctoral Fellow. S.A.M., M.J.L., and R.A.W. are inventors of a patent application in part based on findings described in this manuscript. K.P’s work is supported in part by Novartis, the NIH (CA89393 and CA94074), and the DOD (W81XWH-07-1-0294), M.S is a Susan Komen Foundation postdoctoral fellow (PDF0707996). Work in the laboratory of C.B. is partially supported by a grant from the Swiss Cancer League (OCS-01445-12-2003).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- Briegel KJ. Embryonic transcription factors in human breast cancer. IUBMB life. 2006;58:123–132. doi: 10.1080/15216540600686870. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- Cifone MA, Fidler IJ. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells from a murine fibrosarcoma. Proc Natl Acad Sci U S A. 1980;77:1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer Stem Cells--Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–1278. doi: 10.1016/s1097-2765(01)00260-x. [DOI] [PubMed] [Google Scholar]
- Davies JA. Mesenchyme to epithelium transition during development of the mammalian kidney tubule. Acta Anat (Basel) 1996;156:187–201. doi: 10.1159/000147846. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC, Weinberg RA. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- Hartwell KA, Muir B, Reinhardt F, Carpenter AE, Sgroi DC, Weinberg RA. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc Natl Acad Sci U S A. 2006;103:18969–18974. doi: 10.1073/pnas.0608636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Hay ED. The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn. 2005;233:706–720. doi: 10.1002/dvdy.20345. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annual review of immunology. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, Gifford A, Reinhardt F, Popescu NC, Guo WG, et al. Enrichment of a population of mammary gland cells that forms mammospheres and has in vivo repopulating activity. Cancer Research. 2007 doi: 10.1158/0008-5472.CAN-06-4493. in press. [DOI] [PubMed] [Google Scholar]
- Liu S, Dontu G, Mantle ID, Patel S, Ahn NS, Jackson KW, Suri P, Wicha MS. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci U S A. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes RC, Zhang X, Harrington N, Fung JY, Wu MF, Hilsenbeck SG, Allred DC, Lewis MT. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development (Cambridge, England) 2007;134:1231–1242. doi: 10.1242/dev.02797. [DOI] [PubMed] [Google Scholar]
- O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Oft M, Akhurst RJ, Balmain A. Metastasis is driven by sequential elevation of H-ras and Smad2 levels. Nat Cell Biol. 2002;4:487–494. doi: 10.1038/ncb807. [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- Perez-Pomares JM, Munoz-Chapuli R. Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in Metazoans. Anat Rec. 2002;268:343–351. doi: 10.1002/ar.10165. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Savagner P, Kusewitt DF, Carver EA, Magnino F, Choi C, Gridley T, Hudson LG. Developmental transcription factor slug is required for effective re-epithelialization by adult keratinocytes. Journal of cellular physiology. 2005;202:858–866. doi: 10.1002/jcp.20188. [DOI] [PubMed] [Google Scholar]
- Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, et al. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Sleeman KE, Kendrick H, Ashworth A, Isacke CM, Smalley MJ. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 2006;8:R7. doi: 10.1186/bcr1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI, Eaves CJ. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Bissell MJ. Functional culture models to study mechanisms governing apoptosis in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 1999;4:193–201. doi: 10.1023/a:1018781325716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66:4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: Supplementary information available