Abstract
Acute kidney injury (AKI) is a common condition with significant associated morbidity and mortality. The insensitivity and non-specificity of traditional markers of renal dysfunction prevent timely diagnosis, estimation of the severity of renal injury, and the administration of possible therapeutic agents. Here, we determine the prognostic ability of urinary liver-type fatty acid-binding protein (L-FABP), and further characterize its sensitivity and specificity as a biomarker of AKI. Initial western blot studies found increased urinary L-FABP in patients with confirmed AKI. A more extensive cross-sectional study found significant increases in urinary L-FABP, normalized to urinary creatinine, in 92 patients with established AKI compared with 62 patients without clinical evidence of AKI. In hospitalized patients, the diagnostic performance of urinary L-FABP for AKI, assessed by the area under the receiver operating characteristic curve, was 0.93. This compares favorably with other established biomarkers of AKI such as kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, N-acetyl-β-glucosaminidase, and interleukin-18. Our study shows that age-adjusted urinary L-FABP levels were significantly higher in patients with poor outcome, defined as the requirement for renal replacement therapy or the composite end point of death or renal replacement therapy.
Keywords: acute kidney injury, biomarker, liver-type fatty acid-binding protein
Acute kidney injury (AKI) is a common medical condition with significant associated morbidity and mortality. In adults, AKI has been reported to complicate 1–7%1-6 of all hospital admissions and 1–25%7,8 of intensive care unit (ICU) admissions. Despite impressive progress in the understanding of the molecular and biochemical mechanisms of AKI, as well as in the general care of patients affected, mortality rates in the ICU setting remain between 50–70%,9 with a significant number of survivors exhibiting persistent evidence of renal dysfunction.4,10 Unfortunately, notable improvements in the therapeutics of AKI have been few since the introduction of renal replacement therapy into clinical practice more than 50 years ago.
Over the past 10 years, increased attention has been focused on identifying and addressing impediments to progress in AKI research. One problem area widely recognized is the continued reliance on markers for the diagnosis of AKI that do not reflect actual injury to renal cells, but rather the functional consequences of injury. In current practice, AKI is identified by increases in serum creatinine (SCr) measurements over time. It is well established, however, that SCr is a suboptimal marker that follows renal injury, when levels are often not reflective of the glomerular filtration rate (GFR) because of a number of renal and non-renal influences. In the setting of AKI, the dynamic relationship between SCr and GFR inhibits the ability to accurately estimate the timing of injury and the severity of dysfunction after injury. As dictated by the laws of mass balance, a sudden fall in GFR to a constant low level causes a gradual increase in SCr until a new steady state between generation and excretion is achieved. Thus, the rate of SCr increase after AKI is dependent on many factors, including the new GFR, rate of generation, rate of tubular secretion, and volume of distribution. As a result, severe injury and large changes in GFR may be associated with small, gradual changes in SCr during the first 48 h after injury, resulting in delayed diagnosis, underestimation of the degree of injury, and delayed intervention.
Intensive investigative efforts have led to the identification of a number of urinary proteins, including kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and interleukin-18 (IL-18), which have emerged as markers of AKI. It is anticipated that further characterization and validation of individual biomarkers and/or biomarker panels will enable earlier diagnosis, patient stratification into groups at varying risks of developing AKI, and targeted intervention after the diagnosis of AKI has been made, ultimately resulting in improved patient outcomes.
The fatty acid-binding proteins (FABPs) are small cytoplasmic proteins abundantly expressed in tissues with active fatty acid metabolism. Nine distinct types have been identified, with each named after the tissue in which they were first identified.11 The primary function of FABPs seems to be the facilitation of long-chain fatty acid transport from the plasma membrane to sites for β-oxidation.11 In addition, FABPs may also have a role in the reduction of cellular oxidative stress, binding fatty acid oxidation products, and limiting the toxic effects of oxidative intermediates on cellular membranes.12,13 The putative antioxidant properties of FABPs have stimulated interest in these proteins as potential tissue-specific markers of injury.
Liver-type FABP (L-FABP) was initially identified in hepatocytes and later found to be expressed in the human renal proximal tubule epithelium,11 a nephron segment largely dependent on energy derived from fatty acid metabolism for normal cellular transport processes. In preclinical studies, renal L-FABP expression protected against tubulointerstitial damage in models of proximal tubule protein overload, as well as against unilateral ureteral obstruction.14,15 Clinical studies have established L-FABP as a promising biomarker in both chronic kidney disease16-18 and AKI.19-21 In addition, Nakamura et al.22 recently reported urinary L-FABP levels to be significantly elevated in patients with septic shock and concluded that this was likely a reflection of associated renal tubular injury. They also noted that urinary L-FABP levels were not correlated with the need for dialysis; however, predictive ability for other outcome measures was not reported.
To further establish the utility of urinary L-FABP as a biomarker of AKI, we conducted a cross-sectional study comparing urinary L-FABP excretion in a heterogeneous group of adult patients with established AKI (n=92) with hospitalized and ambulatory control subjects without clinical evidence of kidney injury (n=68). Cross-sectional studies provide important information on kidney biomarker research. They eliminate the inherent uncertainty associated with prospective short-term studies—uncertainty due to the fact that in the absence of a kidney biopsy there is no current gold standard for the diagnosis of AKI, especially injury that is not associated with an increase in SCr. All of the cases in the study defined as AKI were associated with clinical confirmation, as well as with increases in SCr. Our results indicate that urinary L-FABP is a highly sensitive and specific marker for the diagnosis and prognosis of established AKI of varying etiology.
RESULTS
Urinary L-FABP detection by western blot analysis
Western blot analysis (Figure 1) was performed on urine samples from six patients with known AKI (secondary to sepsis and aminoglycoside toxicity), one healthy control, and one ICU control patient who did not have clinical evidence of AKI using conventional metrics. L-FABP pure protein was included as a reference standard. Urinary L-FABP was undetectable in healthy control urine and low levels were observed in the urine of some patients in the critically ill control group. In sharp contrast, all patients with established AKI had easily detectable levels of urinary L-FABP.
Figure 1. Western blot analysis of urine from patients with and without established acute kidney injury (AKI).
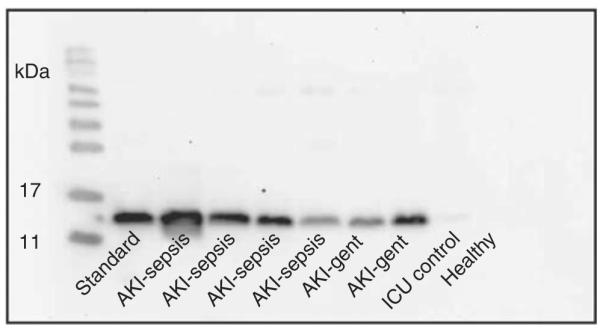
The first lane was loaded with liver-type fatty acid-binding protein (L-FABP) as a reference standard. Remaining lanes were loaded with urine from patients with AKI in the setting of sepsis or aminoglycoside administration, a critically ill (intensive care unit (ICU)) patient without clinical evidence of AKI, and a healthy volunteer. Western blot analysis was carried out with a polyclonal antibody to human L-FABP.
Cross-sectional study of urinary L-FABP in patients with established AKI
A total of 92 patients with established AKI and 68 control subjects were studied. To obtain a diverse control group, healthy volunteers (n=29), critically ill patients without a diagnosis of AKI (n=13), and patients who were scheduled for elective cardiac catheterization without clinical evidence of AKI (n=26) were recruited. These subjects are a large subgroup of those investigated in our recent study showing the comparative value of multiple biomarkers in the diagnosis and prognosis of AKI.23 Clinical characteristics are presented in Table 1.
Table 1.
Subject characteristics
| Healthy volunteers (n=26) |
Precatheterization controls (n=29) |
ICU controls (n=13) |
All controls (n=68) |
AKI (n=92) |
|
|---|---|---|---|---|---|
| Age (±s.d.) | 34.7 (±9.1) |
67.0 (±14.5) |
67.6 (±13.1) |
54.8 (±20.1) |
60.5 (±17.2) |
| Gender | |||||
| Male (%) | 27 | 69 | 69 | 54 | 57 |
| Ethnicity | |||||
| Black (%) | 12 | 7 | 0 | 9 | 11 |
| Cause of AKI a | |||||
| ATN (%) | 30 | ||||
| Sepsis (%) | 33 | ||||
| Nephrotoxins (%) | 7 | ||||
| Contrast (%) | 5 | ||||
| Other (%) | 25 | ||||
| Reason for ICU admission | |||||
| Post-surgery (%) | 54 | ||||
| Trauma (%) | 32 | ||||
| Sepsis (%) | 14 |
Abbreviations: AKI, acute kidney injury; ATN, acute tubular necrosis; ICU, intensive care unit.
A single most likely cause of AKI was determined based on chart review.
Median urinary L-FABP levels normalized to urinary creatinine were significantly higher in patients with AKI than in control subjects, whether analyzed as individual groups (healthy volunteers versus AKI, ICU control versus AKI, or precatheterization versus AKI) or together (all controls versus AKI). Scatterplots of urinary L-FABP levels (log scale) and summary statistics are shown in Figure 2.
Figure 2. Cross-sectional data.
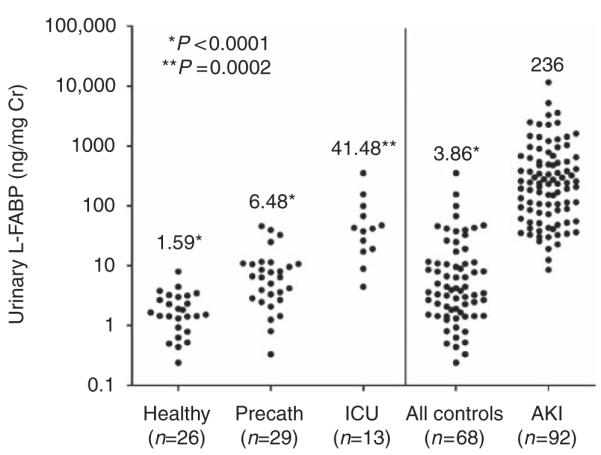
Comparison of urinary liver-type fatty acid-binding protein (L-FABP) in acute kidney injury (AKI) and control patients. Scatterplot of all urine L-FABP measurements by ELISA. Urinary L-FABP levels were significantly elevated in patients with AKI (median 236; interquartile range (IQR) 74–610) when compared with healthy controls (median 1.59; IQR 0.89–3.05; P<0.0001, Mann–Whitney U-Test), precatheterization controls (Precath, median 6.48; IQR 2.78–10.86; P<0.0001), intensive care unit (ICU) controls (median 41.48; IQR 18.25–83.61; P=0.0002), and all controls together (median 3.86; IQR 1.57–11.32; P<0.0001). Cr, creatinine.
Diagnostic ability of urinary L-FABP
Diagnostic performance was assessed by comparing normalized urinary L-FABP levels in patients with AKI versus control subjects using receiver operating characteristic area under the curves (ROC-AUC). Figure 3 shows the ROC curve for AKI versus all hospitalized control subjects (ICU controls and precatheterization controls; AUC-ROC 0.93). Table 2 summarizes the ROC-AUC, sensitivity, specificity, and cutoff values for AKI versus control patients, analyzed together and as individual groups. Urinary L-FABP effectively distinguished control patients from those with AKI (ROC-AUC=0.96), distinguished precatheterization controls from those with AKI (ROC-AUC=0.98), and allowed perfect discrimination between healthy volunteers and those with AKI (AUC=1.0). Diagnostic performance remained high when ICU controls were analyzed alone against those with AKI (AUC=0.82).
Figure 3. Diagnostic performance of urinary liver-type fatty acid-binding protein (L-FABP) for the identification of established acute kidney injury (AKI) in hospitalized patients.
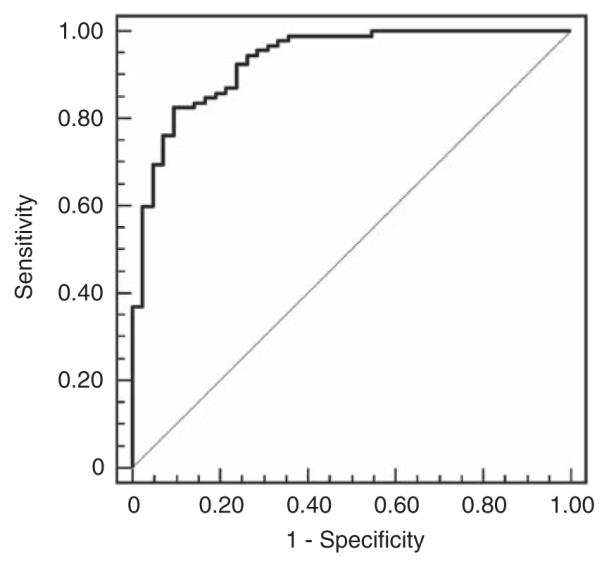
Receiver operating characteristic curve for AKI versus hospitalized controls (intensive care unit controls and precatheterization controls). Area under the curve (AUC) was 0.93 (95% CI 0.88–0.97) using a cutoff of 47.1 ng per mg Cr; sensitivity was 83% (95% CI 73–90%), and specificity was 90% (95% CI 77–97%). CI, confidence intervals; Cr, creatinine.
Table 2.
Diagnostic performance characteristics of urinary L-FABP for the identification of established AKI
| AUC-ROC (95% CI) | Cutoff (ng per mg Cr) | Sensitivity (95% CI) | Specificity (95% CI) | |
|---|---|---|---|---|
| AKI versus hospitalized controlsa | 0.93 (0.88–0.97) | 47.1 | 83% (73–90%) | 90% (77–97%) |
| AKI versus all controls | 0.96 (0.92–0.98) | 26.1 | 95% (88–98%) | 84% (73–92%) |
| AKI versus healthy volunteers | 1.00 (0.97–1.00) | 8.1 | 100% (96–100%) | 100% (87–100%) |
| AKI versus precath. controls | 0.98 (0.94–1.00) | 32.5 | 92% (85–97%) | 93% (77–99%) |
| AKI versus ICU controls | 0.82 (0.74–0.89) | 99.0 | 70% (59–79%) | 85% (55–98%) |
Abbreviations: AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curves; CI, confidence interval; Cr, creatinine; ICU, intensive care unit; L-FABP, liver-type fatty acid-binding protein; Precath, precatheterization.
Hospitalized controls = ICU controls + precatheterization controls.
To determine the diagnostic ability of urinary L-FABP relative to other established biomarkers of AKI, ROC-AUCs were calculated for AKI versus hospitalized controls for urinary NGAL, KIM-1, N-acetyl-β-glucosaminidase (NAG), and IL-18. These results are summarized in Table 3. Urinary L-FABP performed at a level comparable to that of NGAL, NAG, and KIM-1, and exceeded that of IL-18. Pairwise comparisons revealed no significant difference between the AUC-ROCs for L-FABP versus NGAL, KIM-1, and NAG. There was a significant difference between the AUC-ROCs for L-FABP and IL-18 (P=0.005).
Table 3.
Comparative diagnostic performance of urinary L-FABP, NGAL, KIM-1, NAG, and IL-18 for the identification of established AKI in hospitalized patients
| Biomarker | AUC-ROC (95% CI) | Cutoff | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|
| L-FABP (ng per mg Cr) | 0.93 (0.88–0.97) | 47.1 | 83% (73–90%) | 90% (77–97%) |
| NGAL (ng per mg Cr) | 0.92 (0.86–0.96) | 186.8 | 81% (71–89%) | 100% (92–100%) |
| KIM-1 (ng per mg Cr) | 0.89 (0.82–0.94) | 1.7 | 77% (67–85%) | 100% (92–100%) |
| NAG (U per mg Cr) | 0.89 (0.82–0.94) | 0.007 | 99% (94–100%) | 64% (48–78%)) |
| IL-18 (pg per mg Cr) | 0.83* (0.76–0.89) | 2.2 | 71% (60–80%) | 90% (77–93%) |
Abbreviations: AKI, acute kidney injury; AUC-ROC, area under the receiver operating characteristic curve; CI, confidence interval; Cr, creatinine; IL, interleukin; KIM, kidney injury molecule; L-FABP, liver-type fatty acid-binding protein; NAG, N-acetyl-β-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin.
P=0.005 compared with L-FABP.
Prognostic ability of urinary L-FABP
Patients with AKI had an in-hospital mortality rate of 36 and 43% required renal replacement therapy (RRT); 59% reached the composite outcome of death or RRT. In age-adjusted analysis using log-transformed, normalized values, urinary L-FABP emerged as a significant predictor of RRT (P=0.02) and of the composite end point of death/RRT (P=0.03); urinary L-FABP did not predict in-hospital mortality (P=0.26). Normalized urinary L-FABP levels of subjects reaching the combined end point of death/RRT and of those who did not are shown in box and whisker plots in Figure 4. Age-adjusted SCr levels at the time of urine collection were not significantly associated with mortality and/or RRT.
Figure 4. Comparison of urinary liver-type fatty acid-binding protein (L-FABP) levels in patients with established acute kidney injury (AKI) by clinical outcome.
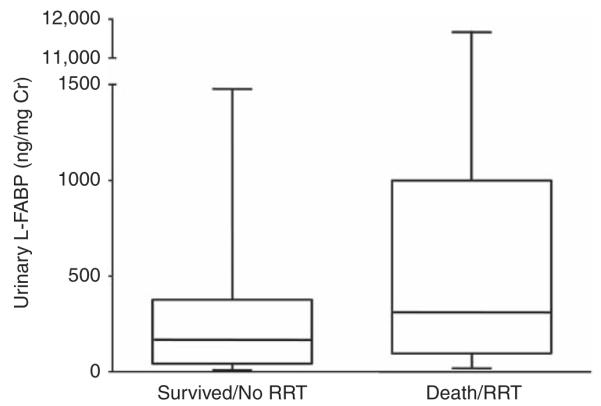
Box and whisker plots of normalized urinary L-FABP levels in patients meeting the composite end point of death/renal replacement therapy (RRT) and those who survived without RRT. Cr, creatinine.
Urinary L-FABP in AKI of different causes
A single diagnosis was established for each patient with AKI on the basis of chart review (acute tubular necrosis (ischemia, post-cardiac surgery, pigment nephropathy) (n=28); sepsis (n=30); nephrotoxin exposure (n=6); contrast nephropathy (n=5) others (obstruction, acute interstitial nephritis, acute glomerulonephritis, multiple myeloma, prerenal, vaso-occlusive disease, tumor lysis syndrome) (n=23). No statistical difference in urinary L-FABP levels among diagnostic categories was observed (Kruskal–Wallis; P=0.15). In general, a trend toward higher levels was observed in acute tubular necrosis and sepsis, and lower levels in contrast nephropathy. Normalized urinary L-FABP levels for each diagnostic category are shown in box and whisker plots in Figure 5.
Figure 5. Comparison of urinary liver-type fatty acid-binding protein (L-FABP) levels in patients with acute kidney injury (AKI) by cause.
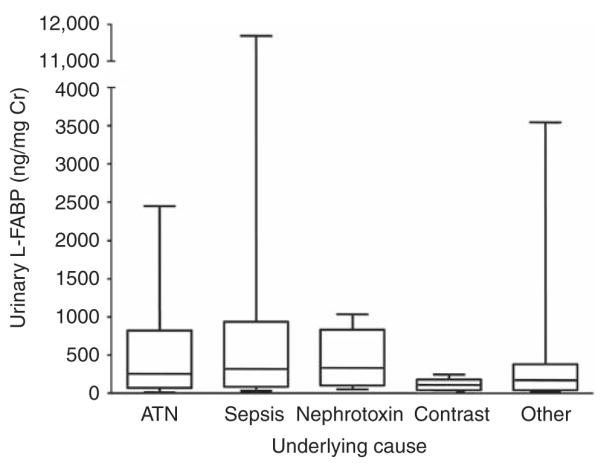
Box and whisker plots of normalized urinary L-FABP levels in patients with established AKI caused by acute tubular necrosis (ATN), sepsis, nephrotoxin exposure, contrast-induced nephropathy, and other underlying disease. Cr, creatinine.
DISCUSSION
Over the past several decades, numerous advances have been made in the understanding of the pathophysiological mechanisms implicated in AKI24,25 and the compensatory mechanisms involved in renal 5recovery. 26,27 In addition, important advances have been made in the treatment of patients with AKI, both in terms of dialytic therapy and supportive ICU care.28-31 Unfortunately, commensurate improvements in patient outcomes have not been observed. Reliance on SCr as a marker of renal injury has contributed to the slow translation of basic science discovery to therapeutically effective approaches in clinical practice.
Recent efforts have led to the identification and characterization of a number of promising biomarkers of AKI, including KIM-1,32-36 NGAL,37-39 and IL-18.40,41 Urinary L-FABP has been studied clinically in a variety of renal pathophysiologic states14,42,43 and has been shown to hold promise in the monitoring of progressive diabetic and non-diabetic chronic kidney disease.17 Recent reports have established urinary L-FABP as a useful biomarker in clinical models of ischemic AKI. Yamamoto et al.21 reported that urinary L-FABP levels correlated well with the ischemic time of the transplanted kidney and with the length of hospital stay in human living related-donor renal transplant recipients. More recently, Portilla et al.20 investigated urinary L-FABP as a biomarker of AKI in pediatric patients after cardiac surgery and found that levels at the 4 h post-operative time point were predictive of those who subsequently developed AKI with an ROC-AUC of 0.810 (sensitivity 0.714; specificity 0.684). In addition, Nakamura et al.19 reported that baseline urinary L-FABP levels were significantly higher in those patients who developed contrast nephropathy after coronary angiography; however, the authors did not evaluate the diagnostic performance of urinary L-FABP in predicting AKI. Our study evaluated a heterogeneous group of adult patients and showed significantly elevated urinary L-FABP levels in established AKI of varying etiology, including acute tubular necrosis, sepsis, and nephrotoxin exposure. Our results suggest that urinary L-FABP has broad utility in the diagnosis of AKI; however, further evaluation is necessary to establish the temporal pattern of excretion in the various forms of AKI, as urine specimens in our study were obtained after the diagnosis of AKI was made.
The study population for this investigation represented a large subset of the subjects described in our recent study showing the comparative value of multiple biomarkers in the diagnosis and prognosis of AKI.23 The diagnostic ability of urinary L-FABP (ROC-AUC 0.93) in hospitalized patients was very good, comparable to other well-described biomarkers of AKI, including NGAL (0.92), KIM-1 (0.89), NAG (0.89), and statistically better than IL-18 (0.83). As expected, urinary L-FABP diagnostic performance characteristics were optimal when AKI was compared with healthy volunteers (ROC-AUC=1.00). Although diagnostic performance remained high when AKI patients were compared against hospitalized patients without a diagnosis of AKI, considerable overlap of urinary L-FABP values was noted, particularly among AKI patients and ICU controls. There are at least two explanations for this phenomenon. It is possible that increased urinary L-FABP levels in acutely ill ICU patients without a diagnosis of AKI are indicative of some other systemic process and therefore reflect decreased specificity for AKI. Alternatively, it is possible that increased urinary L-FABP levels in this setting are due to subtle renal injury that does not result in increased SCr and therefore reflect increased sensitivity for AKI. The difficulty in differ-entiating between these two possibilities will persist as long as new biomarker data are evaluated against a suboptimal reference standard (SCr). It is also interesting that a number of patients who were not identified clinically as having renal disease had elevated urinary L-FABP levels before cardiac catheterization. This suggests that urinary L-FABP may prove to be useful for a more sensitive screening of patients for renal disease when their SCr is within the normal range.
This study did not include blood collections for serum biomarker quantification. As a result, it is not possible to correlate serum and urinary L-FABP levels in this cohort. However, previous studies in patients with chronic kidney disease and sepsis have shown that serum L-FABP levels do not have an influence on urinary levels and that urinary L-FABP levels in patients with liver disease are not significantly higher than those in healthy subjects.17,22 Therefore, in our patients with established AKI, urinary excretion of L-FABP is very likely to reflect a renal and not a hepatic source. To confirm that liver-derived L-FABP does not contribute to urinary levels, particularly in the setting of sepsis and/or shock, future studies should include determination of serum L-FABP levels and liver function tests.
This is the first study to establish urinary L-FABP levels as a predictor of adverse outcome (RRT, death/RRT) in patients with established AKI. Thus, urinary L-FABP may be added to the growing list of biomarkers that promise to aid in prognosis in the setting of AKI. This is notable given that improved AKI prognostic paradigms are critical for appropriate stratification strategies in future studies in the evaluation of novel AKI therapies.
Given the inherent renal heterogeneity and the disparate settings under which kidney injury occurs, a panel of carefully selected biomarkers may prove to be most appropriate in the diagnosis and prognosis of AKI. Development of such a panel will require large, well-designed prospective studies comparing multiple biomarkers in the same set of urine samples over extended time courses. Such studies will allow temporal patterns of biomarker elevation to be established, patterns that may be specific to the mechanism of injury (nephrotoxicant, ischemia, sepsis, allograft rejection, etc.), population of interest (elderly, pediatric, etc.), and/or co-occurring disease states (diabetes, heart disease, sepsis, etc.). Although these prospective studies are crucial, they are not without their own limitations for biomarker validation. Ambiguity of results will persist as long as biomarkers are compared against SCr. For example, a very sensitive biomarker may be increased in mild kidney injury in which SCr remains unchanged. Alternatively, a very specific biomarker may not be increased in a setting in which an increase in SCr is related to functional changes and not kidney tubular injury. This cross-sectional study, in which the diagnosis of AKI is firm, is less susceptible to these ambiguities.
In conclusion, urinary L-FABP is a sensitive and specific marker of AKI in patients. Higher levels predict the need for RRT and the composite end point of death/RRT in patients with AKI. Therefore, urinary L-FABP should be included in future studies evaluating the diagnostic and prognostic significance of urinary biomarker levels in AKI.
MATERIALS AND METHODS
Patient selection for urine collection
Human subjects were recruited from the inpatient nephrology consultation service (established AKI), inpatient cardiology service (precatheterization controls), critical care service (ICU controls), and hospital employees (healthy volunteers) of Brigham and Women’s Hospital. The institutional review board approved the protocols for subject recruitment and specimen collection. Patients with established AKI had ≥50% increase in SCr from admission or known baseline values. Causes of AKI were diverse, including acute tubular necrosis, sepsis, and nephrotoxin exposure (Table 1). Healthy volunteers were excluded if they reported a recent hospitalization, diagnosis of chronic kidney disease, or treatment with nephrotoxic medications (non-steroidal anti-inflammatory drugs were allowed). Patients undergoing cardiac catheterization and those admitted to the ICU were included in the non-AKI cohort if they had a normal urine output, stable SCr during hospitalization, and an estimated GFR >50 ml/min using the Modification of Diet in Renal Disease equation.
ELISA for urinary L-FABP quantification
Urine samples were prepared as described above. All samples were assayed in duplicate using a commercially available sandwich ELISA kit (HK404, Hycult Biotechnology, Uden, The Netherlands). Urinary L-FABP levels were normalized to urinary creatinine, which was measured with Beckman Creatinine Analyzer 2 (Beckman Coulter, Fullerton, CA, USA).
NGAL, KIM-1, NAG, and IL-18 quantification
Urinary NGAL, KIM-1, and IL-18 levels were quantified using a microbead-based sandwich ELISA. KIM-1 and NGAL assays were developed and evaluated as previously described.23 IL-18 assay materials were commercially available from Bio-Rad (Hercules, CA, USA). Urinary NAG was measured spectrophotometrically using a commercially available colorimetric assay (Roche Diagnostics, Basel, Switzerland).
Urine samples
Urine was collected from spontaneous voids or from indwelling Foley catheters and subsequently centrifuged to remove cellular components. The supernatant was aliquoted in 1.8 ml eppendorf tubes and frozen within 2 h of collection at −80 °C. At the time of assay, samples were thawed, vortexed, and centrifuged at 14,000 RPM at 4 °C for 5 min and assays performed in duplicate for biomarker measurement.
Western blot analysis
Urinary total protein was determined using the BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). In an effort to sensitively detect L-FABP in control urines, equal concentrations of urine protein were loaded per well and subjected to standard western blot analysis with affinity-purified goat polyclonal antibody raised against human L-FABP (AF2964, R&D Systems, Minneapolis, MN, USA). Standard human L-FABP protein (Hycult Biotechnology) was used as a reference standard.
Statistics
Continuous variables are expressed as means±standard deviation or medians. Urinary L-FABP levels were compared using the Mann–Whitney U-Test. Diagnostic performance was assessed using the ROC curve. The AUC for a diagnostic test ranges from 0.5 (no better than chance alone) to 1.0 (perfect test, equivalent to the gold standard). Comparative diagnostic performance of urinary L-FABP versus NGAL, KIM-1, NAG, and IL-18 was determined using pairwise comparison of ROC-AUCs.44 The ability of urinary L-FABP to predict adverse outcome in established AKI was tested using logistic regression analysis, adjusting for age. A two-tailed P-value of <0.05 was considered to be statistically significant. JMP version 7.0 (SAS Institute, Cary, NC, USA) and MedCalc Version 9.5.0 (MedCalc Software, Mariakerke, Belgium) statistical software were used for analyses.
ACKNOWLEDGMENTS
A part of this study was presented at the American Society of Nephrology meeting in San Francisco, CA, USA on 3 November 2007. This work was supported by NIH grants DK 39773 and DK 72381 to JVB, NIEHS grant R00 ES016723 to VSV, NIH career development grant DK 075941 to SSW, and by a Research Fellowship from the National Kidney Foundation to MAF.
Footnotes
DISCLOSURE JVB is co-inventor on patents for KIM-1 and is a consultant for Johnson & Johnson and Genzyme. Patents have been licensed to Johnson & Johnson and Genzyme by Partners Health Care.
REFERENCES
- 1.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Wish JB, et al. Hospital-acquired renal insufficiency: a prospective study. Am J Med. 1983;74:243–248. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 3.Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survery. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 4.Nash K, Hafeez A, Hou S. Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002;39:930–936. doi: 10.1053/ajkd.2002.32766. [DOI] [PubMed] [Google Scholar]
- 5.Shusterman N, Strom BL, Murray TG, et al. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83:65–71. doi: 10.1016/0002-9343(87)90498-0. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 8.de Mendonca A, Vincent JL, Suter PM, et al. Acute renal failure in the ICU: risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000;26:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 9.Ympa YP, Sakr Y, Reinhart K, et al. Has mortality from acute renal failure decreased? A systematic review of the literature. Am J Med. 2005;118:827–832. doi: 10.1016/j.amjmed.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 10.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 11.Pelsers MM, Hermens WT, Glatz JF. Fatty acid-binding proteins as plasma markers of tissue injury. Clin Chim Acta. 2005;352:15–35. doi: 10.1016/j.cccn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Gong Y, Anderson J, et al. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 13.Ek-Von Mentzer BA, Zhang F, Hamilton JA. Binding of 13-HODE and 15-HETE to phospholipid bilayers, albumin, and intracellular fatty acid binding proteins. Implications for transmembrane and intracellular transport and for protection from lipid peroxidation. J Biol Chem. 2001;276:15575–15580. doi: 10.1074/jbc.M011623200. [DOI] [PubMed] [Google Scholar]
- 14.Kamijo A, Sugaya T, Hikawa A, et al. Urinary excretion of fatty acid-binding protein reflects stress overload on the proximal tubules. Am J Pathol. 2004;165:1243–1255. doi: 10.1016/S0002-9440(10)63384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamijo-Ikemori A, Sugaya T, Obama A, et al. Liver-type fatty acid-binding protein attenuates renal injury induced by unilateral ureteral obstruction. Am J Pathol. 2006;169:1107–1117. doi: 10.2353/ajpath.2006.060131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamijo A, Kimura K, Sugaya T, et al. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med. 2004;143:23–30. doi: 10.1016/j.lab.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kamijo A, Sugaya T, Hikawa A, et al. Urinary liver-type fatty acid binding protein as a useful biomarker in chronic kidney disease. Mol Cell Biochem. 2006;284:175–182. doi: 10.1007/s11010-005-9047-9. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura T, Sugaya T, Koide H. Angiotensin II receptor antagonist reduces urinary liver-type fatty acid-binding protein levels in patients with diabetic nephropathy and chronic renal failure. Diabetologia. 2007;50:490–492. doi: 10.1007/s00125-006-0545-4. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura T, Sugaya T, Node K, et al. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006;47:439–444. doi: 10.1053/j.ajkd.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Portilla D, Dent C, Sugaya T, et al. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2007;73:465–472. doi: 10.1038/sj.ki.5002721. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto T, Noiri E, Ono Y, et al. Renal L-type fatty acid binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Sugaya T, Koide H. Urinary liver-type fatty acid-binding protein in septic shock: effect of polymyxin B-immobilized fiber hemoperfusion. Shock. 2009;31:454–459. doi: 10.1097/SHK.0b013e3181891131. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya V, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acture kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonventre JV, Weinberg JM. Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol. 2003;14:2199–2210. doi: 10.1097/01.asn.0000079785.13922.f6. [DOI] [PubMed] [Google Scholar]
- 25.Schrier RW, Wang W, Poole B, et al. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 27.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 28.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 29.Liu KD, Matthay MA, Chertow GM. Evolving practices in critical care and potential implications for management of acute kidney injury. Clin J Am Soc Nephrol. 2006;1:869–873. doi: 10.2215/CJN.00450206. [DOI] [PubMed] [Google Scholar]
- 30.Martin GS, Mannino DM, Eaton S, et al. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 31.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 32.Bailly V, Zhang Z, Meier W, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 33.Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 34.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 35.Ichimura T, Hung CC, Yang SA, et al. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- 36.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 37.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 38.Mishra J, Ma Q, Prada A, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 39.Mishra J, Mori K, Ma Q, et al. Neutrophil gelatinase-associated lipocalin: a novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004;24:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 40.Parikh CR, Abraham E, Ancukiewicz M, et al. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16:3046–3052. doi: 10.1681/ASN.2005030236. [DOI] [PubMed] [Google Scholar]
- 41.Parikh CR, Jani A, Melnikov VY, et al. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–414. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Sugaya T, Ebihara I, et al. Urinary liver-type fatty acid-binding protein: discrimination between IgA nephropathy and thin basement membrane nephropathy. Am J Nephrol. 2005;25:447–450. doi: 10.1159/000087826. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki K, Babazono T, Murata H, et al. Clinical significance of urinary liver-type fatty acid-binding protein in patients with diabetic nephropathy. Diabetes Care. 2005;28:2038–2039. doi: 10.2337/diacare.28.8.2038. [DOI] [PubMed] [Google Scholar]
- 44.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]


