Abstract
OBJECTIVES
1) To examine the effects of a month-long nap regimen using one of two durations (45 min, or 2 hr) on nighttime sleep and waking function in a group of healthy older subjects. 2) To assess the degree to which healthy older individuals are willing/able to adhere to such napping regimens.
DESIGN
Three laboratory sessions, with 2-week at-home recording interspersed, using a between-subjects approach.
SETTING
The study was conducted in the Laboratory of Human Chronobiology at Weill Cornell Medical College and in subjects’ homes.
PARTICIPANTS
Twenty-two healthy men and women aged 50–88 years (mean = 70y).
MEASUREMENTS
Polysomnography (sleep EEG), actigraphy, sleep diaries, neurobehavioral performance, sleep latency tests.
RESULTS
With the exception of compliance to the protocol, there were few differences between Short and Long nap conditions. Napping had no negative impact on subsequent nighttime sleep quality or duration, resulting in a significant increase in 24-hour sleep amounts. Such increased sleep was associated with enhanced cognitive performance, but had no impact on simple reaction time. Subjects were generally able to comply better with the 45-minute than the 2-hour nap regimen.
CONCLUSION
A month-long, daily nap regimen may enhance waking function without negatively impacting nighttime sleep. Using 2-hour naps in such a regimen is unlikely to meet with acceptable compliance; a regimen of daily 1-hour naps may be more desireable for both effectiveness and compliance.
Keywords: napping, performance, sleep disturbance, aging, body temperature, compliance
INTRODUCTION
Aging is associated with significant changes in the structure and quality of sleep. Compared to healthy young adults, individuals over the age of about 60 years exhibit reduced amounts of slow wave sleep, a decline in the spectral power of EEG delta activity, shortened REM onset latencies, increases in the number and duration of within-sleep awakenings and significantly earlier terminal waking time. These latter two events result in an average nighttime sleep duration almost 2 hours shorter than that of healthy young adults (1–5).
Efforts to extend the nighttime sleep of older subjects have been largely unsuccessful, leading several authors to conclude that many older individuals may actually be incapable of obtaining more than about 6 hours of sleep per twenty-four hours (3). Such speculation has led us and others to investigate the possibility that 24-hour sleep amounts could be increased significantly by introducing an afternoon nap into the sleep schedules of older subjects (6–11). We found that a two-hour afternoon sleep opportunity resulted in an average increase in 24-hour sleep amounts of 81 minutes (from 6.05 h to 7.4 h). The impact of the nap on subsequent nighttime sleep quality was limited to a slight increase in sleep onset latency (21.8 min. compared to 15.5 min. in the control condition). No changes in sleep efficiency, or amounts or proportions of non-REM or REM sleep were observed.
In a more extended (17 day) study of napping in healthy older subjects, in which subjective and objective data were examined, Monk and co-workers (11) reported mixed results. Sleep logs maintained by subjects at home revealed significantly increased 24-hour sleep amounts, with no significant impact on nighttime sleep duration or quality.
In contrast, lab-based polysomnography (PSG) showed a non-significant decline in 24-hour sleep amounts, as a consequence of significant reductions in nighttime sleep duration and efficiency.
A study by Tanaka and colleagues (8; 9) examined the effects of a month of afternoon naps plus evening exercise on nighttime sleep in eleven elderly individuals with chronic sleep difficulties. Although the relative influence of naps versus exercise could not be teased apart, nighttime sleep was consolidated by the treatment combination, and sleep efficiency increased from an average of 75% at baseline to 90% during the last week of the study.
In our study, the increase in total sleep time per 24 hours was associated with significant improvements in a number of cognitive performance measures immediately following the nap, as well as, throughout the following day. Similar results were reported by Tamaki, et al (7) for a group of healthy, older habitual nappers who, on one occasion, took a 30-minute nap and on another, remained sedentary, but awake, for 30 minutes. Performance on a visual detection task was significantly better in the nap condition compared to the sedentary condition. In contrast to these findings, Monk and co-workers reported no significant effect of naps on several measures of cognitive and psychomotor performance.
In light of these somewhat mixed findings and because of the relative paucity of data addressing the issue of effects of naps in older subjects, we sought to build on our previous study by examining the longer-term impact of a napping regimen on nighttime sleep quality and daytime cognitive performance. We also sought to determine the feasibility of such a longer-term regimen in terms of subject compliance. Here, we describe the results of a six-week protocol that involved an at-home napping regimen interspersed with objective laboratory assessments of sleep and neurobehavioral function.
METHODS
Subjects
The data reported here are from 22 subjects (11 men, 11 women) over the age of 50 (mean age = 70 + 10 years, range 50 – 83 years). The protocol was approved by the Weill Cornell Medical College’s Institutional Review Board. All subjects provided written informed consent and they were compensated for their participation.
After responding to advertisements to the general public targeting older individuals with or without sleep disturbance, age-eligible individuals were screened via phone for additional inclusion/exclusion criteria relating to medication usage, sleep and napping habits, and subjective sleep ratings. This was followed by an in-person physical and mental health screening and a tour of the laboratory facilities.
A total of 37 potential participants underwent the in-person screening interview. Of these, 3 were ineligible because of medication usage. An additional 5 individuals declined to participate. Thus, 29 subjects were enrolled in the study. Two enrolled subjects completed only the baseline session, and 2 were excluded after the first night spent in the laboratory because of suspected, significant periodic limb movement disorder (see below). Of the 25 who completed the protocol, 3 had datasets that were not usable: one because of actiwatch malfunction; one because the subject took melatonin for sleep problems sporadically thoughout the study, and one because she started smoking during the study.
Subjects were in self-reported good physical health and, at the time of the study, were not taking psychotropic medications or any other medications known to interfere with normal sleep. Minor, controlled health problems such as mild hypertension or mild arthritis were not grounds for exclusion from the study. A brief psychiatric screening (17-item Hamilton Depression Rating Scale; HDRS-17; 12) was completed on each subject. A score of <7 was required for participation. In addition, subjects were excluded from participation if they had a score of >5 on the global Pittsburg Sleep Quality Index (PSQI; 1), or >2 on the “sleep latency” or “use of sleeping medication” PSQI subscales.
Only those who did not report habitual napping were enrolled. Eligible participants had to report taking an average of fewer than 2 naps per week in the last 6 months. On the other hand, all those enrolled consented to following the protocol, including the requirement to incorporate a daily nap into their routines.
Although all subjects reported age-related sleep problems, primarily in the form of sleep maintenance difficulties, and/or insufficient sleep duration, a previous diagnosis or current evidence of other sleep disorders (e.g. sleep apnea, periodic limb movement disorder (PLMD), narcolepsy, REM behavior disorder, circadian rhythm sleep disorder, restless legs syndrome, primary insomnia) excluded subjects from participation. An extensive sleep history was obtained during the screening examination, and questionnaires including the Sleep Disorders Questionnaire (13), Epworth Sleepiness Scale (14) and a separate questionnaire probing for symptoms of restless legs syndrome (15) were used to screen out those with significant sleep pathologies. Also, on the first night in the laboratory, pulse oximetry, and bilateral leg EMG recordings were used to further screen subjects for sleep apnea or PLMD (see below).
Procedure
Baseline -- in-home and laboratory session
Subjects maintained daily sleep logs, and actigraphy was recorded continuously for 1–2 weeks prior to the baseline laboratory session. These data were used to confirm the self-reported sleep schedules and frequency of napping, and also to calculate habitual bedtimes and waketimes for use during the lab session. On the day immediately following the conclusion of the in-home baseline phase, subjects reported to the sleep laboratory by 1900, for the baseline laboratory session. This session consisted of 3 consecutive nights and the 2 intervening days, during which subjects remained in the lab. After they settled into private bedrooms, electrodes were applied for polysomnographic (PSG) recording. A trained research assistant then introduced subjects to the neurobehavioral performance assessment battery (PAB). The familiarization session continued until the subject had an understanding of how to perform each task.
Bedtimes and waketimes in the lab were tailored for each subject, as calculated from the average weekday sleep times reported in the sleep logs maintained during the pre-baseline in-home phase. In the laboratory, each subject slept in a private, darkened, sound-attenuated bedroom.
On the first laboratory night, subjects were screened for sleep pathology using pulse oximetry and bilateral leg EMGs. Subjects whose O2 saturation levels dropped below 90%, or who had an index of > 10/hr leg movements associated with an EEG-defined arousal were excluded from continued participation. Based on these criteria, 2 subjects were excluded from the study.
Beginning two hours after awakening from Night 1, and then every 2 hours for the next 10 hours, subjects practiced the performance task battery. Subjects were required to practice the PAB until their trial-to-trial deviation in accuracy was <5% across three consecutive trials, on each of the four individual tasks comprising the battery. The data from one subject who was unable to achieve this level of performance on the logical reasoning task (see Data Analysis, below) were excluded from subsequent analyses including that task.
Sleep on baseline nights 2 and 3 was again recorded polygraphically. Parameters derived from the scored EEG records were averaged across the two nights to yield single baseline measures of sleep composition and quality. Two-night averages were chosen due to the well-documented night-to-night variability in sleep quality frequently exhibited by older individuals (4; 16; 17). It was, therefore, felt that such an approach provided a more accurate reflection of subjects’ typical sleep.
On the day between nights 2 and 3, performance was again measured 2 hours after wake time and each subsequent 2 hours, until 5 trials were completed. Performance measures obtained on this day were used to establish baseline performance levels. One hour after each PAB trial, subjects underwent a 20-min Sleep Latency Test (SLT), in which they were asked to try to fall asleep while lying in bed in a darkened room. Detection of a sleep spindle or K-complex resulted in the termination of the SLT. These measures were employed to establish baseline sleepiness levels.
Between PAB trials and SLTs, subjects were permitted to engage in leisure activities (reading, watching TV or movies, etc.) within the lab area. They were not permitted to nap. Both continuous EEG and closed-circuit TV monitoring were employed to ensure wakefulness. Meals and snacks were available ad lib, except during the performance and sleepiness assessment intervals.
In-Home Napping Phase
Following the baseline lab visit, subjects began the 4-week-long in-home phase of the study. They were randomly assigned to either a 45-minute nap condition (“Short”) or a 2-hour nap condition (“Long”). They were instructed to nap at least 5 days per week and strongly encouraged to nap daily. They were further instructed to nap only one time per day and to complete their naps by no later than 1800h. Subjects completed sleep logs on a twice-daily basis (bedtime and rise time) throughout the 4-week interval.
Mid and End laboratory sessions
Two subsequent laboratory sessions, 2 weeks and 4 weeks after the initial visit ended, were identical to the baseline session, with the exception that a) subjects stayed in the lab for only 2 nights and the intervening day, and b) they napped on the day in the lab. The in-lab nap opportunity was scheduled to start 6.5 hours following wake-up from night 1 and to continue for either 45 minutes, or 2 hours, depending on group assignment. We considered scheduling the in-lab nap at the same time that each subject napped most frequently during the preceding 2 weeks at home, or at their average nap time across the 2 weeks. We also considered allowing subjects to self-select the time of in-lab naps, in order to mimic the in-home regimen. However, scheduling nap times based on time from waking had two important methodological advantages. First, this approach avoided the problem of missed performance trials by subjects who might be napping at the time of a scheduled trial. Second, the approach permitted direct within-subject comparisons of performance and sleepiness data at baseline and following implementation of the napping regimen. That is, all subjects completed 4 PAB trials and 3 SLTs that corresponded to the times of testing at their baseline lab session, anchored to morning wake time.
Data Analysis
Polysomnographic Sleep
All EEG records from nighttime and nap sleep were scored by a trained sleep scorer in 30-sec epochs according to Rechtschaffen and Kales criteria (18), with the exception that no amplitude criterion was applied to evaluate slow wave sleep stages 3 and 4 (19). The following variables were derived from these records: sleep onset latency (SOL – interval from bedtime until the first epoch of stage 2, SWS, or REM), minutes and percentage of stages 1, 2, SWS, and REM during the sleep period time (SPT – interval from sleep onset until scheduled wake time), minutes and percentage of wakefulness after sleep onset (WASO), total sleep time (TST – sum of minutes of sleep stages 1 – REM), and sleep efficiency (SE), calculated as both the ratio of TST during the SPT, and as the ratio of TST during time in bed (TIB – interval from scheduled bedtime until scheduled wake time).
Neurobehavioral performance
The performance assessment battery (PAB) was comprised of 4 tasks from the Automated Neuropsychiatric Assessment Metrics (20) : Logical Reasoning-Symbolic (LOG), Mathematical Processing (MTH), Sternberg 6-letter Memory Search (ST6), and 2-Choice Reaction Time (2CH). The measure of throughput (calculated as response time/accuracy) was used as the outcome variable for each task. For most analyses, a daily average throughput value was calculated for each subject, for each session. To account for high intersubject variability in absolute performance levels, and to evaluate how performance changed across the study, the percent change in throughput from baseline to mid and baseline to end sessions was determined for each subject. For analyses comparing performance before versus after the nap at mid and end sessions, the absolute throughput value of the two pre-nap PAB trials were averaged, and the percent change from the pre-nap “baseline” to the single post-nap PAB trial was calculated for each subject. For correlations between performance and sleep measures (see below), absolute throughput levels were used.
Sleep Latency Tests
As with performance measures, a daily average sleep onset latency was calculated for the SLTs at baseline, mid, and end sessions. If a subject did not fall asleep during the 20-min SLT, a latency of 20 minutes was assigned for that test.
Actigraphic sleep
Actiwatches (Mini-mitter Respironics, Inc.) were worn continuously during the 4-week in-home napping phase of the study. The following sleep variables for both nighttime and nap sleep were obtained from the combination of actigraphy records and daily sleep logs: sleep period duration, total sleep time, and sleep efficiency. (Sleep onset latency was not reliably measured using actigraphy and sleep logs, and was not calculated for either nighttime or nap periods during the in-home study phase). The actigraphy records were analyzed using the Actiware 5.0 algorithm, set at a medium threshold for sleep versus wake detection for all subjects.
Statistical Analyses
Mixed ANOVAs and multivariate ANOVAs were used to compare measures between conditions and across lab sessions (e.g., nighttime PSG, nap PSG, performance), or across intervals of the in-home napping phase (e.g., actigraphy-derived sleep measures from baseline to mid). Significant interactions were followed by post-hoc comparisons. Possible relationships between sleep and performance measures were examined using Pearson product-moment correlations.
RESULTS
Nighttime Sleep in Lab (PSG)
Randomized assignment to the two nap conditions resulted in 11 subjects each in the Short and Long groups. As shown in Table 1, there were no significant differences between the groups with respect to age, gender distribution, or baseline sleep and neurobehavioral performance measures.
Table 1.
Baseline Variables × Condition.
| BASELINE | ||
|---|---|---|
| Short | Long | |
| AGE | 72.3 ± 9.3 | 67.55 ± 10.3 |
| GENDER | 6M, 5F | 5M, 6F |
| BT (hh:mm) | 23:23 ± :56 | 24:02 ± 1:15 |
| WT (hh:mm) | 07:06 ± :48 | 07:45 ± 1:15 |
| Polysomnographic Sleep | ||
| Sleep Efficiency (%) (TST/SPT) | 83.0 ± 9.9 | 82.0 ± 8.4 |
| SOL (min) | 16 ± 13 | 19 ± 12 |
| SPT (min) | 451 ± 68 | 445 ± 38 |
| TST (min) | 370 ± 49 | 364 ± 47 |
| WASO (min / %) | 80 ± 51 / 17.0 ± 9.9 | 81 ± 39 / 18.0 ± 8.4 |
| Stage 1 (min / %) | 24 ± 25 / 5.4 ± 4.7 | 25 ± 14 / 5.5 ± 3.0 |
| Stage 2 (min / %) | 181 ± 57 / 40.0 ± 11.8 | 172 ± 32 / 38.3 ± 5.0 |
| SWS (min / %) | 84 ± 35 / 19.4 ± 8.9 | 85 ± 23 / 19.3 ± 5.9 |
| REM (min / %) | 80 ± 43 / 18.3 ± 10.2 | 83 ± 23 / 18.8 ± 4.6 |
Abbreviations: BT - bedtime; WT - wakeup time; TST - total sleep time; SPT - sleep period time (from sleep onset to wakeup time); WASO - wakefulness after sleep onset; SWS - slow wave sleep; REM - rapid eye movement; SOL - sleep onset latency (to first epoch of Stage 2, SWS, or REM).
Neither long nor short naps had a significant effect on any measure of subsequent nighttime sleep (Figure 2). Post-nap nighttime sleep at mid or end was not significantly from baseline on any measure. Sleep onset latency, sleep efficiency and total sleep time remained essentially unchanged across laboratory sessions. Also, as at baseline, there were no significant differences between the Long and Short nap groups on any measure of nighttime sleep at mid or at end.
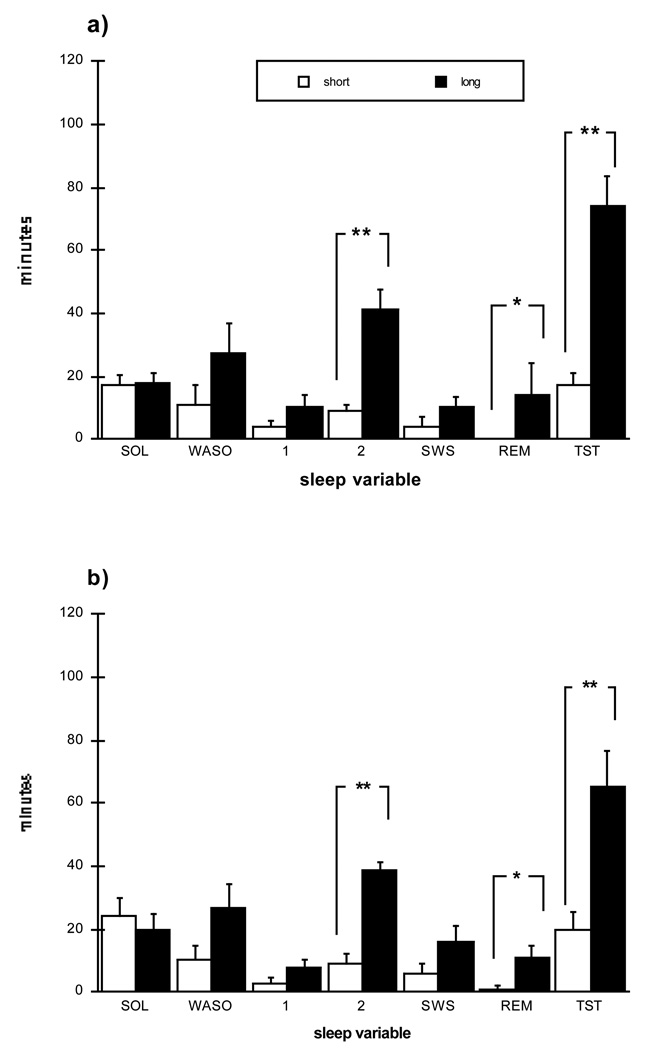
Figure 2.
Polysomnographic sleep variables from naps for Short and Long conditions at (a) mid and (b) end laboratory sessions. Minutes to sleep onset (SOL) and minutes of each sleep stage, including wakefulness after sleep onset (WASO) are presented. Nap opportunity duration was 45 minutes for Short and 120 minutes for Long. Asterisks denote significant differences between conditions (** p < .001; * p < .05).
Naps in Lab (PSG)
Neither the Short nor the Long group showed a significant change in nap sleep measures between the mid and end sessions. As would be expected, at both mid and end sessions, the Long nap subjects obtained significantly more average total sleep during their naps (TST) than did the Short nappers (mid: 74 ± 32 min for Long vs. 17 ± 13 min for Short, p < .001; end: 65 ± 36 min for Long vs. 20 ± 18 min for Short, p < .001). As a consequence of longer sleep times, the Long group also obtained significantly more minutes of REM sleep than the Short group (mid: 14 ± 7 min Long vs. 0 ± 0 min Short, p < .02; end: 11 ± 12 min Long vs. 1 ± 4 min Short, p < .02).
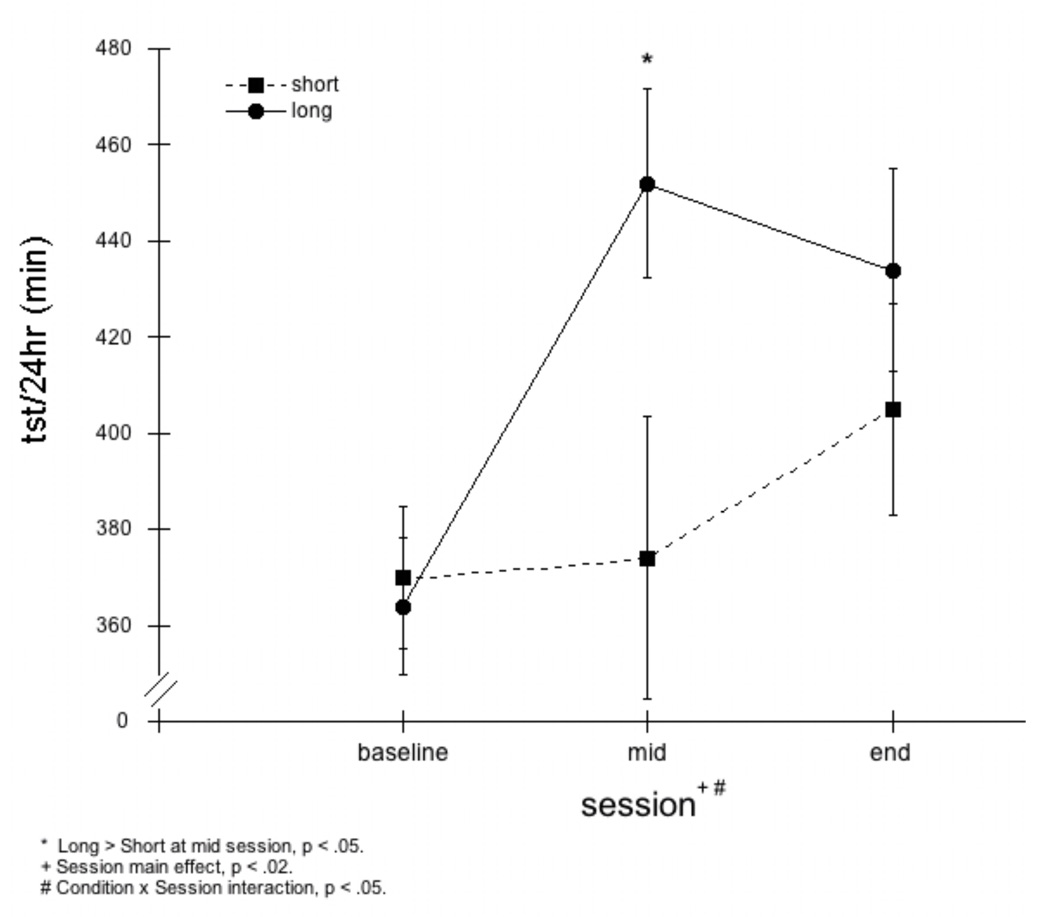
Mixed ANOVA (Condition × Session), revealed that the addition of nap TSTs to nighttime TSTs resulted in significantly greater total sleep time per 24 hours (TST/24) for the Long and the Short nap groups combined, at both mid and end lab sessions (see Figure 3). Post hoc analyses indicated that average TST/24 of the Short group increased significantly over baseline only at the end session (+34.7 min; p< .001), whereas the Long group showed significant increases at both lab sessions (+87.5 min; p <.01 at mid and +70.3 min; p< .001 at end).
Figure 3.
Total sleep time per 24 hours (tst/24hr). A mixed ANOVA (Condition × Session) revealed a main effect for Session (p<0.02) and Condition × Session interaction (p<0.05). Post-hoc comparisons indicated that tst/24hr in Long group increased significantly from baseline to mid and baseline to end, tst/24hr in Short group increased significantly from baseline to end only, and tst/24hr in the Long group was significantly greater than in the Short group at the mid Session (p<0.05), but not at the end Session.
Neurobehavioral Function
The following results are reported for 21 subjects (11 Short, 10 Long) because significant amounts of data from one subject were lost due to equipment difficulties.
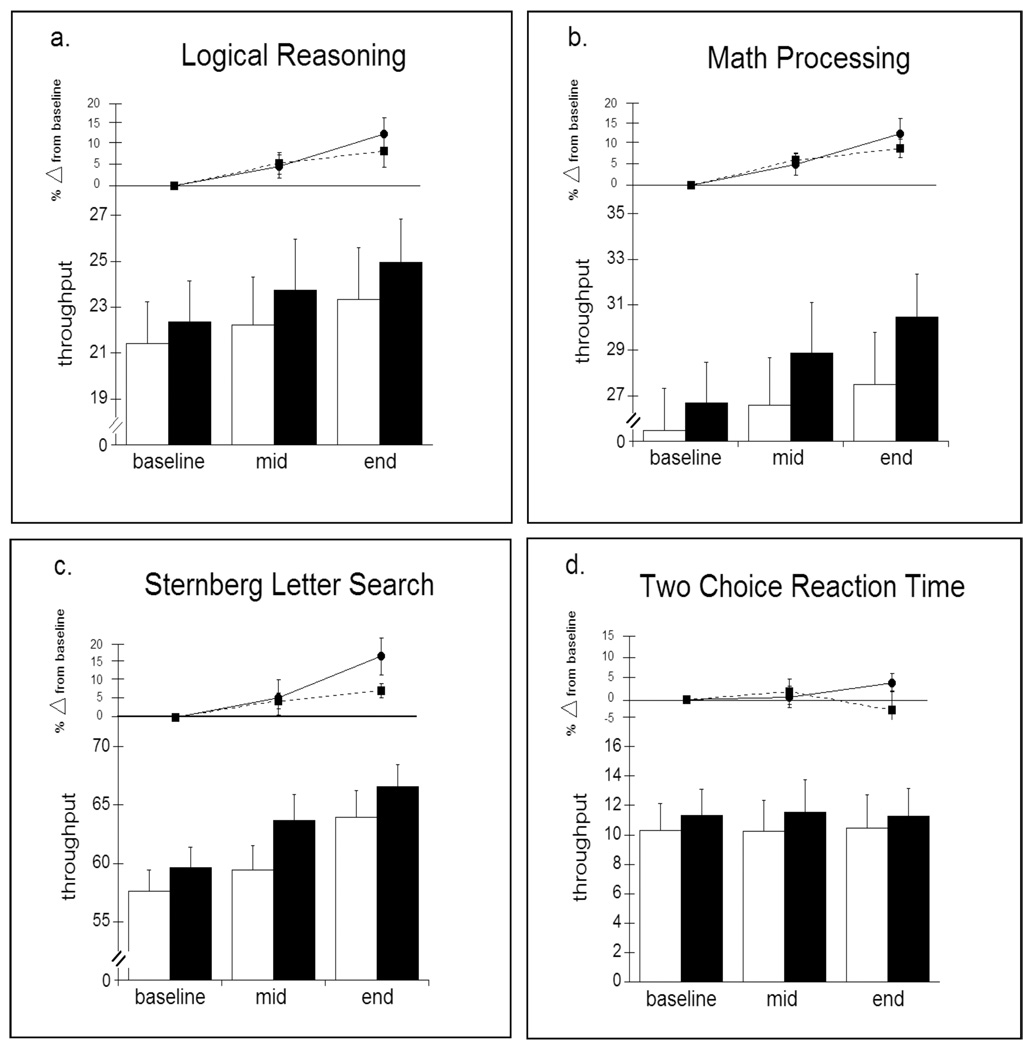
Throughput on the PAB tasks at each session is shown in Figure 3. As was the case for nighttime sleep measures, no significant differences were found between Long and Short nappers at baseline, on any of the tasks comprising the Performance Assessment Battery (PAB). Performance on 3 of the 4 tasks improved significantly from baseline to mid and from baseline to end sessions. There were also significant improvements on these three tasks from mid to end sessions. Two-Choice Reaction Time remained essentially unchanged across the 3 laboratory sessions. These results held for both the Long and Short nap groups, although there was a consistent (non-significant) trend for those in the Long group to show greater improvement at mid and end sessions.
To investigate possible explanations for the improvements in performance, relationships between changes in performance and sleep measures were conducted. These analyses revealed that there were no significant correlations between any performance measures and either nap duration, or 24-hour sleep amounts (i.e. nap + nighttime TST). Likewise, no significant correlations were found between performance and compliance (see below) during the at-home intervals of the study. With respect to the possible influence of sleep architecture on performance measures, improvement on the Logical Reasoning task at the end session was associated with minutes of SWS during naps (r=.64, p < .01). No other correlations between sleep stages and changes in performance were significant. Finally, there were no significant relationships between nap variables (TST, minutes of SWS, minutes of REM) and performance on the single PAB trial that occurred in the late afternoon/early evening hours, 2 hours after the nap opportunity.
Sleep Latency Tests
Although sleep onset latencies (SOL) on the sleep latency tests did not indicate that these subjects were sleepy during the daytime hours, both Short and Long naps increased SOLs in both groups. The mean sleep onset latency (SOL) at baseline for the Short group was 14.0 ± 5.0 min, compared with 15.5 ± 3.5 min for the Long group (n.s.). Average latency to sleep onset for the all subjects, for the entire day (i.e., averaging the 2 pre-nap and 1 post-nap SLTs), was significantly longer at both mid (18.0 ± 5.0 min) and end (18.5 ± 5.0 min) sessions when compared to baseline; these day averages did not differ between Short and Long groups. Both Short and Long nappers exhibited significantly longer sleep onset latencies on the SLT following the nap compared to the average of the two SLTs that preceded the nap (Short mid pre-nap: 14.0 ± 6.0 min vs post nap: 18.0 ± 4.0 min, p<0.001; Long mid pre-nap: 15.0 ± 5.5 min vs. post-nap: 20.0 ± 0 min, p<0.001; Short end pre-nap: 13.0 ± 6.0 in vs. post-nap: 16.5 + 6.5 min, p < 0.001; Long end pre-nap: 14.5 ± 5.0 min vs. post-nap: 20.0 ± 1.0, p<0.001). Indeed, in the Long group, no subjects fell asleep on the post-nap SLT at mid, and only 2 fell asleep during the post-nap SLT at end.
Naps and Nighttime Sleep at Home (Actigraphy/Logs)
According to actigraphy records (combined with sleep log information), subjects in both the Short and Long groups napped approximately 5 times per week (Short: 5.11 ± 1.23 naps / week vs. Long: 5.17 ± 1.24 naps / week, n.s.). Although the group averages for number of naps per week were >5 in both groups, some subjects napped every day, while others napped much less frequently (see Compliance, below). The mean duration of in-home naps was 58 ± 29 minutes for the Short group, compared with 95 ± 35 minutes for the Long group (p < .02). Sleep efficiency of the at-home naps averaged 60 ± 16% for the Short, compared with 56 ± 20% for the Long groups, respectively (n.s.); these sleep efficiencies were similar to PSG naps in the lab sessions.
While at home, the average nap start time was 15:07h ± 2:09 for the Short group and 14:43h ± 1:32 for the Long group (n.s.). These clock times were not significantly different from the clock times at which nap opportunities were scheduled in the lab (i.e., 6.5 hours after Tmin), which were 13:37h ± 0:52 for the Short, and 14:20h ± 1:36 for the Long groups, respectively.
To examine whether any ‘mismatch’ between at-home nap timing and in-lab nap timing affected the nap, the difference between these times were calculated for each subject, separately for mid and end sessions, and then correlated with nap variables. The average mismatch was – 0:59, i.e., the nap at home was initiated, on average, an hour later than the scheduled lab nap. The mismatch amounts did not differ between Short and Long groups, and were not associated with any nap variables (all probability values for correlations > 0.10).
To examine whether naps taken at home affected nighttime sleep at home, the duration and sleep efficiency (SE) of nighttime sleep was compared for nights following naps versus nights following a day when no nap was taken. These analyses confirmed that naps did not negatively impact nighttime sleep. Combining Short and Long groups, nighttime sleep duration post-nap averaged 7:57 ± 1:22 hours versus 8:06 ± 1:49 hours following a nap-free day (n.s.). Nighttime SE averaged 76 ± 8% post-nap versus 78% ± 8% following nap-free days (n.s.). Moreover, there were no significant relationships between nap duration or nap SE and sleep measures on the subsequent night.
Compliance
Compliance to the napping protocol was operationally defined in 3 different dimensions: frequency, duration and timing. Subjects were considered compliant in terms of frequency if they averaged at least 5 naps per week. If at least 80% of naps were within 15 minutes of the prescribed length (45 minutes or 2 hours) subjects were deemed compliant with respect to duration. To be considered compliant with regard to timing 90% of naps had to be initiated between 1000h and 1800h. A subject was considered “super compliant” if all three criteria were met.
Compliance was assessed based on actigraphy data supplemented by information provided by subjects’ daily sleep logs. The following results are based on 21 subjects, as one subject in the Long nap group did not complete daily logs and did not consistently wear an actigraph during the home portion of the study.
The number of subjects meeting the compliance criterion for frequency did not differ between groups (χ2 = 2.39, n.s.). Eighty-two percent (9/11) of subjects in the Short group were compliant for frequency (5.11 ± 1.23 naps per week) compared to 50% (5/10) in the Long group (5.17 ± 1.24 naps per week). Subjects in the Short group showed a non-significant tendency toward better compliance in terms of nap duration, though both groups demonstrated relatively poor compliance (45% vs 30%; χ2 = .53, n.s.). In terms of timing, the Long group showed a tendency, again non-significant, toward better compliance, with 80% of subjects consistently intiating their naps within the prescribed window, compared to only 45% of Short nappers (χ2 = 2.65, p=0.10). Only 2 subjects in each group met criteria for “super compliance”.
DISCUSSION
These results support and extend our previous findings (10) that a daytime nap may improve neurobehavioral functioning in healthy older adults, without negatively affecting subsequent nighttime sleep. Whereas, our previous study examined the effects of a single, 2-hour nap opportunity on performance and nighttime sleep quality, the current study focused on the impact of a longer-term napping regimen, and on the relative effectiveness of a short (45 minute) versus a longer (2 hour) nap opportunity. Because the study was conducted to examine the effectiveness of napping as a possible countermeasure against some of the negative consequences of age-related changes in sleep, we were also interested in the degree to which subjects could adhere to the napping regimen.
Sleep and Alertness
There were few differences between the Short and Long nap groups in terms of their impact on nighttime sleep and daytime sleepiness. Both Short and Long nappers showed significant increases in 24-hour sleep amounts when compared to their baseline sleep times, and neither the short nor long nap had a negative impact on nighttime sleep: sleep onset latency, sleep efficiency, and sleep architecture remained essentially unchanged across the study. This finding is in agreement with a majority of studies that have examined the relationship between napping and subsequent nighttime sleep quality in older individuals (6–11; 21–25) and argues against the frequently-expressed notion that naps should be avoided.
The increase in total sleep time per 24-hours resulted in decreased daytime sleepiness, as reflected in mid and end lab sessions compared to baseline, as well as, within the mid and end sessions when pre- versus post-nap latencies were compared.
Neurobehavioral Function
In both the Short and Long groups, performance on 3 of the 4 tasks comprising the PAB showed significant improvement, with simple reaction time showing no change. There were no significant differences in the degree of improvement between the groups, though the Long group showed a consistent tendency for greater improvement. Significant improvements in performance were observed not only between baseline and each subsequent lab session, but also between the mid and end sessions. That is, performance continued to improve across the entire study.
These findings are in contrast to those by Monk and co-workers (11) who reported no significant effect of napping on performance. It should be noted, however, that performance was measured in laboratory sessions, during which total 24-hour sleep amounts between the nap and no-nap conditions did not differ. This difference, the dissimilar average age of subjects studied, or perhaps differences in the performance tasks employed, may help explain the discrepancy between studies.
On one hand, our findings may indicate that the beneficial effects of napping on neurobehavioral function may not be maximized even after a month of scheduled naps. It is conceivable that the negative effects of years of chronic sleep restriction associated with age-related truncation of nighttime sleep may require a longer time to be fully mitigated.
On the other hand, the results may be viewed as simply reflecting a practice effect. Despite the fact that subjects were thoroughly trained on the PAB and were required to reach strict criteria demonstrating asymptotic accuracy levels on each of the tasks prior to baseline testing (see Procedures), the possibility that subjects simply got better due to repeated exposure to the PAB cannot be ruled out. Perhaps adding support to this interpretation is the finding that changes in performance were not associated with either increased sleep amounts, or the composition of nighttime sleep or naps (with the exception of a significant correlation between SWS in the end nap with enhanced performance on the Logical Reasoning task). By the same token, no measure of compliance was related to change in performance.
Compliance
Based on our operational definition of compliance, which considered frequency, timing and duration of naps, it is clear that most older individuals would have difficulty incorporating a daily 2-hour nap into their schedules: Only half of the subjects in the Long group napped at least 5 times per week, and only 30% showed average nap durations within 15 minutes of the assigned duration, with most naps failing to reach minimum length. Although the Short nap group showed better compliance with regard to frequency, these subjects also showed poor compliance in terms of nap duration, with a large majority exceeding the maximum length. Thus, in terms of compliance to a nap regimen, it appears that a 2-hour nap opportunity may be too long, while a 45-minute window may be too short.
Limitations
Because neurobehavioral function was assessed only at the three laboratory visits (at baseline, after 2 and after 4 weeks of napping), relatively infrequent “snapshots” of effects of napping could be evaluated. It is impossible to determine, therefore, whether the improvements we observed throughout the entire protocol would have continued across additional days/weeks of napping, or whether the positive effects on cognitive performance were attenuated, or leveled out, at some point between the 2nd and 4th weeks of the napping regimen.
Another limitation of our study, with respect to generalizing the findings to other older samples, involves the overall health, in general, of our subjects, and their sleep quality, in particular. All of the subjects studied here were in good physical and emotional health and, although all reported some degree of age-related sleep disturbance (e.g., early morning awakening, more disrupted nighttime sleep), none suffered from a diagnosed sleep disorder, including significant insomnia. Thus, it is unclear whether a napping regimen would be beneficial to older individuals with chronic illnesses and/or sleep disorders.
In summary, these findings add further to a still-limited, but growing body of literature suggesting that a daily afternoon nap may be a safe and effective means of increasing ones 24-hour sleep total and, in so doing, improve waking function. Although a practice effect could not be ruled out, these data support our previous finding that increased 24-hour sleep amounts are accompanied by enhanced neurobehavioral functioning, as well as reduced daytime sleepiness, with little negative impact on nighttime sleep. While there were few differences between the 45-minute and 2-hour naps in terms of their impact on nighttime sleep and daytime functioning, it is clear from the compliance results that a regimen featuring the longer nap would not be widely accepted. From the current data, it is of course not possible to evaluate whether naps shorter than 45 minutes would also be associated with enhanced waking function, though at least one study has reported beneficial effects, in a small group of habitual nappers, of a 30-minute nap (7). In this regard, it is of note that our subjects, who were not habitual nappers before the study, did not respond to the instructions to nap daily by taking “power naps” at home. Rather, the majority of subjects in the Short group preferred a nap of about an hour in duration.
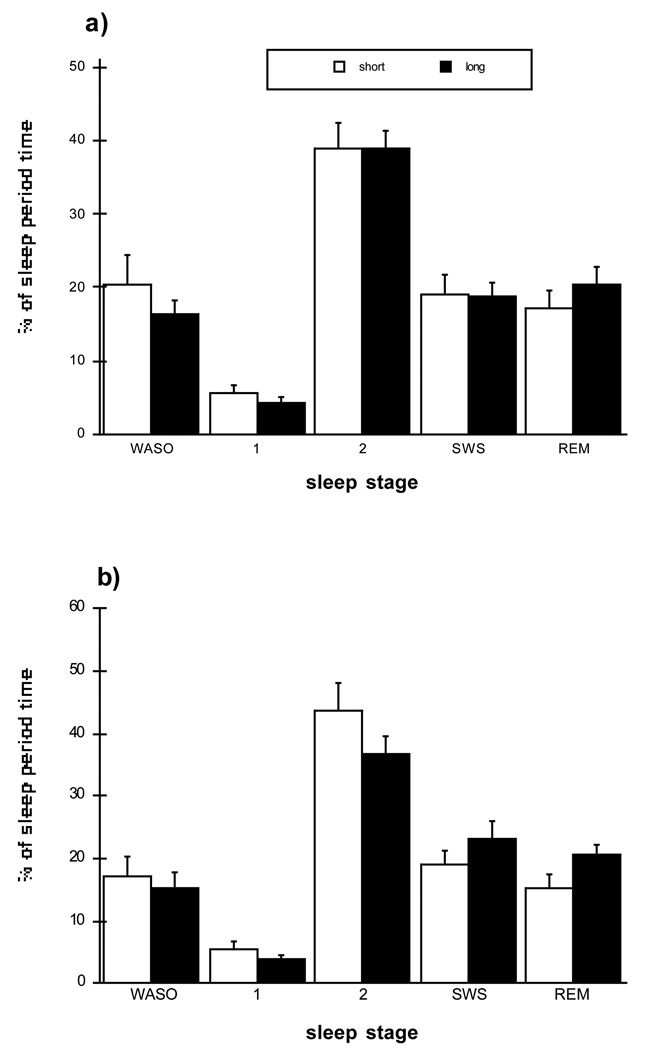
Figure 1.
Post-nap nighttime polysomnographic sleep variables for Short and Long conditions at (a) mid and (b) end laboratory sessions. Sleep stage percentages, including wakefulness after sleep onset, are expressed as a percentage of the interval from sleep onset to wakeup time, or sleep period time (SPT). The duration of nighttime SPT varied among subjects but did not differ between Short and Long groups. A mixed MANOVA (Condition × Session, including all sleep stage percentage variables), n.s. No main effects for Condition or Session, and no interaction effects.
Figure 4.
Throughput ([accuracy × speed] × 100) on 4 neurobehavioral performance tasks (a–d). Top portion of each graph shows % change from baseline for Short (- - • - -) and Long (— ■ —) conditions. Bottom portion of each graph shows absolute levels of throughput for Short (open bars) and Long (solid bars) conditions. Multivariate ANOVAs for Condition × Session including all 4 tasks revealed no main effect for Condition, a significant main effect for Session (Wilks lambda p < 0.01), and no Condition × Session interaction. Univariate ANOVAs indicated that throughput increased significantly across Sessions for all but d) Two Choice Reaction Time. Post-hoc comparisons indicated that throughput increases were significant from baseline to mid, mid to end, and baseline to end on each of the other three tasks.
Acknowledgments
This work was funded by National Institutes of Health grants R01AG12112, R01NS057628 and R01052495.
REFERENCES
- 1.Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991 Aug;14(4):331–338. [PubMed] [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995 Jul;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J American Geriatric Society. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 4.Webb WB. Sleep in older persons: sleep structures of 50- to 60-year-old men and women. J Gerontol. 1982 Sep;37(5):581–586. doi: 10.1093/geronj/37.5.581. [DOI] [PubMed] [Google Scholar]
- 5.Dijk DJ, Duffy JF. Circadian regulation of human sleep and age-related changes in its timing, consolidation and EEG characteristics. Ann. Med. 1999 Apr;31(2):130–140. doi: 10.3109/07853899908998789. [DOI] [PubMed] [Google Scholar]
- 6.Tamaki M, Shirota A, Tanaka H, Hayashi M, Hori T. Effects of a daytime nap in the aged. Psychiatry Clin. Neurosci. 1999 Apr;53(2):273–275. doi: 10.1046/j.1440-1819.1999.00548.x. [DOI] [PubMed] [Google Scholar]
- 7.Tamaki M, Shirota A, Hayashi M, Hori T. Restorative effects of a short afternoon nap (<30 min) in the elderly on subjective mood, performance and eeg activity. Sleep Res Online. 2000;3(3):131–139. [PubMed] [Google Scholar]
- 8.Tanaka H, Taira K, Arakawa M, Toguti H, Urasaki C, Yamamoto Y, Uezu E, Hori T, Shirakawa S. Effects of short nap and exercise on elderly people having difficulty in sleeping. Psychiatry Clin. Neurosci. 2001 Jun;55(3):173–174. doi: 10.1046/j.1440-1819.2001.00813.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka H, Taira K, Arakawa M, Urasaki C, Yamamoto Y, Okuma H, Uezu E, Sugita Y, Shirakawa S. Short naps and exercise improve sleep quality and mental health in the elderly. Psychiatry Clin. Neurosci. 2002 Jun;56(3):233–234. doi: 10.1046/j.1440-1819.2002.00995.x. [DOI] [PubMed] [Google Scholar]
- 10.Campbell SS, Murphy PJ, Stauble TN. Effects of a nap on nighttime sleep and waking function in older subjects. J Am Geriatr Soc. 2005 Jan;53(1):48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- 11.Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon "siesta" naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001 Sep;24(6):680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton M. A rating scale for depression. J Neurol Neurosur Psychiat. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglass AB, Bornstein R, Nino-Murcia G, Keenan S, Miles L, Zarcone VP, Guilleminault C, Dement WC. The Sleep Disorders Questionnaire. I: Creation and multivariate structure of SDQ. Sleep. 1994 Mar;17(2):160–167. doi: 10.1093/sleep/17.2.160. [DOI] [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991 Dec;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Abetz L, Arbuckle R, Allen RP, Garcia-Borreguero D, Hening W, Walters AS, Mavraki E, Kirsch JM. The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical-trial setting. Sleep Med. 2006 Jun;7(4):340–349. doi: 10.1016/j.sleep.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Dement WC, Miles LE, Carskadon MA. ÒWhite paperÓ on sleep and aging. J Am Geriatr Soc. 1982;30(1):25–50. doi: 10.1111/j.1532-5415.1982.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 17.Vitiello M. Sleep in normal aging. Sleep Medicine Clinics. 2006;(1):171–176. doi: 10.1016/j.jsmc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rechtschaffen A, Kales A. Washington D.C.: National Institute of Health; A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed]
- 19.Webb WB, Dreblow LM. A modified method for scoring slow wave sleep of older subjects. Sleep. 1982;5(2):195–199. doi: 10.1093/sleep/5.2.195. [DOI] [PubMed] [Google Scholar]
- 20.Reeves DL, Winter KP, Bleiberg J, Kane RL. ANAM genogram: historical perspectives, description, and current endeavors. Arch Clin Neuropsychol. 2007 Feb;22 Suppl 1:S15–S37. doi: 10.1016/j.acn.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M. The role of prescribed napping in sleep medicine. Sleep Med Rev. 2003 Jun;7(3):227–235. doi: 10.1053/smrv.2002.0241. [DOI] [PubMed] [Google Scholar]
- 22.Evans FJ, Cook MR, Cohen HD, Orne EC, Orne MT. Appetitive and replacement naps: EEG and behavior. Science. 1977 Aug;197(4304):687–689. doi: 10.1126/science.17922. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Browman KE, Monk TH, Reynolds CF, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992 Aug;40(8):779–786. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi M, Ito S, Hori T. The effects of a 20-min nap at noon on sleepiness, performance and EEG activity. Int J Psychophysiol. 1999 May;32(2):173–180. doi: 10.1016/s0167-8760(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 25.Yoon I, Kripke DF, Elliott JA, Langer RD. Naps and circadian rhythms in postmenopausal women. J. Gerontol. A Biol. Sci. Med. Sci. 2004 Aug;59(8):844–848. doi: 10.1093/gerona/59.8.m844. [DOI] [PubMed] [Google Scholar]