Abstract
Intensive research over the past 2 decades has implicated ceramide in the regulation of several cell responses. However, emerging evidence points to dramatic complexities in ceramide metabolism and structure that defy the prevailing unifying hypothesis on ceramide function that is based on the understanding of ceramide as a single entity. Here, we develop the concept that “ceramide” constitutes a family of closely related molecules, subject to metabolism by >28 enzymes and with >200 structurally distinct mammalian ceramides distinguished by specific structural modifications. These ceramides are synthesized in a combinatorial fashion with distinct enzymes responsible for the specific modifications. These multiple pathways of ceramide generation led to the hypothesis that individual ceramide molecular species are regulated by specific biochemical pathways in distinct subcellular compartments and execute distinct functions. In this minireview, we describe the “many ceramides” paradigm, along with the rationale, supporting evidence, and implications for our understanding of bioactive sphingolipids and approaches for unraveling these pathways.
Keywords: Apoptosis, Cancer Therapy, Protein Phosphatase, Signal Transduction, Sphingolipid, Ceramide, Metabolism, Senescence, Sphingosine
Introduction
Studies over the past 2 decades have begun to define critical roles of several sphingolipids, especially ceramide, sphingosine, sphingosine 1-phosphate (S1P),3 and ceramide 1-phosphate as bioactive molecules (1–3). These molecules are now clearly appreciated to function as either intra- or intercellular messengers and as regulatory molecules that play essential roles in signal transduction, inflammation, angiogenesis, diabetes/metabolic syndrome, neurodegeneration, and cancer/cancer therapy (4–18).
Significant research has focused on ceramide as a key bioeffector molecule, resulting in the paradigm that ceramide functions as a stress responder/coordinator, involved in the response of cells to various stress stimuli such as cytokines, ischemia/reperfusion, radiation, and various toxins and chemotherapeutic agents (19, 20). In turn, ceramide is involved in regulating cell responses that include growth arrest, senescence, apoptosis, and, more recently, autophagy (11, 21–24).
Advances in Ceramide Studies and Emerging Complexities
A large body of work has netted several significant advances in the study of the metabolism, regulation, structure, and function of ceramide. These include molecular identification of enzymes of ceramide metabolism, development of in vivo models (e.g. yeast (11, 25), Caenorhabditis elegans (26, 27), Drosophila (28–31), and genetically modified mice (32, 33)) that have led to elucidation of key functions of various genes involved in ceramide metabolism, numerous cell biology advances that permitted defining several ceramide metabolism pathways, development of mass spectroscopy as a major analytical method to define and quantify ceramide and other sphingolipids (34, 35), and the application of novel systems biology approaches to the study of sphingolipids (36). Unwittingly, these advances have highlighted previously unappreciated complexities of ceramide-regulated pathways, and these include the following.
Appreciation of a Multitude of Distinct Metabolic Pathways Involved in Regulating Ceramide
The “textbook” blueprint of sphingolipid metabolism describes basic connectivity of the major sphingolipids in the biosynthetic and degradative pathways (Fig. 1). However, reality is much more complex. For example, current estimates suggest that >28 distinct enzymes exist to act on ceramide as either substrate or product (1, 37). Thus, ceramide is a “hub” in sphingolipid metabolism, serving as a precursor to ceramide phosphate, sphingomyelin, ceramide phosphoethanolamine, and the entire glycosphingolipid family. Moreover, in the degradative pathway, ceramide is the precursor to sphingosine, which in turn is the precursor to S1P. An intensive 20 years of study has led to the molecular identification of all known enzymes of ceramide metabolism. This achievement not only catapulted sphingolipid research into the modern era of cell and molecular biology but also revealed the complexity of ceramide metabolism and the many enzymes involved in conducting the “same” reaction; for example, there are six ceramide synthases (CerSs) (38), five ceramidases (39), and at least four or five sphingomyelinases (SMases) (40–42), all products of distinct genes (thus not including alternative splicing and other mechanisms of generating diversity in protein products).
FIGURE 1.
Basic blueprint of sphingolipid metabolism. Shown are the de novo pathway of ceramide formation, the production of complex sphingolipids from ceramide, the degradation of ceramide to sphingosine, the formation of S1P from sphingosine, and the clearance of S1P through the lyase reaction. CERK, ceramide kinase; GCS, glucosylceramide synthase; GBA, acid glucocerebrosidase; SK, sphingosine kinase; SMS, sphingomyelin synthase; SPP, S1P phosphatase.
Ceramide Metabolism Is Highly Compartmentalized
Because ceramide is highly hydrophobic, it tends to reside in the membranes where it is generated unless it is transported (for reviews on ceramide metabolism, see Refs. 43–47). These pathways are outlined in Fig. 2. In addition to its basic de novo synthetic pathway in the endoplasmic reticulum (ER), ceramide can also be generated in the plasma membrane by the action of SMases and possibly neutral glucocerebrosidase (48, 49). These enzymatic activities also exist in the lysosome and in mitochondria, resulting in compartment-specific ceramide generation. Interestingly, ceramide also can be generated in a slightly more complex mechanism, the salvage pathway, which involves first the breakdown of sphingomyelin and complex sphingolipids into ceramide and then sphingosine in the endolysosomal system. The liberated sphingosine may then be reacylated to ceramide (i.e. salvaged or recycled) (50). It has also been reported that ceramide can be generated by the reverse action of ceramidases (well documented in Saccharomyces cerevisiae) (51).
FIGURE 2.
Compartmentalization of sphingolipid metabolism. Ceramide (Cer) is synthesized de novo in the ER and then is transported either via ceramide transfer protein (CERT) to the Golgi, where it serves as a substrate for the synthesis of sphingomyelin (SM), or is transported by vesicular traffic for the synthesis of glucosylceramide (gluCer) (97). Sphingomyelin and glycosphingolipids (GlycoSL) are, in turn, transported to the plasma membrane through vesicular trafficking, and they also undergo vesicular trafficking in the endosomal system and clearance through lysosomal degradation. Ceramide can also be transformed to galactosylceramide (GalCer) in the ER, a process enriched in neural tissues. SLs, sphingolipids; SMS, sphingomyelin synthase; glySL, glycosphingolipids; dhSph, dihydrosphingosine; aCDase, acid ceramidase; ma-nSMase, mitochondrial associated SMase; aSMase, acid SMase; SK, sphingosine kinase; Sph, sphingosine; CDase, ceramidase; dhCer, dihydroceramide; Mito, mitochondria; Nuc, nucleus.
Thus, ceramide metabolism is subject to “local” metabolism and control. This is well illustrated by the individual SMases and ceramidases, which have distinct subcellular localization with distinct enzymes localizing to the plasma membrane, lysosomes, mitochondria, Golgi, and ER (41, 52). Likewise, there are six distinct CerSs (23), and although they appear to reside primarily in the ER, they may have more specific localization. Indeed, two of the six CerSs, CerS1 and CerS6, show perinuclear staining when overexpressed, with CerS1 colocalizing morphologically with lamin B (53, 54). CerS1 has also been shown to undergo regulated translocation to the Golgi (55). Recently, CerS4 and CerS6 have been shown to reside in mitochondria (56, 57). Other enzymes of ceramide metabolism such as sphingomyelin synthases and glucocerebrosidases also have multiplicity and compartment-specific localization (48, 58). Functionally, the existence of these compartment-specific pathways clearly suggests high specialization of these pathways, which in turn suggests specific mechanisms of regulation and, equally as likely, distinct functions and mechanisms of action of their lipid products.
Sphingolipid Metabolism Is Highly Connected
Our classical understanding of how metabolites participate in signaling and cell regulation has been shaped to a large extent by conceptualization of distinct “modules” of signaling regulators (initially, modeled after the cAMP pathway). There are clear-cut advantages in studying sphingolipid-mediated cell regulation as a set of modular processes, as this allows dissection of individual components of these processes and their mechanisms of regulation (i.e. a classical reductionist approach). Indeed, significant progress has been achieved in the past few years in understanding specific pathways mediated by specific enzymes such as acid and neutral SMases (59), the de novo pathway (60), and the savage pathway (1, 61). However, it should be equally recognized that the interconnection of lipid metabolites immediately generates a second layer of organization. Thus, although one stimulus (e.g. TNF) may activate one enzyme (e.g. SMase) to generate the first metabolite (ceramide in this case), a second enzyme (e.g. ceramidase or ceramide kinase) may act on this metabolite to generate an additional signal (e.g. sphingosine or ceramide 1-phosphate, respectively). These subsequent metabolites may then mediate their own specific actions. Each of the secondary metabolites is itself capable of affecting the levels of its own set of metabolites and so on. These interconnections therefore result in “metabolic ripple effects” that complicate attempts at dissecting specific pathways of ceramide metabolism and function. As such, implicating a specific enzyme in a cell response, which can be defined through use of biochemical, genetic, and pharmacological studies, does not immediately equate with implicating the immediate product of the reaction as the direct mediator of the process. For example, studies that implicate SMase in TNF action should not conclude that it is the ceramide that mediates the response. Indeed, there are now a few examples for which S1P appears to be the likely mediator of TNF actions on growth and induction of endothelial NOS downstream of neutral SMase activation (62, 63). In another example, knockdown of one of the CerSs leads to reciprocal changes in other CerSs (64, 65).
Ceramide Is a Family of Molecules
Mass spectroscopy-based analysis of ceramide has disclosed that cells and tissues may harbor dozens of distinct ceramide molecular species that are distinguished by specific components and/or modifications (Fig. 3A) (66). For example, ceramide may contain a 4,5-cis-double bond introduced by dihydroceramide desaturase (DES) 1 (67), a hydroxyl at the 4-position introduced by DES2 (Syr2 in yeast; for the trivial designation of phytoceramide) (68), or neither (for the designation of dihydroceramide). Fatty acid 2-hydroxylase introduces an α-hydroxy on the amide-linked fatty acid (69–71). The six CerSs show distinct substrate preference for incorporation of fatty acids of different chain lengths in amide linkage (38, 72). More recently, appreciation has grown that serine palmitoyltransferase (SPT) can employ fatty acids other than palmitate, depending on the subunit composition of SPT (with SPTLC3 preferring myristate) (73) and/or the presence of regulatory subunits (with yeast Tsc3 and its mammalian ortholog steering toward stearate as the substrate) (74, 75). SPT can also employ alanine as a substrate to generate a 1-deoxysphingoid backbone, an effect that is exaggerated in the SPT mutants in type I hereditary sensory and autonomic neuropathy (76), and may even employ glycine to generate a 1-de(OH-methyl)sphingoid backbone (77).
FIGURE 3.
Many ceramides. A, complexity of ceramide structure. Ceramide is a family of closely related molecules. Distinct enzymes control the introduction of OH on the acyl chain (1), resulting in two variants, an OH on the sphingoid base or a double bond in the sphingoid base (2; total of three variants); the chain length and desaturation of the acyl chain (3; at least 10 variants); the length of the sphingoid base (4; at least three major variants); and the OH at the 1-position (5; two variants). Because these are independent modifications (at each of the sites), one can calculate the upper limit of possible ceramides as the product of these modifications (i.e. 360), but not all these ceramides necessarily exist (e.g. some modifications may preclude others because of enzyme specificities). On the other hand, any new discovery of additional variations would enlarge this number. B, partial representation of the metabolic domains of distinct ceramides. Each box represents a structurally distinct ceramide and the sphingolipids (SLS) derived from that particular ceramide. Box 1 illustrates the formation of C16-dihydroceramide with a C18-sphingoid backbone (18C16dhCer). Likewise, each of the other boxes illustrates the combinatorial action of unique enzymes/subunits to effect the formation of unique ceramides and subsequent sphingolipids. Given the structural uniqueness of each ceramide, we recommend the shorthand designations shown, where the initial prefix designates the length of the sphingoid base and the number of double bonds in it (e.g. 18:1), followed by Cx indicating the length of the acyl chain, followed by an indication of acyl chain modifications (e.g. 2′-OH for hydroxylation at the 2-position of the fatty acid). These nine boxes are representative of all individual ceramide species, of which >100–150 can be detected using current LC-MS/MS technology. SPTLC, subunits of SPT; FA2H, fatty acid 2-hydroxylase; Pal, palmitoyl-CoA; Myr, myristoyl-CoA; Sph, sphingosine; C1P, ceramide 1-phosphate.
Although heterogeneity in molecular composition of lipids (especially in the chain length of the fatty acyl groups of glycerolipids) has been noted for more than 3 decades, it was assumed mostly to be of minimal biological significance, and its existence was implicitly attributed to lack of fidelity of enzymes of glycerolipid metabolism and/or the relative availability of specific fatty acids as substrates. In contrast, the many ceramides are the product of combinatorial synthesis, with several enzymes collaborating to produce >200 distinct mammalian ceramides (Fig. 3A). Thus, the action of any specific enzyme combination results in the formation of one or a few ceramides; for example, the action of CerS1, fatty acid 2-hydroxylase, and SPT3 may result in formation of α-hydroxy-C18:1-ceramide with a C16-sphingoid backbone (Fig. 3B). These considerations also raise questions about how we define lipid molecular species (Fig. 4). Moreover, each of these distinct ceramides becomes a founding member of its own “world” of complex sphingolipids based on this ceramide structure, thus compounding the molecular complexity of sphingolipids (Fig. 3B).
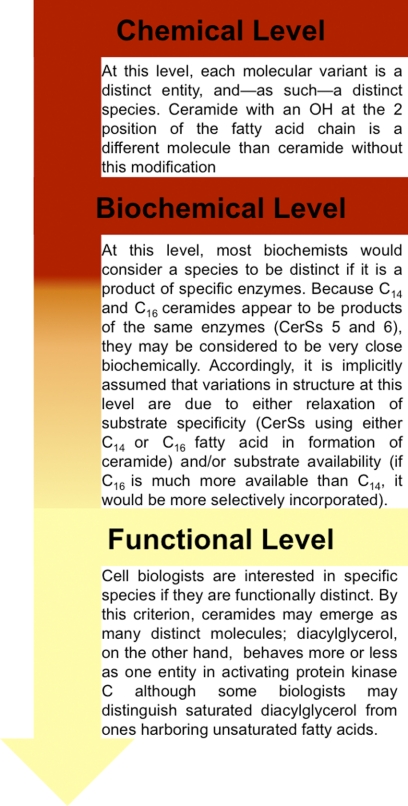
FIGURE 4.
Defining a lipid species. Three levels of “definition” must be considered as shown in the figure.
Complexity and Confusion in Determining “Ceramide Function”
In the unitary approach to ceramide function, studies often revealed not only several functions for ceramide but also, at times, contradictory ones, depending on cell type and other variables, thereby rendering a unified understanding of ceramide function difficult if not contrived. For example, ceramide has been implicated in mediating apoptotic responses both downstream and upstream of caspases or mitochondrial dysfunction. Although some of these discrepant effects may be the result of the action of subsequent ceramide metabolites (as discussed above), emerging evidence implicates distinct ceramides in distinct responses (as discussed below).
Many Ceramides
Thus, a unitary conceptualization of ceramide function is no longer tenable; rather, individual ceramide molecular species are likely regulated by specific biochemical pathways in distinct subcellular compartments and execute distinct functions. This represents a significant paradigm shift away from the singular function of ceramide to the “many ceramides” model (loosely analogous to the “many worlds” interpretation of quantum mechanics as proposed by Hugh Everett and further developed by Bryce DeWitt, which in essence posits that alternative outcomes predicted by quantum mechanics are all realized but in different “worlds”). According to this analogy, the many ceramides hypothesis posits that (in the extreme case) every structural change in ceramide leads to its own world of derived sphingolipids with possibly distinct subcellular localization and possibly distinct functions (as discussed below).
Examples of Distinct Ceramide Pathways
The era of deciphering functional differences for distinct ceramides was enabled by the development of mass spectrometric quantitative and semiquantitative analytical approaches over the past 10 years. It should be noted, however, that the functional significance of specific modifications in ceramide structure was hinted at by nearly 2-decade-old studies that defined a functional role for the 4,5-cis-double bond in ceramide; thus, ceramides but not dihydroceramides could induce apoptotic and other cell responses (78).
In one of the earliest studies to examine molecular ceramide species in the context of cell responses, it was observed that triggering of the B-cell receptor in lymphocytes induced a biphasic elevation in ceramides (79). The early phase (over minutes/hours) consisted primarily of C16-ceramide and preceded the onset of activation-induced cell death. The accumulation of this ceramide was inhibited by fumonisin B, an inhibitor of CerSs, which also prevented activation of caspases, mitochondrial damage, and induction of cell death. The later phase of ceramide involved accumulation of C24-ceramide, and in contradistinction to the first phase, this accumulation required activation of caspases. Functionally, this was linked to activation of the proteasome (80). In a historical aside, the very first observation of specific elevations of C16-ceramide was probably that reported by Watts et al. (81); however, the authors dismissed the significance of that change because it required 1–2 h of cell stimulation.
Insight into ceramide-specific functions has arisen from a molecularly driven line of investigation focusing on members of the CerS family that form ceramides with distinct acyl chain lengths. In one study, Koybasi et al. (82) discovered that, of the various ceramides, only C18:0-ceramide was selectively down-regulated in head and neck cancer tissues. Because this ceramide is a specific product of CerS1, the authors subsequently implicated CerS1 in mediating growth regulation (53).
In another study, Min et al. (83) provided evidence that CerS1 increased sensitivity of HEK293 cells to several chemotherapeutic agents, whereas CerS4 did not appear to modulate drug sensitivity. Mechanistically, these results were traced to selective activation of the p38 MAPK by CerS1 and not the other CerSs.
Voelkel-Johnson and co-workers (54) found that colon cancer cells had extremely reduced CerS6 levels and were defective in the C16-ceramide response to TRAIL-induced apoptosis. Expressing CerS6 was sufficient to restore the apoptotic response to TRAIL (54). Additional studies by Dent and co-workers (84) implicated CerS6 in regulating calcium release, reactive oxygen species production, and cell death induced by MDA-7/IL-24. Another recent study specifically implicated C16-ceramide, most likely generated through the salvage pathway, in contributing to celecoxib-induced cytotoxicity (85). Kolesnick and co-workers (56) recently implicated CerS5 and CerS6 in mediating cytotoxic responses to ionizing radiation, whereas CerS2, probably acting in mitochondria, offered partial protection. Very recent work from our laboratories has also implicated CerS5 and CerS6 in the generation of long chain ceramides by the salvage pathway, which were necessary for regulating membrane permeability in the programmed cell death execution phase in MCF-7 breast cancer cells in response to UV radiation (86). Thus, taken together, studies on CerSs not only implicate each of these enzymes in the formation of specific ceramides and perhaps in specific compartments but also clearly suggest ceramide species-specific functions.
Other recent studies implicated the specific production of dihydroceramide in the induction of autophagy. Zheng et al. (87) showed that autophagy was induced in prostate cancer cells treated with C2-dihdydroceramide, one of the first studies to demonstrate biological activities of the dihydroceramide class. Signorelli et al. (88) reported that resveratrol induced autophagy in gastric cancer cells, and this was accompanied by inhibition of DES and accumulation of dihydroceramide. Importantly, direct inhibition of DES with XM462 caused the accumulation of dihydroceramide and was sufficient to induce autophagy.
It should be noted that several additional recent studies have disclosed specific functional and pathobiological roles of specific enzymes of ceramide metabolism. For example, knock-out of CerS2 results in severe liver pathology (38), mutations in the fatty acid 2-hydroxylase have been implicated in inherited human leukodystrophy (89), and mutations in DES have been related to amelioration of diabetic complications (4). At this point, these results do not yet distinguish roles of specific ceramides versus roles of subsequent metabolites. As such, these approaches allow us to make conclusions about functional roles of specific modifications in the ceramide backbone that also affect all downstream sphingolipids, and further studies are required to define the specific lipid mediator(s). Thus, this approach has promise for yielding significant results and insights.
Implications of This Paradigm Shift
It is understandable that tackling the major questions about how ceramide is regulated and how it functions would have been much easier had ceramide been a single entity generated by a single pathway. However, the reality of the complexities of ceramide formation and the multitude of distinct enzymes of ceramide metabolism and distinct ceramide species has become undeniable. In turn, this enforces a re-examination of how studies on ceramide should evolve and how results are to be interpreted.
First and foremost, the many ceramides approach negates the current prevailing paradigm that ceramide can be understood in terms of regulation and function as a single entity. It is quite unlikely that ceramide formed in the lysosome by the action of acid SMase should exert the same specific effects as ceramide formed in the Golgi or the plasma membrane by the regulation of ceramidases or neutral SMase. Thus, at the very least, mechanistic studies on ceramide function and regulation should focus on specific pathways of formation. This may be termed the “enzyme-centric” approach. Given the specific subcellular localizations of these enzymes, this approach is by necessity also a “compartment-specific” approach.
A poorly studied aspect of ceramide compartmentalization relates to membrane topology, sidedness, and submembrane organization of ceramide metabolism and action. Current results show that ceramide can flip-flop readily in artificial membranes and in red blood cells (consistent with its physicochemical properties of lack of charge in a small hydrophobic molecule) (90). However, at this point, we cannot rule out sidedness to ceramide metabolism and function in more complex biological membranes. Moreover, there is strong evidence from over 4 decades of research that sphingomyelin partitions into membrane subdomains (91) that may also exist in biological membranes as “rafts” (92) and that ceramide may regulate the formation of these domains (93).
As a corollary, this conceptualization of compartment-specific metabolism of many ceramides raises the issue of whether “ceramide” serves as a hub in sphingolipid metabolism. What is emerging is that distinct ceramides in distinct compartments serve as local minihubs of sphingolipid metabolism. In turn, protein-mediated transfer and vesicular transport of sphingolipids then serve to connect these “many hubs” (Fig. 2). At this point, it is not clear if individual ceramide species reside either in distinct membranes or within distinct subdomains of membranes. Unfortunately, there is a paucity of tools to evaluate this critical issue, which would require advanced subcellular fractionation coupled with mass spectrometry and, even better, the development of high affinity probes for specific ceramides.
A major quest in understanding bioactive lipids is the need to understand the mechanisms of their formation, but also equally important is the need to understand their mechanisms of action. As such, defining the “active” lipid species becomes of obvious critical importance in discerning the downstream targets and mechanisms involved in mediating the specific functions of the enzyme under study. Therefore, we propose a two-track approach in elucidating lipid-dependent pathways. First, an enzyme-centric approach could define the function of the specific enzyme. This builds on the availability of molecular tools; animal models; and biochemical, pharmacological, and genetic approaches. Next and because of the interconnectivity of lipid mediators, a “lipid-centric” approach could define which specific lipid is implicated and then its mechanisms of action. This can also employ genetic approaches; one could aim to “triangulate the lipid” by specific modulation of enzymes through gain- or loss-of-function studies. This lipid-centric approach can also avail itself of analytical tools to quantify lipids and correlate lipids with specific functions as well as biochemical and pharmacological approaches. One such recent example from our group employed exogenously applied purified bacterial enzymes of sphingolipid metabolism (SMase, ceramidase, and SMase D) to define a specific role for ceramide in the plasma membrane and not sphingomyelin or other metabolites in the regulation of ezrin dephosphorylation (94). Moreover, it is expected that bioinformatic approaches will also help in the quest to define lipid-specific functions. In such an approach, functions for yeast sphingoid base phosphates were elucidated by “deconvoluting” gene-based transcriptomic data to lipid-based regulation by simultaneously analyzing transcriptomic and lipidomic results (95). Another very recent approach helps visualize sphingolipidomic results and correlates those with functional changes in transcriptomic data on sphingolipid enzymes (96).
At a mechanistic level, this emerging paradigm has significant implications for molecular mechanisms of action of ceramide. As a sum of all of its species, ceramide is present in cells at levels that usually range from 0.1 to 1.0% of total phospholipids. If all ceramides regulate one specific target and, in turn, that target reacts to the total relative concentration of ceramides, then such interactions would be of moderate affinity. As such, ceramide would be expected to more closely resemble diacylglycerol in its interactions with protein kinase C. However, regulation of distinct species of ceramide in specific subcellular compartments would involve very low abundance lipids. The relative concentrations of individual ceramide species span many orders of magnitude, with some of the minor yet detectable species now present as <0.001% of total lipids, concentrations that are more similar to those of many eicosanoids and S1P, all with high affinity receptors. Accordingly, one would expect that if these individual ceramides are bioactive, they may have high affinity targets.
Concluding Remarks
In conclusion, tackling the biology of the many ceramides model has become significantly complicated by the emerging complexities in our understanding of ceramide metabolism, the many enzymes involved, the compartmentalization of these pathways, the interconnectivity of lipid metabolism, and the distinct species of ceramide molecular species. Paradoxically, we propose that by tackling ceramide as a family of perhaps >200 distinct molecules, we will better clarify the conceptual underpinnings, although, understandably, the volume of data required will multiply, which is another challenge. Still, the principles will be easier to develop and assimilate. Such simplification following complexity has been observed previously (e.g. in the study of the eicosanoids). With the many ceramides approach, this promises equally rewarding and exciting discoveries and progress. For example, it is currently difficult to predict whether an observed change in ceramide will lead to apoptosis or ER stress or senescence. According to the new hypothesis, defining the pathway regulating ceramide, where in the cell this occurs, and what specific species of ceramide accumulates will result in a much more robust understanding of that specific pathway of cell regulation and in predicting its function. Clearly, the study of ceramides is unfolding into a world of its own that requires substantial investigation and promises novel and unique insights into lipid metabolism and function.
Acknowledgments
We thank the many members of the Obeid and Hannun laboratories over the years who have contributed to much of the knowledge in this minireview. We also thank Dr. Jennifer Schnellmann for reviewing the manuscript.
This work was support, in whole or in part, by National Institutes of Health Grants R01AG016583 and R01GM062887 (to L. M. O.) and R01GM43825, R01GM63265, and P01CA097132 from NCI (to Y. A. H.) and in part by a MERIT Award (to L. M. O.) from the Office of Research and Development, Department of Veterans Affairs, Ralph H. Johnson Veterans Affairs Medical Center (Charleston, SC). This minireview will be reprinted in the 2011 Minireview Compendium, which will be available in January, 2012.
- S1P
- sphingosine 1-phosphate
- CerS
- ceramide synthase
- SMase
- sphingomyelinase
- ER
- endoplasmic reticulum
- DES
- dihydroceramide desaturase
- SPT
- serine palmitoyltransferase.
REFERENCES
- 1. Hannun Y. A., Obeid L. M. (2008) Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 2. Merrill A. H., Jr., Wang M. D., Park M., Sullards M. C. (2007) Trends Biochem. Sci 32, 457–468 [DOI] [PubMed] [Google Scholar]
- 3. Maceyka M., Milstien S., Spiegel S. (2009) J. Lipid Res. 50, S272–S276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holland W. L., Summers S. A. (2008) Endocr. Rev. 29, 381–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pettus B. J., Kitatani K., Chalfant C. E., Taha T. A., Kawamori T., Bielawski J., Obeid L. M., Hannun Y. A. (2005) Mol. Pharmacol. 68, 330–335 [DOI] [PubMed] [Google Scholar]
- 6. Nixon G. F. (2009) Br. J. Pharmacol. 158, 982–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang W. C., Chen C. L., Lin Y. S., Lin C. F. (2011) J. Lipids 2011, 565316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Modrak D. E., Gold D. V., Goldenberg D. M. (2006) Mol. Cancer Ther. 5, 200–208 [DOI] [PubMed] [Google Scholar]
- 9. Ryland L. K., Fox T. E., Liu X., Loughran T. P., Kester M. (2011) Cancer Biol. Ther. 11, 138–149 [DOI] [PubMed] [Google Scholar]
- 10. Saba J. D., Hla T. (2004) Circ. Res. 94, 724–734 [DOI] [PubMed] [Google Scholar]
- 11. Dickson R. C. (2008) J. Lipid Res. 49, 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pyne N. J., Pyne S. (2010) Nat. Rev. Cancer 10, 489–503 [DOI] [PubMed] [Google Scholar]
- 13. Ponnusamy S., Meyers-Needham M., Senkal C. E., Saddoughi S. A., Sentelle D., Selvam S. P., Salas A., Ogretmen B. (2010) Future Oncol. 6, 1603–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X., Becker K. A., Zhang Y. (2010) Cell Physiol. Biochem. 26, 41–48 [DOI] [PubMed] [Google Scholar]
- 15. Haughey N. J., Bandaru V. V., Bae M., Mattson M. P. (2010) Biochim. Biophys. Acta 1801, 878–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beckham T. H., Elojeimy S., Cheng J. C., Turner L. S., Hoffman S. R., Norris J. S., Liu X. (2010) Expert Opin. Ther. Targets 14, 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gangoiti P., Camacho L., Arana L., Ouro A., Granado M. H., Brizuela L., Casas J., Fabriás G., Abad J. L., Delgado A., Gómez-Muñoz A. (2010) Prog. Lipid Res. 49, 316–334 [DOI] [PubMed] [Google Scholar]
- 18. Stancevic B., Kolesnick R. (2010) FEBS Lett. 584, 1728–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hannun Y. A. (1996) Science 274, 1855–1859 [DOI] [PubMed] [Google Scholar]
- 20. Nikolova-Karakashian M. N., Rozenova K. A. (2010) Adv. Exp. Med. Biol. 688, 86–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pattingre S., Bauvy C., Levade T., Levine B., Codogno P. (2009) Autophagy 5, 558–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guillas I., Jiang J. C., Vionnet C., Roubaty C., Uldry D., Chuard R., Wang J., Jazwinski S. M., Conzelmann A. (2003) J. Biol. Chem. 278, 37083–37091 [DOI] [PubMed] [Google Scholar]
- 23. Stiban J., Tidhar R., Futerman A. H. (2010) Adv. Exp. Med. Biol. 688, 60–71 [DOI] [PubMed] [Google Scholar]
- 24. Goldkorn T., Filosto S. (2010) Am. J. Respir. Cell Mol. Biol. 43, 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matmati N., Hannun Y. A. (2008) J. Lipid Res. 49, 922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Menuz V., Howell K. S., Gentina S., Epstein S., Riezman I., Fornallaz-Mulhauser M., Hengartner M. O., Gomez M., Riezman H., Martinou J. C. (2009) Science 324, 381–384 [DOI] [PubMed] [Google Scholar]
- 27. Deng X., Yin X., Allan R., Lu D. D., Maurer C. W., Haimovitz-Friedman A., Fuks Z., Shaham S., Kolesnick R. (2008) Science 322, 110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kraut R. (2011) J. Neurochem. 116, 764–778 [DOI] [PubMed] [Google Scholar]
- 29. Yang Q., Gong Z. J., Zhou Y., Yuan J. Q., Cheng J., Tian L., Li S., Lin X. D., Xu R., Zhu Z. R., Mao C. (2010) Cell. Mol. Life Sci. 67, 1477–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vacaru A. M., Tafesse F. G., Ternes P., Kondylis V., Hermansson M., Brouwers J. F., Somerharju P., Rabouille C., Holthuis J. C. (2009) J. Cell Biol. 185, 1013–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fyrst H., Zhang X., Herr D. R., Byun H. S., Bittman R., Phan V. H., Harris G. L., Saba J. D. (2008) J. Lipid Res. 49, 597–606 [DOI] [PubMed] [Google Scholar]
- 32. Kawamori T. (2010) Adv. Exp. Med. Biol. 688, 109–117 [DOI] [PubMed] [Google Scholar]
- 33. Kono M., Allende M. L., Proia R. L. (2008) Biochim. Biophys. Acta 1781, 435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. (2010) Adv. Exp. Med. Biol. 688, 46–59 [DOI] [PubMed] [Google Scholar]
- 35. Sullards M. C., Allegood J. C., Kelly S., Wang E., Haynes C. A., Park H., Chen Y., Merrill A. H., Jr. (2007) Methods Enzymol. 432, 83–115 [DOI] [PubMed] [Google Scholar]
- 36. Alvarez-Vasquez F., Sims K. J., Cowart L. A., Okamoto Y., Voit E. O., Hannun Y. A. (2005) Nature 433, 425–430 [DOI] [PubMed] [Google Scholar]
- 37. Hannun Y. A., Obeid L. M. (2002) J. Biol. Chem. 277, 25847–25850 [DOI] [PubMed] [Google Scholar]
- 38. Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brügger B., Sachsenheimer T., Wieland F., Prieto M., Merrill A. H., Jr., Futerman A. H. (2010) J. Biol. Chem. 285, 10902–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mao Z., Sun W., Xu R., Novgorodov S., Szulc Z. M., Bielawski J., Obeid L. M., Mao C. (2010) J. Biol. Chem. 285, 29078–29090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ledesma M. D., Prinetti A., Sonnino S., Schuchman E. H. (2011) J. Neurochem. 116, 779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke C. J., Guthrie J. M., Hannun Y. A. (2008) Mol. Pharmacol. 74, 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang Y., Cheng Y., Hansen G. H., Niels-Christiansen L. L., Koentgen F., Ohlsson L., Nilsson A., Duan R. D. (2011) J. Lipid Res. 54, 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van Echten-Deckert G., Herget T. (2006) Biochim. Biophys. Acta 1758, 1978–1994 [DOI] [PubMed] [Google Scholar]
- 44. Gault C. R., Obeid L. M., Hannun Y. A. (2010) Adv. Exp. Med. Biol. 688, 1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riboni L., Giussani P., Viani P. (2010) Adv. Exp. Med. Biol. 688, 24–45 [DOI] [PubMed] [Google Scholar]
- 46. Hanada K. (2010) Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 86, 426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sonnino S., Prinetti A., Mauri L., Chigorno V., Tettamanti G. (2006) Chem. Rev. 106, 2111–2125 [DOI] [PubMed] [Google Scholar]
- 48. Boot R. G., Verhoek M., Donker-Koopman W., Strijland A., van Marle J., Overkleeft H. S., Wennekes T., Aerts J. M. (2007) J. Biol. Chem. 282, 1305–1312 [DOI] [PubMed] [Google Scholar]
- 49. Milhas D., Clarke C. J., Hannun Y. A. (2010) FEBS Lett. 584, 1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kitatani K., Idkowiak-Baldys J., Hannun Y. A. (2008) Cell. Signal. 20, 1010–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mao C., Xu R., Bielawska A., Obeid L. M. (2000) J. Biol. Chem. 275, 6876–6884 [DOI] [PubMed] [Google Scholar]
- 52. Mao C., Obeid L. M. (2008) Biochim. Biophys. Acta 1781, 424–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Senkal C. E., Ponnusamy S., Rossi M. J., Bialewski J., Sinha D., Jiang J. C., Jazwinski S. M., Hannun Y. A., Ogretmen B. (2007) Mol. Cancer Ther. 6, 712–722 [DOI] [PubMed] [Google Scholar]
- 54. White-Gilbertson S., Mullen T., Senkal C., Lu P., Ogretmen B., Obeid L., Voelkel-Johnson C. (2009) Oncogene 28, 1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sridevi P., Alexander H., Laviad E. L., Min J., Mesika A., Hannink M., Futerman A. H., Alexander S. (2010) Exp. Cell Res. 316, 78–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mesicek J., Lee H., Feldman T., Jiang X., Skobeleva A., Berdyshev E. V., Haimovitz-Friedman A., Fuks Z., Kolesnick R. (2010) Cell. Signal. 22, 1300–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Novgorodov S. A., Chudakova D. A., Wheeler B. W., Bielawski J., Kindy M. S., Obeid L. M., Gudz T. I. (2011) J. Biol. Chem. 286, 4644–4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tafesse F. G., Ternes P., Holthuis J. C. (2006) J. Biol. Chem. 281, 29421–29425 [DOI] [PubMed] [Google Scholar]
- 59. Perrotta C., Clementi E. (2010) Physiology 25, 64–71 [DOI] [PubMed] [Google Scholar]
- 60. Bose R., Verheij M., Haimovitz-Friedman A., Scotto K., Fuks Z., Kolesnick R. (1995) Cell 82, 405–414 [DOI] [PubMed] [Google Scholar]
- 61. Ogretmen B., Pettus B. J., Rossi M. J., Wood R., Usta J., Szulc Z., Bielawska A., Obeid L. M., Hannun Y. A. (2002) J. Biol. Chem. 277, 12960–12969 [DOI] [PubMed] [Google Scholar]
- 62. Tellier E., Nègre-Salvayre A., Bocquet B., Itohara S., Hannun Y. A., Salvayre R., Augé N. (2007) Mol. Cell. Biol. 27, 2997–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. De Palma C., Meacci E., Perrotta C., Bruni P., Clementi E. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 99–105 [DOI] [PubMed] [Google Scholar]
- 64. Mullen T. D., Spassieva S., Jenkins R. W., Kitatani K., Bielawski J., Hannun Y. A., Obeid L. M. (2011) J. Lipid Res. 52, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Spassieva S. D., Mullen T. D., Townsend D. M., Obeid L. M. (2009) Biochem. J. 424, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Merrill A. H., Jr., Stokes T. H., Momin A., Park H., Portz B. J., Kelly S., Wang E., Sullards M. C., Wang M. D. (2009) J. Lipid Res. 50, S97–S102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ternes P., Franke S., Zähringer U., Sperling P., Heinz E. (2002) J. Biol. Chem. 277, 25512–25518 [DOI] [PubMed] [Google Scholar]
- 68. Omae F., Miyazaki M., Enomoto A., Suzuki M., Suzuki Y., Suzuki A. (2004) Biochem. J. 379, 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hama H. (2010) Biochim. Biophys. Acta 1801, 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mizutani Y., Kihara A., Chiba H., Tojo H., Igarashi Y. (2008) J. Lipid Res. 49, 2356–2364 [DOI] [PubMed] [Google Scholar]
- 71. Grilley M. M., Stock S. D., Dickson R. C., Lester R. L., Takemoto J. Y. (1998) J. Biol. Chem. 273, 11062–11068 [DOI] [PubMed] [Google Scholar]
- 72. Mizutani Y., Kihara A., Igarashi Y. (2005) Biochem. J. 390, 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hornemann T., Penno A., Rütti M. F., Ernst D., Kivrak-Pfiffner F., Rohrer L., von Eckardstein A. (2009) J. Biol. Chem. 284, 26322–26330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cowart L. A., Hannun Y. A. (2007) J. Biol. Chem. 282, 12330–12340 [DOI] [PubMed] [Google Scholar]
- 75. Han G., Gupta S. D., Gable K., Niranjanakumari S., Moitra P., Eichler F., Brown R. H., Jr., Harmon J. M., Dunn T. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 8186–8191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Penno A., Reilly M. M., Houlden H., Laurá M., Rentsch K., Niederkofler V., Stoeckli E. T., Nicholson G., Eichler F., Brown R. H., Jr., von Eckardstein A., Hornemann T. (2010) J. Biol. Chem. 285, 11178–11187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zitomer N. C., Mitchell T., Voss K. A., Bondy G. S., Pruett S. T., Garnier-Amblard E. C., Liebeskind L. S., Park H., Wang E., Sullards M. C., Merrill A. H., Jr., Riley R. T. (2009) J. Biol. Chem. 284, 4786–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bielawska A., Crane H. M., Liotta D., Obeid L. M., Hannun Y. A. (1993) J. Biol. Chem. 268, 26226–26232 [PubMed] [Google Scholar]
- 79. Kroesen B. J., Pettus B., Luberto C., Busman M., Sietsma H., de Leij L., Hannun Y. A. (2001) J. Biol. Chem. 276, 13606–13614 [DOI] [PubMed] [Google Scholar]
- 80. Kroesen B. J., Jacobs S., Pettus B. J., Sietsma H., Kok J. W., Hannun Y. A., de Leij L. F. (2003) J. Biol. Chem. 278, 14723–14731 [DOI] [PubMed] [Google Scholar]
- 81. Watts J. D., Gu M., Patterson S. D., Aebersold R., Polverino A. J. (1999) Cell Death Differ. 6, 105–114 [DOI] [PubMed] [Google Scholar]
- 82. Koybasi S., Senkal C. E., Sundararaj K., Spassieva S., Bielawski J., Osta W., Day T. A., Jiang J. C., Jazwinski S. M., Hannun Y. A., Obeid L. M., Ogretmen B. (2004) J. Biol. Chem. 279, 44311–44319 [DOI] [PubMed] [Google Scholar]
- 83. Min J., Mesika A., Sivaguru M., Van Veldhoven P. P., Alexander H., Futerman A. H., Alexander S. (2007) Mol. Cancer Res. 5, 801–812 [DOI] [PubMed] [Google Scholar]
- 84. Yacoub A., Hamed H. A., Allegood J., Mitchell C., Spiegel S., Lesniak M. S., Ogretmen B., Dash R., Sarkar D., Broaddus W. C., Grant S., Curiel D. T., Fisher P. B., Dent P. (2010) Cancer Res. 70, 1120–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schiffmann S., Ziebell S., Sandner J., Birod K., Deckmann K., Hartmann D., Rode S., Schmidt H., Angioni C., Geisslinger G., Grösch S. (2010) Biochem. Pharmacol. 80, 1632–1640 [DOI] [PubMed] [Google Scholar]
- 86. Mullen T. D., Jenkins R. W., Clarke C. J., Bielawski J., Hannun Y. A., Obeid L. M. (2011) J. Biol. Chem. 286, 15929–15942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zheng W., Kollmeyer J., Symolon H., Momin A., Munter E., Wang E., Kelly S., Allegood J. C., Liu Y., Peng Q., Ramaraju H., Sullards M. C., Cabot M., Merrill A. H., Jr. (2006) Biochim. Biophys. Acta 1758, 1864–1884 [DOI] [PubMed] [Google Scholar]
- 88. Signorelli P., Munoz-Olaya J. M., Gagliostro V., Casas J., Ghidoni R., Fabriàs G. (2009) Cancer Lett. 282, 238–243 [DOI] [PubMed] [Google Scholar]
- 89. Kruer M. C., Paisán-Ruiz C., Boddaert N., Yoon M. Y., Hama H., Gregory A., Malandrini A., Woltjer R. L., Munnich A., Gobin S., Polster B. J., Palmeri S., Edvardson S., Hardy J., Houlden H., Hayflick S. J. (2010) Ann. Neurol. 68, 611–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. López-Montero I., Rodriguez N., Cribier S., Pohl A., Vélez M., Devaux P. F. (2005) J. Biol. Chem. 280, 25811–25819 [DOI] [PubMed] [Google Scholar]
- 91. Oldfield E., Chapman D. (1972) FEBS Lett. 21, 303–306 [DOI] [PubMed] [Google Scholar]
- 92. Simons K., Ikonen E. (1997) Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 93. Cremesti A. E., Goni F. M., Kolesnick R. (2002) FEBS Lett. 531, 47–53 [DOI] [PubMed] [Google Scholar]
- 94. Canals D., Jenkins R. W., Roddy P., Hernández-Corbacho M. J., Obeid L. M., Hannun Y. A. (2010) J. Biol. Chem. 285, 32476–32485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cowart L. A., Shotwell M., Worley M. L., Richards A. J., Montefusco D. J., Hannun Y. A., Lu X. (2010) Mol. Syst. Biol. 6, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Momin A. A., Park H., Portz B. J., Haynes C. A., Shaner R. L., Kelly S. L., Jordan I. K., Merrill A. H., Jr. (2011) J. Lipid Res. 52, 1073–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kolter T., Proia R. L., Sandhoff K. (2002) J. Biol. Chem. 277, 25859–25862 [DOI] [PubMed] [Google Scholar]