Abstract
c-Myc promotes cell growth by enhancing ribosomal biogenesis and translation. Deregulated expression of c-Myc and aberrant ribosomal biogenesis and translation contribute to tumorigenesis. Thus, a fine coordination between c-Myc and ribosomal biogenesis is vital for normal cell homeostasis. Here, we show that ribosomal protein L11 regulates c-myc mRNA turnover. L11 binds to c-myc mRNA at its 3′ untranslated region (3′-UTR), the core component of microRNA-induced silencing complex (miRISC) argonaute 2 (Ago2), as well as miR-24, leading to c-myc mRNA reduction. Knockdown of L11 drastically increases the levels and stability of c-myc mRNA. Ablation of Ago2 abrogated the L11-mediated reduction of c-myc mRNA, whereas knockdown of L11 rescued miR-24-mediated c-myc mRNA decay. Interestingly, treatment of cells with the ribosomal stress-inducing agent actinomycin D or 5-fluorouracil significantly decreased the c-myc mRNA levels in an L11- and Ago2-dependent manner. Both treatments enhanced the association of L11 with Ago2, miR-24, and c-myc mRNA. We further show that ribosome-free L11 binds to c-myc mRNA in the cytoplasm and that this binding is enhanced by actinomycin D treatment. Together, our results identify a novel regulatory paradigm wherein L11 plays a critical role in controlling c-myc mRNA turnover via recruiting miRISC in response to ribosomal stress.
INTRODUCTION
The c-myc proto-oncogene product c-Myc regulates expression of a large number of genes involved in the control of cell growth, proliferation, apoptosis, differentiation, angiogenesis, stem cell renewal, and metabolism, as well as ribosomal biogenesis and translation (1, 62). However, deregulated overexpression of c-Myc occurs in a wide range of human cancers (43). Transgenic animal studies have clearly shown the oncogenic potential of c-Myc (45). Thus, the levels and activity of c-Myc must be precisely regulated in normal cells.
The c-Myc activity seen in promoting cell proliferation and tumorigenesis is thoroughly integrated with its role in enhancing ribosomal biogenesis (13, 62). Ribosomal biogenesis is a multistep cellular process, which includes synthesis of rRNA and ribosomal proteins (RPs), rRNA processing, and the assembly of the mature ribosome subunits in the nucleolus followed by their transport into the cytoplasm (50). This process requires coordinated transcription catalyzed by all three RNA polymerases (RNA Pol I, II, and III). c-Myc enhances RNA Pol I-catalyzed synthesis of rRNA (3, 24, 25) and Pol III-catalyzed synthesis of 5S rRNA and tRNAs (23). c-Myc also promotes Pol II-catalyzed transcription of ribosomal proteins and translation initiation and elongation factors, as well as many nucleolar proteins required for rRNA processing and ribosome subunit assembly and transport (62). Collectively, c-Myc potentiates ribosomal biogenesis and translation activity.
Accumulating evidence has suggested that enhanced ribosomal biogenesis and translation contribute to cell transformation (13, 54). For example, overexpression of RNA Pol III-specific transcription factor Brf1 or RNA Pol III-transcribed tRNAiMet alone is sufficient to induce cell transformation and tumorigenicity in mice (40). Likewise, overexpression of translation initiation factors such as eukaryotic translation initiation factors 4E (eIF4E) (53), eIF4G (20), and eIF3s (66) promotes transformation in cells. Thus, c-Myc-enhanced ribosomal biogenesis and translation conceivably contribute to its oncogenic activity and should be tightly regulated during normal cell homeostasis.
We have previously demonstrated that RP L11 binds to c-Myc and inhibits the recruitment of the key coactivator TRRAP at c-Myc target gene promoters, leading to direct inhibition of c-Myc-driven gene transcription by all the three RNA Pols (12, 17). We have also found that L11 regulates c-Myc levels (12, 15). However, the underlying mechanism is not clear. Interestingly, L11, together with several other RPs, also plays a key role in transmitting ribosomal stress signals to p53-dependent cell cycle checkpoints via suppressing MDM2, a major p53 negative regulator (10, 14, 18, 28, 36, 44, 69, 70). Ribosomal stress, also called nucleolar stress because it is often accompanied by nucleolar disruption (52), is triggered by perturbation of any of the steps involved in ribosomal biogenesis (68), such as treatment of cells with a low dose of actinomycin D (Act D) (4, 14, 18), 5-fluorouracil (5-FU) (57), or mycophenolic acid (58), expression of the dominant-negative mutant of the Bop1 rRNA processing factor (56), serum starvation or contact inhibition (4), genetic disruption of the TIF-IA Pol I transcription initiation factor (65), or knockdown of certain ribosomal proteins (21, 59). In response to ribosomal stress, these RPs, including L11, are released from the nucleolus or from intact ribosomes to suppress MDM2 (68). However, whether L11 suppresses c-Myc in response to ribosomal stress is not known.
MicroRNAs (miRNAs) are evolutionarily conserved small noncoding RNAs that are ∼22 nucleotides (nt) in length and have emerged as key posttranscriptional regulators of gene expression (33). The mature miRNA is incorporated into an RNA-induced silencing complex (RISC) called miRNA-induced silencing complex (miRISC) and pairs with the 3′ untranslated region (3′-UTR) of target mRNAs, leading to their translational inhibition and/or mRNA degradation (33). In this study, we found that L11 regulates c-myc mRNA stability via a miRNA-mediated pathway. L11 binds to c-myc mRNA at its 3′-UTR, recruits a miR-24-loaded miRISC to the c-myc mRNA, and subsequently promotes c-myc mRNA degradation. Interestingly, ribosomal stress induced by treatment of cells with a low dose of Act D or 5-FU drastically reduced the levels of c-myc mRNA in an L11- and argonaute 2 (Ago2)-dependent manner. Both treatments enhance the association of L11 with Ago2, miR-24, and c-myc mRNA. These results reveal a novel regulatory paradigm wherein L11 plays a critical role in controlling c-myc mRNA stability in response to ribosomal stress.
MATERIALS AND METHODS
Cell culture, antibodies, plasmids, and reagents.
Human lung non-small cell carcinoma H1299 cells, human osteosarcoma U2OS cells, and human embryonic kidney epithelial 293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 50 U of penicillin/ml, and 0.1 mg of streptomycin/ml at 37°C in a 5% CO2 humidified atmosphere (18). Human diploid lung fibroblast WI38 cells were cultured in DMEM supplemented with 15% FBS and MEM nonessential amino acids (Gibco). To observe the c-Myc regulation by overexpression of L11, cells were transfected and cultured in DMEM containing 0.2% FBS. Anti-Flag (M2; Sigma), rabbit polyclonal anti-Ago2 (Millipore), mouse monoclonal anti-Ago2 (Abcam), mouse monoclonal anti-Myc (9E10; Zymed), and mouse polyclonal anti-Myc (Y69; Abcam) antibodies were purchased. Rabbit polyclonal anti-L11 (12) and anti-L23 (18) antibodies were previously described. Act D and 5-FU were purchased from Sigma. The Flag-tagged L11 (Flag-L11) plasmid was previously described (12). To construct a pGL3-myc3′UTR luciferase reporter, the c-myc 3′-UTR was amplified from U2OS cell mRNA by reverse transcriptase PCR (RT-PCR) with primers 5′-CGCTCTAGAGGAAAAGTAAGGAAAACGATTCCTTC-3′ and 5′-CGCTCTAGATTGGCTCAATGATATATTTGCCAG-3′, where the underlined sequences represent the XbaI site. The PCR product was then cloned into pGL3-promoter plasmid (Promega) at the XbaI site and sequenced. The luciferase reporters containing different fragments of c-myc 3′-UTR were cloned by inserting PCR products into the pGL3-promoter plasmid at the XbaI site.
Transfection, immunoblot, and coimmunoprecipitation analyses.
Cells were transfected with plasmids by the use of TransIT-LT1 reagents following the manufacturer's protocol (Mirus Bio Corporation). Cells were harvested at 48 h posttransfection and lysed in lysis buffer consisting of 50 mM Tris-HCl (pH 8.0), 0.5% Nonidet P-40, 5 mM EDTA, 150 mM NaCl, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM dithiothreitol (DTT), 1 μg of pepstatin A/ml, and 1 mM leupeptin. Equal amounts of cell lysates were used for immunoblot (IB) analysis as described previously (16). Coimmunoprecipitation (co-IP) was conducted as described previously (14, 16). Sequential co-IP of L11 and c-Myc was conducted as described previously (12, 17). RNA was extracted from the immunoprecipitates and subjected to RT-quantitative PCR (RT-qPCR) assays as described below. For analysis of the RNA dependency of L11-Ago2 interaction (see Fig. 3), RNase A (200 μg/ml) was added to cell lysates followed by IP.
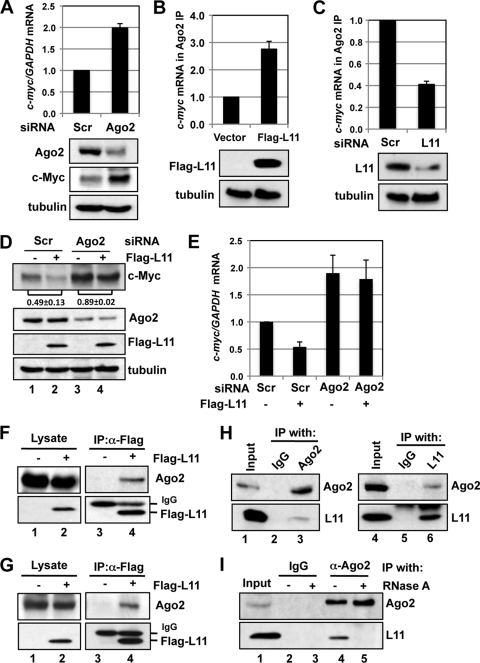
Fig. 3.
Mutual dependency of L11 and ago2 in regulating c-myc mRNA. (A) Ago2 regulates c-myc mRNA levels. U2OS cells transfected with scrambled or Ago2 siRNA were assayed for expression of c-Myc protein (bottom panels) and mRNA (top panel). (B) Overexpression of L11 increases association of Ago2 with c-myc mRNA. U2OS cells were transfected with control or Flag-L11 plasmid. The cell lysates were immunoprecipitated with anti-Ago2 antibodies followed by an RT-qPCR assay to determine the levels of c-myc mRNA. The protein expression results are shown in the bottom panels. (C) Knockdown of L11 reduces the association of Ago2 with c-myc mRNA. Lysates from U2OS cells transfected with scrambled or L11 siRNA were immunoprecipitated with anti-Ago2 antibodies followed by an RT-qPCR assay to determine the levels of c-myc mRNA. The protein expression results are shown in the bottom panels. (D and E) L11 suppression of c-myc mRNA requires Ago2. U2OS cells transfected with control or Flag-L11 plasmid together with scrambled or Ago2 siRNA were assayed for c-Myc protein expression by immunoblotting (IB) (D) and for mRNA expression by RT-qPCR assays (E). The c-Myc bands were quantified and normalized to tubulin. The ratios of lane 2 to lane 1 and of lane 4 to lane 3 for the results from three independent experiments are indicated in panel D. (F and G) Ectopically expressed L11 interacts with Ago2. 293 (F) or U2OS (G) cells transfected with control or Flag-L11 plasmid were subjected to IP with anti-Flag antibody followed by IB using anti-Ago2 antibodies. (H) Endogenous L11 interacts with endogenous Ago2. 293 cell lysates were immunoprecipitated with control mouse IgG or anti-Ago2 (left panels) or rabbit IgG or anti-L11 (right panels) antibody followed by IB detection performed using anti-L11 or anti-Ago2 antibodies. (I) Interaction of L11 with Ago2 requires RNA. U2OS cell lysates were immunoprecipitated with control mouse IgG or anti-Ago2 antibodies in the absence (lanes 2 and 4) or presence (lanes 3 and 5) of RNase followed by IB detection performed using anti-L11 or anti-Ago2 antibodies.
[35S]methionine pulse-labeling.
To examine c-Myc translation upon L11 knockdown, cells were prestarved in methionine-free medium supplemented with dialyzed 10% FBS for 30 min followed by pulse-labeling with 50 μCi of [35S]methionine/ml for 15 min. Cells were lysed, and the cleared cell lysates were immunoprecipitated using anti-c-Myc (C33; Santa Cruz Biotechnology) monoclonal antibody-conjugated beads. After washing, half of the immunoprecipitate was separated using a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. The gel was incubated in Amplify solution (Amersham Biosciences) for 10 min, dried, and exposed to X-ray film. The other half was subjected to IB using anti-c-Myc (Y69) antibodies. Radiolabeled and total c-Myc were quantified, and the ratio of radiolabeled to total c-Myc represents the relative translational activity of c-Myc. Equal amounts of total proteins were also loaded onto an SDS-PAGE gel to examine total 35S-labeled cellular proteins.
Immunoprecipitation of protein-associated RNAs (RNA IP).
Immunoprecipitation of RNA-protein complexes was performed as previously described, with minor modifications (37, 60). Briefly, cells were lysed in polysome lysis buffer (PLB) (100 mM KCl, 5 mM MgCl2, 10 mM HEPES [pH 7.0], 0.5% Nonidet P-40, 1 mM DTT, 100 U of RNase inhibitor/ml) supplemented with 20 mM EDTA and protease inhibitors on ice for 20 min followed by centrifugation. The supernatants were precleared with protein A-Sepharose beads. The cleared supernatants were then diluted (1:10 [vol/vol]) in NT2 buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM MgCl2, 0.05% Nonidet P-40, 1 mM DTT, 100 U of RNase inhibitor/ml) supplemented with 20 mM EDTA and protease inhibitors and incubated with primary antibodies at 4°C for 4 h, followed by incubation of protein A/G beads for an additional 2 h at 4°C. The beads were washed five times with NT2 buffer supplemented with protease inhibitors. The bead-bound protein-RNA complexes were then treated with DNase I and proteinase K and eluted twice with NT2 buffer containing 0.1% SDS. RNAs were extracted from the elution with phenol-chloroform and ethanol precipitation and subjected to RT-qPCR assays as described below.
Luciferase reporter assays.
Cells were transfected with pCMV–β-galactoside (β-gal) and luciferase reporter plasmid pGL3, with pGL3-myc3′UTR or its fragments, together with control or Flag-L11 plasmid, or with scrambled or L11 small interfering RNA (siRNA), as indicated in the figures. Luciferase activity was determined and normalized by calculating β-gal activity in the same assay as described previously (12).
RT-qPCR analysis.
Total RNA was isolated from cells by the use of TRIzol reagent (Invitrogen). Reverse transcriptions were performed as described previously (59). qPCR was performed using an ABI StepOne real-time PCR system (Applied Biosystems) and SYBR green mix (Bio-Rad) for mRNA expression determinations as described previously (59). Analysis of expression of mature miRNAs was performed using a TaqMan miRNA assay kit (Applied Biosystems) following the manufacturer's protocol. All reactions were carried out in triplicate. Relative gene expression levels were calculated using the ΔCτ method following the manufacturer's instructions. The primers for c-myc and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were previously described (12). The primers for other genes were 5′-CATCGTTGACCGCCTGAAGT-3′ and 5′-GGAGCAAGATGGATTCCAATTC-3′ (for luciferase); 5′-TCAACGCGCAGGACTTCTG-3′ and 5′-CAGTGACCGTGGGAATGAAGTT-3′ (c-fos); 5′-GCAAAGATGGAAACGACCTTCT-3′ and 5′-GCTCTCGGACGGGAGGAA-3′ (c-jun); 5′-GCCTGCGACATCTGTGGAA-3′ and 5′-CGCAAGTGGATCTTGGTATGC-3′ (egr1); 5′-AGGCCTTGGAACTCAAGGAT-3′ and 5′-TGAGTCAGGCCCTTCTGTCT-3′ (p53); 5′-ATGAATCCCCCCCTTCCAT-3′ and 5′-CAGGAAGCCAATTCTCACGAA-3′ (mdm2); and 5′-GCCTTGAGGAAGGATTGGTA-3′ and 5′-TCGACAATCAGGGACATCAT-3′ (mdmx).
RNA interference (RNAi) and miRNA overexpression.
The 21-nt siRNA duplexes with a 3′ dTdT overhang were synthesized by Dharmacon Inc. (Lafayette, CO). The target sequences for L11 and control scramble II RNA were previously described (12). L11 siRNA-1 was used in all the experiments except where indicated. The target sequence for Ago2 was 5′-GCACGGAAGUCCAUCUGAA-3′. The miR-24 mimics and control cel-miR-67 were purchased from Dharmacon Inc. These siRNA duplexes (100 nM) and miRNA mimics (25 to 50 nM) were introduced into cells by the use of SilentFect lipid reagent (Bio-Rad) following the manufacturer's protocol. The cells were analyzed 48 h after transfection.
Cell fractionations.
To isolate the cytoplasmic and nuclear fractions, cells were resuspended in hypotonic buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.5 mM DTT) in the presence of protease inhibitors. Cell membranes were then broken using a Dounce homogenizer (tight pestle) and 10 up-and-down strokes. After centrifugation, the supernatant was collected as the cytoplasmic fraction and supplemented with a 0.1 volume of 10× buffer B (0.3 M Tris-HCl [pH 7.9], 1.4 M KCl, 30 mM MgCl2). The nuclear pellets were washed with buffer A and then resuspended in buffer C (20 mM HEPES [pH 7.9], 420 mM NaCl, 0.2 mM EDTA, 1.5 mM MgCl2, 0.5 mM DTT, 25% glycerol) in the presence of protease inhibitors and sonicated. The nuclear fraction (supernatant) was collected by centrifugation.
For isolation of the nucleolus fraction, the nuclear pellets were resuspended in buffer S1 containing 0.25 M sucrose and 10 mM MgCl2, layered over buffer S2 containing 0.35 M sucrose and 0.5 mM MgCl2, and centrifuged at 1,430 × g for 10 min at 4°C. The pelleted nuclei were resuspended in buffer S2 followed by sonication. The sonicated nuclei were then layered over buffer S3 containing 0.88 M sucrose and 0.5 mM MgCl2 and centrifuged at 3,000 × g for 10 min at 4°C. The pellet contained purified nucleoli, and the supernatant represented the nucleoplasm (2, 4).
Ribosome fractionation.
For separation of ribosome-free L11 from the total ribosome volume, cells were lysed in polysome lysis buffer. The extracts were overlaid on a 20% (wt/vol) sucrose cushion and centrifuged at 150,000 × g for 2 h. The polysome-containing pellet and the nonribosomal supernatant were collected separately (42) and immunoblotted with anti-L11 antibody. The nonribosomal supernatant was also used for RNA IP assays.
RESULTS
L11 regulates the levels and stability of c-myc mRNA.
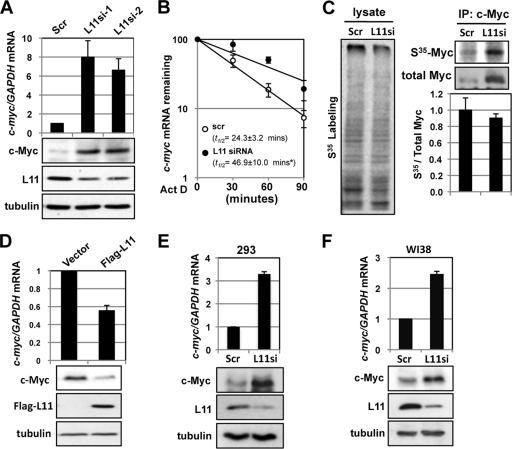
We previously showed that L11 directly suppresses c-Myc transactivation activity (12). Interestingly, L11 also regulates c-Myc levels. Knockdown of L11 drastically increased the levels of c-Myc protein (12) (bottom panels of Fig. 1 A) and c-myc mRNA (15) in U2OS cells. RT-qPCR assays revealed that the levels of c-myc mRNA were markedly increased upon knockdown of L11 (top panel of Fig. 1A). This effect was unlikely to have been an off-target effect, as similar results were observed when two different siRNAs against L11 were used. To examine how c-myc mRNA was induced by knockdown of L11, we determined the half-life (t1/2) of c-myc mRNA. As shown in Fig. 1B, knockdown of L11 significantly prolonged the half-life of c-myc mRNA, suggesting that knockdown of L11 stabilizes c-myc mRNA. To examine whether knockdown of L11 affects c-Myc translation, we performed [35S]methionine pulse-labeling assays. Consistent with a previous report (4), transient knockdown of L11 did not significantly affect global translation (left panel of Fig. 1C). It also appears that knockdown of L11 did not affect c-Myc translation, as levels of nascent synthesized c-Myc were increased proportionally to total cellular c-Myc levels (right panels of Fig. 1C). Therefore, we conclude that transient knockdown of L11 increases c-Myc levels by stabilizing c-myc mRNA without affecting c-Myc translation. Consistently, overexpression of L11 reduced the levels of c-myc mRNA and protein (Fig. 1D) in U2OS cells. Similarly, knockdown of L11 also increased the levels of c-myc mRNA and protein in 293 cells (Fig. 1E), human normal fibroblast WI38 cells (Fig. 1F), and H1299 cells (data not shown), suggesting that the regulation of c-myc mRNA by L11 was not specific to cell type. Taken together, these results demonstrate that L11 regulates c-myc mRNA turnover.
Fig. 1.
L11 regulates c-myc mRNA levels and stability. (A) Knockdown of L11 increases the levels of c-myc mRNA and c-Myc protein. U2OS cells were transfected with scrambled (Scr) siRNA or one of two L11 siRNAs (L11si-1 or L11si-2). The cells were assayed for expression of c-myc mRNA by the use of RT-qPCR and of c-Myc protein by the use of IB assays. (B) Knockdown of L11 stabilizes c-myc mRNA. U2OS cells transfected with scrambled or L11 siRNA were treated with 2 μM Act D. The cells were harvested at the indicated time points and assayed for relative levels of c-myc mRNA normalized to the expression of GAPDH mRNA by the use of RT-qPCR assays. The average c-myc mRNA half-life value is shown. *, P < 0.01 compared to t1/2 of c-myc mRNA in scrambled-RNA transfected cells. (C) Knockdown of L11 does not affect c-myc mRNA translation. U2OS cells transfected with scrambled or L11 siRNA were pulse-labeled with [35S]methionine. Equal amounts of cell lysates were immunoprecipitated with anti-c-Myc (C33) antibody-conjugated beads followed by autography (top right panel) and IB with anti-c-Myc antibody (Y69) (middle right panel). The total lysates were also loaded on an SDS-PAGE gel followed by autography (left panel). The relative translation efficiency of c-myc mRNA was calculated based on the ratio of radiolabeled to total immunoprecipitated c-Myc protein and is plotted in the bottom right panel. (D) Overexpression of L11 reduces the levels of c-myc mRNA and c-Myc protein. U2OS cells transfected with control or Flag-L11 vector were assayed for expression of c-myc mRNA by the use of RT-qPCR and of protein by the use of IB assays. (E and F) Knockdown of L11 increases the levels of c-myc mRNA and c-Myc protein in 293 and WI38 cells. 293 (E) and WI38 (F) cells were transfected with scrambled or L11 siRNA. The cells were assayed for expression of c-myc mRNA by the use of RT-qPCR and of c-Myc protein by the use of IB assays.
L11 binds to c-myc at its 3′-UTR.
To understand how L11 regulates c-myc mRNA stability, we examined whether L11 physically associates with c-myc mRNA by the use of RNA immunoprecipitation (IP) assays. Lysates from 293 or U2OS cells transfected with Flag-L11 were immunoprecipitated with control IgG or anti-Flag antibody, followed by detection of c-myc mRNA by the use of RT-qPCR assays. As shown in Fig. 2 A, c-myc mRNA was specifically detected in anti-Flag but not control IgG immunoprecipitates from both cell lines, suggesting that L11 binds to c-myc mRNA in cells.
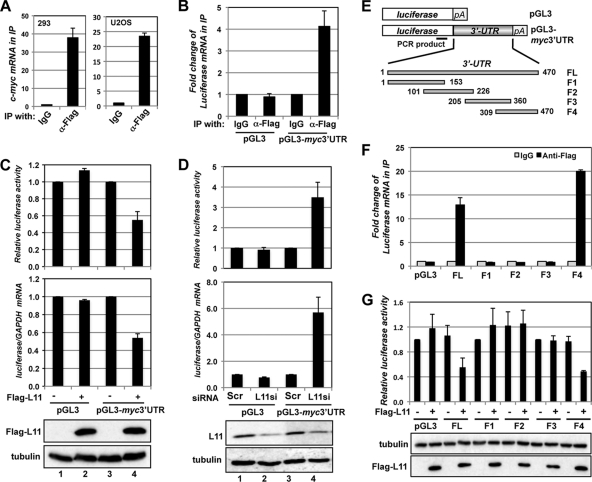
Fig. 2.
L11 regulates c-myc by targeting the 3′-UTR of c-myc mRNA. (A) L11 binds to c-myc mRNA. Lysates from 293 (left panel) and U2OS (right panel) cells transfected with Flag-L11 were immunoprecipitated with control mouse IgG or anti-Flag antibody. RNA extracted from the immunoprecipitates was retrotranscribed and assayed for expression of c-myc mRNA by the use of RT-qPCR assays. (B) L11 binds to the c-myc 3′-UTR. U2OS cells transfected with Flag-L11 together with control pGL3 or pGL3-myc3′UTR vector were immunoprecipitated with anti-Flag or mouse IgG. The immunoprecipitates were assayed for expression of the luciferase mRNA by RT-qPCR assays. (C) L11 reduction of luciferase mRNA levels and suppression of luciferase activity are dependent on the c-myc 3′-UTR. 293 cells transfected with the indicated plasmids together with β-gal plasmid were assayed for relative luciferase activity levels normalized to β-gal expression (top panel) and relative luciferase mRNA levels normalized to GAPDH mRNA by the use of RT-qPCR assays (middle panel). The protein expression results are shown in the bottom panels. (D) Knockdown of L11 increases the levels of luciferase mRNA and luciferase activity in a manner dependent on the c-myc 3′-UTR. U2OS cells transfected with β-gal and pGL3 or pGL3-myc3′UTR plasmids and siRNAs were assayed for relative luciferase activity levels normalized to β-gal expression (top panel) and relative luciferase mRNA levels normalized to GAPDH mRNA by the use of RT-qPCR assays (middle panel). The protein expression results are shown in the bottom panels. (E) Diagram of the control pGL3, pGL3-myc3′UTR, and pGL3-myc3′UTR fragment (F1, F2, F3, and F4) vectors. The relative positions of the full-length (FL) c-myc 3′UTR and its fragments (F1 through F4) are indicated, with the first nucleotide after the stop codon labeled “1.” The position of the PCR product used to examine expression of the luciferase mRNA is indicated in the coding region of the luciferase gene. pA indicates a poly(A) tail. (F) L11 binds to the 3′ end of the c-myc 3′-UTR. 293 cells transfected with Flag-L11 together with control pGL3 or pGL3-myc3′UTR or its fragments were immunoprecipitated with anti-Flag or mouse IgG. The immunoprecipitates were assayed for expression of luciferase mRNA by RT-qPCR assays. (G) L11 suppression of luciferase activity is dependent on the 3′ end of the c-myc 3′-UTR. 293 cells transfected with the indicated plasmids together with a β-gal plasmid were assayed for relative luciferase activity levels normalized to β-gal expression. The protein expression results are shown in the bottom panels.
c-myc mRNA turnover is fast and highly regulated in cells, with a normal half-life of about 15 to 30 min (19) (Fig. 1B). The c-myc 3′-UTR contains several AU-rich elements (AREs) and plays an important role in the regulation of c-myc mRNA turnover (30). Several ARE binding proteins, including AUF1 (67), HuR (32), and tristetraprolin (TTP) (39), have been found to bind to c-myc AREs and regulate c-myc mRNA stability. To test whether L11 binds to c-myc mRNA through its 3′-UTR, we generated a luciferase reporter that contained a c-myc 3′-UTR in the 3′ portion of the luciferase gene (pGL3-myc3′UTR; Fig. 2E). U2OS cells were transfected with Flag-L11 together with pGL3-myc3′UTR or control pGL-3 vector. The cell lysates were immunoprecipitated with anti-Flag antibody or control IgG followed by RT-qPCR detection of the luciferase gene. As shown in Fig. 2B, luciferase mRNA was specifically coimmunoprecipitated with Flag-L11 in cells transfected with c-myc 3′-UTR-containing but not control luciferase plasmid. Similar results were also observed in 293 cells (Fig. 2F; see also Fig. 8). These results demonstrate that L11 binds to the c-myc 3′-UTR in cells.
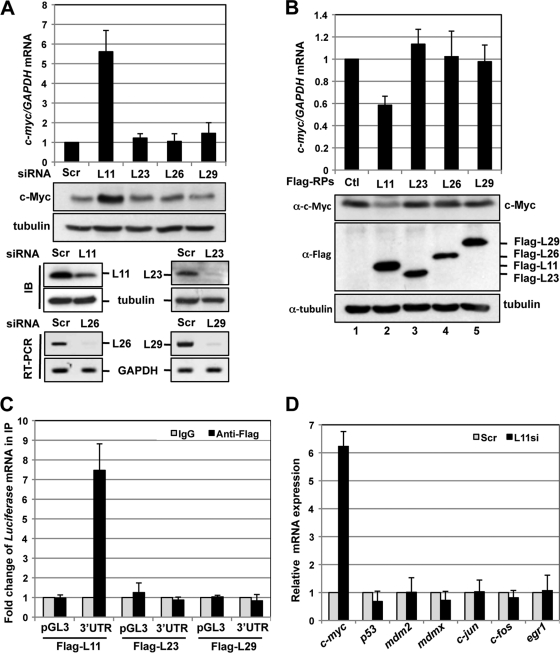
Fig. 8.
L11 regulation of c-myc mRNA turnover is specific. (A) Knockdown of L23, L26, or L29 does not enhance c-Myc levels. U2OS cells transfected with scrambled, L23, L26, or L29 siRNA were subjected to detection of c-myc mRNA by the use of RT-qPCR and of c-Myc protein by the use of IB assays. The results of knockdown of the individual RPs shown in the bottom panels were determined by IB (L11 and L23) or RT-PCR (L26 and L29). (B) Overexpression of L23, L26, or L29 does not reduce c-Myc levels. U2OS cells transfected with control (Ctl), Flag-L23, Flag-L26, or Flag-L29 plasmid were subjected to detection of c-myc mRNA by the use of RT-qPCR (top panel) and of c-Myc protein by the use of IB assays (bottom panels). (C) L23 and L29 do not bind to c-myc mRNA in cells. 293 cells were transfected with control pGL3 or pGL3-myc3′UTR together with Flag-L11, Flag-L23, or Flag-L29. The cell lysates were immunoprecipitated with anti-Flag antibody or control IgG, followed by RT-qPCR detection of luciferase mRNA. (D) Knockdown of L11 does not evoke upregulation of a set of other mRNAs. U2OS cells transfected with scrambled or L11 siRNA were subjected to detection of the indicated mRNAs by the use of RT-qPCR assays.
L11 regulates c-myc mRNA levels through the c-myc 3′-UTR.
Next, we examined whether L11 regulates c-myc mRNA levels through the c-myc 3′-UTR. 293 cells were transfected with pGL3 or pGL3-myc3′UTR together with control or Flag-L11 plasmid. The cells were assayed for relative luciferase activity levels as well as expression of luciferase mRNA by RT-qPCR. As shown in Fig. 2C, overexpression of L11 specifically suppressed the luciferase activity (top panel) and reduced luciferase mRNA levels (middle panel) in pGL3-myc3′UTR but not control pGL3 transfected cells. Similar results were also observed in H1299 cells (data not shown). Conversely, knockdown of endogenous L11 significantly increased the luciferase activity and the luciferase mRNA levels in pGL3-myc3′UTR (compare lane 4 to lane 3) but not control pGL3 (compare lane 2 to lane 1) transfected U2OS cells (Fig. 2D). Together, these results suggest that L11 regulates c-myc mRNA levels by acting on the c-myc 3′-UTR.
To demonstrate the specificity of L11 regulation of c-myc mRNA through the c-myc 3′-UTR, we constructed a panel of luciferase reporter plasmids containing overlapping fragments of the c-myc 3′-UTR (Fig. 2E) and mapped the L11-binding site at the c-myc 3′-UTR. 293 cells were transfected with Flag-L11 together with control pGL-3 and pGL3-myc3′UTR or its deletion fragments followed by RNA IP using control IgG or anti-Flag antibodies. As shown in Fig. 2F, Flag-L11 was associated specifically with the luciferase mRNA containing the full-length (FL) c-myc 3′-UTR and the fragment consisting of nt 309 to 470 (F4) but not other fragments encompassing nt 1 to 360. Furthermore, overexpression of L11 significantly suppressed the luciferase activity in cells transfected with pGL3-myc3′UTR-F4 but not those transfected with other c-myc 3′-UTR fragments containing luciferase reporter plasmids (F1, F2, and F3) (Fig. 2G). These results suggest that L11 regulates c-myc mRNA through nt 361 to 470 of the c-myc 3′-UTR.
L11-mediated c-myc mRNA reduction requires Ago2.
miRNAs have emerged as key regulators of gene expression by suppressing translation and/or inducing mRNA decay of target genes through the 3′-UTR (47, 61). Therefore, we asked whether L11 regulates c-myc mRNA turnover through a miRNA-mediated pathway. As Ago2 is a core component of miRISC (47, 61) and, consistent with a previous report (32), knockdown of endogenous Ago2 significantly increased the levels of c-Myc protein and mRNA (Fig. 3 A), we tested whether Ago2 is involved in L11-mediated c-myc mRNA reduction. We first asked whether L11 could facilitate the recruitment of Ago2 to the c-myc mRNA. To this end, we performed RNA IP followed by RT-qPCR assays and found that overexpression of L11 increased the binding of Ago2 to the c-myc mRNA (Fig. 3B), whereas knockdown of L11 significantly reduced the Ago2 binding to the c-myc mRNA (Fig. 3C), suggesting that L11 facilitates the recruitment of Ago2 to the c-myc mRNA. Furthermore, knockdown of Ago2 extensively rescued the L11-mediated reduction of c-Myc protein (compare the ratio of lane 4 to lane 3 with the ratio of lane 2 to lane 1 in Fig. 3D) and c-myc mRNA (Fig. 3E) in U2OS cells. Together, these results suggest that L11 decreases c-myc mRNA levels by recruiting Ago2 to the c-myc mRNA.
L11 associates with Ago2.
Given that L11 regulates Ago2 targeting of c-myc mRNA (Fig. 3B and C), we tested whether L11 physically associates with Ago2. 293 cells were transfected with control or Flag-L11 followed by co-IP assays using anti-Flag antibodies. As shown in Fig. 3F, endogenous Ago2 was specifically coimmunoprecipitated with Flag-L11 in 293 cells. Similar results were also observed in U2OS cells (Fig. 3G) and H1299 cells (data not shown). Also, endogenous Ago2 and endogenous L11 were specifically coimmunoprecipitated with each other using either anti-Ago2 or anti-L11 antibodies, but not control IgG, in 293 cells (Fig. 3H) and U2OS cells (Fig. 3I and data not shown). RNase treatment abolished the interaction between L11 and Ago2 (Fig. 3I). These results suggest that the interaction between L11 and Ago2 in cells is dependent on the presence of cellular RNA.
L11 recruits miR-24 to target c-myc mRNA.
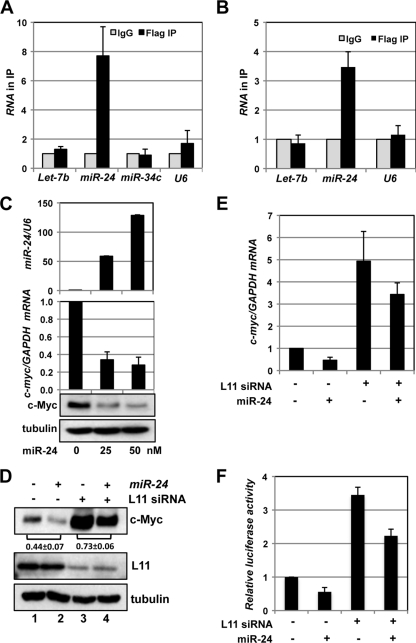
We then asked whether L11 regulates c-myc mRNA through the activity of miRNAs. At least four miRNAs, including Let-7 (32), miR-145 (55), miR-24 (34), and miR-34c (7), have been previously shown to downregulate c-Myc by either repressing c-Myc translation (let-7 and miR-34c) or reducing c-myc mRNA levels (miR-24 and miR-145). Thus, we examined whether L11 associates with these miRNAs in cells. Lysates from 293 or U2OS cells transfected with Flag-L11 were immunoprecipitated with anti-Flag antibodies or control IgG followed by RT-qPCR detection of the miRNAs described above. U6 snRNA was used as a control. As shown in Fig. 4 A, miR-24, but not let-7b, miR-34c, and U6 snRNA, was significantly enriched in the Flag-L11 immunoprecipitates compared to control IgG in 293 cells. Similar results were also observed in U2OS cells except that miR-34c was undetectable in the immunoprecipitates (Fig. 4B). We were unable to detect miR-145 in the immunoprecipitates from both 293 and U2OS cells (data not shown). These results clearly indicate that L11 specifically associates with miR-24 but not with other known c-myc targeting miRNAs.
Fig. 4.
L11 recruits miR-24 to target c-myc mRNA. (A and B) L11 binds to miR-24 in cells. Lysates from 293 (A) or U2OS (B) cells transfected with Flag-L11 plasmid were immunoprecipitated with anti-Flag antibody or control IgG followed by detection of the indicated miRNAs by the use of RT-qPCR assays. (C) Overexpression of miR-24 decreases c-Myc levels. U2OS cells transfected with a control or with different doses of miR-24 mimics were assayed for the relative levels of expression of miR-24 normalized with U6 snRNA (top panel) and of c-myc mRNA normalized with GAPDH mRNA (middle panel) by the use of RT-qPCR assays as well as c-Myc protein (bottom panel) by the use of IB. (D and E) Knockdown of L11 attenuates c-myc downregulation by miR-24. U2OS cells transfected with scrambled or L11 siRNA together with control or miR-24 mimics were assayed for c-Myc protein levels by IB (D) and c-myc mRNA levels by the use of RT-qPCR (E). The c-Myc bands were quantified and normalized to tubulin. The ratios of lane 2 to lane 1 and of lane 4 to lane 3 for the results from three independent experiments are indicated in panel D. (F) Knockdown of L11 attenuates the miR-24-mediated suppression of luciferase activity. U2OS cells were transfected with pGL3-myc3′UTR and β-gal expression plasmids together with siRNA and/or miRNAs as indicated. The cells were assayed for relative luciferase activity levels normalized to β-gal expression.
Next, we examined the role of miR-24 in L11-mediated c-myc mRNA decay. Consistent with a previous report (34), overexpression of miR-24 significantly reduced the levels of c-Myc protein and mRNA in a dose-dependent manner (Fig. 4C). We then transfected U2OS cells with different combinations of scrambled or L11 siRNA and control (cel-miR-67) or miR-24 mimics. The cells were analyzed for expression of c-Myc protein and mRNA. Interestingly, knockdown of L11 drastically rescued the miR-24-mediated reduction of c-Myc protein levels (compare the ratio of lane 4 to lane 3 with the ratio of lane 2 to lane 1; Fig. 4D) and c-myc mRNA levels (Fig. 4E). Similarly, knockdown of L11 significantly abolished the miR-24-mediated reduction of relative luciferase activity in cells transfected with pGL3-myc3′UTR reporter plasmid (Fig. 4F). These results suggest that L11 plays a critical role in miR-24-mediated downregulation of c-myc mRNA, supporting the notion that L11 regulates c-myc mRNA by recruiting miR-24-loaded miRISC to the c-myc 3′-UTR.
c-myc mRNA is reduced in response to ribosomal stress.
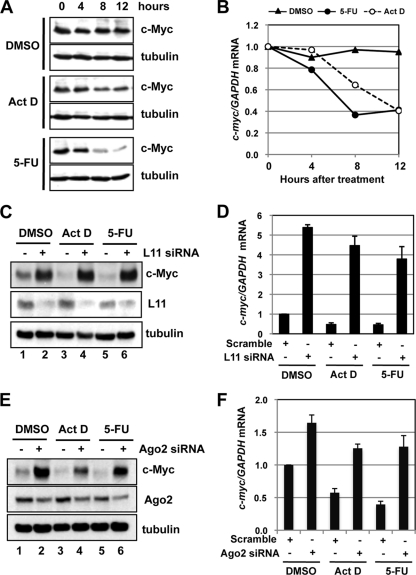
To examine the physiological relevance of the L11 regulation of c-myc mRNA turnover, we asked whether L11 is involved in c-myc regulation in cells in response to ribosomal stress, as L11 plays a key role in p53-mediated cell cycle control in response to such stress (4, 36, 57–59, 69). Ribosomal stress is triggered following perturbation of ribosomal biogenesis, including inhibition of rRNA synthesis by treatment with a low dose of Act D (5 nM) (4, 14, 18) and inhibition of rRNA processing by treatment with 5-FU (57). c-Myc has recently been shown to be downregulated in response to DNA damage (7, 48). To test whether c-Myc levels are also downregulated by ribosomal stress, we treated U2OS cells with a low dose of either Act D or 5-FU followed by the detection of c-Myc expression. As shown in Fig. 5 A, treatment of cells with either Act D or 5-FU significantly reduced the c-Myc protein levels in a time-dependent manner. This reduction correlates with the reduction of c-myc mRNA levels (Fig. 5B), suggesting that ribosomal stress induces c-myc mRNA degradation.
Fig. 5.
L11 downregulates c-myc mRNA in response to ribosomal stress. (A and B) c-Myc is downregulated by treatment with Act D or 5-FU. U2OS cells were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for the indicated hours. The cells were assayed for c-Myc protein expression by IB (A) and c-myc mRNA expression by RT-qPCR (B) assays. (C and D) Knockdown of L11 rescues the downregulation of c-Myc by treatment with Act D or 5-FU. U2OS cells transfected with scrambled or L11 siRNA were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h. The cells were assayed for c-Myc protein expression by IB (C) and c-myc mRNA expression by RT-qPCR (D) assays. (E and F) Knockdown of Ago2 rescues the downregulation of c-Myc by treatment with Act D or 5-FU. U2OS cells transfected with scrambled or Ago2 siRNA were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h. The cells were assayed for c-Myc protein expression by IB (E) and c-myc mRNA expression by RT-qPCR (F) assays.
L11 and Ago2 are required for c-myc mRNA downregulation in response to ribosomal stress.
Next, we asked whether L11 is involved in c-Myc downregulation in response to ribosomal stress. U2OS cells were transfected with scrambled or L11 siRNA followed by treatment with control dimethyl sulfoxide (DMSO), Act D, or 5-FU. As shown in Fig. 5C, knockdown of L11 completely abolished the c-Myc protein reduction caused by the Act D or 5-FU treatment. Again, this occurred at the mRNA level, as knockdown of L11 rescued the reduction of c-myc mRNA levels caused by Act D or 5-FU treatment (Fig. 5D). To evaluate the involvement of miRNA-mediated silencing in the downregulation of c-myc mRNA by ribosomal stress, we performed Ago2 knockdown experiments. Indeed, knockdown of Ago2 significantly rescued the downregulation of c-Myc protein (Fig. 5E) and mRNA (Fig. 5F) levels caused by treatment with Act D or 5-FU. Thus, L11 and Ago2 are required for c-myc mRNA downregulation by ribosomal stress.
L11 recruits miR-24-loaded miRISC to c-myc mRNA in response to ribosomal stress.
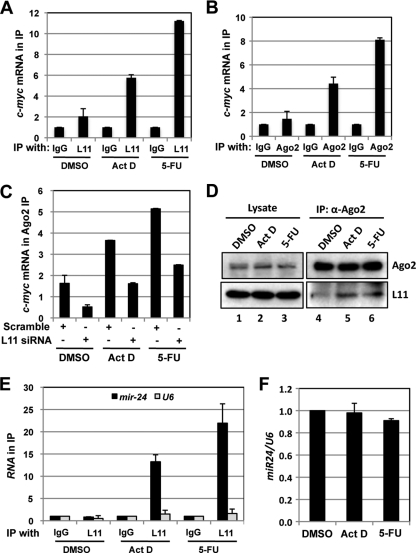
As L11 has been previously shown to dissociate from intact ribosomes into the ribosome-free pool (free L11) in response to ribosomal stress (4, 57), we reasoned that free L11 might be able to associate with c-myc mRNA. Thus, U2OS cells treated with Act D, 5-FU, or DMSO were subjected to RNA IP using anti-L11 antibodies, followed by RT-qPCR assays. As shown in Fig. 6 A, L11 binding to c-myc mRNA significantly increased in cells treated with Act D or 5-FU compared to the control results, indicating that ribosomal stress enhances L11 targeting of c-myc mRNA.
Fig. 6.
L11 recruits miRISC to c-myc mRNA in response to ribosomal stress. (A) Treatment with Act D or 5-FU increases the binding of L11 to c-myc mRNA. U2OS cells were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h. The cell lysates were immunoprecipitated with control IgG or anti-L11 antibodies followed by RT-qPCR detection of c-myc mRNA. (B) Treatment with Act D or 5-FU increases the binding of Ago2 to c-myc mRNA. U2OS cells were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h. The cell lysates were immunoprecipitated with anti-Ago2 antibodies or control IgG followed by RT-qPCR detection of c-myc mRNA. (C) Knockdown of L11 abolishes Ago2 binding to c-myc mRNA in response to ribosomal stress. U2OS cells transfected with scrambled or L11 siRNA were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h. The cell lysates were immunoprecipitated with anti-Ago2 antibodies or control IgG followed by RT-qPCR detection of c-myc mRNA. The data were normalized to the c-myc mRNA in IP with control IgG. (D) Treatment with Act D or 5-FU increases the binding of L11 to Ago2. U2OS cells were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h. The cell lysates were immunoprecipitated with anti-Ago2 antibodies followed by IB using anti-L11 antibodies. (E) Treatment with Act D or 5-FU increases the association of L11 with miR-24. U2OS cells were treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h and subjected to IP with anti-L11 or control IgG, followed by RT-qPCR detection of miR-24 and U6 snRNA. (F) Treatment with Act D or 5-FU does not change the total levels of miR-24 in cells. U2OS cells treated with DMSO, Act D (5 nM), or 5-FU (10 μg/ml) for 12 h were examined for expression of miR-24 normalized to that of U6 snRNA by the use of RT-qPCR assays.
Next, we examined whether L11 recruits miRISC to c-myc mRNA in response to ribosomal stress. The cell lysates from U2OS cells treated with DMSO, Act D, or 5-FU were immunoprecipitated with anti-Ago2 antibodies or control IgG. As shown in Fig. 6B, treatment of cells with Act D or 5-FU markedly increased the association of Ago2 with c-myc mRNA. This increase requires L11, as knockdown of L11 significantly reduced the binding of Ago2 with c-myc mRNA in cells treated with Act D or 5-FU (Fig. 6C). To confirm that L11 interacts with Ago2 under conditions of ribosomal stress, we performed co-IP assays using anti-Ago2 antibodies. As shown in Fig. 6D, treatment of cells with Act D or 5-FU significantly increased the binding of L11 to Ago2. These data demonstrate that L11 recruits Ago2 to c-myc mRNA in response to ribosomal stress. Lastly, we examined whether L11 recruits miR-24 in response to ribosomal stress. As shown in Fig. 6E, treatment of cells with either Act D or 5-FU drastically increased the binding of L11 to miR-24 but not U6 snRNA. In contrast, the total level of miR-24 was not changed following both treatments (Fig. 6F). Together, these data strongly suggest that L11 mediates c-myc mRNA decay by recruiting miR-24-loaded miRISC to c-myc mRNA in response to ribosomal stress.
L11 associates with c-myc mRNA in the cytoplasm.
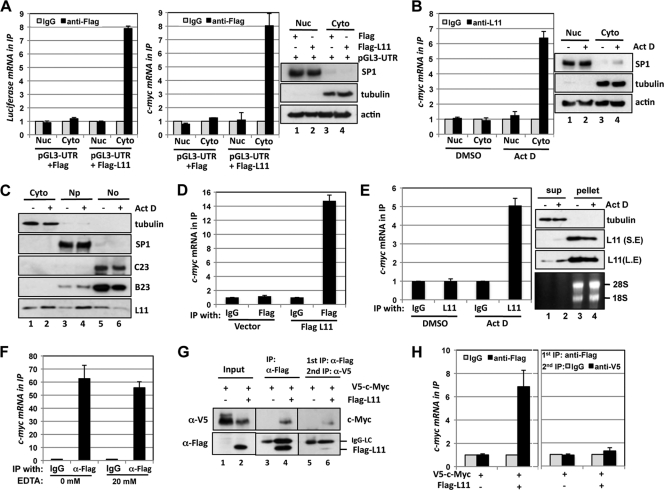
To test where L11 associates with c-myc mRNA in cells, we performed cell fractionation assays. First, 293 cells transfected with pGL3-myc3′UTR together with control Flag or Flag-L11 vector were then fractionated into cytoplasmic and nuclear fractions (right panel of Fig. 7 A). RNA IP was conducted using the cytoplasmic and nuclear lysates with control IgG or anti-Flag antibodies followed by detection of luciferase and c-myc mRNAs. As shown in Fig. 7A, L11 specifically associates with exogenous luciferase mRNA (left panel) and endogenous c-myc mRNA (middle panel) in the cytoplasm but not in the nucleus. Next, U2OS cells treated with DMSO or Act D were fractionated into cytoplasmic and nuclear lysates (right panel of Fig. 7B) followed by RNA IP using anti-L11 or control IgG. Again, Act D treatment significantly increased the association of endogenous L11 with c-myc mRNA in the cytoplasm (left panel, Fig. 7B). Thus, these results indicate that L11 associates with c-myc mRNA in the cytoplasm and that this association is markedly increased following ribosomal stress.
Fig. 7.
Ribosome-free L11 associates with c-myc mRNA in the cytoplasm. (A) L11 binds to the c-myc 3′-UTR in the cytoplasm. 293 cells transfected with pGL3-myc3′UTR together with control Flag or Flag-L11 vector were fractionated into the cytoplasm (Cyto) and the nucleus (Nuc) fractions, followed by IP with anti-Flag antibody or mouse IgG. The immunoprecipitates were assayed for expression of luciferase mRNA (left panel) and c-myc mRNA (middle panel) by the use of RT-qPCR assays. Cellular fractionation was verified by detecting the nuclear SP1 and cytoplasmic tubulin by the use of IB (right panels). Actin was used as a loading control. (B) Act D treatment enhances the interaction of endogenous L11 with the c-myc mRNA in the cytoplasm. The cytoplasmic and the nuclear fractions were isolated from U2OS cells treated with DMSO or Act D (5 nM) for 12 h, followed by IP with anti-L11 antibodies or control rabbit IgG. The immunoprecipitates were assayed for expression of c-myc mRNA by the use of RT-qPCR assays (left panel). Cellular fractionation was verified by IB detection of SP1 and tubulin proteins (right panels). (C) Treatment with Act D releases L11 from the nucleolus into the nucleoplasm and the cytoplasm. U2OS cells treated with DMSO or Act D (5 nM) for 12 h were subjected to isolation of the cytoplasm (Cyto), nucleoplasm (Np), and the nucleolus (No) fractions, followed by IB detection of the indicated proteins. Nucleophosmin (B23) and nucleolin (C23) were used as nucleolar markers. (D) Ribosome-free L11 binds to c-myc mRNA. Nonribosome supernatants containing free RPs were isolated from 293 cells transfected using control or Flag-L11 plasmid and sucrose centrifugation as described in Materials and Methods. The supernatants were immunoprecipitated with anti-Flag antibody or mouse IgG followed by RT-qPCR detection of c-myc mRNA in the immunoprecipitates. (E) Act D treatment enhances the interaction of free endogenous L11 with c-myc mRNA. Nonribosome supernatants containing free RPs were isolated from U2OS cells treated with DMSO or Act D (5 nM) for 12 h. The supernatants were immunoprecipitated with anti-L11 antibodies or control IgG followed by RT-qPCR detection of c-myc mRNA (left panel). The L11 protein was detected using IB (upper right panels). S.E, short exposure; L.E, longer exposure. The rRNAs from polysome pellets as shown in the lower right panel were revealed by ethidium bromide staining. (F) L11 interacts with c-myc mRNA in the presence of EDTA. U2OS cells transfected with Flag-L11 were subjected to IP in the absence or presence of 20 mM EDTA. RNA IPs were conducted using anti-Flag antibody or control IgG followed by RT-qPCR detection of c-myc mRNA. (G) Sequential co-IP of the L11–c-Myc protein complex. 293 cells were transfected with V5–c-Myc in the presence or absence of Flag-L11. The cell lysates were first immunoprecipitated with anti-Flag antibody. The Flag-L11-associated protein complexes were eluted with Flag peptide. Half of the elution was assayed for V5–c-Myc and Flag-L11 proteins by the use of IB. The other half was subjected to a second IP using anti-V5 antibody followed by IB detection of V5–c-Myc and Flag-L11 proteins. (H) Sequential co-IP of the L11–c-myc mRNA complex. 293 cells were transfected with V5–c-Myc in the presence or absence of Flag-L11. The cell lysates were first immunoprecipitated with anti-Flag antibody or control IgG followed by elution with Flag peptide. Half of the elution was assayed for c-myc mRNA by the use of RT-qPCR assays. The other half was subjected to a second IP using anti-V5 antibody or control IgG, followed by RT-qPCR detection of the c-myc mRNA.
Ribosome-free L11 associates with c-myc mRNA.
It has been proposed that L11 is released from the nucleolus and intact ribosomes to suppress MDM2 in the nucleoplasm, leading to p53 activation, in response to ribosomal stress (68). To further observe the L11 localization following ribosomal stress, we fractionated U2OS cells treated with control DMSO or Act D into cytoplasmic, nuclear, and nucleolar fractions. As shown in Fig. 7C, the levels of L11 in the nucleolus were reduced whereas its levels in both the nucleus and the cytoplasm increased in response to Act D treatment. To test whether it is the ribosome-free L11 that interacts with c-myc mRNA in the cytoplasm, we performed ribosome fractionation assays using sucrose centrifugation to isolate free RPs (nonribosomal supernatant) from ribosomes and polysomes (42). Nonribosomal supernatant isolated from 293 cells transfected with control or Flag-L11 plasmid was immunoprecipitated with control IgG or anti-Flag antibodies, followed by detection of c-myc mRNA. As shown in Fig. 7D, ribosome-free Flag-L11 specifically associated with c-myc mRNA in cells. Similar experiments were also conducted in U2OS cells treated with DMSO or Act D. As shown in Fig. 7E, Act D treatment significantly increased the association of free endogenous L11 with c-myc mRNA in cells (left panel). Of note, the level of free L11 was increased whereas that of L11 in the polysome pellet was decreased by Act D treatment (right panels of Fig. 7E). Taken together, these results suggest that ribosome-free L11 interacts with c-myc mRNA in cells.
Our RNA IP assays were performed in the presence of EDTA, which disrupts the 80S ribosomes and polysomes (51), suggesting that L11 binding to c-myc mRNA is independent of the translating c-myc mRNA. This notion was further supported by the results showing that L11 associated with the c-myc mRNA regardless of the presence or absence of 20 mM EDTA (Fig. 7F). To further confirm this notion while also testing whether L11 associates with c-myc mRNA through binding to translating c-Myc protein in a ribosome–c-myc mRNA complex, we conducted sequential co-IP followed by RT-qPCR assays. 293 cells transfected with V5–c-Myc in the presence or absence of Flag-L11 were subjected to a first co-IP with anti-Flag antibodies. As shown in Fig. 7G and consistent with our previous reports (12, 17), c-Myc was coimmunoprecipitated with Flag-L11 (lane 4). The Flag-L11-associated protein complex was eluted with Flag peptides. The elution was then subjected to a second co-IP using anti-V5 antibodies. The anti-V5 immunoprecipitates (lane 6, Fig. 7G) specifically represent the c-Myc-associated L11. RNA was extracted from the elution from the first IP with anti-Flag antibody or control IgG as well as the immunoprecipitates from the second IP with anti-V5 antibodies or control IgG, followed by detection of c-myc mRNA by the use of RT-qPCR. As shown in Fig. 7H, c-myc mRNA was detected only in the immunoprecipitates from the first anti-Flag and not in those from the second anti-V5 IP. These results clearly demonstrate that L11 association with c-myc mRNA is independent of its interaction with c-Myc protein (including nascent synthesized c-Myc protein), further supporting the notion that L11 association with c-myc mRNA was not due to ribosome translation of c-myc mRNA. Given all of these observations, we conclude that free L11 interacts with c-myc mRNA in the cytoplasm.
L11 regulation of c-myc mRNA is specific.
To determine whether L11 regulation of c-myc mRNA turnover is a general effect of all individual RPs, we first examined whether knockdown of several other RP genes, including L23, L26, and L29, would also affect c-myc mRNA and protein levels. As shown in Fig. 8 A, unlike knockdown of L11, knockdown of L23, L26, or L29 did not affect the levels of c-myc mRNA (top panel) and protein (middle panel) in U2OS cells. Consistent with those results, overexpression of these RPs did not reduce the levels of c-myc mRNA and protein either (Fig. 8B). Also, neither knockdown nor overexpression of S12 affected the levels of c-myc mRNA and protein (data not shown). Furthermore, RNA IPs were performed using anti-Flag antibodies in cells transfected with Flag-L23 or Flag-L29. As shown in Fig. 8C, unlike L11, neither L23 nor L29 was associated with c-myc mRNA in cells. Taken together, these results suggest that L11 downregulation of c-myc mRNA and protein is a specific rather than a general effect of all individual RPs.
To test whether L11 regulation of c-myc mRNA is also specific to c-myc mRNA rather than a general effect on global mRNA turnover, we examined the levels of a number of other mRNAs upon L11 knockdown. These included the tumor suppressor gene p53, oncogenes mdm2 and mdmx, two labile early-response genes that contain AREs in their 3′-UTR similar to that in c-myc 3′-UTR, c-fos and c-jun (8, 9, 46), and the early-response gene egr1. As shown in Fig. 8D, unlike the results seen with c-myc mRNA, none of these genes were upregulated upon knockdown of L11. Thus, L11 regulation of c-myc mRNA is also not a general effect for all cellular transcripts, although we cannot exclude the possibility that knockdown of L11 might affect the turnover of some other untested mRNAs.
DISCUSSION
c-Myc promotes cell growth and proliferation by enhancing ribosomal biogenesis and translation. Deregulated ribosomal biogenesis and translation contribute to malignant transformation and conceivably play a key role in c-Myc-driven tumorigenesis. Our previous studies revealed that ribosomal protein L11, whose transcription is induced by c-Myc, directly suppresses c-Myc activity, thus coordinating c-Myc activity with ribosomal biogenesis. In this study, we found that L11 destabilizes c-myc mRNA through a novel miRNA-mediated mechanism. Importantly, L11-mediated c-myc mRNA decay is required for c-Myc downregulation in response to ribosomal stress.
Exposure of cells to external or intrinsic insults, if not properly dealt with, can lead to genomic instability. p53-dependent cell cycle checkpoints play a key role in maintaining genomic instability in response to diverse stressors (63, 64). Recent studies have shown that deregulated overactivation of c-Myc induces genomic instability, which could contribute to its oncogenic potential (27, 41, 49). For this reason, c-Myc should be tightly controlled under stress conditions. Indeed, it has recently been shown that c-Myc levels are reduced in cells following DNA damage (6, 7, 48). However, whether and how c-Myc is regulated in response to ribosomal stress are not clear. Treatment of cells with a low dose of Act D or 5-FU suppresses ribosomal biogenesis and causes ribosomal stress, leading to p53 activation via enhancing RP-MDM2 interaction (4, 14, 18, 57). Here, we show for the first time that c-Myc is significantly reduced at mRNA levels following ribosomal stress mediated by treatment with Act D or 5-FU, implying that c-Myc downregulation might also be an important cellular response following ribosomal stress. Failure of that response might contribute to genomic instability and tumorigenesis.
Accumulating data suggest that miRNAs play a crucial role in gene regulation in response to stress (35). However, most studies have focused on the changes in miRNA levels or the molecular ratio of miRNAs to target mRNAs following stress (35). Certain RNA-binding proteins (RBPs) can modulate the function of miRNAs. For example, TTP promotes tumor necrosis factor alpha (TNF-α) mRNA decay caused by miR-16 (29) and HuR facilitates the targeting of c-myc mRNA by let-7 (32). Conversely, dead end 1 (Dnd1) suppresses miRNA access to target mRNAs (31). However, whether RBPs regulate miRNA functions in response to stress has not been investigated as fully. Bhattacharyya et al. (5) showed that HuR interacts with cationic amino acid transporter 1 (CAT-1) mRNA and relieves its translational repression by miR-122 following amino acid starvation. Here, we report for the first time that L11 plays a key role in miR-24-mediated c-myc mRNA decay in response to ribosomal stress. Treatment of cells with Act D or 5-FU markedly increased the binding of L11 and Ago2 to the c-myc mRNA as well as the association of L11 with Ago2 and miR-24 (Fig. 6). L11 is required for the increased binding of Ago2 to c-myc mRNA following the treatments described above (Fig. 6C). Knockdown of either L11 or Ago2 abolished c-myc mRNA reduction in response to the treatments described above (Fig. 5). Interestingly, binding of HuR to either c-myc mRNA or let-7b was not increased in response to treatment with Act D or 5-FU (data not shown). Thus, ribosomal stress might trigger a specific L11-mediated c-myc mRNA decay pathway involving the miR-24-loaded miRISC.
How L11 recruits miRISC to the c-myc mRNA is currently not clear. Ago2 is a core component of miRISC (47, 61) and directly interacts with mature miRNAs. L11 interacts with Ago2 in cells, but this interaction requires RNA. Thus, it is likely that by binding to miR-24 and c-myc mRNA, L11 may act as an accessory factor to facilitate miRISC loading onto c-myc mRNA or to stabilize the miR-24-loaded miRISC-c-myc mRNA complex. It is also likely that L11 binding changes the c-myc 3′-UTR into a conformation that favors its targeting by miRISC. Another unanswered question is how L11 specifically associates with miR-24 among the tested miRNAs that target c-myc mRNA (Fig. 4A and B). miR-24 seems to strongly target a “seedless” sequence (nt 447 to 468) at the 3′ end of the c-myc 3′-UTR, although it also targets an additional site (nt 255 to 276) (34). Our mapping results showed that L11 binds to the 3′ end of the c-myc 3′-UTR (Fig. 2E), suggesting that L11 may change the local 3′-UTR conformation to expose the miR-24 binding region and facilitate the miR-24 targeting of the c-myc 3′-UTR. Otherwise, L11-miRNA sequence-specific interactions may contribute to this specificity. Further studies that aim to identify other L11-associated miRNAs that target c-myc are planned.
miR-24 has recently been shown to block the G1/S transition by targeting the cell cycle regulatory network, among the components of which c-Myc is one of the key direct targets of miR-24 (34). Consistent with these results, miR-24 is upregulated during terminal differentiation in diverse cell types. miR-24 targets c-myc mRNA through binding to several seedless 3′-UTR miRNA recognition elements. This miRNA-target mRNA pairing, though imperfect, could still lead to c-myc mRNA decay (34). In fact, it has recently been shown that mRNA degradation contributes to most (>84%) of the miRNA-mediated gene silencing effect in mammalian cells (26). Our data support the notion of a role for miR-24 in downregulating c-myc mRNA levels and negatively regulating cell cycle progression. It should be interesting to examine whether ribosomal stress can elicit L11- and miR-24-dependent suppression of other genes in the cell cycle regulatory network in addition to c-myc.
Our further data revealed that ribosome-free L11 binds to c-myc mRNA in the cytoplasm. First, cytoplasmic but not nuclear L11 bound to c-myc mRNA in cell fractionation assays (Fig. 7A). Second, free L11 from nonribosome supernatants bound to c-myc mRNA efficiently in our sucrose centrifugation assays (Fig. 7D). Third, L11 binds to c-myc mRNA efficiently when the 80S ribosomes and polysomes are disrupted, as shown by adding EDTA into cell lysates (Fig. 7F). Furthermore, ribosomal stress induces the release of nucleolar L11 into both the cytoplasm and the nucleus (Fig. 7C) and enhances the free L11 (Fig. 7E) binding to c-myc mRNA in the cytoplasm (Fig. 7B). It is therefore likely that ribosome-free L11 plays a dual role in regulating c-Myc: suppressing c-Myc transactivation activity in the nucleus (12) and promoting c-myc mRNA decay by recruiting miRISC to the c-myc 3′-UTR in the cytoplasm (Fig. 9). These two effects are mutually exclusive, as our sequential RNA-IP assays showed that c-Myc protein-associated L11 does not bind to c-myc mRNA (Fig. 7H). These results also exclude the possibility that L11 binding to c-myc mRNA occurs through binding to the nascent translating c-Myc protein.
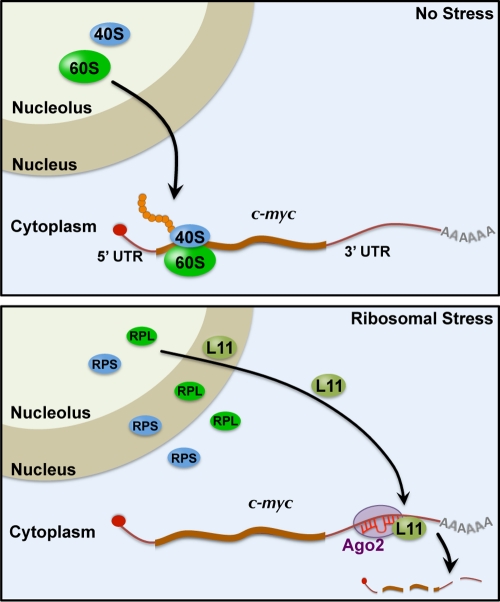
Fig. 9.
Schematic model of L11 regulation of c-myc mRNA decay via miRISC in response to ribosomal stress. Under normal conditions (top panel), the 40S and 60S mature ribosome subunits are assembled in the nucleolus and transported into the cytoplasm to mediate translation of c-myc mRNA. Upon perturbation of ribosomal biogenesis (ribosomal stress; bottom panel), individual small (RPS) and large (RPL) ribosomal proteins, including L11, are released from the nucleolus into the nucleoplasm, where L11 binds to c-Myc protein and suppresses its transactivation activity (not shown), and into the cytoplasm, where L11 recruits miR-24-loaded miRISC to the c-myc 3′-UTR, leading to c-myc mRNA decay.
Interestingly, the regulation of c-myc mRNA by L11 is specific to L11, as neither knockdown nor overexpression of other tested RPs, including L23, L26, L29, and S12 (Fig. 8A and B and data not shown), changed the levels of c-myc mRNA and protein. Unlike L11, neither L23 nor L29 associated with the c-myc mRNA (Fig. 8C). Thus, not all individual RPs would regulate c-myc mRNA. On the other hand, L11 regulation of c-myc mRNA is also specific to c-myc mRNA and not a general effect on global mRNA turnover, as knockdown of L11 did not affect the levels of several tested mRNAs (Fig. 8D). In particular, and in similarity to the results seen with c-myc mRNA, c-fos and c-jun are labile mRNAs that contain AREs in their 3′-UTR (8, 9, 46). However, neither of the mRNAs exhibited an increased level upon knockdown of L11. Although we cannot rule out the possibility that other untested RPs may regulate c-myc mRNA or that L11 may regulate other untested mRNAs, our data strongly suggest a key role for L11 in controlling c-Myc levels (and thus activity during normal homeostasis as well as in response to stress).
We have previously shown that L11 suppresses c-Myc-driven target gene transcription mediated by all three RNA polymerases and cell proliferation whereas transient knockdown of L11 enhances these c-Myc activities (12, 17). Also, knockdown of L11 attenuates ribosomal stress-induced cell cycle arrest by preventing p53 activation (4, 16, 57–59). However, prolonged ablation of L11 would eventually impair ribosomal biogenesis and cause cell growth arrest. In that case, upregulated c-Myc could cause replicative stress or some other form of metabolic stress and lead to cell cycle exit from and/or cell death in the S phase. Supporting this notion, mutations in the L11 gene have been reported in patients with Diamond-blackfan anemia, an inherited cancer-related disease characterized by defective erythropoiesis and developmental defects (11, 22). Future studies would aim to test this hypothesis.
In summary, our current observations, together with the results of previous studies (12, 17), suggest that L11, whose transcription is induced by c-Myc (12, 38), tightly coordinates c-Myc activity and levels with ribosomal biogenesis, forming an elegant feedback regulatory loop. Conceivably, when ribosomal biogenesis is overactive following, for example, overexpression of oncogenes, including c-Myc, L11 is induced and may subsequently target c-Myc to maintain normal homeostatic levels of c-Myc in cells. Together, our data here reveal a critical role for L11 in controlling c-Myc levels in response to ribosomal stress. Previous studies have firmly established the role for L11 in transmitting ribosomal stress to p53 signaling (68). Thus, L11 acts as a key ribosomal “stress sensor” in monitoring the integrity of ribosomal biogenesis by activating both p53 and suppressing c-Myc signals.
ACKNOWLEDGMENTS
This work was supported by NIH/NCI grant R00CA127134, a grant from Department of Defense (Peer Reviewed Cancer Research Program) under award number W81XWH-10-1-1029, and the startup fund from Oregon Health & Science University to M.-S.D. M.-S.D. is a recipient of Cancer Research Development Award from the OHSU Knight Cancer Institute.
Views and opinions of and endorsements by the author(s) do not reflect those of the U.S. Army or the Department of Defense.
Footnotes
Published ahead of print on 1 August 2011.
REFERENCES
- 1. Adhikary S., Eilers M. 2005. Transcriptional regulation and transformation by Myc proteins. Nat. Rev. Mol. Cell Biol. 6:635–645 [DOI] [PubMed] [Google Scholar]
- 2. Andersen J. S., et al. 2002. Directed proteomic analysis of the human nucleolus. Curr. Biol. 12:1–11 [DOI] [PubMed] [Google Scholar]
- 3. Arabi A., et al. 2005. c-Myc associates with ribosomal DNA and activates RNA polymerase I transcription. Nat. Cell Biol. 7:303–310 [DOI] [PubMed] [Google Scholar]
- 4. Bhat K. P., Itahana K., Jin A., Zhang Y. 2004. Essential role of ribosomal protein L11 in mediating growth inhibition-induced p53 activation. EMBO J. 23:2402–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhattacharyya S. N., Habermacher R., Martine U., Closs E. I., Filipowicz W. 2006. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125:1111–1124 [DOI] [PubMed] [Google Scholar]
- 6. Britton S., Salles B., Calsou P. 2008. c-MYC protein is degraded in response to UV irradiation. Cell Cycle 7:63–70 [DOI] [PubMed] [Google Scholar]
- 7. Cannell I. G., et al. 2010. p38 MAPK/MK2-mediated induction of miR-34c following DNA damage prevents Myc-dependent DNA replication. Proc. Natl. Acad. Sci. U. S. A. 107:5375–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen C. Y., Shyu A. B. 1995. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem. Sci. 20:465–470 [DOI] [PubMed] [Google Scholar]
- 9. Chen C. Y., Xu N., Shyu A. B. 1995. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol. Cell. Biol. 15:5777–5788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen D., et al. 2007. Ribosomal protein S7 as a novel modulator of p53-MDM2 interaction: binding to MDM2, stabilization of p53 protein, and activation of p53 function. Oncogene 26:5029–5037 [DOI] [PubMed] [Google Scholar]
- 11. Cmejla R., et al. 2009. Identification of mutations in the ribosomal protein L5 (RPL5) and ribosomal protein L11 (RPL11) genes in Czech patients with Diamond-Blackfan anemia. Hum. Mutat. 30:321–327 [DOI] [PubMed] [Google Scholar]
- 12. Dai M. S., Arnold H., Sun X. X., Sears R., Lu H. 2007. Inhibition of c-Myc activity by ribosomal protein L11. EMBO J. 26:3332–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dai M. S., Lu H. 2008. Crosstalk between c-Myc and ribosome in ribosomal biogenesis and cancer. J. Cell. Biochem. 105:670–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai M. S., Lu H. 2004. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J. Biol. Chem. 279:44475–44482 [DOI] [PubMed] [Google Scholar]
- 15. Dai M. S., Sears R., Lu H. 2007. Feedback regulation of c-Myc by ribosomal protein L11. Cell Cycle 6:2735–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai M. S., Sun X. X., Lu H. 2008. Aberrant expression of nucleostemin activates p53 and induces cell cycle arrest via inhibition of MDM2. Mol. Cell. Biol. 28:4365–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dai M. S., Sun X. X., Lu H. 2010. Ribosomal protein L11 associates with c-Myc at 5 S rRNA and tRNA genes and regulates their expression. J. Biol. Chem. 285:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dai M. S., et al. 2004. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol. Cell. Biol. 24:7654–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dani C., et al. 1985. Increased rate of degradation of c-myc mRNA in interferon-treated Daudi cells. Proc. Natl. Acad. Sci. U. S. A. 82:4896–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fukuchi-Shimogori T., et al. 1997. Malignant transformation by overproduction of translation initiation factor eIF4G. Cancer Res. 57:5041–5044 [PubMed] [Google Scholar]
- 21. Fumagalli S., et al. 2009. Absence of nucleolar disruption after impairment of 40S ribosome biogenesis reveals an rpL11-translation-dependent mechanism of p53 induction. Nat. Cell Biol. 11:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gazda H. T., et al. 2008. Ribosomal protein L5 and L11 mutations are associated with cleft palate and abnormal thumbs in Diamond-Blackfan anemia patients. Am. J. Hum. Genet. 83:769–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez-Roman N., Grandori C., Eisenman R. N., White R. J. 2003. Direct activation of RNA polymerase III transcription by c-Myc. Nature 421:290–294 [DOI] [PubMed] [Google Scholar]
- 24. Grandori C., et al. 2005. c-Myc binds to human ribosomal DNA and stimulates transcription of rRNA genes by RNA polymerase I. Nat. Cell Biol. 7:311–318 [DOI] [PubMed] [Google Scholar]
- 25. Grewal S. S., Li L., Orian A., Eisenman R. N., Edgar B. A. 2005. Myc-dependent regulation of rRNA synthesis during Drosophila development. Nat. Cell Biol. 7:295–302 [DOI] [PubMed] [Google Scholar]
- 26. Guo H., Ingolia N. T., Weissman J. S., Bartel D. P. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herold S., Herkert B., Eilers M. 2009. Facilitating replication under stress: an oncogenic function of MYC? Nat. Rev. Cancer 9:441–444 [DOI] [PubMed] [Google Scholar]
- 28. Jin A., Itahana K., O'Keefe K., Zhang Y. 2004. Inhibition of HDM2 and activation of p53 by ribosomal protein L23. Mol. Cell. Biol. 24:7669–7680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jing Q., et al. 2005. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120:623–634 [DOI] [PubMed] [Google Scholar]
- 30. Jones T. R., Cole M. D. 1987. Rapid cytoplasmic turnover of c-myc mRNA: requirement of the 3′ untranslated sequences. Mol. Cell. Biol. 7:4513–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kedde M., et al. 2007. RNA-binding protein Dnd1 inhibits microRNA access to target mRNA. Cell 131:1273–1286 [DOI] [PubMed] [Google Scholar]
- 32. Kim H. H., et al. 2009. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 23:1743–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krol J., Loedige I., Filipowicz W. 2010. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 11:597–610 [DOI] [PubMed] [Google Scholar]
- 34. Lal A., et al. 2009. miR-24 inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to “seedless” 3′UTR microRNA recognition elements. Mol. Cell 35:610–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leung A. K., Sharp P. A. 2010. MicroRNA functions in stress responses. Mol. Cell 40:205–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lohrum M. A., Ludwig R. L., Kubbutat M. H., Hanlon M., Vousden K. H. 2003. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell 3:577–587 [DOI] [PubMed] [Google Scholar]
- 37. Lópezde Silanes I., et al. 2005. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol. Cell. Biol. 25:9520–9531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Macias E., et al. 2010. An ARF-independent c-MYC-activated tumor suppression pathway mediated by ribosomal protein-Mdm2 interaction. Cancer Cell 18:231–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Marderosian M., et al. 2006. Tristetraprolin regulates Cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling. Oncogene 25:6277–6290 [DOI] [PubMed] [Google Scholar]
- 40. Marshall L., Kenneth N. S., White R. J. 2008. Elevated tRNA(iMet) synthesis can drive cell proliferation and oncogenic transformation. Cell 133:78–89 [DOI] [PubMed] [Google Scholar]
- 41. Meyer N., Penn L. Z. 2008. Reflecting on 25 years with MYC. Nat. Rev. Cancer 8:976–990 [DOI] [PubMed] [Google Scholar]
- 42. Mukhopadhyay R., et al. 2008. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol. Cell 32:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nesbit C. E., Tersak J. M., Prochownik E. V. 1999. MYC oncogenes and human neoplastic disease. Oncogene 18:3004–3016 [DOI] [PubMed] [Google Scholar]
- 44. Ofir-Rosenfeld Y., Boggs K., Michael D., Kastan M. B., Oren M. 2008. Mdm2 regulates p53 mRNA translation through inhibitory interactions with ribosomal protein L26. Mol. Cell 32:180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pelengaris S., Khan M., Evan G. 2002. c-MYC: more than just a matter of life and death. Nat. Rev. Cancer 2:764–776 [DOI] [PubMed] [Google Scholar]
- 46. Peng S. S., Chen C. Y., Shyu A. B. 1996. Functional characterization of a non-AUUUA AU-rich element from the c-jun proto-oncogene mRNA: evidence for a novel class of AU-rich elements. Mol. Cell. Biol. 16:1490–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peters L., Meister G. 2007. Argonaute proteins: mediators of RNA silencing. Mol. Cell 26:611–623 [DOI] [PubMed] [Google Scholar]
- 48. Popov N., Herold S., Llamazares M., Schulein C., Eilers M. 2007. Fbw7 and Usp28 regulate myc protein stability in response to DNA damage. Cell Cycle 6:2327–2331 [DOI] [PubMed] [Google Scholar]
- 49. Prochownik E. V. 2008. c-Myc: linking transformation and genomic instability. Curr. Mol. Med. 8:446–458 [DOI] [PubMed] [Google Scholar]
- 50. Rodnina M. V., Wintermeyer W. 2009. Recent mechanistic insights into eukaryotic ribosomes. Curr. Opin. Cell Biol. 21:435–443 [DOI] [PubMed] [Google Scholar]
- 51. Röther S., Strasser K. 2007. The RNA polymerase II CTD kinase Ctk1 functions in translation elongation. Genes Dev. 21:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rubbi C. P., Milner J. 2003. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. EMBO J. 22:6068–6077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruggero D., et al. 2004. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat. Med. 10:484–486 [DOI] [PubMed] [Google Scholar]
- 54. Ruggero D., Pandolfi P. P. 2003. Does the ribosome translate cancer? Nat. Rev. Cancer 3:179–192 [DOI] [PubMed] [Google Scholar]
- 55. Sachdeva M., et al. 2009. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. U. S. A. 106:3207–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Strezoska Z., Pestov D. G., Lau L. F. 2000. Bop1 is a mouse WD40 repeat nucleolar protein involved in 28S and 5. 8S RRNA processing and 60S ribosome biogenesis. Mol. Cell. Biol. 20:5516–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun X. X., Dai M. S., Lu H. 2007. 5-Fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J. Biol. Chem. 282:8052–8059 [DOI] [PubMed] [Google Scholar]
- 58. Sun X. X., Dai M. S., Lu H. 2008. Mycophenolic acid activation of p53 requires ribosomal proteins L5 and L11. J. Biol. Chem. 283:12387–12392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sun X. X., Wang Y. G., Xirodimas D. P., Dai M. S. 2010. Perturbation of 60S ribosomal biogenesis results in ribosomal protein L5 and L11-dependent p53 activation. J. Biol. Chem. 285:25812–25821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tenenbaum S. A., Lager P. J., Carson C. C., Keene J. D. 2002. Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods 26:191–198 [DOI] [PubMed] [Google Scholar]
- 61. Tolia N. H., Joshua-Tor L. 2007. Slicer and the argonautes. Nat. Chem. Biol. 3:36–43 [DOI] [PubMed] [Google Scholar]
- 62. van Riggelen J., Yetil A., Felsher D. W. 2010. MYC as a regulator of ribosome biogenesis and protein synthesis. Nat. Rev. Cancer 10:301–309 [DOI] [PubMed] [Google Scholar]
- 63. Vogelstein B., Lane D., Levine A. J. 2000. Surfing the p53 network. Nature 408:307–310 [DOI] [PubMed] [Google Scholar]
- 64. Vousden K. H., Prives C. 2009. Blinded by the light: the growing complexity of p53. Cell 137:413–431 [DOI] [PubMed] [Google Scholar]
- 65. Yuan X., et al. 2005. Genetic inactivation of the transcription factor TIF-IA leads to nucleolar disruption, cell cycle arrest, and p53-mediated apoptosis. Mol. Cell 19:77–87 [DOI] [PubMed] [Google Scholar]
- 66. Zhang L., Pan X., Hershey J. W. 2007. Individual overexpression of five subunits of human translation initiation factor eIF3 promotes malignant transformation of immortal fibroblast cells. J. Biol. Chem. 282:5790–5800 [DOI] [PubMed] [Google Scholar]
- 67. Zhang W., et al. 1993. Purification, characterization, and cDNA cloning of an AU-rich element RNA-binding protein, AUF1. Mol. Cell. Biol. 13:7652–7665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang Y., Lu H. 2009. Signaling to p53: ribosomal proteins find their way. Cancer Cell 16:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y., et al. 2003. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol. Cell. Biol. 23:8902–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu Y., et al. 2009. Ribosomal protein S7 is both a regulator and a substrate of MDM2. Mol. Cell 35:316–326 [DOI] [PMC free article] [PubMed] [Google Scholar]