Abstract
Background
Mitral regurgitation (MR) has been associated with adverse outcomes after myocardial infarction (MI). Without structural valve disease, functional MR has been related to left ventricular (LV) remodeling and geometric deformation of the mitral apparatus. The aims of this study were to elucidate the mechanistic components of MR after high-risk MI and to identify predictors of MR progression during follow-up.
Methods
The Valsartan in Acute Myocardial Infarction Echo substudy prospectively enrolled 610 patients with LV dysfunction, heart failure, or both after MI. MR at baseline, 1 month, and 20 months was quantified by mapping jet expansion in the left atrium in 341 patients with good-quality echocardiograms. Indices of LV remodeling, left atrial size, and diastolic function and parameters of mitral valve deformation, including tenting area, coaptation depth, anterior leaflet concavity, annular diameters, and contractility, were assessed and related to baseline MR. The progression of MR was further analyzed, and predictors of worsening among the baseline characteristics were identified.
Results
Tenting area, coaptation depth, annular dilatation, and left atrial size were all associated with the degree of baseline MR. Tenting area was the only significant and independent predictor of worsening MR; a tenting area of 4 cm2 was a useful cutoff to identify worsening of MR after MI and moderate to severe MR after 20 months.
Conclusions
Increased mitral tenting and larger mitral annular area are determinants of MR degree at baseline, and tenting area is an independent predictor of progression of MR after MI. Although LV remodeling itself contributes to ischemic MR, this influence is directly dependent on alterations in mitral geometry.
Keywords: Mitral regurgitation, Myocardial infarction, Mitral geometry, Tenting area, Left ventricular remodeling
Mitral regurgitation (MR) is a powerful predictor of adverse prognosis after myocardial infarction (MI) with increased risk for death and heart failure and provides incremental prognostic information over conventional known clinical and echocardiographic predictors of risk after MI.1–5 Moreover, progression of MR in the chronic phase after MI is associated with an increased likelihood of cardiovascular morbidity.1 Although MR can be caused by discrete papillary muscle disruption after MI, this occurs rarely.6 Rather, the majority of cases of post-MI MR develop in the absence of structural mitral disease and are caused as a result of apical and posterior papillary muscle (PPM) displacement with consequent mitral valve tethering and incomplete valve closure due to the restriction of leaflet motion, resulting in functional MR.7–9 In addition, dilatation and loss of contraction of the mitral annulus contribute to increased valvular malcoaptation, therefore increasing the severity of MR.10,11
To understand the mechanistic components and the echocardiographic determinants of baseline MR as well as the progression of MR during follow-up after MI, we studied patients enrolled in the Valsartan in Acute Myocardial Infarction (VALIANT) Echo substudy. We hypothesized that the effects of left ventricular (LV) remodeling on mitral geometry deformation are the dominant factors underlying the genesis of post-MI MR and its evolution.
METHODS
Study Population
VALIANT was designed to test the efficacy and safety of long-term treatment with valsartan, captopril, and their combination after acute MI (between 0.5 and 10 days previously) complicated by clinical or radiologic signs of heart failure, evidence of LV systolic dysfunction (ejection fraction [EF] ≤ 0.35 on echocardiography or contrast angiography and ≤ 0.40 on radionuclide ventriculography), or both. The main criteria for exclusion were a previous intolerance or contraindication to an angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker and another disease known to limit life expectancy severely.12 A total of 14,703 patients were eligible for this study.
The VALIANT Echo substudy was designed prospectively to test the hypothesis that valsartan, either alone or in combination with captopril, could attenuate progressive LV enlargement or improve LV function to a greater extent than captopril alone. Entry criteria were identical to those for the main VALIANT study. All the clinical sites participating in the main VALIANT study were invited to enroll patients in the VALIANT Echo substudy. From 94 clinical sites in 13 different countries, a total of 610 echocardiographic studies were sent to the core laboratory at Brigham and Women’s Hospital, where they were reviewed for quality.13 Baseline characteristics of patients enrolled in the VALIANT Echo substudy were similar to those who were not, as previously reported.13 Patients with echocardiographic images of insufficient quality, absence of color-flow Doppler images, or color-flow Doppler images of insufficient quality were excluded from the VALIANT MR Echo cohort, and the final population consisted of 496 patients. For 341 of these patients, it was possible to prospectively evaluate the evolution of MR after 20 months. Of the others, 84 patients died, and for 71 patients, the 20-month echocardiographic study was not available or had MR color-flow Doppler images of insufficient quality.
Echocardiographic examinations were performed at a mean of 4.9 ± 2.5 days after MI (before randomization), at 1 month, and at 20 months. The median follow-up time was 24.7 months. The majority of patients were hemodynamically stable at the time of the echocardiographic assessment. Except for minor differences, the VALIANT MR Echo cohort was similar to the overall VALIANT cohort.1 Patients provided informed consent for inclusion in the VALIANT Echo substudy, and the protocol was approved by the appropriate institutional review boards.
Baseline Clinical Characteristics
The following baseline clinical characteristics were analyzed in the VALIANT MR Echo cohort: age; race; gender; blood pressure; heart rate; body mass index; Killip class; history of MI, hypertension, diabetes, heart failure, or smoking; anterior or inferior MI site; Q-wave or non Q-wave MI type; reperfusion therapy with thrombolysis or primary percutaneous transluminal coronary intervention; previous therapy with angiotensin-converting enzyme inhibitors; and treatment with aspirin, β-blockers, or statins at randomization.1,13
Echocardiographic Analysis
All echocardiographic studies were analyzed in the core laboratory at Brigham and Women’s Hospital. Echocardiograms from videotape were digitized, and analyses were performed with the use of quantitative analysis software.
LV endocardial borders were manually traced at end-diastole and end-systole in apical four-chamber and two-chamber views. LV volumes and EF were derived according to the modified biplane Simpson’s method.13,14 The LV sphericity indices were calculated as the ratio of LV volumes (end-diastolic and end-systolic) and the volume of a sphere with a diameter equal to the LV (end-diastolic and end-systolic) long axis (sphericity index = 6V/πL3).14 LV volumes and sphericity indices were considered parameters of global LV remodeling.
MR was assessed using a semiquantitative method tracing the area of the maximum systolic jet expansion in the left atrium in four-chamber and two-chamber views. Left atrial (LA) area was also measured in the same frame with the maximum regurgitant jet. According to current guidelines, color-flow Doppler images of regurgitant jet were acquired using a Nyquist limit (aliasing velocity) of 50 to 60 cm/sec and a color gain that just eliminated random color speckle from nonmoving regions. All the color-flow Doppler images used the same color map (red for flow toward the transducer and blue for flow away from the transducer, with the shade of color indicating velocity up to the Nyquist limit). The MR jet comprised a mosaic of many colors indicating turbulence. The protocol specified that the same ultrasound instruments, transducers, and settings of the baseline acquisition should be used for the follow-up examinations.
MR was then categorized by calculating MR jet/LA area ratio. MR was considered mild when the regurgitant jet area occupied >5% and <20% of LA area, moderate when regurgitant jet area occupied >20% and <40% of LA area, and severe when regurgitant jet area occupied >40% of LA area.1,15,16 The presence of an eccentric jet raised the grade of MR by one degree on the basis of evidence of reduced color-flow jet areas due to a loss of momentum in jets adjacent to chamber walls.17,18 An eccentric jet was defined as a regurgitant jet, which impinges on the LA wall immediately beyond the mitral valve.
The following indices of geometric mitral valve deformation were measured from parasternal long-axis view (Figure 1): tenting area, defined as the area enclosed between the annular plane and the mitral leaflets in mid-systole; coaptation depth, defined as the distance between leaflet coaptation and the mitral annular (MA) plane in mid-systole7–9; anterior mitral leaflet (AML) shape, visually assessed as concave or convex configuration of the leaflet toward the left atrium in mid-systole (with convexity representing the normal condition and concavity an expression of tethering of mitral valve leaflets)19; and AML diastolic restricted motion, defined as maximal opening angle between the AML and the annulus equal or inferior to the angle between the annulus and a line connecting the PPM head and intervalvular fibrosa.20 Moreover, maximum and minimum MA diameters were measured in four-chamber and two-chamber views at end-diastole and end-systole, and MA areas were calculated using an ellipsoid assumption (MA area = d1 × d2 × π/4); MA contraction was calculated as the ratio of the difference between MA end-diastolic area and MA end-systolic area to MA end-diastolic area.1,8,21
Figure 1.
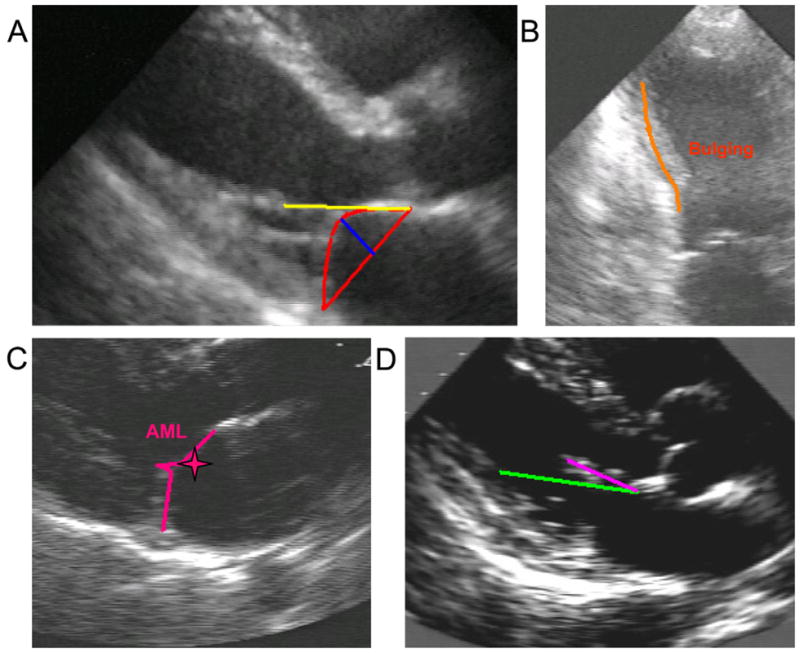
Indices of geometric mitral valve deformation and LV remodeling. (A) Tenting area (red triangle), coaptation depth (blue line), MA diameter (basis of the red triangle), and annular-papillary distance (yellow line). (B) Bulging of inferior wall (orange line). The PPM attachment was outwardly displaced because of bulging of the inferior wall after necrosis. (C) Convexity of the AML toward the left atrium in mid-systole (pink star). (D) AML diastolic normal motion. The AML (pink line) in diastole moves forward the green line connecting PPM head and intervalvular fibrosa.
Outward displacement of the PPM, an index of global and local LV remodeling, was quantified by annular-papillary distance, defined as the distance between the PPM head and intervalvular fibrosa in the parasternal long-axis view in systole.8 Local remodeling of the LV wall at the PPM attachment was evaluated by the presence of thinning or bulging of inferior wall.22
LA volume was measured by the biplane area-length method from apical four-chamber and two-chamber views at end-systole, and LA volume was indexed to body surface area.23 Diastolic function was evaluated by transmitral pulsed-wave Doppler from the apical four-chamber view, and a restrictive pattern was defined as E/A peak wave ratio > 2 and deceleration time < 140 msec.24 All the echocardiographic measurements were repeated in three separate cardiac cycles.
Statistical Analysis
Baseline continuous data are expressed as mean ± SD, and categorical variables are expressed as absolute numbers of patients and percentages. At baseline, patients were divided into three groups according to MR degree (none, mild, and moderate to severe), and a nonparametric test derived from Wilcoxon’s rank-sum test was used to examine trends in baseline clinical and echocardiographic characteristics. During 20 months of follow-up, the patients were divided into a group with no MR changes or improvement and a group with MR increase of at least one grade; clinical and echocardiographic characteristics in these two groups were compared using t tests for continuous variables and χ2 tests for discrete variables. Backward and forward stepwise multivariate linear regression analysis was performed to assess the baseline predictors of baseline MR jet/LA area ratio and the overall change in MR jet/LA area ratio, using two different models. The first model considered only echocardiographic variables, while the second one added the clinical variables that were significant by univariate analyses to echocardiographic variables. The relationship between baseline tenting area and MR severity at baseline and after 20 months was tested using a logistic regression. The cutoff value of tenting area that was most sensitive and specific in predicting MR was determined by means of receiver operating characteristic curve analysis. Change in MR jet/LA area was compared with changes in LV and LA remodeling over time using Pearson’s correlations.
Intraobserver reproducibility was tested in 15% of patients randomly selected, in a blinded fashion. The mean differences and the SD of the differences between measurements were calculated (Bland-Altman method), and variability was calculated as the SD of the difference divided by the mean measurement.13 The overall coefficients of variability of the continuous echocardiographic parameters were as follows: MR area, 1%; LA volume index, 5.4%; LV end-diastolic volume, 8.3%; diastolic sphericity index, 6.2%; annular-papillary distance, 7.2%; tenting area, 6%; coaptation depth, 6.4%; and MA diastolic area, 5.5%. All P values were two sided; P values < .05 were considered statistically significant. All statistical analyses were performed using Stata version 8 (StataCorp LP, College Station, TX).
RESULTS
Baseline MR
Among the 496 patients with baseline echocardiographic evaluations of MR, 231 patients (46%) did not have MR, 202 patients (41%) had mild MR, and 63 patients (13%) had moderate to severe MR. MR severity at baseline was associated with older age (P < .001), female sex (P < .001), prior MI (P < .01), hypertension (P = .02), diabetes (P < .01), heart failure (P = .001), and non-Q-wave MI (P = .01).1
Echocardiographic measures of ventricular and mitral geometry associated with MR degree are shown in Table 1. Patients with higher MR degrees had worse LV global remodeling, with higher LV volumes and sphericity indices and lower EFs1; moreover, they had greater annular-papillary distances and were more likely to have worse local remodeling of the inferior wall. MR severity was also associated with increased geometric deformation of the mitral apparatus (higher tenting area, coaptation depth, and MA areas with lower MA contraction; presence of diastolic restricted motion and concavity of the AML). Higher MR degree was associated with larger left atria and restrictive filling. Twenty-five patients presented at baseline with eccentric jets. Considering only the 471 patients (95%) with central jets, all the considered echocardiographic parameters were still significant determinants of higher MR degree, with the exception of a restrictive diastolic pattern and restrictive motion of the mitral leaflet.
Table 1.
Echocardiographic characteristics according to baseline MR degree (n = 496)
| Variable | Baseline MR degree | P for trend | ||
|---|---|---|---|---|
| None or trace (n = 231 [46%]) | Mild (n = 202 [41%]) | Moderate to severe (n = 63 [13%]) | ||
| LV remodeling | ||||
| LV EDV (mL) | 112 ± 28 | 123 ± 29 | 139 ± 43 | <.001 |
| LV ESV (mL) | 67 ± 20 | 77 ± 23 | 88 ± 33 | <.001 |
| LV EF (%) | 40 ± 5 | 38 ± 6 | 38 ± 7 | .001 |
| Sphericity index (%) | ||||
| Diastolic | 42 ± 10 | 47 ± 10 | 50 ± 10 | <.001 |
| Systolic | 36 ± 10 | 40 ± 11 | 43 ± 9 | <.001 |
| Annular-papillary distance (cm) | 3.8 ± 0.6 | 4.1 ± 0.6 | 4.1 ± 0.5 | .028 |
| Thinning-bulging of inferior wall | 15% | 24% | 29% | .011 |
| Mitral valve geometry | ||||
| Tenting area (cm2) | 3.8 ± 0.7 | 4.2 ± 0.7 | 4.1 ± 0.8 | <.001 |
| Coaptation depth (cm) | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | .008 |
| Mitral annulus | ||||
| Diastolic area (cm2) | 7.1 ± 1 | 8.2 ± 1.2 | 9.6 ± 1.4 | <.001 |
| Systolic area (cm2) | 5.5 ± 0.9 | 6.5 ± 1.2 | 7.7 ± 1.4 | <.001 |
| Annular contraction (%) | 22 ± 6 | 21 ± 6 | 20 ± 6 | .004 |
| Concavity of AML | 31% | 35% | 56% | .003 |
| Diastolic restricted motion | 14% | 19% | 36% | .003 |
| Diastolic function and left atrium | ||||
| Restrictive pattern | 9% | 15% | 21% | .013 |
| LA volume index (mL/m2) | 20 ± 5 | 26 ± 7 | 35 ± 10 | <.001 |
EDV, End-diastolic volume; ESV, end-systolic volume.
Data are expressed as mean ± SD or as percentages.
In a multivariate model adjusted for echocardiographic variables, the degree of baseline MR was related to parameters of altered mitral valve geometry (tenting area, P = .009; coaptation depth, P = .046; MA diastolic area, P < .001) and larger left atria (P = .016); LV remodeling indices were not independently associated with baseline MR degree. By adding to the previous multivariate model the clinical variables that were significant by univariate analyses, tenting area, coaptation depth, MA diastolic area, and LA volume index remained independently associated with degree of baseline MR severity (P = .006, P = .011, P < .001, and P = .013, respectively), while female sex was the only clinical factor independently associated with MR degree (P = .006).
MR Progression
In 341 patients with completed echocardiographic follow-up, MR worsened by one degree in 78 patients (23%) and by two degrees in 10 patients (3%) at 20 months compared with baseline. Forty-seven patients (14%) experienced improvements in MR by one degree, and in 206 patients (60%), the presence and severity of MR were unchanged. At 20 months, 150 patients (44%) had no detectable MR, 139 patients (41%) had mild MR, and 52 patients (15%) had moderate to severe MR.
During the first month after MI, early MR progression was significantly greater than late progression during the remaining follow-up period (1.9 ± 0.3% vs 0.4 ± 0.3% increase in MR jet/LA area ratio during the first month and between 1 and 20 months, respectively, P < .001). Patients with worsening by one or more MR degrees during the first month after MI experienced the greatest increase in MR jet/LA area ratio during follow-up (11.4 ± 9.3% vs 0.01 ± 6.7%, P < .001) and were more likely to have moderate or greater MR degrees at 20 months (69% vs 31%, P < .001).
Among the clinical baseline characteristics, only systolic blood pressure was significantly increased in the group of patients who developed worsening in MR degree after 20 months (124 ± 15 vs 119 ± 14 mm Hg, P = .011). There was no difference in degree of MR worsening by treatment group.
Echocardiographic baseline characteristics according to worsening versus unchanged or improved MR degree after 20 months are shown in Table 2. Greater baseline tenting area and coaptation depth predicted MR progression in univariate analyses. Tenting area remained a predictor of MR progression in multivariate analyses, in models including either echocardiographic measures or a combination of echocardiographic and clinical variables (P = .023). Adding 1-month MR data to this model, both tenting area and worsening by at least one degree during the early phase after MI were significant and independent predictors (P = .018 and P < .001, respectively). Sixteen of these patients presented with eccentric jets. Considering only the 325 patients (95%) with central jets, tenting area and coaptation depth were confirmed to be predictors of worsening MR.
Table 2.
Echocardiographic characteristics according to worsening MR
| Variable | Overall population (n = 341) | No MR changes or improvement (n = 253 [74%]) | Worsening MR of at least one grade (n = 88 [26%]) | P |
|---|---|---|---|---|
| LV remodeling | ||||
| LV EDV (mL) | 118 ± 30 | 118 ± 32 | 117 ± 26 | .740 |
| LV ESV (mL) | 72 ± 24 | 72 ± 25 | 71 ± 20 | .592 |
| LV EF (%) | 39 ± 6 | 39 ± 6 | 39 ± 6 | .684 |
| Sphericity index (%) | ||||
| Diastolic | 44 ± 11 | 44 ± 10 | 45 ± 11 | .748 |
| Systolic | 38 ± 11 | 37 ± 10 | 39 ± 13 | .l28 |
| Annular-papillary distance (cm) | 3.9 ± 0.6 | 3.8 ± 0.5 | 4.0 ± 0.6 | .101 |
| Thinning-bulging of inferior wall | 18% | 19% | 16% | .331 |
| Mitral valve geometry | ||||
| Tenting area (cm2) | 3.9 ± 0.6 | 3.8 ± 0.6 | 4.2 ± 0.7 | <.001 |
| Coaptation depth (cm) | 1.6 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.2 | .032 |
| Mitral annulus | ||||
| Diastolic area (cm2) | 7.8 ± 1.3 | 7.8 ± 1.3 | 7.7 ± 1.1 | .448 |
| Systolic area (cm2) | 6.1 ± 1.2 | 6.1 ± 1.3 | 6.0 ± 1.1 | .283 |
| Annular contraction (%) | 22 ± 6 | 22 ± 6 | 22 ± 6 | .275 |
| Concavity of AML | 35% | 35% | 37% | .795 |
| Diastolic restricted motion | 18% | 17% | 21% | .459 |
| Diastolic function and left atrium | ||||
| Restrictive pattern | 13% | 14% | 9% | .250 |
| LA volume index (mL/m2) | 23 ± 7 | 23 ± 8 | 22 ± 7 | .180 |
EDV, End-diastolic volume; ESV, end-systolic volume.
Data are expressed as mean ± SD or as percentages.
LV and LA remodeling during the overall follow-up are shown in Table 3. MR worsening was significantly correlated with the increases in LV and LA volumes and with the reduction in LV function, although these correlations were weak.
Table 3.
Correlation between changes in MR jet/LA area ratio and LV and LA remodeling during 20 month follow-up
| Variable | Baseline | 20 months | Coefficient of correlation | P |
|---|---|---|---|---|
| LV EDV (mL) | 119 ± 32 | 120 ± 30 | 0.12 | .027 |
| LV ESV (mL) | 73 ± 24 | 72 ± 25 | 0.15 | .005 |
| LV EF (%) | 39 ± 6 | 41 ± 7 | −0.11 | .035 |
| Sphericity index (%) | ||||
| Diastolic | 45 ± 11 | 50 ± 11 | 0.097 | .078 |
| Systolic | 38 ± 11 | 44 ± 11 | 0.095 | .084 |
| LA volume index (mL/m2) | 24 ± 8 | 26 ± 9 | 0.49 | <.001 |
EDV, End-diastolic volume; ESV, end-systolic volume.
Tenting Area as a Predictor of MR Severity and Worsening
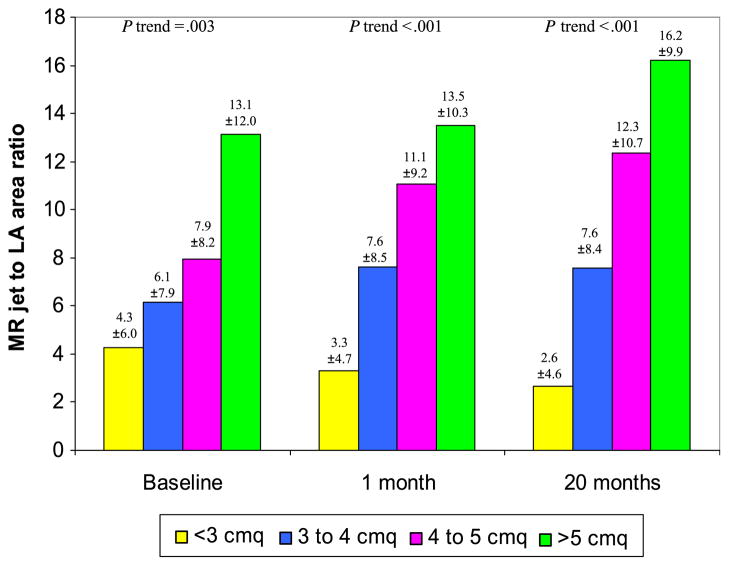
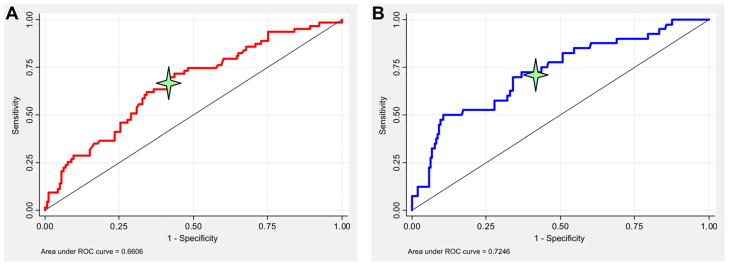
Each 1-cm2 increase in tenting area was associated with greater MR not only at baseline but also at 1 and 20 months (Figure 2). Receiver operating characteristic curve analysis demonstrated that a tenting area of 4 cm2 was the optimal cutoff in predicting MR severity (Figure 3). For each 1-cm2 increase in tenting area beyond 4 cm2, the odds ratio of worsening MR degree during follow-up was 3.60 (95% confidence interval, 1.68–7.73), and the odds ratio of having moderate or greater MR at 20 months was 5.79 (95% confidence interval, 2.70–12.39) (Figure 4).
Figure 2.
Relationship between MR severity at baseline and after 1 and 20 months and each 1-cm2 increase in baseline tenting area.
Figure 3.
Receiver operating characteristic (ROC) curve analysis showing the tenting area cutoff level distinguishing worsening MR of at least one degree from unchanged or improved MR during follow-up (A, red line) and moderate to severe MR from none to mild MR at 20 months (B, blue line).
Figure 4.
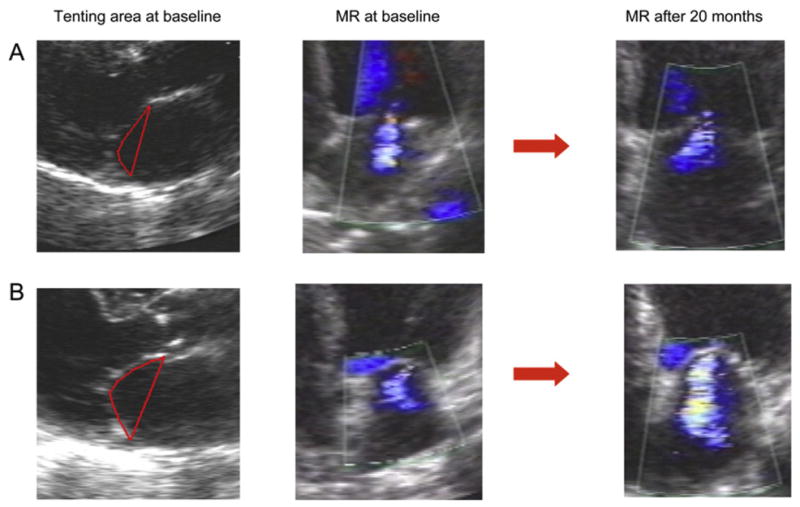
(A) A patient with mild MR at baseline and a tenting area of 3.7 cm2 who experienced no MR progression during follow-up. (B) A patient with mild MR at baseline and a tenting area of 4.5 cm2 who worsened to moderate MR at 20 months.
Discussion
We demonstrated that alterations in mitral geometry, indicated by mitral tenting, coaptation depth, and diastolic MA area, as well as LA size, were associated with degree of baseline MR after a high-risk MI. LV volume, sphericity and EF—expressions of global LV remodeling—were associated with baseline MR in univariate analyses only and were not independently related to the degree of MR in the early post-MI period or the progression of MR over time. In contrast, baseline tenting area remained an independent predictor of worsening MR over time. Therefore, mitral tenting, as a potential expression of localized remodeling affecting mitral valve apparatus, seems to be superior to the classical indices of global LV remodeling, both as determinant of baseline MR and as a predictor of MR progression.
Our results are consistent with recent studies demonstrating the central role of mitral geometric deformation in the genesis of ischemic MR7–9 and potentially provide new insights regarding echocardiographic predictors of ischemic MR evolution. The main novelty of this study is the demonstration that mitral tenting has a predictive value in identifying patients with ischemic MR in whom progression is more likely to occur during follow-up.
Functional MR, defined as abnormal mitral valve function in the absence of structural abnormalities of the mitral apparatus, is commonly observed after MI and is an independent predictor of mortality and cardiovascular morbidity.1–5,25,26 In normal hearts, papillary muscles are parallel to the LV long axis and perpendicular to the leaflets and can balance forces generated by ventricular pressure on the leaflet surface. Distortion of ventricular geometry caused by infarction displaces the papillary muscles toward the apex and the posterolateral wall, which results in a disadvantageous alignment of the papillary muscle apparatus relative to the mitral leaflets. Consequently, mitral leaflets are drawn and tethered into the ventricle and their motion is restricted from closure. Moreover, worse LV systolic function and annular contraction contribute to decrease mitral valve closing forces and increase valvular malcoaptation.7,11,27
We and others have previously shown that after high-risk MI, baseline MR is associated with worse LV function, greater LV enlargement and chamber distortion, and worse MA contraction.1,11 Global contractile dysfunction leads to functional MR only in presence of a dilated left ventricle.28 Previous studies have indicated that increased sphericity in dilated left ventricles could result in displaced papillary muscles, leading to MR.29 However, recent studies have raised the question of whether global LV remodeling represents the main determinant of post-MI MR, because similar degrees of LV dysfunction or enlargement are associated with widely different degrees of MR.8,30 Indeed, localized remodeling of the posterior walls leads to papillary muscle displacement and results in increased mitral tenting independently of LV volumes.8 Moreover, varying degrees of leaflet adaptation to LV remodeling may play a role in the development of functional MR.9,31
This study provides further confirmation of the central role of mitral geometric deformation in the genesis of ischemic MR at baseline. These new results highlight the importance of geometric changes of the mitral apparatus in the pathophysiology of post-MI MR. Indeed, we found that increased mitral tenting, coaptation depth, and MA area seem to be the main determinants of ischemic MR. Even if LV dysfunction, enlargement, and altered shape are determinants of MR, they lose their predictive power in the multivariate analyses adjusted for parameters of mitral geometry. Although geometric changes of the mitral apparatus are related to LV enlargement and dysfunction, these appear to lead to excess valvular tenting independently of global LV remodeling.
These results are consistent with the notion that mitral competence depends on the balance of different forces acting on mitral leaflets, including tethering forces generated by papillary muscle displacement, annular forces, and left ventricle–generated closing forces.7,23 The final common pathway of all these forces may be the mitral tenting determining the level of leaflet coaptation. Tethering forces acting on mitral leaflets lead to insufficient systolic leaflet body displacement toward the annulus, with coaptation limited to leaflet tips31 and consequent increase of the mitral tenting area. Annular dilatation has an adjunctive role in inadequate mitral coaptation. When the leaflet bodies can reach the annular level in systole, considerable annular dilatation would be required to result in inadequate mitral co-aptation, because the ratio of leaflets to the annular surface area is >2.32 However, enlarged MA area further increases the insufficiency of leaflet coaptation in the presence of augmented tethering. LV dilatation and function are important determinants of baseline MR after MI as well. Indeed, in a malfunctioning left ventricle, closing forces are unable to oppose tethering, and global LV remodeling influences mitral geometry.
Most prior studies have focused on MR in the early phase after MI; in the present study, we had the advantage of being able to follow patients and changes in MR over 20 months after MI. In multivariate analyses, altered mitral geometry expressed by tenting area was the only independent predictor of worsening regurgitation. Global LV dysfunction, dimensions, and sphericity were not direct predictors of changes in ischemic MR during long-term follow-up. These data suggest a central role for mitral geometric alteration on MR progression and that mitral tenting area in particular was the crucial determinant of not just early post-MI MR but of progression of MR over time. A comprehensive assessment of MR severity, mitral geometry, and LV local and global remodeling in the acute phase after MI and during the following months may improve patient risk stratification and might help identify patients who might best benefit from particular types of surgical approaches to functional MR.33
Some limitations of this study should be noted. MR degree was evaluated with a semiquantitative method, mapping regurgitant jet expansion within the left atrium by color flow Doppler. More rigorous quantitative methods such as proximal isovelocity surface area and effective regurgitant orifice area are more challenging and less likely to be possible in international, multicenter clinical trials such as VALIANT.1 Nevertheless, the mitral jet area/LA area ratio has been shown to be well correlated with quantitative methods to evaluate MR and to be a highly sensitive technique to detect even mild degrees of ischemic MR.33
Our analysis is subject to survivor bias, as many patients with worse degrees of MR and greater LV remodeling at baseline did not survive to undergo 20-month echocardiographic assessments.1,13 Therefore, the severity of MR progression may be underestimated because of patient dropout.
Mitral valve surgery was not a prespecified outcome in VALIANT, and data on this were not collected. However, only 10 patients in the VALIANT Echo cohort underwent subsequent cardiac surgery, suggesting that the number of patients who might have had mitral valve surgery is likely to be very low.23
Finally, the tenting area cutoff of 4 cm2 obtained from a receiver operating characteristic curve analysis has not been prospectively tested in an independent second population.
CONCLUSIONS
Alteration in mitral geometry, expressed by an increased tenting and dilated mitral annulus, is an independent predictor of baseline MR after high-risk MI, while tenting area also predicts worsening in MR during the following period. These data suggest that increased mitral tenting is the final pathway by which LV remodeling alters mitral geometry and thus influences the extent of regurgitation.
Acknowledgments
The VALIANT trial was funded by a grant from Novartis Pharmaceuticals Corporation (East Hanover, NJ). Drs. Køber, Velazquez, McMurray, Pfeffer, Califf, and Solomon have received research support from Novartis Pharmaceuticals Corporation.
Abbreviations
- AML
Anterior mitral leaflet
- EF
Ejection fraction
- LA
Left atrial
- LV
Left ventricular
- MI
Myocardial infarction
- MR
Mitral regurgitation
- PPM
Posterior papillary muscle
- VALIANT
Valsartan in Acute Myocardial Infarction
References
- 1.Amigoni M, Meris A, Thune JJ, Mangalat D, Skali H, Bourgoun M, et al. Mitral regurgitation in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: prognostic significance and relation to ventricular size and function. Eur Heart J. 2007;28:326–33. doi: 10.1093/eurheartj/ehl464. [DOI] [PubMed] [Google Scholar]
- 2.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, et al. Survival and Ventricular Enlargement Investigators. Clinical significance of mitral regurgitation after acute myocardial infarction. Circulation. 1997;96:827–33. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 3.Grigioni F, Enriquez-Sarano M, Zehr K, Bailey K, Tajik A. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–64. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 4.Bursi F, Enriquez-Sarano M, Nkomo VT, Jacobsen SJ, Weston SA, Meverden RA, et al. Heart failure and death after myocardial infarction in the community: the emerging role of mitral regurgitation. Circulation. 2005;11:295–301. doi: 10.1161/01.CIR.0000151097.30779.04. [DOI] [PubMed] [Google Scholar]
- 5.Grigioni F, Detaint D, Avierinos JF, Scott C, Tajik J, Enriquez-Sarano M. Contribution of ischemic mitral regurgitation to congestive heart failure after myocardial infarction. J Am Coll Cardiol. 2005;45:260–77. doi: 10.1016/j.jacc.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Uemura T, Otsuji Y, Nakashiki K, Yoshifuku S, Maki Y, Yu B, et al. Papillary muscle dysfunction attenuates ischemic mitral regurgitation in patients with localized basal inferior left ventricular remodeling: insights from tissue Doppler strain imaging. J Am Coll Cardiol. 2005;46:113–9. doi: 10.1016/j.jacc.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 7.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–58. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 8.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: A quantitative clinical study. Circulation. 2000;102:1400–6. doi: 10.1161/01.cir.102.12.1400. [DOI] [PubMed] [Google Scholar]
- 9.Silbiger JJ. Mechanistic insights into ischemic mitral regurgitation: echocardiographic and surgical implications. J Am Soc Echocardiogr. 2011;24:707–19. doi: 10.1016/j.echo.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Boltwood CM, Tei C, Wong M, Shah PM. Quantitative echocardiography of the mitral complex in dilated cardiomyopathy: the mechanism of functional mitral regurgitation. Circulation. 1983;68:498–508. doi: 10.1161/01.cir.68.3.498. [DOI] [PubMed] [Google Scholar]
- 11.Bursi F, Enriquez-Sarano M, Jacobsen SJ, Roger VL. Mitral regurgitation after myocardial infarction: a review. Am J Med. 2006;119:103–12. doi: 10.1016/j.amjmed.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. New Engl J Med. 2003;349:1893–906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 13.Solomon SD, Skali H, Anavekar NS, Bourgoun M, Barvik S, Ghali JK, et al. Changes in ventricular size and function in patients treated with valsartan, captopril, or both after myocardial infarction. Circulation. 2005;111:3411–9. doi: 10.1161/CIRCULATIONAHA.104.508093. [DOI] [PubMed] [Google Scholar]
- 14.Lamas GA, Vaughan DE, Parisi AF, Pfeffer MA. Effects of left ventricular shape and captopril therapy on exercise capacity after anterior wall acute myocardial infarction. Am J Cardiol. 1989;63:1167–73. doi: 10.1016/0002-9149(89)90173-2. [DOI] [PubMed] [Google Scholar]
- 15.Helmcke F, Nanda NC, Hsiung MC, Soto B, Adey CK, Goyal RG, et al. Color Doppler assessment of mitral regurgitation with orthogonal planes. Circulation. 1987;75:175–83. doi: 10.1161/01.cir.75.1.175. [DOI] [PubMed] [Google Scholar]
- 16.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 17.Chen CG, Thomas JD, Anconina J, Harrigan P, Mueller L, Picard MH, et al. Impact of impinging wall jet on color Doppler quantification of mitral regurgitation. Circulation. 1991;84:712–20. doi: 10.1161/01.cir.84.2.712. [DOI] [PubMed] [Google Scholar]
- 18.McCully RB, Enriquez-Sarano M, Tajik AJ, Seward JB. Overestimation of severity of ischemic/functional mitral regurgitation by color Doppler jet area. Am J Cardiol. 1994;74:790–3. doi: 10.1016/0002-9149(94)90436-7. [DOI] [PubMed] [Google Scholar]
- 19.Nesta F, Otsuji Y, Handschumacher MD, Messas E, Leavitt M, Carpentier A, et al. Leaflet concavity: a rapid visual clue to the presence and mechanism of functional mitral regurgitation. J Am Soc Echocardiogr. 2003;16:1301–8. doi: 10.1067/j.echo.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Otsuji Y, Gilon D, Jiang L, He S, Leavitt M, Roy MJ, et al. Restricted diastolic opening of the mitral leaflets in patients with left ventricular dysfunction: evidence for increased valve tethering. J Am Coll Cardiol. 1998;32:398–404. doi: 10.1016/s0735-1097(98)00237-x. [DOI] [PubMed] [Google Scholar]
- 21.Vijayaraghavan G, Boltwood CM, Tei C, Wong M, Shah PM. Simplified echocardiographic measurement of the mitral annulus. Am Heart J. 1986;112:985–91. doi: 10.1016/0002-8703(86)90310-8. [DOI] [PubMed] [Google Scholar]
- 22.Liel-Cohen N, Guerrero JL, Otsuji Y, Handschumacher MD, Rudski LG, Hunziker PR, et al. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation. 2000;101:2756–63. doi: 10.1161/01.cir.101.23.2756. [DOI] [PubMed] [Google Scholar]
- 23.Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Køber L, et al. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study. Eur Heart J. 2009;30:56–65. doi: 10.1093/eurheartj/ehn499. [DOI] [PubMed] [Google Scholar]
- 24.Pratali L, Otasevic P, Rigo F, Gherardi S, Neskovic A, Picano E. The additive prognostic value of restrictive pattern and dipyridamole-induced contractile reserve in idiopathic dilated cardiomyopathy. Eur J Heart Fail. 2005;7:844–51. doi: 10.1016/j.ejheart.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Carabello BA. The management of functional mitral regurgitation. Curr Cardiol Rep. 2007;9:112–7. doi: 10.1007/BF02938337. [DOI] [PubMed] [Google Scholar]
- 26.Otsuji Y, Handschumacher MD, Kisanuki A, Tei C, Levine RA. Functional mitral regurgitation. Cardiologia. 1998;43:1011–6. [PubMed] [Google Scholar]
- 27.Kaul S, Spotnitz WD, Glasheen WP, Touchstone DA. Mechanism of ischemic mitral regurgitation: an experimental evaluation. Circulation. 1991;84:2167–80. doi: 10.1161/01.cir.84.5.2167. [DOI] [PubMed] [Google Scholar]
- 28.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 29.Kono T, Sabbah HN, Rosman H, Alam M, Jafri S, Goldstein S. Left ventricular shape is the primary determinant of functional mitral regurgitation in heart failure. J Am Coll Cardiol. 1992;20:1594–8. doi: 10.1016/0735-1097(92)90455-v. [DOI] [PubMed] [Google Scholar]
- 30.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, et al. Mitral leaflet adaptation to ventricular remodeling. Occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–52. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perloff JK, Roberts WC. The mitral apparatus: functional anatomy of mitral regurgitation. Circulation. 1972;46:227–39. doi: 10.1161/01.cir.46.2.227. [DOI] [PubMed] [Google Scholar]
- 32.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875–83. doi: 10.1056/NEJMoa041451. [DOI] [PubMed] [Google Scholar]
- 33.Magne J, Sénéchal M, Dumesnil JG, Pibarot P. Ischemic mitral regurgitation: a complex multifaceted disease. Cardiology. 2008;112:244–59. doi: 10.1159/000151693. [DOI] [PubMed] [Google Scholar]