Abstract
Polybrominated diphenyl ethers (PBDEs) are widely used flame retardants that have become pervasive environmental contaminants and may contribute to adverse health outcomes. We evaluated in mice the developmental neurotoxicity of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), one of the most abundant PBDE congeners detected in animal and human tissues. Female C57BL/6J mice were exposed to daily doses of 0, 0.03, 0.1 or 1 mg/kg beginning 4 weeks prior to conception, continuing through gestation and lactation, and ending at weaning on postnatal day (PND) 21. Levels of BDE-47 in blood, brain, liver and adipose tissues of dams were markedly increased after 4 weeks of exposure, around the time of mating, and continued to increase through the time of parturition. Blood levels of BDE-47 in the dosed dams were within the range reported in humans. BDE-47 tissue levels in the dams decreased between parturition and weaning, possibly reflecting mobilization during lactation. Brain BDE-47 levels in the offspring at PND 1 approached those of the dams at parturition. Perinatal exposure to BDE-47 resulted in significant dose dependent growth retardation, slower motor performance in several behavioral tests, and mice exposed to 1 mg/kg/day BDE-47 showed altered performance in the Morris water maze. There were no differences between groups in the numbers of pyramidal neurons in hippocampus CA1. These results document accumulation of BDE-47 in several organ systems following exposure to low-levels of BDE-47, and provide evidence that such exposure is associated with early behavioral deficits in exposed neonates.
Keywords: Flame retardant, BDE-47, Polybrominated diphenyl ether, Neurotoxicity, Behavior, Hippocampus
1. Introduction
Polybrominated diphenyl ethers (PBDEs) are a family of flame-retardant chemicals used worldwide in a variety of products, including plastics and textiles, building materials, carpets, vehicles and computers. Due to the inability of PBDEs to covalently bind to polymers in these products, they readily enter the environment where they bioaccumulate in human and animal tissues (Hites, 2004). Exposure to BDE-47 is now world-wide, and levels of it have been detected in tissue samples from Sweden (Noren and Meironyte, 2000), India (Kumar et al., 2006), Australia (Toms et al., 2007), Pacific Northwest (She et al., 2007), United States (McDonald, 2005; Schecter et al., 2008, 2007, 2005a, 2005b, 2003), Taiwan (Chao et al., 2007), Poland (Jaraczewska et al., 2006; Wang et al., 2006), Japan (Eslami et al., 2006) and China (Bi et al., 2006, 2007). This situation raises concerns that measurable levels of PBDEs could pose significant health risks to humans. Studies on a similarly structured chemical family, polychlorinated biphenyls (PCBs), have identified a wide range of toxic effects which include genotoxicity, endocrine disruption, and in particular developmental toxicity (Pessah et al., 2009). Along with structure PBDEs share many physical characteristics with PCBs (Wang et al., 2006), which were originally banned for their dioxin-like toxicity (Carpenter, 2006).
Out of the 209 possible PBDE congeners available we focused our study on BDE-47, a tetra-brominated congener. BDE-47 has been detected in human adipose tissue (Fernandez et al., 2007; Petreas et al., 2003), breast milk (Inoue et al., 2006; Tsydenova et al., 2007), serum (Fangstrom et al., 2005; Inoue et al., 2006; Petreas et al., 2003), and in the liver of fetuses and newborns (Schecter et al., 2007) at concentrations higher than those reported for other congeners. This bioaccumulation in humans is of particular concern because PBDEs, including BDE-47, can be transferred to the fetus from the maternal circulatory system or to the infant through breast milk (Darnerud and Risberg, 2006; Schecter et al., 2003). Although BDE-47 is ubiquitous in the environment (Schecter et al., 2010), there is a poor understanding regarding its toxicological effects.
Several studies in mice have shown that neonatal exposure to PBDEs can disrupt normal brain development. A single exposure to 8 mg/kg of 2,2′,4,4′,5-penta-BDE (BDE-99) on postnatal day (PND) 10 alters spontaneous motor behavior in adulthood (Eriksson et al., 2001), and decreases locomotor activity in response to nicotine (Viberg et al., 2002). Exposure to 12 mg/kg of BDE-99 on PND 10 in mice altered levels of proteins associated with neurodegeneration and neuroplasticity in the striatum, and altered energy metabolism in the hippocampus (Alm et al., 2006). Neonatal exposures to PBDEs with higher bromination, such as congeners 153, 183, 203, 206, and 209, at concentrations ranging from 0.4 to 20.1 mg/kg have also been associated with developmental neurotoxicity (Dingemans et al., 2007; Viberg et al., 2007, 2003a, 2002, 2006, 2008).
Recent behavioral studies conducted on BDE-47 have reported that neonatal exposure results in hyperactivity and decreased habituation to anovel environment (Eriksson et al., 2001; Gee and Moser, 2008; Suvorov et al., 2009a). Altered levels of thyroid hormone have also been reported with exposure to BDE-47 (Costa and Giordano, 2007; Darnerud et al., 2007) and hydroxylated metabolites of BDE-47 have been shown to bind to thyroid carrier proteins with a higher affinity than thyroxine (Hamers et al., 2008; Meerts et al., 2000). These findings paired with the fact that thyroid hormone plays a critical role in early brain development, especially in regards to the cerebellum and hippocampus (Williams, 2008), provide reasons for concern.
Considered together, these studies indicate that neonatal exposure to PBDEs can affect regions of the brain that are important for learning and memory such as the hippocampus, as well as regions involved in locomotor behaviors such as the striatum and cerebellum. However, the majority of previous toxicological studies on PBDEs have examined acute, high dose exposures, such as 30 mg/kg for 7 days beginning at PND 6 (Dufault et al., 2005), or 20.1 mg/kg on PND 3 (Viberg et al., 2007), or chronic high dose exposure such as 16 mg/kg/day from gestational day (GD) 6 to PND 21 (Branchi et al., 2005). As a result, little is currently known about the potential risks of chronic low-dose exposure to PBDEs during early development which should better approximate human exposure conditions. In the present study, we examined effects of perinatal exposure to chronic low doses of BDE-47 (0.03, 0.1 or 1.0 mg/kg/day) in C57BL/6J mice. Somatic growth, indices of early development, sensory and motor function, and learning and memory were assessed following perinatal exposure. In addition, tissue levels of BDE-47 were measured in order to establish the relationship between exposure levels and neurotoxic effects.
2. Materials and methods
2.1. Chemical reagents and standards
Chemicals used throughout the animal exposure and analytical processes to determine the bioaccumulation of BDE-47 in tissue samples were A.C.S. Reagent Grade and solvents used for tissue extraction were HPLC grade. Columns used to purify extracted samples were made with polypropylene and packed with silica gel (45 μm particle size, 60 Å pore size; Supelclean, Supelco, Bellefonte, PA). Whatman cellulose extraction thimbles used in the Soxhlet extractor were purchased from Fisher Scientific (Pittsburgh, PA). Solutions of BDE-47 used for dosing mice were made from certified BDE-47 (AccuStandard, Inc., New Haven, CT, Cat. No. BDE-047N, Lot. #100901MT). A concentrated stock solution was made by sonicating 5 mg of pure BDE-47 in 250 μl DMSO until dissolved. Aliquots from this stock solution were then further diluted with Mazola® corn oil (100% pure, cholesterol free) to yield solutions containing 0.03, 0.1 or 1.0 mg/ml and stored in amber Pyrex bottles. Dosing was accomplished by applying BDE-47 to 1–2 cornflakes (Kellogg’s®, Battle Creek, MI) and feeding these directly to the mice. Unlabeled BDE-47 solutions used as standards for gas chromatography–mass spectrography (GC–MS) were made by dissolving certified pure BDE-47 (AccuStandard, Inc., New Haven, CT) in hexane to produce concentrations of (in ng/ml) 0.1, 0.3, 1, 3, 10, 30, 100, 300, 600 or 1000. These solutions were used to obtain a BDE-47 standard curve. Isotopically labeled 13C12-2,2′,4,4′-tetrabrominated diphenyl ether (BDE-47L) was obtained as a stock solution of 50 μg/ml 13C12-BDE-47 in nonane from Cambridge Isotope Laboratories (Cambridge, MA). The BDE-47L stock was diluted in hexane to produce a 120 ng/ml internal standard solution. 250 μl of this solution was added to each tissue sample extract, and the amount recovered was used to calculate and correct for percent recovery of BDE-47 during tissue extraction.
2.2. Tissue extraction and sample preparation
Tissue samples from BDE-47-exposed mice were finely minced, transferred to 20 ml glass vials and spiked with the labeled BDE-47L as an internal standard. The samples were then homogenized in 5 ml of HPLC-grade water (CHROMASOLV® for HPLC, Sigma-Aldrich, St. Louis, MO) using a polytron (PT 1200, Kinematica AG, Switzerland). Once homogenized, the samples were frozen immediately, lyophilized to a dry powder and stored at −20 °C. All glassware was washed and tested to ensure that background levels of BDE-47 were below detection limits by GC–MS. Later extraction of BDE-47 from the powdered tissue was performed overnight (i.e., at least 15 h) in 125 ml hexane/acetone (3:1, v/v) using an automated Soxhlet extractor (Organomation Associates, Inc., Berlin, MA). Extracted samples were then filtered through a solid-phase extraction cartridge pre-packed with silica (Supelclean-LC-Si-SPE, Sigma-Aldrich) to remove lipid, and then eluted with 20 ml hexane. The eluted samples were placed into a vacuum concentrator (RC10.22, Jouan, Winchester, VA) to remove solvent. This final extracted residue was re-dissolved in 200 μl hexane and transferred to an amber deactivated-glass vial with PTFE/Silicon septa (Waters Corp., Milford, MA) for GC-MS analysis.
2.3. Chemical analysis–gas chromatography and mass spectrometry (GC–MS)
Levels of BDE-47 from tissue extracts were analyzed by GC–MS using an Agilent 6890 gas chromatograph (GC) coupled with an Agilent 5973 mass spectrometer (MS) (Agilent Technologies, Inc., Santa Clara, CA). A 25 m × 0.22 mm × 0.25 μm HT-8 capillary column (SGE Analytical Science, Austin, TX) designed specifically for analysis of PCBs and PBDEs was used to measure BDE-47 levels in tissue extracts. One microliter (1 μl) of the final purified extract was injected into the heated inlet (280 °C) of the GC–MS, with the injector operated in splitless mode. Helium at constant flow rate of 0.8 ml/min was used as the carrier gas. The oven temperature program started from 90 °C, held for 1.5 min, then increased 30 °C/min to 190 °C, held 0.5 min; increased 5 °C/min to 280 °C, held 0.5 min; and then increased 25 °C/min to 320 °C and held 15 min. Ion source, quadrupole, and interface temperatures were set at 230, 150, and 300 °C, respectively. The electron ionization (EI) mode was used to quantify BDE-47 in select ion monitoring (SIM) mode at m/z 486 and 498 for BDE-47 and BDE-47L, with dwell time set at 100 ms. Using the quadrupole mass analyzer (m/z=0–800 mass range), a full scan of pure BDE-47 and BDE-47L were obtained to verify the purity of standards, to determine their retention times, and to acquire mass spectra.
Mass spectral data were analyzed using ChemStation software (Agilent Technologies, Inc., Santa Clara, CA). An internal standard curve of pure BDE-47 and BDE-47L in the range from 1 to 1000 ng/ml was used to quantify the extracted samples. The amount of BDE-47 present in each sample and amount of the internal standard were quantified by integrating the area under the curve. The detected concentrations of BDE-47 were corrected using recovery data from the internal standard. The reliable limit of detection (LOD) of BDE-47 in individual samples was determined by accepting only concentrations that had a signal/noise (S/N) of ≥5 and limit of quantification (LOQ) was established at S/N=10. The S/N ratio measured at the lowest BDE-47-spiked standard sample was used to establish the standard curve. This approach was then used to quantify the concentration of BDE-47 within the samples and included any possible contribution from the procedural blank. The LOD for BDE-47 in tissue was 0.1 ng/g tissue weight (i.e., 0.1 ng/g=0.1 ppb). Using the full scan mode of the single quadrupole mass analyzer (m/z of 0–800 mass range), the chromatogram and mass spectra parameters of BDE-47 and BDE-47L were acquired. The analysis showed that the retention times for BDE-47 and BDE-47L are 22.24 and 24.25 min, respectively. For quantification specified in the protocol, their corresponding molecular ions were at m/z 486 and m/z 498, respectively.
2.4. Animals
Adult male and female C57BL/6J mice used in this study were from Jackson Laboratory (Jackson Laboratory-West, Sacramento, CA). Upon arrival, the general health of these animals was checked and mice were maintained in the small-animal facility at the Center for Laboratory Animal Research (CLAS), University of California, Davis. Mice were fed standard mouse chow (LabDiet, 5001 Rodent Diet, Purina, Farmington, MA) and were maintained on a 14 h light/10 h dark cycle, with the light cycle between 7 AM to 9 PM. Ambient temperature was maintained between 68±2 °F and humidity between 40 and 60%.
2.4.1. BDE-47 exposure
Following arrival, female mice were allowed one-week acclimation before the start of exposure to BDE-47. The night prior to the first dosing, 3–4 cornflakes per mouse were left in their home cages to accustom them to the novel food. Female mice were weighed daily, and then fed 1–2 cornflakes that were dosed with BDE-47 (0.03, 0.1, or 1.0 mg/kg/day) or corn oil vehicle (Vehicle). The daily BDE-47 dosage was determined individually for each mouse based on body weight. Mice readily ate BDE-47-dosed cornflakes and consumption was verified by direct observation.
2.4.2. Breeding
After four weeks of exposure, mice were bred to untreated male C57BL/6J mice. Female and male C57BL/6J mice were randomly assigned (randomizer.org) to breeding pairs and all male mice used for breeding were approximately the same age as the females. Daily dosing of the dams continued throughout gestation and subsequent weaning of pups on PND 21. This dosing regimen resulted in 70–80 days of total exposure of the dams to BDE-47, depending on the number of days required for successful mating as determined by the presence of a copulatory plug.
2.4.3. Tissue levels of BDE-47 in dams and pups
At weaning after 70 days of exposure to Vehicle (n=9), 0.03 (n=10), 0.1 (n=9) or 1.0 (n=7) mg/kg/day BDE-47, female mice were sacrificed along with a group of experimentally naïve female mice (n=5), and BDE-47 levels were measured in blood, brain, liver and fat. Subsets of offspring from these dams were sacrificed on PND 1 (n=9), 7 (n=6), 14 (n=4) and 21 (n=4) for analysis of BDE-47 in blood and brain tissues. Tissue levels in dams exposed to 1 mg/kg/day BDE-47 were similarly measured at approximately the time of mating (i.e., after 27 days of exposure; n=3), and at parturition (i.e., after 54 days of exposure; n=3) to provide information on accumulation of BDE-47 in blood, brain, liver and fat over the course of exposure.
2.4.4. Offspring for behavioral testing
Offspring used for behavioral testing were from dams exposed to Vehicle (n=9), 0.03 (n=9), 0.1 (n=11) or 1.0 (n=10) mg/kg/day BDE-47. Offspring were sexed at birth, marked with a non-toxic identifying foot tattoo, coded for treatment group and returned to the litter. Experimenters were blinded to the exposure history of the pups. Each dam and litter was housed together until weaning on PND 21 when the juvenile mice were ear-notched, weighed and measured for body length. They remained with their litter mates until PND 28 when they were re-caged according to sex, treatment condition and with litter mates when possible. The numbers of animals were kept to a minimum by arranging for the distribution of animals across experiments so that animals from each litter could be used for behavioral analysis and histology.
2.5. Behavioral test battery
The behavioral tests are described in the order that they were conducted (Table 1), along with the PND or postnatal week at which they were tested. All tests were conducted during the light phase of the light/dark cycle. One male and one female pup from each litter were randomly selected for behavioral testing using the behavioral tests listed under Track 1. Different pairs of male and female pups were selected from each litter and behaviorally tested using the tests listed under Track 2. This was done to maximize the use of the mice bred for these experiments and to include sex as a variable in the statistical analyses. Therefore, groups for behavioral testing under Tracks 1 and 2 were as follows: Vehicle (n=9♀ and 9♂), 0.03 (n=9♀ and 9♂), 0.1 (n=11♀ and 11♂) or 1.0 (n=10♀ and 10♂) mg/kg/day BDE-47.
Table 1.
Behavioral test schedule (age of testing).
| Growth and reflex (PND 8, 12, 14, 16, 18) | |
| Ultrasonic vocalization (PND 9, 11, 13, 17) | |
| Wean with litter (PND 21) | |
| Track 1 (1♀,1♂ from each litter) |
Track 2 (1♀,1♂ from each litter) |
|
| |
| Elevated Plus Maze (PND 35) |
Social dyadic interaction (PND 25–26) |
| Acoustic Startle & Prepulse Inhibition (PND 42) |
Rotarod (PND 35–36) |
| Fear Conditioning (8Weeks) |
Locomotor Activity (PND 42) |
| Locomotor Activity (Integra) (PND 60) |
Morris Water Maze (8Weeks) |
| Euthanized for Histology (PND 70) |
|
2.5.1. Sensory and motor development (PNDs 8–18)
Sensory and motor development were assessed every other day using the Wahlsten test battery for all preweanling mice (Golub et al., 2004; Wahlsten, 1974). Pups were gently removed from the litter and placed in holding cups on a warming pad. They were then randomly selected for scoring of maturational landmarks. These included righting reflex, cliff aversion, needle grasp, visual placing, vibrissa placing, eye opening, ear opening, ear twitch response, screen pull, screen cling/climb, narrow and wide, auditory startle and popcorn behavior (startle response to puff of air directed to the face). Mean composite scores for motor development and sensory development were computed as previously described (Wahlsten, 1974).
2.5.2. Ultrasonic vocalization (PNDs 9, 11, 13 and 17)
Pups and dams were transported to the testing room and allowed to acclimate at least 30 min prior to testing. Each pup was individually isolated from the mother and placed in a plastic cup that positioned the pup directly under the microphone of the ultrasonic vocalization (USV) recording system (UltraVox, Noldus IT, Tacoma, WA). The sound-attenuated testing chamber door was gently closed and a 2-min recording period began within 15 s after the pup was removed from its mother. After the recording session ended, the pup was placed in a plastic container on a warming pad separated from the rest of the litter until all pups in the litter were tested. The USV detector was set at 40 kHz, the audio filter on top of the ultrasonic vocalization chamber was set at 8.5 and temperature inside the testing chamber was recorded at the end of each session. Vocalizations were analyzed if they were longer than 10 ms in duration, with a minimum of 1 ms between individual vocalizations. Total number of USVs, total USV duration and duration of individual vocalizations were analyzed using the Noldus UltraVox software.
2.5.3. Social dyadic interaction (PNDs 25–26)
Social interactions were characterized using a behavioral ethogram and behavioral scoring procedures developed by this laboratory (Jaubert et al., 2007). Briefly, an experimental mouse from one of the treatment groups was paired with an unfamiliar same strain, same sex, experimentally näive mouse for 10 min in a 10×10×10 cm Plexiglas chamber. The dyadic interactions between the two mice were video-recorded for later scoring using Noldus Observe 4.1 software programmed with codes reflecting the behaviors specified by the ethogram. The duration and frequency of social, non-social and aggressive behaviors were measured using the ethogram as previously described (Jaubert et al., 2007). The animal initiating each behavioral event was also determined. Video recording was preceded by an acclimation period in which each mouse was alone in a similar box for 5 min.
2.5.4. Elevated plus maze (PND 35)
Fear and anxiety were assessed using the elevated plus maze (File, 2001; Lister, 1987). The maze was made of black Plexiglas, with two open (30×5×0.25 cm) and two closed (30×5×6 cm) arms emanating from a central platform (5×5 cm), and elevated 60 cm above the floor. Each mouse was placed onto the central platform and allowed 5 min of free exploration in the apparatus. Distance traveled, number of entries into each arm and into the central platform, time in open vs. closed arm and latency to first arm entry were recorded and analyzed by a video-tracking system (SMART, San Diego Instruments, San Diego, CA). All testing sessions were directly observed by an investigator blinded to treatment condition. Increased time spent on the closed versus open arms of the maze was used as a measure of anxiety and fear.
2.5.5. Rotarod (PNDs 35–36)
A rotarod (Rota-Rod 7600, Ugo Basile Biological Res., Malvern, PA) was used to measure balance and motor coordination as described previously (Golub et al., 2004). Mice were given two 2-min training sessions 2 h apart on 1 day with the rotarod at 16 revolutions/minute (rpm). On the following day the rotarod was set at 24 rpm and mice were given a single 2-min test. During training, the mouse was replaced on the rod when it fell. During testing, the trial terminated when the mouse fell. The time to first fall, the number of falls, and instances of rotating with the rod (‘passive rotation’ or ‘flipping’) were recorded.
2.5.6. Acoustic startle and pre-pulse inhibition (PND 42)
Prepulse inhibition (PPI) of the acoustic startle response was measured to assess sensorimotor gating (Dulawa and Geyer, 2000; Graham, 1975). Mice were placed individually into the acoustic startle apparatus (SR-LAB, SD Instruments, San Diego, CA) and allowed to acclimate to background white noise for 5 min. This was followed by a 20-min PPI session consisting of 50 test trials, 10 each for five different trial types presented in a pseudorandom order with variable inter-trial intervals of 5–20 ms. Trial types included: 120 dB acoustic stimulus alone, 120 dB stimulus with a 74, 82, or 90 dB pre-pulse acoustic stimulus or a measure of baseline activity (i.e., no acoustic stimulus). All pre-pulses were 20 ms long and were presented 100 ms before the 120 dB stimulus. Broadband pink noise was used for the acoustic stimulus.
2.5.7. Locomotor activity (PND 42)
Locomotor activity was tested on PND 42 using a TruScan Photo Beam open field activity arena for mice (Coulbourn Instruments, Allentown, PA) as described previously (Berman et al., 2008). The apparatus (27.5×27.5×37.5 cm) detects movements in the three geometric planes by recording infrared beam breaks. One male and one female from each litter were individually tested by placing the mouse in the center of the apparatus and activity measurements (e.g., horizontal and vertical movements and distance) were collected for 60 min and analyzed by the TruScan software.
2.5.8. Fear conditioning (Week 8)
Contextual fear conditioning procedures were modified from previously published protocols (Goddyn et al., 2008; Paradee et al., 1999). The experiment was carried out over 3 consecutive days during Postnatal Week 8. On day 1, mice were placed in a testing chamber constructed of steel and Plexiglas with a metal grid floor, and allowed to acclimate for 5 min. On day 2, animals were placed in the testing chamber and allowed to freely explore the chamber for 2 min to establish baseline activity levels. They were then presented with a 30 s acoustic conditioned stimulus (CS; 440 Hz tone). Two seconds before termination of the CS, a 2 s, 0.3 mA foot-shock, the unconditioned stimulus (US), was given. After 30 s, the mice were given a second CS-US pairing, and activity was measured for another 30 s. On day 3, animals were placed in the chamber in the absence of the acoustic CS and freezing was scored for 5 min (i.e., “context trial”). “Freezing” was defined as an absence of limb or head movements (vibrissa and tail movements could still occur). Mice were removed from the chamber and chamber context was changed by covering the grid floor with a hard plastic plate, putting in a curved white wall that spanned 3 walls of the chamber, and covering the floor with CareFresh® bedding (Absorption Corp., Ferndale, WA). Ninety minutes later, mice were placed in the chamber with the new context and freezing was measured for 3 min in the absence of the tone (pre-CS freezing), followed by measurement of freezing for 3 min in the presence of the tone (freezing to the CS). Freezing was sampled every 10 s. Thus, 12 freezing measurements were taken during the 2 min baseline period, 12 during foot shock trials, 30 during context trials, and 18 during pre-CS and CS trials. Only the first 18 observations during the context trials were used for analysis. The number of instances of freezing relative to the total number of observations under each condition was used to calculate a percent freezing score.
2.5.9. Locomotor activity (PND 60)
Locomotor activity was evaluated on PND 60 in a different apparatus (Integra, Accuscan, Columbus, OH) and in different pairs of male and female mice (i.e., Track 1) from those used to test locomotor activity on PND 42 (i.e., Track 2). Mice were individually placed in the center of the automated activity apparatus (40 × 40 × 15 cm) and vertical and horizontal activity were analyzed for 60 min as previously described (Golub et al., 2004).
2.5.10. Spatial memory and learning in the water maze (Postnatal Week 8)
Spatial memory and learning was tested in a Morris water maze (Jaubert et al., 2007). The maze was 90 cm in diameter with a hidden 6 cm square platform submerged 1 cm below the surface of the water. Water temperature was maintained at 21 °C. Maze performance was monitored using an automated tracking system (Polytrack, San Diego Instruments, CA). Before training began, a single visible platform trial was performed in which animals were able to see the platform, raised 2 cm above the water surface, and were allowed to swim to the platform and escape from the water. This was then followed by four 90 s test trials with the platform submerged. Training was repeated over four consecutive days, and the average of the four trials on each day was used for statistical analysis. Briefly, mice were placed in the maze at one of three positions contained within the quadrants not containing the platform, and were allowed to locate and mount the escape platform. Latency to mount the platform, swim distance, swim speed, and time spent floating were measured. Animals who failed to reach the platform during the 90 s training session received the maximum 90 s for latency. Inter-trial intervals were 5 min long during which the mouse waited in a warming cage. On the fifth day, a “probe trial” was given in which the platform was removed. Mice were released at the center of the quadrant opposite the quadrant where the platform was, and the animals were allowed to swim freely for 90 s. Time spent swimming in the former escape platform quadrant was used to assess memory for spatial location of the platform.
2.6. Histological procedures
At the end of behavioral testing at 10 weeks of age, all mice were sacrificed (100 mg/kg i.p. Euthasol®, Virbac AH, Inc., Fort Worth, TX). Mice used for histological evaluation (n=8 per treatment group) were perfused with 20 ml of 0.1 M phosphate buffer (pH 7.4), followed by 30 ml 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Brains were removed, post-fixed for 1 h in 4% paraformaldehyde, cryoprotected in 10% sucrose solution for 1 h and in 30% sucrose solution for 24 h, frozen, and sectioned at 50 μm on a sliding microtome (model 860, American Optical, Buffalo, NY). All slices were preserved in 0.1% sodium azide in 0.1 M phosphate buffer, and sections were mounted, air dried and Nissl stained. These sections were used for unbiased stereological analysis of numbers of neurons within the CA1 subregion of the hippocampus (StereoInvestigator, Microbrightfield, Williston, VT).
2.7. Statistical analysis
Data in figures represent mean±standard error of the mean (SEM). Statistical analyses were carried out using version 18 of SPSS (SPSS, Chicago, IL) and version 9.2 of the SAS programming language (SAS Institute, Cary, NC). Litter means were used for analyses of neonatal body weight, sensory and locomotor development (Wahlsten test battery), and ultrasonic vocalization tests. For all other statistical analyses, data from no more than one male and one female mouse from each litter were used for statistical analyses, with treatment and sex (and treatment×sex when appropriate) used as fixed effects variables and litter as a random effects variable. A mixed effects repeated measures ANOVA was used when appropriate. Repeated measurements over time for each pup were considered by using the autoregressive-1 (AR(1)) covariance structure. The Kenward–Rogers method of calculating degrees of freedom was used. The numbers of pyramidal neurons in the CA1 subregion of the hippocampus were analyzed by a univariate ANOVA. Individual post hoc group comparisons were made using the Tukey–Kramer test for multiple comparisons. Data were examined for homogeneity of variance, and when assumptions of homogeneity of variance were not met, data were analyzed using the Kruskal–Wallace nonparametric analysis, followed by individual post hoc group comparisons using the Mann–Whitney U adjusted for multiple comparisons. The minimum level set for statistical significance was p<0.05. Frequencies of behavioral events in the social dyad and fear conditioning were analyzed by Chi-square tests.
3. Results
3.1. Tissue levels of BDE-47
Table 2 shows the levels of BDE-47 in blood, brain, liver and fat in dams after 27, 54 and 70 days of exposure to 1 mg/kg/day, compared to naïve and Vehicle groups. The three time-points selected for examination correspond approximately to the time of mating (i.e., 27 days exposure), birth of pups (i.e., 54 days exposure) and pup weaning (i.e., 70 days of exposure). As shown in Table 2, naïve and Vehicle exposed mice had low mean baseline levels of BDE-47 in blood, brain, liver and fat (i.e., less than 5 ng/g tissue). However, relatively high levels were found in all tissues from dosed dams at 27 days of exposure (time of mating). Further increases in BDE-47 levels in blood, liver and fat between 27 and 54 days of dosing were relatively modest, while brain levels roughly doubled (i.e., 162 versus 306 ng/g). After 70 days of exposure, corresponding to the end of lactation at the time of pup weaning, BDE-47 tissue levels had decreased, although they remained above levels found in naïve and Vehicle treated dams. The decrease occurred even though dams continued to be dosed with BDE-47 throughout the preweaning period. This finding may be related to mobilization of BDE-47 into milk stores during lactation, as considered in the Discussion. Similar data were not collected in this study for the two lower doses (i.e., 0.03 and 0.1 mg/kg/day).
Table 2.
BDE-47 tissue levels (ng/g tissue; ppb) of dams orally exposed to a dose of 1 mg/kg/day for 27, 54, or 70 days, corresponding to the times of breeding, parturition and weaning, respectively.
| Duration of exposure | Blood (ppb) | Brain (ppb) | Liver (ppb) | Fat (ppb) |
|---|---|---|---|---|
| Naïve (n=5)a |
2.1±1.3 | 3.3±2.2 | 2.4±0.1 | 1.4±1.1 |
| Vehicle (n=5) |
4.9±4.9 | 1.3±1.1 | 1.2±0.1 | LODb |
| 27 days (n=3) |
36.8±6.1c | 162±23c | 1510±60c | 20,800±500c |
| 54 days (n=3) |
56.9±14.4c | 306±60c | 1560±90c | 20900c,d |
| 70 days (n=7) |
9.6±3.8 | 55.2c±11.7 | 505c±115 | 6880±2090c |
Number of animals for each group.
Below limit of detection (LOD).
Significantly different from both Naïve and Vehicle groups (p<0.0001).
No SE calculated because n=2 after one outlier removed.
Table 3 shows BDE-47 levels in blood, brain, liver and fat in dams after 70 days exposure to 0.03, 0.1 or 1.0 mg/kg/day BDE-47 or Vehicle. Levels of BDE-47 in brain (p<0.01), liver (p<0.001), and fat (p<0.001) from dams dosed with 1.0 mg/kg/day BDE-47 were significantly elevated compared to the Vehicle group, with the highest levels in fat stores. Tissue levels in fat and liver with the two lower doses (e.g., 0.03 and 0.1 mg/kg/day) were also significantly elevated compared to that of Vehicle controls (p<0.01). However, the two lower doses did not differ from Vehicle in blood or brain tissue at this time point. Since levels of BDE-47 dropped in all tissues from the dams over the period of lactation (Table 2), it is likely that tissue levels for the two lower doses (i.e., 0.03 and 0.1 mg/kg/day) were substantially higher before the start of lactation, but data for those doses before the start of lactation were not collected in this study.
Table 3.
BDE-47 tissue levels (ng/g tissue; ppb) of dams exposed to Vehicle, 0.03, 0.1, or 1 mg/kg/day BDE-47 for 70 days corresponding to the time of weaning.
| Group | Blood (ppb) | Brain (ppb) | Liver (ppb) | Fat (ppb) |
|---|---|---|---|---|
| Vehicle (n=9)a |
1.70±1.3 | LODb | 1.98±1.4 | 6.6±2.5 |
| 0.03 mg/kg/day (n=10) |
1.57±1.0 | 4.25±2.1 | 22.07±6.3c | 120.5±50.6c |
| 0.1 mg/kg/day (n=9) |
2.06±1.9 | 1.81±1.0 | 36.13+8.3c | 103.0±33.3c |
| 1 mg/kg/dayd (n=7) |
9.60±3.8 | 55.2±11.7c | 505.2±115c | 6876±2087c |
Number of animals for each treatment group.
Below limit of detection (LOD).
p<0.01 versus Vehicle.
Data from Table 2 from the 1 mg/kg/day group.
Table 4 shows elevated brain levels of BDE-47 on PNDs 1, 7, 14 and 21 in offspring of dams exposed to 1 mg/kg/day BDE-47. High levels of BDE-47 were also found in blood on PNDs 7, 14 and 21. Blood levels of BDE-47 were not measured on PND 1 due to the small volume of blood obtainable from these pups. Pup brain levels of BDE-47 were between 150 and 350 ng/g throughout the preweaning period. Although BDE-47 blood levels appeared to decrease over the period of lactation, the apparent differences across ages did not reach statistical significance. Note that the range of brain levels of BDE-47 in these offspring during the preweaning period was similar to brain levels in dams exposed to 1 mg/kg/day at the time pups were born (i.e., Table 2; 54 days exposure). Similar data for offspring of dams exposed to the two lower levels of BDE-47 (i.e., 0.03 and 0.1 mg/kg/day) were not collected in this study. Tissue levels of BDE-47 were below the level of detection for pups from the Vehicle-treated dams.
Table 4.
BDE-47 brain and blood levels (ng/g tissue; ppb) on postnatal day (PND) 1, 7, 14, and 21 from pups of dams exposed to 1 mg/kg/day BDE-47.
| Group | Brain (ppb) | Blood (ppb) |
|---|---|---|
| PND1 (n=9) |
366±117 | NA |
| PND7 (n=6) |
166±61 | 516±326 |
| PND14 (n=4) |
293±86 | 442±228 |
| PND21 (n=4) |
202±158 | 249±93 |
3.2. Maternal and offspring development
As shown in Table 5, exposure to BDE-47 did not significantly affect maternal weight gain, duration of gestation or litter size. There were also no effects of BDE-47 exposure on pup sex ratio (χ2=0.45, df=3, p=0.93). At weaning on PND 21, a litter-based analysis of crown-rump length showed a difference among groups which approached statistical significance (F3,32=2.84, p=0.053), with post hoc analyses showing that pups in the 0.1 mg/kg/day group had significantly reduced body length compared to pups in the Vehicle group (p<0.05). The ponderal index (weight/crown-rump length) was not significantly different among treatment groups (F3,32=1.68, p=0.19).
Table 5.
Maternal and litter data.
| Maternal and litter data | Perinatal BDE-47 exposure group |
|||
|---|---|---|---|---|
| Vehicle | 0.03 mg/kg/ day |
0.1 mg/kg/ day |
1.0 mg/kg/ day |
|
| Maternal weight gain (%) | 52.9±1.1 | 54.8±0.5 | 55.5±1.1 | 53.8±1.2 |
| Days gestation (days) | 19.8±0.2 | 19.5±0.3 | 19.9±0.6 | 20.0±0.3 |
| Litter size | 6.5±0.9 | 6.9±0.3 | 6.4±0.3 | 6.6±0.3 |
| Females per litter | 3.5±0.5 | 2.5±0.3 | 3.0±0.6 | 3.2±0.3 |
| Males per litter | 2.8±0.4 | 2.5±0.4 | 3.0±0.6 | 2.8±0.4 |
| Sex ratio (F/M) | 1.2±0.4 | 1.1±0.2 | 0.9±0.2 | 1.2±0.4 |
| Ponderal index on PND 21 | 1.13±0.04 | 1.15±0.04 | 1.04±0.03 | 1.11±0.05 |
As shown in Fig. 1, there was a statistically significant treatment×-day interaction for body weight gain from PNDs 8–18 (F15,155=2.99, p<0.001) and a significant increase in body weight over days across groups (F5,155=312.1, p<0.0001). Post hoc analyses on individual days showed that body weight for the 0.1 mg/kg/day group was significantly lower than that of the Vehicle group on PNDs 14, 16 and 18 (p<0.05) and lower than the 0.03 mg/kg/day group on PNDs 14 and 16 (p<0.05). No other group comparisons reached statistical significance on any PND.
Fig. 1.

Body weight gain from postnatal days 8–18 for the four treatment groups. *p<0.05 versus Vehicle; #p<0.05 versus 0.03 mg/kg/day.
3.3. Behavioral tests
3.3.1. Sensory and motor development
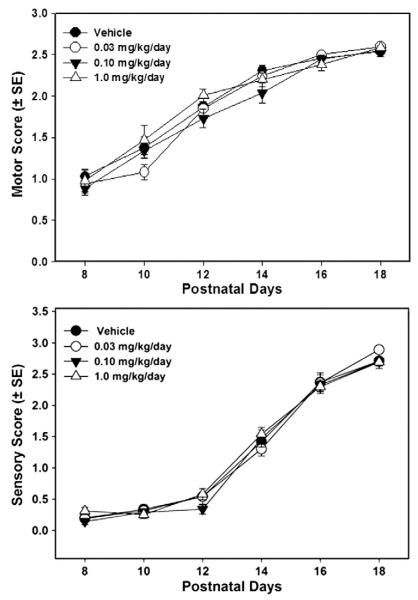
Composite sensory and motor developmental scores from the Wahlsten test battery between PNDs 8 and 18 are shown in Fig. 2. Statistical analyses did not reveal significant differences among treatment groups for motor development (F3,32=1.09, p=0.37) or sensory development (F3,32=0.54, p=0.66).
Fig. 2.
Motor and sensory development scores across postnatal days 8–18. No significant differences were seen among treatment groups.
3.3.2. Ultrasonic vocalization
For total duration of USV, there was a significant difference across postnatal days (F3,105=5.31, p<0.01) and a significant treatment×-day interaction (F9,105=2.26, p<0.05). As shown in Fig. 3A, mice in the 1.0 mg/kg/day dose group had a longer total duration of USV on PND 13 than mice in all other groups (p<0.05). There was also a significant difference in the average duration of individual bouts of USV across PNDs (F3,105=57.4, p<0.001) and a significant treatment×day interaction (F3,105=3.11, p<0.01). Post hoc analysis showed that on PNDs 9 and 13 (Fig. 3B), the mean duration of USV bouts was significantly longer for mice in the 1.0 mg/kg/day group compared to the Vehicle control group (p<0.05) and longer than the 0.03 and 0.1 mg/kg/day mice on PND 13 (p<0.01). There were also significant differences across PNDs in the number of USVs across all groups (F9,105=11.1, p<0.001), but the apparent difference between treatment groups in the number of USVs was not statistically significant for any PND (Fig. 3C).
Fig. 3.
Effects of perinatal exposure to BDE-47 on ultrasonic vocalization during separation of the pups from their litter. (A) Total duration of USVs were significantly increased on PND 13 for offspring exposed to 1.0 mg/kg/day BDE-47 compared to all other groups (*p<0.05). (B) Mean duration of USV bouts was significantly longer for mice in the 1.0 mg/kg/day exposure group compared to mice in the Vehicle group (*p<0.05), and longer than that of the 0.03 and 0.1 mg/kg/day exposed mice on PND 13 (#p<0.05). (C) The number of USVs did not differ significantly between treatment groups.
3.3.3. Social dyadic interaction
There were no significant differences among treatment groups in percent time in social interactions (F3,35=0.20, p=0.90) or in the non-social state (F3,35=0.20, p=0.90), and aggressive behaviors in this test in young mice occurred at too low a frequency to analyze statistically as previously reported (Jaubert et al., 2007). There were no statistically significant differences among treatment groups across the individual social, non-social or aggressive behaviors examined in this test of social dyadic interaction.
3.3.4. Elevated plus maze
There were no significant group differences for any behaviors examined in the elevated plus maze, including percent time in open arm (F3,26=0.52, p=0.67) or number of entries into the open arm (p=0.09, Kruskal–Wallis test) (data not shown).
3.3.5. Rotarod performance
There were no significant differences between treatment groups in the number of falls (F3,52=0.35, p=0.80) or time to fall (F3,52=0.38, p=0.77) during the training and test sessions (data not shown).
3.3.6. Acoustic startle and pre-pulse inhibition
There were no significant differences among treatment groups in maximum acoustic startle amplitude (F3,58=0.19, p=0.90), or pre-pulse inhibition (F3,51=1.03, p=0.34) across the three pre-pulse amplitudes (i.e., 74, 82 and 90 dB) (data not shown).
3.3.7. Fear conditioning
There were no significant differences among treatment groups in freezing to the chamber context (pre-CS freezing; F3,57=0.32, p=0.81) or to the CS tone (freezing to the CS; F3,57=1.45, p=0.24) following fear conditioning.
3.3.8. Locomotor activity
There were no statistically significant differences between groups in any measure of locomotor activity when tested at 42 days of age in the Coulbourn TruScan activity apparatus. However, as shown in Fig. 4, animals tested at 60 days of age in the Integra activity apparatus showed a significant treatment×sex interaction for total distance traveled (F3,26=4.69, p<0.01). Individual group comparisons showed that female mice in the 0.1 and 1.0 mg/kg/day treatment groups traveled a significantly shorter distance than Vehicle controls (p<0.05) or mice in the 0.03 mg/kg/day group (p<0.01). There was also a treatment×sex interaction for distance traveled in the center of the locomotor apparatus (F3,26=4.55, p<0.05), with females in all three BDE-47 treatment groups showing less center activity than Vehicle controls (p<0.05) (data not shown). These treatment group differences were not found for male mice.
Fig. 4.

Total distance traveled in the open field on postnatal day 60 in the Integra activity apparatus. Female mice in the 0.1 and 1.0 mg/kg/day exposure groups traveled significantly shorter distances than female mice in the Vehicle control group (*p<0.05) and 0.03 mg exposure group (#p<0.01). Males did not differ significantly between groups in this locomotor test.
3.3.9. Spatial memory and learning in the water maze
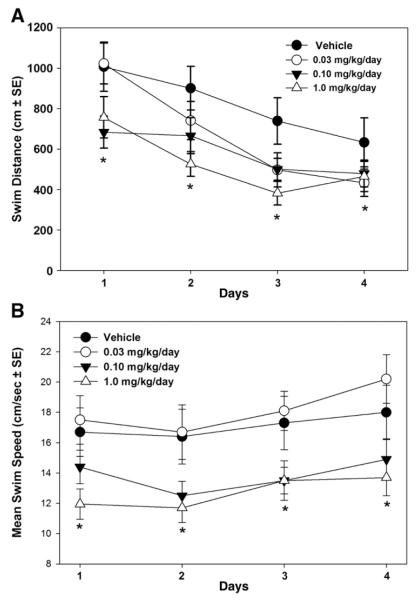
During the visible trial test in the water maze, there was a significant treatment × sex interaction (F3,21=3.68, p<0.05). As shown in Fig. 5, female mice in the 1.0 mg/kg/day group had a slower mean swim speed than Vehicle control female mice (p<0.05), and male mice in the 1.0 mg/kg/day group had a slower mean swim speed than male mice in the 0.03 mg/kg/day group (p<0.05). Performance in the water maze during training to locate the hidden escape platform is shown in Fig. 6A and B. Statistical analysis showed a significant group difference in distance swum (F3,33=3.15, p<0.05). Individual group comparisons showed that the 1.0 mg/kg/day treatment group swam a shorter distance to the platform than the Vehicle control group across all training days (p<0.05; Fig. 6A). There was also a significant treatment effect for swim speed (F3,32=3.06, p<0.05; Fig. 6B). Group comparisons showed that the 1.0 mg/kg/day group swam significantly slower than the 0.03 mg/kg/day group (p<0.01) and the Vehicle control group (p<0.05). Latency to reach the platform did not differ significantly across treatment groups (F3,35=1.27, p=0.30). There were no significant sex differences or sex by treatment interactions.
Fig. 5.

Swim speed in the Morris water maze during the visible trial test. Female mice in the 1.0 mg/kg/day treatment groups swam significantly slower than female mice in the Vehicle control group (*p<0.05), and male mice in the 1.0 mg/kg/day group swam significantly slower than mice in the 0.03 mg/kg/day treatment group (#p<0.05).
Fig. 6.
Mean swim distance and swim speed during acquisition training in the Morris water maze. (A) Mice in the 1 mg/kg/day exposure group swam a shorter distance over training days to find the submerged escape platform compared to Vehicle treated controls (*p<0.05). (B) Mice in the 1 mg/kg/day exposure group also showed a slower mean swim speed compared to Vehicle controls (*p<0.05) and mice in the 0.03 mg/kg/day exposure group (*p<0.01).
During the probe trial with the escape platform removed, there were no significant differences among treatment groups in the time spent in the platform quadrant, latency to enter the platform quadrant or mean swim speed.
3.4. Hippocampal cytoarchitecture
No gross morphological abnormalities in the hippocampus were found from examination of cresyl violet-stained brain sections, and the treatment groups did not differ significantly in the number of pyramidal neurons in the CA1 subregion of the hippocampus (F3,28=2.21, p=0.11). The mean (±SEM) numbers of pyramidal neurons in the CA1 subregion of the hippocampus based on unbiased stereological procedures for the treatment groups were as follows: Vehicle (68,280±4058), 0.03 mg/kg/day (67,733±4877), 0.1 mg/kg/day (79,566±3845) and 1.0 mg/kg/day BDE-47 (76,648±3017).
4. Discussion
Chronic, perinatal, low-dose exposure to BDE-47 in C57BL/6J dams resulted in substantial accumulation in all tissues measured. At the time of birth (PND 1) which was equivalent to dam exposure day 54, levels of BDE-47 in the brains of offspring from dams dosed with 1 mg/kg/day BDE-47 averaged 366 ng/g tissue weight (Table 4). This is approximately 20% higher than the 306 ng/g tissue weight found in the brains of their BDE-47 exposed dams (Table 2). The finding of higher brain levels in newborn pups compared to their dams is consistent with earlier reports by Staskal et al. that 10 day-old pups develop higher tissue levels than adults when exposed to the same amounts of BDE-47 (Staskal et al., 2006a). Because differences in brain levels between pups and dams were found on PND 1, before the start of lactational exposure, such levels could also have been present in utero suggesting an in utero risk posed by PBDEs to the developing fetus. Delayed body weight gain was seen in pups exposed to 0.1 mg/kg/day BDE-47 suggesting an effect on somatic growth, possibly due to the effects of this class of compounds on endocrine function (Zoeller, 2010; Zoeller et al., 2002). Pups exposed to the highest 1.0 mg/kg/day dose showed increased durations of USV when separated from their dams and littermates on PND 13 indicating increased distress. Pups exposed prenatally to BDE-47 also showed slower swim speeds in the water maze, and female offspring showed less total activity (i.e., distance traveled) in the open field. Interestingly, slower swim speeds were accompanied by shorter swim distances to locate the hidden escape platform in the water maze, and could reflect better spatial learning. However, other mechanisms could also explain the results, including improved attention to spatial cues, motivational effects, or possibly better visual perception. Finally, unbiased stereological analysis of numbers of neurons in the CA1 of the hippocampus did not reveal any loss of neurons or abnormal morphology in exposed pups. Taken together, these results indicate that chronic perinatal exposure to BDE-47 can have lasting effects on development, including somatic growth, motor function and performance in the spatial water maze. Each of these findings is discussed below.
4.1. Blood and tissue levels of BDE-47
As previously reported, lipophilic tissues such as brain, adipose tissue and liver, readily accumulate BDE-47 following chronic, low-dose oral exposure (Staskal et al., 2006a). This was true for both exposed dams and their offspring. Similar tissue levels for the 1.0 mg/kg/day dose was found after 27 and 54 days of exposure, suggesting that relatively stable tissue levels were achieved before mating using the present oral daily dosing regimen.
Chronic exposure (i.e., 54 days) of dams to a dose of 1 mg/kg/day resulted in levels of BDE-47 in blood (56.9 ng/g), brain (306 ng/g), liver (1560 ng/g) and adipose tissue (20,900 ng/g) that were in approximately the same ranges reported by Staskal et al. measured 5 days after a single 10 mg/kg (p.o.) dose of BDE-47 in mice for blood (178.7 ng/g), brain (109 ng/g), liver (1688 ng/g) and adipose tissue (17,712 ng/g) (Staskal et al., 2006b). In that same study, ten daily oral doses of 1.0 mg/kg/day also resulted in an accumulation of BDE-47 in all tissues examined 5 days after the end of dosing, albeit at lower levels than with a single 10 mg/kg bolus injection. As in the present study, Staskal et al. found high levels of BDE-47 in adipose tissue and brain (Staskal et al., 2006b).
Reported levels of BDE-47 in human blood samples range from 0.25 ng/g (lw) to 46 ng/g (lw) in North America, levels that are approximately one order of magnitude higher than levels observed in Europe (0.24–2.4 ng/g lw) (Frederiksen et al., 2009). The levels of BDE-47 in blood samples from mouse dams dosed with 1 mg/kg/day were 36.8 and 56.9 ng/g after 27 and 54 days of exposure, respectively. These blood levels in dosed mice are in the upper range of levels reported in human blood. This means that blood levels for the two lower doses used in mice (i.e., 0.03 and 0.1 mg/kg/day) are also within the range of reported human blood levels. Levels of BDE-47 in mouse adipose tissue from this study (i.e., ~20,000 ng/g) were many orders of magnitude higher than the 0.52 to 29.3 ng/g PBDE levels reported in human adipose tissue (Frederiksen et al., 2009). However, relatively few studies have reported levels of BDE-47 in human adipose tissue, and most of these reports have been from countries with some of the lowest reported PBDE exposure levels. Therefore, they may not accurately reflect BDE-47 adipose levels in the US population. In addition, these data indicate that blood levels may not accurately reflect the levels of PBDEs in other tissues, and that current exposure paradigms used in many rodent studies are likely to be producing much higher tissue levels than those occurring in humans. The present results do highlight the high degree to which BDE-47 can accumulate in tissues during chronic exposure.
Dams dosed with 1.0 mg/kg/day BDE-47 in the present study showed a marked decrease in tissue levels between the beginning and end of the lactation period (i.e., 54 and 70 days exposure, respectively). One explanation is that over the course of lactation, BDE-47 may have been mobilized from fat stores into milk. Although BDE-47 in milk of exposed dams was not measured in this study, previous research has shown BDE-47 to be one of the major congeners found in human breast milk, and that breast milk is likely to be a major source of exposure to infants (Lignell et al., 2009; Zhu et al., 2009). Studies by Darnerud et al. and Oskarsson et al. demonstrated that PBDEs are present in the breast milk of lactating mouse dams after exposure (Darnerud and Risberg, 2006; Oskarsson and Moller, 2004). This possibility is also supported by the report that 20% and 24% of a single i.v. administration of BDE-85 and BDE-99, respectively, were transferred to the litter through lactation (Darnerud and Risberg, 2006). Oskarsson et al. examined the partitioning of BDE-99 between serum and milk of lactating mouse dams following an i.v. administration of 310 μg/kg on either lactational day (LD) 3 or LD 10. They found that the milk:plasma ratio after administration of a single i.v. dose was 40, and that the majority of the administered dose was excreted via breast milk over 7 days. The maximum concentration was found 4 h after administration on LD 3, and at 10 h following administration on LD 10 (Oskarsson and Moller, 2004). Additionally, they documented that the concentration of BDE-99 in the pups was higher than that in the dams. These findings demonstrate that PBDEs are readily transferred into milk from the dam and support our hypothesis that the drop in concentration of BDE-47 in the dams over lactation was a result of its transfer to milk during lactation. We are currently measuring BDE-47 transfer to milk in lactating mouse dams to better understand the phenomenon.
The blood level of BDE-47 in 7-day old pups born to dams exposed to 1 mg/kg/day of BDE-47 was 516 ng/g, and this was approximately 10-fold higher that blood levels in the dams at the start of lactation (i.e., 56.9 ng/g after 54 days of exposure). This again demonstrates that neonates can accumulate very high levels of BDE-47 through transfer from exposed dams, although at 7 days of age exposure was through a combination of in utero and lactational exposure due to the relatively long terminal half-life of BDE-47 (Staskal et al., 2005). At birth, brain levels of BDE-47 in pups from dams exposed to 1 mg/kg/day were very similar to brain levels in the dams. This shows that even before the start of lactational exposure, pup brains were exposed to approximately the same levels of BDE-47 as the dams, and highlights the risk of the developing fetal brain to these environmental agents.
4.2. Developmental and behavioral effects of perinatal BDE-47 exposure
Pups exposed to 0.1 mg/kg/day BDE-47 had significantly lower body weights than Vehicle controls between PNDs 14–18, and pups exposed to 0.03 mg/kg/day on PNDs 14–16. This dose response function was unexpected, but other dose–response profiles that do not follow an expected dose–response pattern have been reported for other PBDEs. For example, a low and a high dose of BDE-209 were reported to decrease serum triiodothyronine (T3) levels, while an intermediate dose was without apparent effect (Tseng et al., 2008). Additional studies on endocrine disrupting chemicals have also reported U-shaped and/or inverted U-shaped dose–response effects (Ahn et al., 2005; Almstrup et al., 2002). The underlying cellular mechanisms for this phenomenon were not examined in this study and will be the focus of future studies.
Chronic low-dose exposure to BDE-47 in the present study did not affect early developmental milestones for sensory and motor development measured using the Wahlsten test battery (Wahlsten, 1974). This is consistent with the general findings of previous studies that also failed to detect effects of BDE-47 exposure on similar developmental milestones (Suvorov et al., 2009b), although developmental delays have been reported following neonatal exposure to the more highly brominated decabromodiphenyl ether (Rice et al., 2007). Offspring exposed perinatally to the 1.0 mg/kg/day dose of BDE-47 did show an increase in duration of USV when separated from their dams and litters on PND 13. Increased USV in mouse pups may reflect primitive separation anxiety that may predict later adult emotionality (Fish et al., 2000; Nastiti et al., 1991; Scattoni et al., 2009; Winslow et al., 2000), and suggests that perinatal exposure to BDE-47 may affect aspects of emotional development. These effects may also reflect altered responsiveness of the exposed dams to the pup’s vocalizations (D’Amato et al., 2005). It should be noted that USVs have been argued to simply reflect a thermoregulation process in isolated pups (Blumberg and Sokoloff, 2001). In addition, no significant effects of perinatal BDE-47 exposure were found in adult mice in the social dyadic interaction test.
Although early measures of motor development were not altered by exposure to BDE-47, evidence for hypoactivity was seen in pups exposed perinatally to BDE-47 when tested as adults. This was observed in the open field for the female pups exposed to 0.1 or 1.0 mg/kg/day, and in swim speed in the water maze for male and female pups exposed to 1.0 mg/kg/day BDE-47 when compared to Vehicle controls and the low 0.03 mg/kg/day dose group. This effect is similar to results in an earlier study by Eriksson et al. where mice exposed to BDE-47 on PND 10 showed less activity and less rearing than controls during the first 20 min in an open field, although they showed more activity than controls during the last 20 min (Eriksson et al., 2001). These authors concluded that BDE-47 exposure reduced habituation in the open field, but an initial hypoactivity was also clear in their study. A more recent study by Gee and Moser reported increased rearing in an open field in mice exposed to 1 mg/kg, but not to 10 or 30 mg/kg BDE-47 on PND 10 when tested at 32 days of age (Gee and Moser, 2008). Mice in all three exposure groups showed increased rearing at 4 months of age, with no differences across dose levels (Gee and Moser, 2008). Suvorov et al. injected dams (i.v.) with 0.002, 0.02 or 0.2 mg/kg BDE-47 every fifth day from GD 15 to PND 20 and tested pups for activity in the open field on PNDs 15, 20 and 25 (Suvorov et al., 2009b). The lowest exposure group in their study showed significantly increased activity compared to controls, with the highest number of significantly different locomotor parameters seen on PND 20. There was no effect of exposure on rotarod performance which is consistent with the present results. Although the results from the studies described above for locomotor effects of BDE-47 exposure are not consistent, they do indicate that motor function may be a sensitive domain of PBDE developmental toxicity.
The hypoactivity seen in our data is also interesting in view of an epidemiological report that levels of PBDEs, including BDE-47, in human umbilical cord serum at the time of birth were significantly correlated with psychomotor deficits during early development (Herbstman et al., 2010). Similarly, a recent study by Roze et al. (2009) reported that PBDEs and other organohalogens measured in cord serum during the 35th week of gestation were correlated to later neurodevelopmental outcomes. In general, PBDEs were significantly correlated with poor fine manipulative abilities and attention, and better coordination, visual perception and behavior (Roze et al., 2009). These results in humans may be related to the present findings that exposure to BDE-47 appeared to result in mild motor deficits, and shorter swim distances during learning in the water maze. While hippocampal function has been associated with performance in the water maze, unbiased stereological counts of pyramidal neurons in the CA1 subregion of the hippocampus did not reveal any effects of BDE-47 exposure, or any gross cytoarchitectural changes in the hippocampus or other brain regions examined. However, changes may have occurred in other brain structures that were not examined in the present study or at a finer level of structural analysis (e.g., dendrites and synapses).
While both differences and similarities between the present results and previously published studies in mice are apparent, it is difficult to compare studies because the dosing levels, routes and patterns of drug administration, age of treatment, and behavioral endpoints differ so widely. However, taken together, previous studies and the present results are consistent in finding evidence for developmental neurotoxicity from BDE-47 exposure in mice. It is interesting that for many of the behavioral categories examined in this study, and at the doses used, there was little evidence that performance was altered by perinatal exposure for BDE-47. This included tests of social interaction, anxiety, acoustic startle and pre-pulse inhibition, and contextual fear conditioning. Rather, the behavioral domains most affect by neonatal exposure were motor and learning, consistent with the majority of previous reports on BDE exposure, and exposure to BDE-47 in particular.
4.3. Mechanisms of PBDE toxicity
The mechanisms underlying the neurodevelopmental effects of PBDEs, including BDE-47, are not well understood. One possible mechanism is related to the signaling of thyroid hormones (TH) (Zoeller, 2010; Zoeller et al., 2002). PBDEs and their metabolites have a higher binding affinity for TH carrier proteins than the natural ligand and may act as agonists to TH effector proteins leading to altered downstream signaling effects (Meerts et al., 2000; Richardson et al., 2008). Recent studies by Kodavanti et al. and Szabo et al. showed that perinatal exposure to DE-71, a mixture of PBDEs, caused a significant decrease in levels of circulating thyroid hormone, as well as disrupted TH homeostasis by altering deiodination, active transport, glucuronidation and sulfonation in rats (Kodavanti et al., 2010; Szabo et al., 2009). Behavioral studies in animals deficient in TH during early development show impaired learning and changes in activity levels (Negishi et al., 2005). These findings parallel to a degree the findings from the current study with perinatal PBDE exposure, and measurement of TH function would strengthen future studies. Additionally abnormalities in the development of both the hippocampus and cerebellum have been found with hypothyroidism (Anderson, 2008; Kilby, 2003; Williams, 2008). PBDEs may also have more direct actions on the developing nervous system. Eriksson et al. have reported changes in cholinergeric receptors in the hippocampus (Eriksson et al., 2002; Viberg et al., 2003a), as well as altered levels of CaMKII, GAP-43, and BDNF following early postnatal exposure to PBDEs (Viberg et al., 2003b, 2008). BDE-47 exposure alone has been shown to reduce long-term potentiation (LTP) in the hippocampus of postnatally exposed mice, possibly by altering phosphorylation of CaMKII and the expression of glutamate receptor subunits (Dingemans et al., 2007). Additionally, in vitro studies have shown that PBDEs are capable of causing protein kinase C (PKC) translocation, stimulate arachidonic acid release, alter calcium signaling and uptake, increase production of reactive oxygen species (ROS) and induce apoptosis in cultured neuronal cells (Coburn et al., 2008; Dingemans et al., 2010; He et al., 2008; Kim et al., 2010; Kodavanti and Derr-Yellin, 2002; Kodavanti and Ward, 2005; Kodavanti et al., 2005).
4.4. Summary
Perinatal exposure to BDE-47 delayed growth and altered ultrasonic vocalization in neonatal offspring of exposed dams. Exposure to BDE-47 also altered locomotor activity in the open field and escape learning in the Morris water maze when mice were tested as adults. Substantial accumulation of BDE-47 was found in brain, liver and adipose tissue in both dams and their offspring. The results provide additional evidence for developmental neurotoxicity of BDE-47, and indicate the need to further evaluate the risk to human health and development posed by environmental contamination with PBDEs.
Acknowledgments
This study is supported by grant number NIEHS 1 P01 ES11269, the U.S. Environmental Protection Agency (U.S. EPA) through the Science to Achieve Results (STAR) program award numbers R833292 and R829388, the Environmental Protection Agency and the Analytical Core of Superfund Research Program 5P42 ES04699NIEHS. Support for CMK was provided by NIEHS T32ES0007059. The content is solely the responsibility of the investigators and does not necessarily represent the official views of the National Institute of Environmental Health Sciences, the National Institutes of Health or the Environmental Protection Agency. The authors wish to acknowledge Jerome McDonald, Mathew Brown, Binh Ta, Ramona von Leden, Huong Bui, Ryan Keating, Dexter Morin and Lee Rognlie-Howes for their excellent assistance.
Footnotes
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- Ahn NS, Hu H, Park JS, Park JS, Kim JS, An S, et al. Molecular mechanisms of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced inverted U-shaped dose responsiveness in anchorage independent growth and cell proliferation of human breast epithelial cells with stem cell characteristics. Mutat Res. 2005;579:189–99. doi: 10.1016/j.mrfmmm.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Alm H, Scholz B, Fischer C, Kultima K, Viberg H, Eriksson P, et al. Proteomic evaluation of neonatal exposure to 2,2,4,4,5-pentabromodiphenyl ether. Environ Health Perspect. 2006;114:254–9. doi: 10.1289/ehp.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almstrup K, Fernandez MF, Petersen JH, Olea N, Skakkebaek NE, Leffers H. Dual effects of phytoestrogens result in u-shaped dose–response curves. Environ Health Perspect. 2002;110:743–8. doi: 10.1289/ehp.02110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GW. Thyroid hormone and cerebellar development. Cerebellum. 2008;7:60–74. doi: 10.1007/s12311-008-0021-4. [DOI] [PubMed] [Google Scholar]
- Berman RF, Pessah IN, Mouton PR, Mav D, Harry J. Low-level neonatal thimerosal exposure: further evaluation of altered neurotoxic potential in SJL mice. Toxicol Sci. 2008;101:294–309. doi: 10.1093/toxsci/kfm265. [DOI] [PubMed] [Google Scholar]
- Bi X, Qu W, Sheng G, Zhang W, Mai B, Chen D, et al. Polybrominated diphenyl ethers in South China maternal and fetal blood and breast milk. Environ Pollut. 2006;144:1024–30. doi: 10.1016/j.envpol.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Bi X, Thomas GO, Jones KC, Qu W, Sheng G, Martin FL, et al. Exposure of electronics dismantling workers to polybrominated diphenyl ethers, polychlorinated biphenyls, and organochlorine pesticides in South China. Environ Sci Technol. 2007;41:5647–53. doi: 10.1021/es070346a. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Sokoloff G. Do infant rats cry? Psychol Rev. 2001;108:83–95. doi: 10.1037/0033-295x.108.1.83. [DOI] [PubMed] [Google Scholar]
- Branchi I, Capone F, Vitalone A, Madia F, Santucci D, Alleva E, et al. Early developmental exposure to BDE 99 or Aroclor 1254 affects neurobehavioural profile: interference from the administration route. Neurotoxicology. 2005;26:183–92. doi: 10.1016/j.neuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carpenter DO. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev Environ Health. 2006;21:1–23. doi: 10.1515/reveh.2006.21.1.1. [DOI] [PubMed] [Google Scholar]
- Chao HR, Wang SL, Lee WJ, Wang YF, Papke O. Levels of polybrominated diphenyl ethers (PBDEs) in breast milk from central Taiwan and their relation to infant birth outcome and maternal menstruation effects. Environ Int. 2007;33:239–45. doi: 10.1016/j.envint.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Coburn CG, Curras-Collazo MC, Kodavanti PR. In vitro effects of environmentally relevant polybrominated diphenyl ether (PBDE) congeners on calcium buffering mechanisms in rat brain. Neurochem Res. 2008;33:355–64. doi: 10.1007/s11064-007-9430-x. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology. 2007;28:1047–67. doi: 10.1016/j.neuro.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amato FR, Scalera E, Sarli C, Moles A. Pups call, mothers rush: does maternal responsiveness affect the amount of ultrasonic vocalizations in mouse pups? Behav Genet. 2005;35:103–12. doi: 10.1007/s10519-004-0860-9. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Risberg S. Tissue localisation of tetra- and pentabromodiphenyl ether congeners (BDE-47, -85 and -99) in perinatal and adult C57BL mice. Chemosphere. 2006;62:485–93. doi: 10.1016/j.chemosphere.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, Hallgren S. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere. 2007;67:S386–92. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, Ramakers GM, Gardoni F, van Kleef RG, Bergman A, Di Luca M, et al. Neonatal exposure to brominated flame retardant BDE-47 reduces long-term potentiation and postsynaptic protein levels in mouse hippocampus. Environ Health Perspect. 2007;115:865–70. doi: 10.1289/ehp.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, Bergman A, Westerink RH. Calcium-related processes involved in the inhibition of depolarization-evoked calcium increase by hydroxylated PBDEs in PC12 cells. Toxicol Sci. 2010;114:302–9. doi: 10.1093/toxsci/kfp310. [DOI] [PubMed] [Google Scholar]
- Dufault C, Poles G, Driscoll LL. Brief postnatal PBDE exposure alters learning and the cholinergic modulation of attention in rats. Toxicol Sci. 2005;88:172–80. doi: 10.1093/toxsci/kfi285. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Geyer MA. Effects of strain and serotonergic agents on prepulse inhibition and habituation in mice. Neuropharmacology. 2000;39:2170–9. doi: 10.1016/s0028-3908(00)00030-7. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Jakobsson E, Fredriksson A. Brominated flame retardants: a novel class of developmental neurotoxicants in our environment? Environ Health Perspect. 2001;109:903–8. doi: 10.1289/ehp.01109903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Viberg H, Jakobsson E, Orn U, Fredriksson A. A brominated flame retardant, 2,2′,4,4′,5-pentabromodiphenyl ether: uptake, retention, and induction of neurobehavioral alterations in mice during a critical phase of neonatal brain development. Toxicol Sci. 2002;67:98–103. doi: 10.1093/toxsci/67.1.98. [DOI] [PubMed] [Google Scholar]
- Eslami B, Koizumi A, Ohta S, Inoue K, Aozasa O, Harada K, et al. Large-scale evaluation of the current level of polybrominated diphenyl ethers (PBDEs) in breast milk from 13 regions of Japan. Chemosphere. 2006;63:554–61. doi: 10.1016/j.chemosphere.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Fangstrom B, Hovander L, Bignert A, Athanassiadis I, Linderholm L, Grandjean P, et al. Concentrations of polybrominated diphenyl ethers, polychlorinated biphenyls, and polychlorobiphenylols in serum from pregnant Faroese women and their children 7 years later. Environ Sci Technol. 2005;39:9457–63. doi: 10.1021/es0513032. [DOI] [PubMed] [Google Scholar]
- Fernandez MF, Araque P, Kiviranta H, Molina-Molina JM, Rantakokko P, Laine O, et al. PBDEs and PBBs in the adipose tissue of women from Spain. Chemosphere. 2007;66:377–83. doi: 10.1016/j.chemosphere.2006.04.065. [DOI] [PubMed] [Google Scholar]
- File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–7. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- Fish EW, Sekinda M, Ferrari PF, Dirks A, Miczek KA. Distress vocalizations in maternally separated mouse pups: modulation via 5-HT(1A), 5-HT(1B) and GABA(A) receptors. Psychopharmacology. 2000;149:277–85. doi: 10.1007/s002130000370. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs–a review of levels and sources. Int J Hyg Environ Health. 2009;212:109–34. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Gee JR, Moser VC. Acute postnatal exposure to brominated diphenylether 47 delays neuromotor ontogeny and alters motor activity in mice. Neurotoxicol Teratol. 2008;30:79–87. doi: 10.1016/j.ntt.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Goddyn H, Callaerts-Vegh Z, Stroobants S, Dirikx T, Vansteenwegen D, Hermans D, et al. Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol Learn Mem. 2008;123:103–11. doi: 10.1016/j.nlm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–70. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Graham FK. Presidential address, 1974. The more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–48. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJ, et al. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Mol Nutr Food Res. 2008;52:284–98. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- He P, He W, Wang A, Xia T, Xu B, Zhang M, et al. PBDE-47-induced oxidative stress, DNA damage and apoptosis in primary cultured rat hippocampal neurons. Neurotoxicology. 2008;29:124–9. doi: 10.1016/j.neuro.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, et al. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–9. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ Sci Technol. 2004;38:945–56. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Inoue K, Harada K, Takenaka K, Uehara S, Kono M, Shimizu T, et al. Levels and concentration ratios of polychlorinated biphenyls and polybrominated diphenyl ethers in serum and breast milk in Japanese mothers. Environ Health Perspect. 2006;114:1179–85. doi: 10.1289/ehp.9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaraczewska K, Lulek J, Covaci A, Voorspoels S, Kaluba-Skotarczak A, Drews K, et al. Distribution of polychlorinated biphenyls, organochlorine pesticides and polybrominated diphenyl ethers in human umbilical cord serum, maternal serum and milk from Wielkopolska region, Poland. Sci Total Environ. 2006;372:20–31. doi: 10.1016/j.scitotenv.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF, et al. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–54. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Kilby MD. Thyroid hormones and fetal brain development. Clin Endocrinol (Oxf) 2003;59:280–1. doi: 10.1046/j.1365-2265.2003.01804.x. [DOI] [PubMed] [Google Scholar]
- Kim KH, Bose DD, Ghogha A, Riehl J, Zhang R, Barnhart CD, et al. Para- and ortho-substitutions are key determinants of polybrominated diphenyl ether activity toward ryanodine receptors and neurotoxicity. Environ Health Perspect. 2011 doi: 10.1289/ehp.1002728. [Epub ahead of print] doi:10.1289/ehp.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Derr-Yellin EC. Differential effectcs of polybrominated diphenyl ethers and polychlorinated biphenyls on [3H]arachidonic acid release in rat cerebellar granule neurons. Toxicol Sci. 2002;68:451–7. doi: 10.1093/toxsci/68.2.451. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR. Differential effects of commercial polybrominated diphenyl ether and polychlorinated biphenyl mixtures on intracellular signaling in rat brain in vitro. Toxicol Sci. 2005;85:952–62. doi: 10.1093/toxsci/kfi147. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS. Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure–activity relationships. Toxicol Sci. 2005;88:181–92. doi: 10.1093/toxsci/kfi289. [DOI] [PubMed] [Google Scholar]
- Kodavanti PR, Coburn CG, Moser VC, MacPhail RC, Fenton SE, Stoker TE, et al. Developmental exposure to a commercial PBDE mixture, DE-71: neurobehavioral, hormonal, and reproductive effects. Toxicol Sci. 2010;116:297–312. doi: 10.1093/toxsci/kfq105. [DOI] [PubMed] [Google Scholar]
- Kumar A, Baroth A, Soni I, Bhatnagar P, John PJ. Organochlorine pesticide residues in milk and blood of women from Anupgarh, Rajasthan, India. Environ Monit Assess. 2006;116:1–7. doi: 10.1007/s10661-006-7463-2. [DOI] [PubMed] [Google Scholar]
- Lignell S, Aune M, Darnerud PO, Cnattingius S, Glynn A. Persistent organochlorine and organobromine compounds in mother’s milk from Sweden 1996–2006: compound-specific temporal trends. Environ Res. 2009;109:760–7. doi: 10.1016/j.envres.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- McDonald TA. Polybrominated diphenylether levels among United States residents: daily intake and risk of harm to the developing brain and reproductive organs. Integr Environ Assess Manag. 2005;1:343–54. [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, et al. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Nastiti K, Benton D, Brain PF. The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav Pharmacol. 1991;2:121–8. [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Sekiguchi S, Ishii Y, Kyuwa S, Kuroda Y, et al. Attention-deficit and hyperactive neurobehavioural characteristics induced by perinatal hypothyroidism in rats. Behav Brain Res. 2005;159:323–31. doi: 10.1016/j.bbr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Noren K, Meironyte D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40:1111–23. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Oskarsson A, Moller N. A method for studies on milk excretion of chemicals in mice with 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) as a model. Toxicol Lett. 2004;151:327–34. doi: 10.1016/j.toxlet.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94:185–92. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ. Minding the calcium store: ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol Ther. 2010;125:260–85. doi: 10.1016/j.pharmthera.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, et al. High body burdens of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in California women. Environ Health Perspect. 2003;111:1175–9. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC, Reeve EA, Herlihy A, Zoeller RT, Thompson WD, Markowski VP. Developmental delays and locomotor activity in the C57BL6/J mouse following neonatal exposure to the fully-brominated PBDE, decabromodiphenyl ether. Neurotoxicol Teratol. 2007;29:511–20. doi: 10.1016/j.ntt.2007.03.061. [DOI] [PubMed] [Google Scholar]
- Richardson VM, Staskal DF, Ross DG, Diliberto JJ, DeVito MJ, Birnbaum LS. Possible mechanisms of thyroid hormone disruption in mice by BDE 47, a major polybrominated diphenyl ether congener. Toxicol Appl Pharmacol. 2008;226:244–50. doi: 10.1016/j.taap.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ Health Perspect. 2009;117:1953–8. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–15. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect. 2003;111:1723–9. doi: 10.1289/ehp.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Joseph JE, Tung KC. Polybrominated diphenyl ethers (PBDEs) in U.S. computers and domestic carpet vacuuming: possible sources of human exposure. J Toxicol Environ Health A. 2005a;68:501–13. doi: 10.1080/15287390590909715. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J Occup Environ Med. 2005b;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J Toxicol Environ Health A. 2007;70:1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- Schecter A, Harris TR, Shah N, Musumba A, Papke O. Brominated flame retardants in US food. Mol Nutr Food Res. 2008;52:266–72. doi: 10.1002/mnfr.200700166. [DOI] [PubMed] [Google Scholar]
- Schecter A, Haffner D, Colacino J, Patel K, Papke O, Opel M, et al. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect. 2010;118:357–62. doi: 10.1289/ehp.0901345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She J, Holden A, Sharp M, Tanner M, Williams-Derry C, Hooper K. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in breast milk from the Pacific Northwest. Chemosphere. 2007;67:S307–17. doi: 10.1016/j.chemosphere.2006.05.154. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, DeVito MJ, Birnbaum LS. Toxicokinetics of BDE 47 in female mice: effect of dose, route of exposure, and time. Toxicol Sci. 2005;83:215–23. doi: 10.1093/toxsci/kfi018. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Birnbaum LS. Disposition of BDE 47 in developing mice. Toxicol Sci. 2006a;90:309–16. doi: 10.1093/toxsci/kfj098. [DOI] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, Birnbaum LS. Impact of repeated exposure on the toxicokinetics of BDE 47 in mice. Toxicol Sci. 2006b;89:380–5. doi: 10.1093/toxsci/kfj038. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Battista MC, Takser L. Perinatal exposure to low-dose 2,2′,4,4′-tetrabromodiphenyl ether affects growth in rat offspring: what is the role of IGF-1? Toxicology. 2009a;260:126–31. doi: 10.1016/j.tox.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Girard S, Lachapelle S, Abdelouahab N, Sebire G, Takser L. Perinatal exposure to low-dose BDE-47, an emergent environmental contaminant, causes hyperactivity in rat offspring. Neonatology. 2009b;95:203–9. doi: 10.1159/000155651. [DOI] [PubMed] [Google Scholar]
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicol Sci. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms LM, Harden FA, Symons RK, Burniston D, Furst P, Muller JF. Polybrominated diphenyl ethers (PBDEs) in human milk from Australia. Chemosphere. 2007;68:797–803. doi: 10.1016/j.chemosphere.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Tseng LH, Li MH, Tsai SS, Lee CW, Pan MH, Yao WJ, et al. Developmental exposure to decabromodiphenyl ether (PBDE 209): effects on thyroid hormone and hepatic enzyme activity in male mouse offspring. Chemosphere. 2008;70:640–7. doi: 10.1016/j.chemosphere.2007.06.078. [DOI] [PubMed] [Google Scholar]
- Tsydenova OV, Sudaryanto A, Kajiwara N, Kunisue T, Batoev VB, Tanabe S. Organohalogen compounds in human breast milk from Republic of Buryatia, Russia. Environ Pollut. 2007;146:225–32. doi: 10.1016/j.envpol.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2′,4,4′,5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol Sci. 2002;67:104–7. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003a;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Jakobsson E, Orn U, Eriksson P. Neurobehavioral derangements in adult mice receiving decabrominated diphenyl ether (PBDE 209) during a defined period of neonatal brain development. Toxicol Sci. 2003b;76:112–20. doi: 10.1093/toxsci/kfg210. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicol Sci. 2006;92:211–8. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209) Neurotoxicology. 2007;28:136–42. doi: 10.1016/j.neuro.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Viberg H, Mundy W, Eriksson P. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29:152–9. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. A developmental time scale for postnatal changes in brain and behavior of B6D2F2 mice. Brain Res. 1974;72:251–64. doi: 10.1016/0006-8993(74)90863-4. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhao C, Ma W, Liu H, Wang T, Jiang G. Quantitative structure–activity relationship for prediction of the toxicity of polybrominated diphenyl ether (PBDE) congeners. Chemosphere. 2006;64:515–24. doi: 10.1016/j.chemosphere.2005.11.061. [DOI] [PubMed] [Google Scholar]
- Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20:784–94. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–55. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Zhu L, Ma B, Li J, Wu Y, Gong J. Distribution of polybrominated diphenyl ethers in breast milk from North China: implication of exposure pathways. Chemosphere. 2009;74:1429–34. doi: 10.1016/j.chemosphere.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Zoeller TR. Environmental chemicals targeting thyroid. Hormones (Athens) 2010;9:28–40. doi: 10.14310/horm.2002.1250. [DOI] [PubMed] [Google Scholar]
- Zoeller TR, Dowling AL, Herzig CT, Iannacone EA, Gauger KJ, Bansal R. Thyroid hormone, brain development, and the environment. Environ Health Perspect. 2002;110(Suppl 3):355–61. doi: 10.1289/ehp.02110s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]