Abstract
Hedgehog signalling—an essential pathway during embryonic pancreatic development, the misregulation of which has been implicated in several forms of cancer—may also be an important mediator in human pancreatic carcinoma1–8. Here we report that sonic hedgehog, a secreted hedgehog ligand, is abnormally expressed in pancreatic adenocarcinoma and its precursor lesions: pancreatic intraepithelial neoplasia (PanIN). Pancreata of Pdx–Shh mice (in which Shh is misexpressed in the pancreatic endoderm) develop abnormal tubular structures, a phenocopy of human PanIN-1 and -2. Moreover, these PanIN-like lesions also contain mutations in K-ras and overexpress HER-2/neu, which are genetic mutations found early in the progression of human pancreatic cancer. Furthermore, hedgehog signalling remains active in cell lines established from primary and metastatic pancreatic adenocarcinomas. Notably, inhibition of hedgehog signalling by cyclopamine induced apoptosis and blocked proliferation in a subset of the pancreatic cancer cell lines both in vitro and in vivo. These data suggest that this pathway may have an early and critical role in the genesis of this cancer, and that maintenance of hedgehog signalling is important for aberrant proliferation and tumorigenesis.
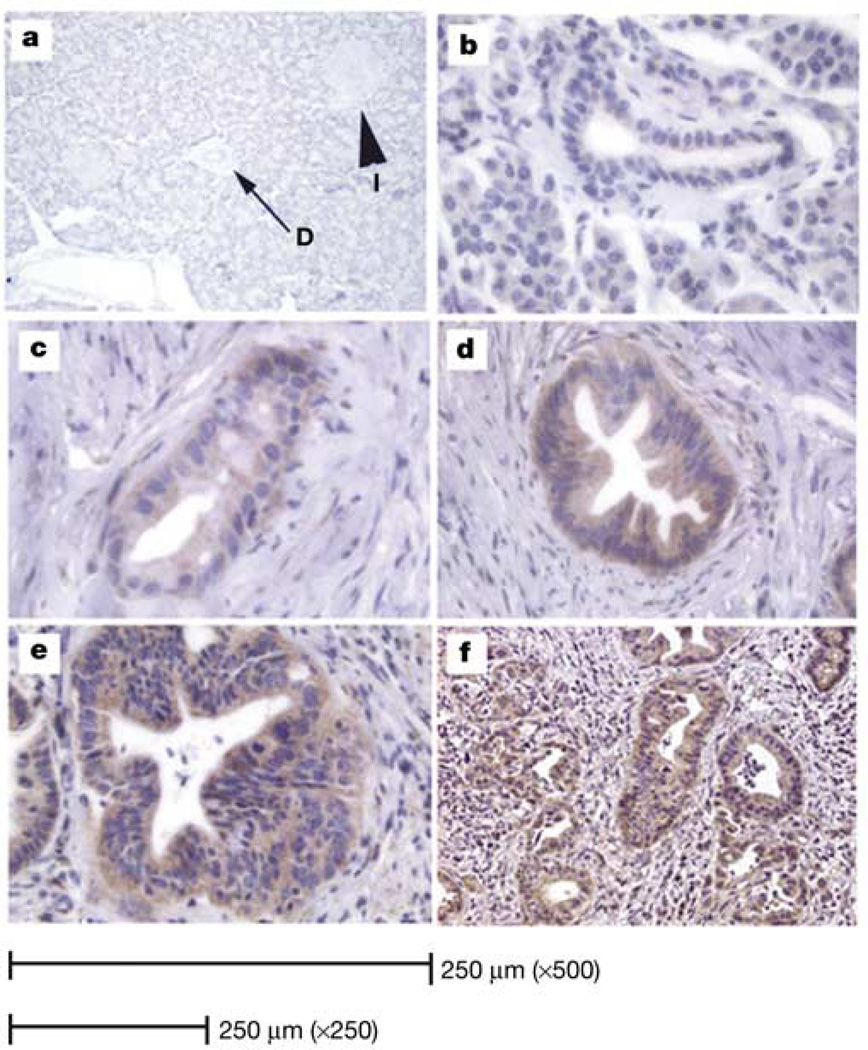
Sonic hedgehog (SHH) is misexpressed in human adenocarcinoma and its precursor lesions. SHH expression was determined using in situ hybridization to detect SHH messenger RNA and immunohistochemistry (IHC) to detect the protein with an antibody directed against SHH9. Pancreatic tissues were obtained from 20 specimens resected for pancreatic cancer. Control pancreatic tissues with no evidence of abnormality or autolysis upon histological evaluation were obtained from autopsy specimens or from pancreatic resections for trauma. In normal adult human pancreata, no SHH was detected in the islets, acini or ductal epithelium (Fig. 1a). However, evaluation of pancreata from patients with adenocarcinoma reveals that SHH is aberrantly expressed in 70% of specimens. Normal ductal epithelium does not express detectable levels of SHH (Fig. 1b); however, as the ductal epithelium shows increasing degrees of atypia, PanIN-1 to -3 (Fig. 1c–e), a higher expression of SHH is observed. SHH expression is also detected in the malignant epithelium of adenocarcinoma samples (Fig. 1f). This expression pattern was also confirmed by our in situ hybridization for SHH mRNA (Supplementary Fig. 1).
Figure 1.
Immunohistochemical identification of SHH. a, Normal human pancreas. No specific staining for SHH protein was identified in acini, islets (I) or ducts (D) (×125 magnification). b, No SHH expression is detected in normal ductal epithelium (×500 magnification). c, PanIN-1 expresses minimal amounts of SHH (×500). d, PanIN-2 expresses moderate levels of SHH (×250). e, PanIN-3 (×250) and f, invasive cancer (×125): moderate to high levels of SHH.
Loss of regulation in this pathway has been implicated in several human cancers10,11. Thus in order to determine the potential role of SHH misexpression in the adult human pancreas, pancreata from transgenic mice (gift of H. Edlund) in which Shh misexpression was driven by the pancreatic-specific Pdx-1 promoter were histologically and immunohistochemically analysed.
A total of four pancreata from three-week-old Pdx–Shh mice were histologically evaluated by a gastrointestinal pathologist (G.Y.L.). All four expressed a significant intestinal phenotype of the pancreatic epithelium. All four also exhibited abnormal tubular complex formations of the epithelium resembling pancreatic cancer precursor lesions (PanINs). These abnormal epithelial changes were characterized and classified according to the pathological classification scheme for human ductal lesions of the pancreas12.
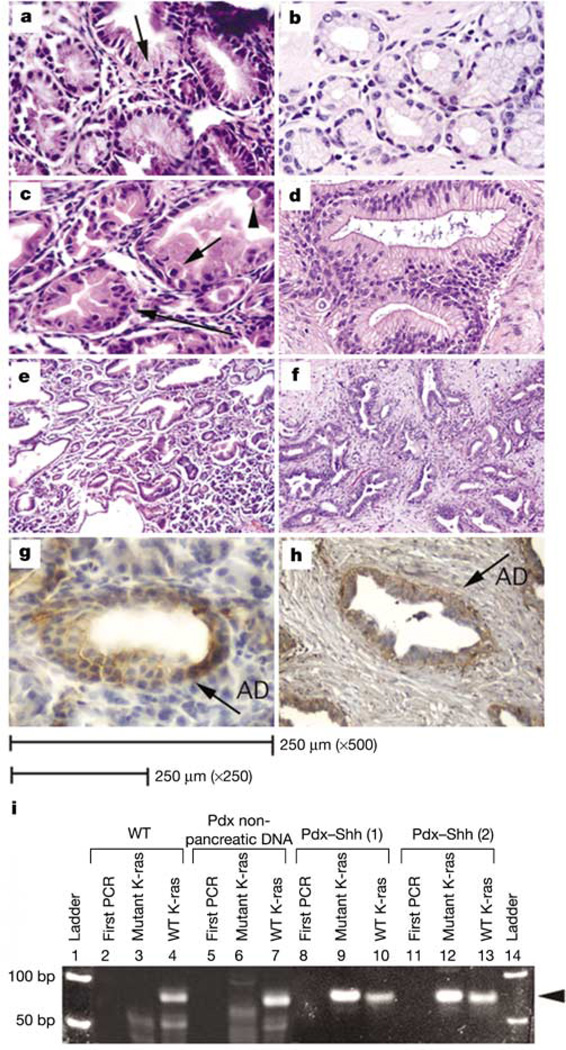
The transgenic pancreata display a mixed phenotype characterized by the transformation of acini into glandular structures lined by tall intestinal-type epithelium embedded in cellular spindle stroma. Focally, the metaplastic epithelium is composed of columnar cells with basally located nuclei and abundant supranuclear mucin (Fig. 2a). This phenotype is similar to human PanIN-1 (Fig. 2b), an early lesion in the stepwise sequence of pancreatic carcinogenesis. Furthermore, some glands are characterized by a lesser degree of mucin production but a higher grade of atypia, with nuclear disarray and stratification, mitosis and apoptosis (Fig. 2c), representing a higher degree of dysplastic change, similar to human PanIN-2 (Fig. 2d). According to the tumour progression model of pancreatic carcinoma, these features are suggestive of an increasing neoplastic potential.
Figure 2.
Histological comparison of human PanINs and Pdx–Shh mouse pancreata. a, Pdx–Shh pancreata display abnormal intestinal epithelial changes (arrow identifies abundant supranuclear mucin) that resemble human PanIN-1 (b) (×500). c, Pdx–Shh pancreata (×500) also exhibit a higher degree of atypia, with nuclear disarray and stratification (long arrow), mitosis (short arrow) and apoptosis (arrowhead), resembling human PanIN-2 (d) (×250). e, f, Low-magnification (×125) view of Pdx–Shh pancreata (e) and tubular complexes seen in human adenocarcinoma (f). g, Pdx–Shh mice (×500) and human PanINs (h) (×250) overexpress HER-2/neu, identified by a brown stain (AD; abnormal ductal epithelium). i, Mutant-allele-specific PCR amplification using mismatched primers (mutant K-ras) to detect codon 12 K-ras mutation. Wild-type primer (WT K-ras) is used as control. Lanes 1 and 14, ladder; 2, 5, 8, 11, first round of PCR (first PCR) reveals no detectable band, as expected. Wild-type mice express only wild-type K-ras (lanes 3, 4). Abnormal pancreatic epithelium of two Pdx–Shh mice expresses a TGT codon 12 mutation of K-ras (lanes 9, 12) and wild-type K-ras (lanes 10, 13), whereas the extrapancreatic tissues of Pdx–Shh mice outside the Shh expression domain express only wild-type K-ras (lanes 6, 7). The arrowhead identifies the 70-bp amplicon.
The histological progression model of pancreatic adenocarcinoma is also associated with progressive genetic alterations13. The transgenic mice pancreata, which histologically resemble PanIN-1 and -2, also express some of the early genetic alterations observed in the human progression model. Three pancreata overexpress HER-2/neu in the epithelium (Fig. 2g). In addition, preliminary data indicate that a codon 12 mutation in K-ras was present in two pancreata (Fig. 2i): changes noted to occur in human PanIN-1 and -2 lesions14,15. Thus the Pdx–Shh mice develop abnormal pancreata with morphological and genetic changes that resemble human ductal PanIN.
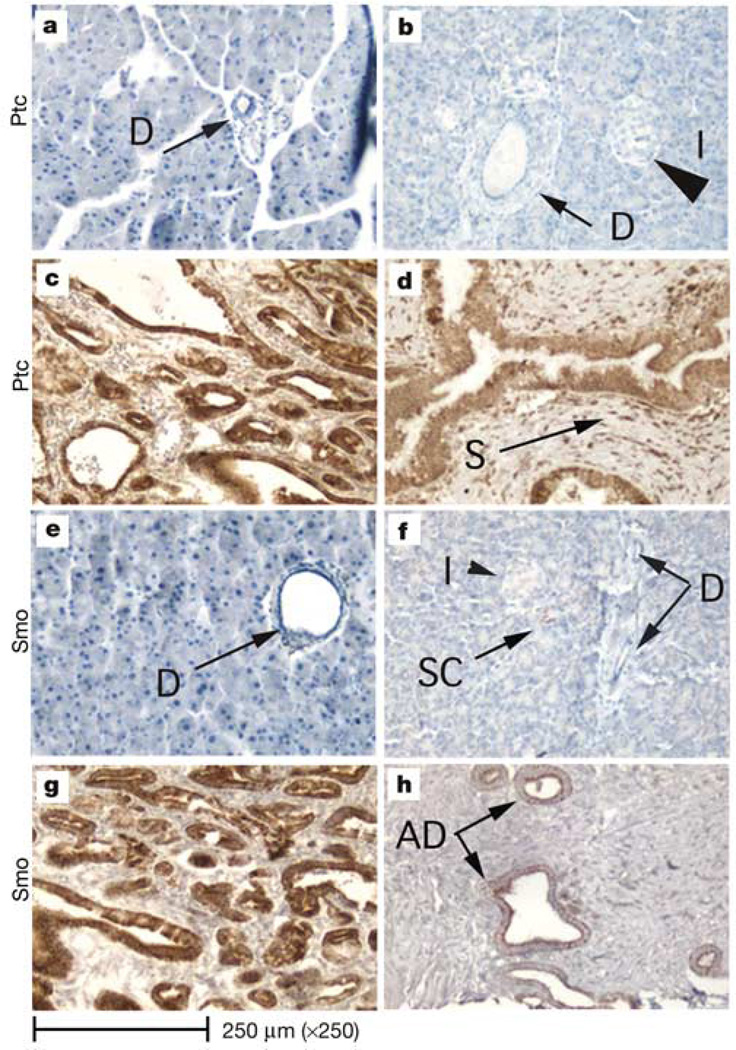
Hedgehog (Hh) signalling is activated through binding of Shh to a membrane receptor, Ptc. This ligand–receptor interaction inhibits Ptc function, thereby allowing the de-repression of another transmembrane protein, smoothened (Smo), which ultimately leads to the activation of the Hh pathway and to the expression of Hh-specific genes, one of which is Ptc1 (ref. 16). Ptc1 expression (determined by IHC) is not detected in normal mouse or human pancreata (Fig. 3a, b). However, Ptc1 protein expression was detected throughout the abnormal epithelium in both Pdx–Shh mice (Fig. 3c) and human neoplastic pancreata (Fig. 3d). Furthermore, in human neoplastic pancreata, PTC1 was also detected in the reactive mesenchymal cells that surround the abnormal epithelium (Fig. 3d).
Figure 3.
Hedgehog signalling pathway. a–d, Immunohistochemical identification of Ptc1 receptor. The Ptc1 receptor is not identified in normal acini, ducts or islets in either mouse (a) or human (b) pancreata. Ptc1 (brown stain) is markedly overexpressed in the abnormal pancreatic epithelium of both Pdx–Shh mice (c) and neoplastic human pancreata (d). In humans, PTC1 is also overexpressed throughout the reactive stroma (S) that surrounds the epithelium. e–h, Immunohistochemical identification of smoothened (Smo). Smoothened is not detected in normal duct epithelium (D) of normal mouse (e) or human (f) pancreata. Very infrequently Smo is detected in scattered cells (SC) in human pancreata; however, Smo (brown stain) is detected in the abnormal epithelium of Pdx–Shh mice (g) and neoplastic human pancreata (h) (arrows). The magnification is ×250 for all images.
Another member of the Hh pathway that is similarly overexpressed in both human neoplastic and mouse transgenic pancreata is Smo. In normal human and mouse pancreata Smo is not generally detected by IHC in the ducts or acini. Very infrequently Smo can be detected in an occasional duct cell or scattered acinar cell within the normal pancreas (Fig. 3f). However, Smo was overexpressed in the abnormal pancreatic epithelium found in Pdx–Shh mice (Fig. 3g), as well as in the epithelium of human adenocarcinoma and precursor lesions (Fig. 3h). The identification of Hh signalling members overexpressed in both mesenchyme and abnormal epithelium suggests that misexpression of Shh may be acting through both a paracrine and an autocrine mechanism to cause many of the morphological changes that characterize pancreatic cancer. To determine whether the HH pathway has an equally important role in the malignant biological behaviour of this tumour, we undertook work with pancreatic cancer cell lines.
Twenty-six human adenocarcinoma lines were screened for expression of HH signalling components. These cell lines were derived either from primary tumours (for example, the Panc series Panc 01.28 to 10.05 (ref. 17)) or from liver, lymph node and spleen metastases (CFPAC1, Hs 766T and SW 1990, respectively). All lines tested expressed two or more components of HH signalling, including PTCH1, SMO, HIP and GLI1 (Supplementary Fig. 2). Thus, sustained HH signalling activity is detected in pancreatic adenocarcinoma cell lines isolated from both primary and metastatic tumours.
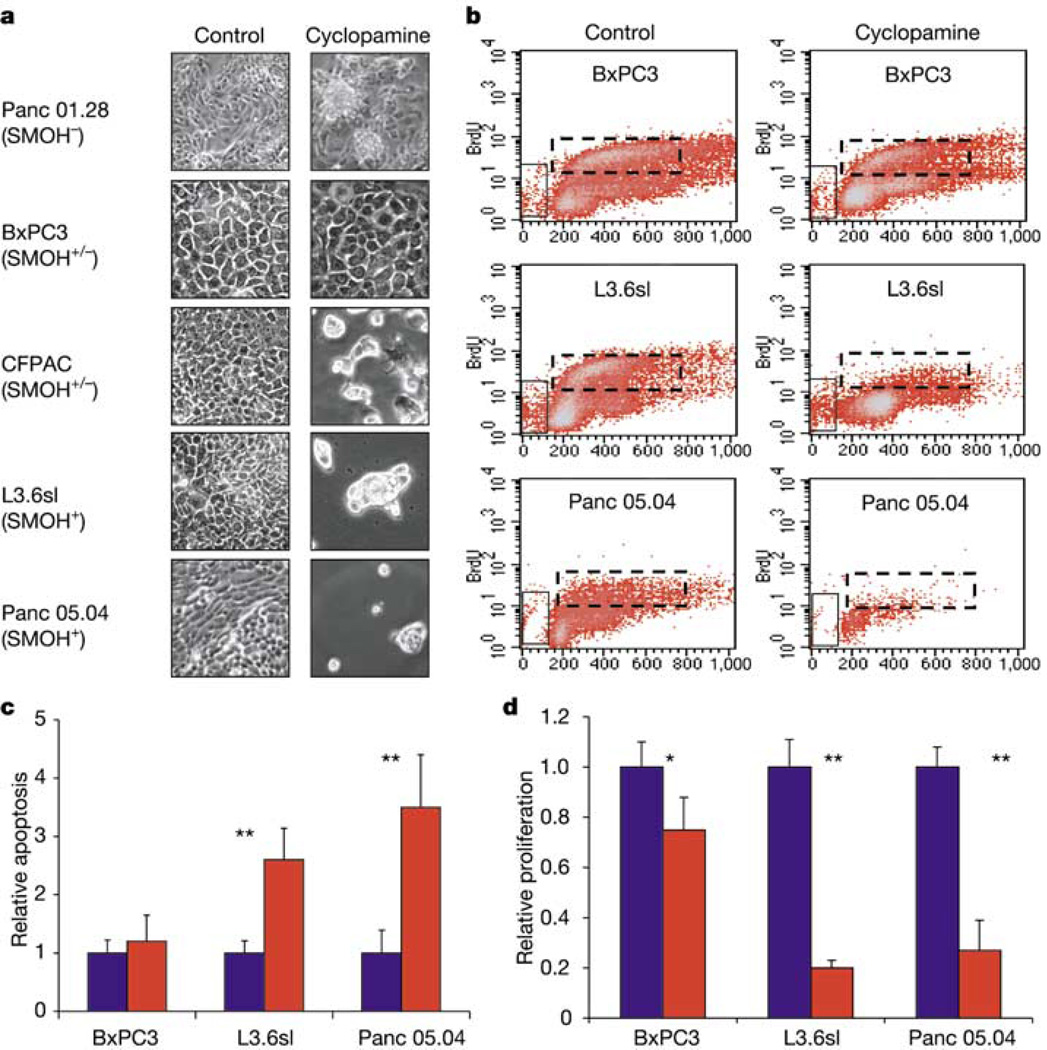
Cyclopamine, a steroidal alkaloid that inhibits Hh signalling through direct interaction with Smo18–20, was used to test whether pancreatic adenocarcinoma cells require HH signalling for proliferation and survival. Initially five pancreatic cancer cell lines—BxPC3, Panc 01.28, CFPAC, L3.6sl and Panc 05.04—were qualitatively assessed. After 7 days of treatment, cyclopamine produced no change in cell density or morphology in control samples that included either untreated cells or cells treated with tomatidine, an inactive cyclopamine analogue. By contrast, a marked reduction in cell density was noted in cell lines CFPAC, L3.6sl and Panc 05.04. Morphologically, the majority of these cells detached from tissue culture plates during the treatment period, and the few remaining cells appeared aplastic (Fig. 4a, and data not shown).
Figure 4.
Effects of cyclopamine treatment on pancreatic adenocarcinoma cells. a, Cell cultures of five pancreatic adenocarcinoma cell lines untreated (control) or treated with 10 µM cyclopamine for 7 days. b, Representative FACS histograms of three cell lines after BrdU incorporation (n = 4). The x axes show DNA content whereas the y axes show BrdU level; vertical boxes mark apoptotic cells; dashed horizontal boxes mark proliferating cells. c, Quantification of apoptotic cells measured in b (n = 4). To compare treated and untreated cells, the number of apoptotic cells in control samples was adjusted to 1. d, Quantification of proliferating cells measured in b (n = 4). To compare treated and untreated cells, the number of proliferating cells in control samples was adjusted to 1. Error bars indicate standard deviation. Asterisk, P < 0.05; double asterisks, P < 0.01. SMOH−, no expression; SMOH+/−, low level expression; SMOH+, strong expression.
Three representative pancreatic cancer cell lines—BxPC3-SMOlow, L3.6sl-SMOhigh and Panc 05.04-SMOhigh—were then used to quantitatively determine the effects of cyclopamine on proliferation and apoptosis by means of FACS analysis after 5-bromodeoxyuridine (BrdU) labelling. Cyclopamine did not induce apoptosis and only marginally reduced proliferation in the BxPC3-SMOlow control cell line. By contrast, apoptosis increased 2.5- to 3.5-fold accompanied by a 75–80% reduction in proliferation in the SMOhigh cyclopamine-responsive cell lines L3.6sl and Panc 05.04 (Fig. 4b–d).
Our initial studies with five cell lines showed that only a subset of pancreatic cancer cell lines are susceptible to cyclopamine treatment. This could be the result of activating mutations of the HH pathway downstream of SMO, which should not be inhibited by this antagonist. To determine the percentage of cancer cell lines susceptible to cyclopamine treatment, we expanded our analysis to a total of 13 lines. Twelve of thirteen lines analysed expressed SMO; half of these (50%) responded to cyclopamine treatment. As predicted, growth of SMO-negative Panc 01.28 cells was not affected (Supplementary Fig. 3). Thus our data suggest that mutations in the HH pathway downstream of SMO might contribute to the aberrant proliferation of these cells. However, we cannot exclude the possibility that mutations in other, HH-independent, pathways regulate proliferation in cyclopamine-resistant pancreatic cancer cell lines.
To demonstrate that cyclopamine-induced inhibition of cancer cell proliferation is secondary to inactivation of the HH pathway, a Gli-luciferase reporter construct containing Gli-binding sites upstream of a TK minimal promoter21 was transfected into control and cyclopamine-responsive cell lines. GLI1 is a downstream transcription factor and transcriptional target of the Hh pathway22. All cell lines tested expressed GLI1 (Supplementary Fig. 2), and transfection of the Gli-reporter construct resulted in significant elevation of luciferase activity (Supplementary Fig. 4a), further indicating that Hh signalling is active in adenocarcinoma cells. Treatment of transfected cells with cyclopamine abolishes luciferase activity in responsive cell lines, whereas control cells display only an insignificant reduction in luciferase activity. Active HH signalling thus appears to be essential for cell survival and proliferation in a subset of cultured human pancreatic cancer cell lines.
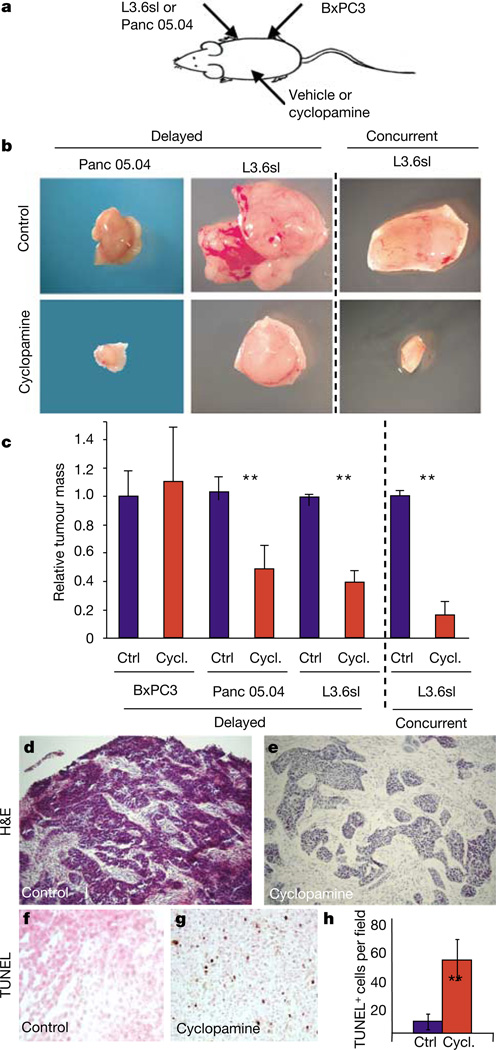
As a more definitive test of the ability of cyclopamine to inhibit tumour cell proliferation, control (BxPC3) and cyclopamine-responsive cells (L3.6sl and Panc 05.04) were injected subcutaneously into immunocompromised nude mice (Fig. 5a). Two treatment models were used—concurrent or delayed treatments—in which cyclopamine was given either at the time of tumour implantation (concurrent) or when the subcutaneous tumours were palpable (delayed). Cyclopamine or carrier control was given for 7 days, at which time the tumours were excised and weighed. In the delayed treatment model, no difference in weight was noted between control and cyclopamine-treated BxPC3-SMOlow tumours (Fig. 5c). By contrast, a 50–60% decrease in tumour mass was observed in Panc 05.04- and L3.6sl-derived tumours, respectively (Fig. 5b, c)—an even more marked effect was noted in the concurrent treatment model, which revealed an 84% reduction in tumour mass of L3.6sl-derived tumours (Fig. 5b, c).
Figure 5.
Cyclopamine treatment blocks tumour formation of human pancreatic adenocarcinoma cells after transplantation into nude mice. a, Schematic indicating sites of tumour cell and cyclopamine/vehicle injections. b, Isolated tumours derived from control or cyclopamine-treated L3.6sl and Panc 05.04 adenocarcinoma cells. Cyclopamine/vehicle injections were initiated either after palpable tumours had formed (delayed) or simultaneously with injection of tumour cells (concurrent). All pictures are shown at the same magnification. c, Weight of isolated tumours. Untreated control tumours of each cell line were adjusted to 1 to allow comparison of relative change in tumour mass. For delayed cyclopamine/vehicle injections, the values are: BxPC3, control 1 (n = 6), cyclopamine 1.1 (n = 5); Panc 05.04, control 1 (n = 5), cyclopamine 0.48 (n = 4); L3.6sl, control 1 (n = 5), cyclopamine 0.39 (n = 4). The values for concurrent cyclopamine/vehicle injections are: L3.6sl, control 1 (n = 4), cyclopamine 0.16 (n = 4). Error bars indicate standard deviation. Double asterisks, P < 0.01. d–h, Histological analysis of the effect of cyclopamine treatment on L3.6sl-derived tumours. d, e, Haematoxylin/eosin staining of sections through the peripheral tumour regions. f, g, TUNEL staining of apoptotic cells in control (f) and cyclopamine-treated (g) tumours. h, Quantification of TUNEL-positive cells in control (blue) and cyclopamine-treated (red) tumours. Error bars indicate s.e.m. Double asterisks, P < 0.01.
Histological analysis of L3.6sl-derived tumours (delayed treatment) revealed a compact mass of epithelial cells in untreated controls, whereas treated tumours appeared as loose epithelial cell aggregates with a larger amount of interspersed mesenchymal cells (Fig. 5d, e). TdT-mediated dUTP nick end labelling (TUNEL) assays demonstrated that cyclopamine treatment resulted in a sixfold increase in apoptotic cells compared with untreated controls (Fig. 5f–h). Therefore both in vitro and in vivo experiments reveal that HH signalling is important for proliferation and survival and thus may be an important mediator of the malignant biological behaviour of pancreatic adenocarcinomas in humans.
The observation that HH signalling has a critical and central role in pancreatic development and results in malignant transformation when mutated prompted us to ask about its potential role in pancreatic cancer16,23–25. Our experiments lead us to believe that misregulation of HH signalling has a critical early role in the initiation and a later role in the maintenance of human pancreatic ductal adenocarcinoma, a hypothesis that is supported by findings in the accompanying paper31. Our evidence is based on comparative analysis of pancreata from Pdx1–Shh transgenic mice and of human adenocarcinoma. We find that HH signalling components are undetectable in normal human ductal epithelium but are strongly expressed in pancreatic precursor (PanIN) and invasive lesions. When Shh is misexpressed in Pdx–Shh mice, we detected the presence of abnormal pancreata with features that histologically, immunohistochemically and genetically resemble human PanIN lesions. Moreover, our analysis of human pancreatic cancer cell lines indicates that the HH signalling pathway is required for both cell division and the suppression of apoptosis. An important but unresolved question concerns the identity of the pancreatic cells that become transformed in response to increased HH signalling. Although pancreatic stem cells have not yet been isolated, results from studies in other tissues suggest that increased HH signalling in progenitor cells can elicit cancer formation. Future studies will be needed to elucidate the role of HH activity in stem-cell-mediated tissue regeneration and pancreatic adenocarcinomas.
Pancreatic cancer, the fourth highest cause of death from cancer in the United States, remains an incurable and rapidly lethal disease, with a 5-yr survival rate of less than 3%. Many factors contribute to this poor prognosis, but ultimately it is our lack of knowledge of the molecular determinants involved in the pathogenesis and progression of this tumour that has limited our ability to design effective treatments. Identification of a role for the HH pathway in the initiation and maintenance of pancreatic cancer suggests that this pathway may hold promise for new diagnostic and therapeutic approaches.
Methods
Tissue preparation
Human tissue specimens were collected in accordance with IRB approval from the Massachusetts General Hospital. Archived specimens were fixed with formalin and paraffin-embedded according to standard institutional protocol. Wild type and Pdx–Shh mouse tissues were fixed in 4% paraformaldehyde, then paraffin-embedded as described above. Tissues were cut into 4–6-µm sections and applied to charged ProbeOn Plus slides (Fisher).
Transgenic mice
Initial pancreatic tissue (gift of H. Edlund) was in a B6/CBA background. Transient transgenic mice (founder mice) were generated by pronuclear injection of a Not1/BamH1 expression cassette containing a 4.5-kilobase (kb) Not1–Nae1 genomic fragment of the Pdx promoter cloned in front of a 2.6-kb Xho1 fragment of full-length rat Shh complementary DNA (vector also a gift of H. Edlund) into a B6/C3F1 background as previously described26.
Immunohistochemistry
Single-antibody detection was accomplished as previously described26, with the following protocol modifications: endogenous peroxidase activity was blocked using 3% H2O2 in methanol for 10 min at room temperature. Antigen retrieval was achieved by boiling tissue in 0.01 M sodium citrate, pH 2.0, 6.0, or 8.0 for 10 min. Tissues were blocked first with 10% NGS in TBS for 40 min at room temperature, then with streptavidin followed by biotin for 20 min each at 37 °C. All primary antibodies were incubated overnight at 4 °C. Primary antibodies used were: Shh, Ptc1 and Smo (1:250; Santa Cruz). Secondary biotinylated antibodies (Vector Laboratories) were applied in 1:500 dilution for 1 h at room temperature. Detection of protein was visualized by brown pigmentation via standard DAB protocol. Slides were lightly counterstained with haematoxylin.
Detection of K-ras codon 12 mutation
The mutant-allele-specific amplification method was used for detection of K-ras codon 12 mutation as previously described27,28. DNA was extracted from microdissected pancreata. Primers used for first-round PCR amplification for K-ras were K12 forward primer 5′-CGCGGCGGCTGAATGACTGA-3′ and K12 reverse primer 5′ -TCGTAGGGTCATACTCATCC -3′. PCR conditions were: 94 °C for 1 min, 54 °C for 1 min, and 72 °C for 0.5 min for 20 cycles. Then a second PCR was performed on 2.0 µl of the first reaction for an additional 40 cycles under similar conditions, using as the new upstream primer either a mismatched primer to amplify the specific mutant band (GGT to TGT), or a wild-type primer, producing a 72-base-pair (bp) mutated or 71-bp wild-type fragment. The second PCR downstream primer sequence is K12 R nest-in 5′ -CCACAAAGTGATTCTGAATTA-3′. Mismatched upstream primer sequence is mutant K12 (TGT) 5′ -TTGTGGTGGTTGGAGCTT-3′; and wild-type sequence is WT K12 5′ -TGTGGTGGTTGGAGCTGG-3′. Amplicons were run on a 10% TBE polyacrylamide gel and stained with SYBR green (Molecular Probes).
Cell culture
Human pancreatic adenocarcinoma cell lines HPAC, SW1990, Mpanc-96, SU86.86, PL45, Panc 10.05, Panc 8.13 and Panc 2.03 were obtained from the American Tissue Culture Collection; cell lines MiaPaCa2, Panc-1, CFPAC1, HPAFII, Capan-2, AsPC1, Hs766T and BxPC3 were a gift from Schering Plough. The cell lines COLO357, L3.3, L3.6sl and L3.6pl were a gift from I. Fidler; cell lines Panc 3.07, Panc 5.04, Panc 2.13, Panc 6.03, Panc 4.21 and Panc 1.28 were a gift from E. Jaffee. BxPC3 and all the Panc cell lines were grown in RPMI medium (Gibco) supplemented with 10% fetal bovine serum (Gibco), l-glutamine and penicillin/streptomycin; medium for Panc cell lines was also supplemented with insulin–transferrin–selenium (Gibco). The CFPAC, Panc1, L3.6sl and L3.6pl cell lines were grown in DMEM without phenol red (CellGro), supplemented with 10% fetal calf serum. To test for cyclopamine responsiveness, cells were grown for 7 days in control medium containing tomatidine (Sigma) or DMSO alone or experimental medium containing cyclopamine (10 µM, Toronto Research Chemicals; the cyclopamine dose–response is shown in Supplementary Fig. 4b). We changed the medium every 2 days. Pictures showing cell morphology were taken with a Nikon Eclipse TE300.
BrdU incorporation assay
Cells were grown for 3 or 4 days in medium containing tomatidine (control, Sigma) or cyclopamine (10 µM, Toronto Research Chemicals). The medium was changed every 48 h. Cells were pulsed with 10 µM BrdU during the final 2 h of culture. BrdU was detected with a fluorescein isothiocyanate-conjugated anti-BrdU antibody (BD Biosciences); total DNA was stained with 7-AAD. FACS analysis was performed according to the BD Biosciences BrdU flow kit instruction manual. Cells in S phase were defined as a cell population that had incorporated BrdU, with DNA content comprising between 2N and 4N. According to the manual, apoptotic cells were defined as a subpopulation of G0/G1 cells with DNA content lower than the diploid amount.
Allograft treatment in vivo
Allograft treatment in vivo was performed according to ref. 19 with minor modifications. A total of 0.1 ml Hanks’ balanced salt solution and matrigel (1:1) containing 2 × 106 cells was injected subcutaneously into CD-1 nude mice. Tumours were grown for 4 days to a minimum volume of 125 mm3; treatment was initiated simultaneously for all subjects. Mice were injected subcutaneously with vector alone (triolein:ethanol 4:1 v/v) or a cyclopamine suspension (1.2 mg per mouse in triolein:ethanol 4:1 v/v) daily for 7 days. At the end of the treatment period, tumours were excised from mice, weighed and then fixed for 3 h at 4 °C with 4% paraformaldehyde, embedded in paraffin wax and sectioned (6 µm). Apoptotic cells were identified by TUNEL using recombinant Tdt (Invitrogen) as previously described29. Sections were then counterstained with eosin. Eight ×20-magnified fields from regions corresponding to the exterior, middle and interior of two control and two cyclopamine-treated tumours were chosen at random. We counted the number of TUNEL-positive nuclei manually. Haematoxylin/eosin staining was done as previously described30.
Supplementary Material
Acknowledgements
S.P.T. and co-workers are grateful to D. A. Melton and P. A. Donahoe for discussion and review of the manuscript; H. Edlund for supplying the construct and initial pancreatic tissue for the Pdx–Shh transgenic mouse; and C. Tabin for the Shh in situ probe. We also thank N. Frost for editorial assistance. This work was supported in part by a grant from the Lustgarten Foundation to S.P.T. M.H. and co-workers thank C. Basbaum, D. Hanahan, I. Herskowitz, T. Kornberg, M. Tempero and members of the Hebrok laboratory for discussions and comments on the manuscript. We thank L. Jaffee for provision of the Panc series of cells. We are grateful to L. Spector for editorial assistance. This work was supported by grants to M.H. from the Juvenile Diabetes Foundation and the National Institutes of Health.
Footnotes
Supplementary Information accompanies the paper on www.nature.com/nature.
Authors’ contributions S.P.T. and M.H. are senior authors.
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Capdevila J, Estrada MP, Sanchez-Herrero E, Guerrero I. The Drosophila segment polarity gene patched interacts with decapentaplegic in wing development. EMBO J. 1994;13:71–82. doi: 10.1002/j.1460-2075.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardcastle Z, Mo R, Hui C-C, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–2811. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- 3.Marigo V, Tabin C. Regulation of patched by sonic hedgehog in the developing neural tube. Proc. Natl Acad. Sci. USA. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 5.St-Jacques B, et al. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- 6.Roberts DJ. In: Development of the Gastrointestinal Tract. Sanderson IR, Walker WA, editors. Decker, Hamilton, Ontario: 2000. pp. 1–12. [Google Scholar]
- 7.Hebrok M. Hedgehog signaling in pancreas development. Mech. Dev. 2003;120:45–47. doi: 10.1016/s0925-4773(02)00331-3. [DOI] [PubMed] [Google Scholar]
- 8.Watkins DN, et al. Hedgehog signaling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 9.van den Brink GR, et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121:317–328. doi: 10.1053/gast.2001.26261. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RL, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 11.Wicking C, Smyth I, Bale A. The hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844–7851. doi: 10.1038/sj.onc.1203282. [DOI] [PubMed] [Google Scholar]
- 12.Hruban R, et al. Pancreatic intraepithelial neoplasia. Am. J. Surg. Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Wilentz RE, et al. Loss of expression of Dpc4 in pancreatic epithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60:2002–2006. [PubMed] [Google Scholar]
- 14.Day JD, et al. Immunohistochemical evaluation of HER-2/neu oncogene expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum. Pathol. 1996;27:119–124. doi: 10.1016/s0046-8177(96)90364-0. [DOI] [PubMed] [Google Scholar]
- 15.Wilentz RE, et al. Inactivation of the p16 (INK4A) tumor-suppressor gene in pancreatic duct lesions: loss of intranuclear expression. Cancer Res. 1998;58:4740–4744. [PubMed] [Google Scholar]
- 16.Hebrok M, Kim SK, St-Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- 17.Jaffee EM, et al. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. Cancer J. Sci. Am. 1998;4:194–203. [PubMed] [Google Scholar]
- 18.Taipale J, et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406:1005–1009. doi: 10.1038/35023008. [DOI] [PubMed] [Google Scholar]
- 19.Berman DM, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 20.Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3 beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Platt KA, Censullo P, Ruiz I Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 23.Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes Dev. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts DJ, Smith DM, Goff D, Tabin C. Epithelial-mesenchymal signaling during the regionalization of the chick gut. Development. 1998;125:2791–2801. doi: 10.1242/dev.125.15.2791. [DOI] [PubMed] [Google Scholar]
- 25.Ramalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development. 2000;127:2763–2772. doi: 10.1242/dev.127.12.2763. [DOI] [PubMed] [Google Scholar]
- 26.Apelqvist A, Ahlegren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr. Biol. 1997;7:801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa Y, et al. Detection of K-ras mutations in DNAs isolated from feces of patients with colorectal tumors by mutant-allele-specific amplification (MASA) Oncogene. 1995;10:1441–1445. [PubMed] [Google Scholar]
- 28.Ito Y, et al. Frequent detection of K-ras mutation in stool samples of colorectal carcinoma patients after improved DNA extraction: Comparison with tissue samples. Int. J. Oncol. 2002;20:1263–1268. [PubMed] [Google Scholar]
- 29.Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1:339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- 30.Sander M, et al. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 31.Berman DM, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. doi: 10.1038/nature01972. (this issue) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.