Abstract
Objectives
Chronic kidney disease (CKD) is common in HIV; CKD is associated with mortality. Urinary markers of tubular injury have been associated with future kidney disease risk, but associations with mortality are unknown.
Methods
We evaluated the association of urinary interleukin-18(IL-18), liver fatty acid binding protein(L-FABP), kidney injury molecule-1(KIM-1), neutrophil gelatinase-associated lipocalin(NGAL), albumin-to-creatinine ratio(ACR) with 10-year, all-cause death in 908 HIV-infected women. Kidney function was estimated using cystatin C (eGFRcys).
Results
There were 201 deaths during 9,269 person-years of follow-up. After demographic adjustment, compared to the lowest tertile, highest tertiles of IL-18 (HR 2.54,95%CI 1.75–3.68), KIM-1 (2.04,1.44–2.89), NGAL(1.50,1.05–2.14), and ACR(1.63,1.13–2.36) were associated with higher mortality. After multivariable adjustment including eGFRcys, only the highest tertiles of IL-18, (1.88,1.29–2.74) and ACR (1.46,1.01–2.12) remained independently associated with mortality. Findings with KIM-1 were borderline (1.41, 0.99–2.02). We found a J-shaped association between L-FABP and mortality. Compared to persons in the lowest tertile, HR for middle tertile of L-FABP was 0.67 (0.46–0.98) after adjustment. Findings were stronger when IL-18, ACR and L-FABP were simultaneously included in models.
Conclusions
Among HIV-infected women, some urinary markers of tubular injury are associated with mortality risk, independently of eGFRcys and ACR. These markers represent potential tools to identify early kidney injury in persons with HIV.
Keywords: HIV, IL-18, KIM-1, L-FABP, NGAL, urinary biomarkers
Introduction
The prevalence of chronic kidney disease (CKD), defined as an estimated glomerular filtration rate (eGFR) <60ml/min/1.73m2, is high among persons with HIV, and CKD is known to be associated with higher mortality risk in the general population and in the setting of HIV. (1–3) Persons with HIV are also at higher risk for death compared to non-HIV infected persons. (4) However, accurate detection of CKD, particularly among HIV-infected persons, remains challenging in clinical practice. Some studies have shown that CKD may be underdiagnosed among persons with HIV because serum creatinine, the clinical standard to evaluate kidney function, is biased by muscle mass. (5) Cystatin C, an alternative filtration marker, is a stronger predictor of mortality among HIV infected persons compared with creatinine. (1, 6) However, both filtration markers are only elevated after the estimated glomerular filtration rate (eGFR) is reduced, and thus kidney disease is already established. The ability of biomarkers to detect kidney damage prior to reductions in eGFR is largely understudied in this population.
Proteinuria, measured either by urinary dipstick or the albumin to creatinine ratio (ACR), has been proposed as an early marker of kidney disease among persons with HIV. The presence of proteinuria has been associated with higher risk of death in this population. (1, 7, 8) However, reliance on albuminuria to detect early kidney damage is limited because it typically reflects glomerular injury, and may not capture damage to other sites of the nephron. Biopsy studies have shown that HIV associated kidney disease can present with a multitude of pathological abnormalities, including tubular and interstitial damage. (9) Recently, several urinary biomarkers that represent injury to the renal tubular cells have been described. Some of these markers, including kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18), liver fatty acid binding protein (L-FABP), and neutrophil gelatinase-associated lipocalin (NGAL) have been identified as markers of acute kidney injury (AKI) in humans. (10–14) More recent work from our group and others has shown that elevated levels of some of these markers may also identify ambulatory persons who are at risk for future kidney function decline and incident CKD. For example, in a case-control study from the Multi-Ethnic Study of Atherosclerosis (MESA), elevated KIM-1 levels were associated with kidney function decline. (15) In the Women’s Interagency HIV Study (WIHS), we showed that elevated levels of IL-18 and KIM-1 were associated with kidney function decline, independently of eGFRcys or albuminuria. (16) Whether levels of these urinary biomarkers are associated with mortality risk has not been studied.
This research question is important because these biomarkers may allow identification of persons with incipient kidney disease, who may already be at risk for complications associated with CKD. Accurate risk stratification of persons with HIV prior to reductions in eGFR may open the window for primary prevention strategies. Moreover, an association of these markers with mortality may provide clues to the underlying mechanisms by which CKD is associated with mortality. Therefore, we designed this study to evaluate the association of levels of urinary markers of kidney injury with all-cause death among HIV infected women participating in the Women’s Interagency HIV Study (WIHS). We hypothesized that subclinical kidney damage captured by higher levels of urinary biomarkers would be independently associated with higher mortality risk.
Methods
Participants
We included 908 HIV infected women participating in the HIV Kidney Aging substudy of the Women’s Interagency HIV Study (WIHS). WIHS is a large observational study designed to understand risk factors for the progression of HIV in women. The WIHS study design and methods have been described previously. (17, 18) In brief, 3,766 women (2,791 HIV-infected and 975 HIV-uninfected) were enrolled in either 1994–1995 (n=2,623) or 2001–2002 (n=1,143). Women were recruited to be representative of the HIV-infected population at the time of enrollment in the following 6 U.S. communities (Bronx/Manhattan, Brooklyn, Chicago, Los Angeles, San Francisco, and Washington, DC). Participants are interviewed and examined every six months. Serum specimens were stored in a −80°C freezer until biomarker measurement.
For these analyses, we utilized data from the WIHS HIV Kidney Aging study. This substudy is a retrospective cohort designed to investigate the onset of kidney disease in the setting of HIV, utilizing stored urine and serum specimens. All HIV-infected women with available specimens were included in this substudy. Single, baseline measures were collected between October 1999 and March 2000. Although 908 HIV-infected and 289 HIV-uninfected women were included in the substudy, only HIV-infected women were included in these analyses (N=908). All 908 HIV infected women had urinary samples from baseline. WIHS was approved by the relevant institutional review boards at all study sites. The ancillary study of kidney injury was also approved by the University of California, San Francisco, San Francisco Veterans Affairs Medical Center, and Yale committees on human research.
Predictors
The urinary injury biomarkers ACR, IL-18, L-FABP, KIM-1, and NGAL were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory. Urine microalbumin and creatinine were measured by immunoturbidimetry and colorimetric enzyme assay, respectively, using a Siemens Dimension Xpand plus HM clinical analyzer (Siemens, Munich, Germany). Urine IL-18 and L-FABP were measured using commercially available ELISA kits (Medical & Biological Laboratories Co., Nagoya, Japan and CMIC Co., Tokyo, Japan, respectively) per manufacturer’s instructions. The urine KIM-1 ELISA was constructed using commercially available reagents (R & D Systems, Inc., Minneapolis, MN). (19) Urine NGAL was assayed using a human-specific commercially available ELISA (AntibodyShop, Grusbakken, Denmark). (20) All urine specimens were in continuous storage without prior freeze-thaw. Laboratory personnel were blinded to clinical information about the participants. Coefficients of variation for the urine measures were: albumin, 5.9%; creatinine, 4.1%; IL-18, 5.2%; L-FABP, 8.9%; KIM-1, 5.2%; and NGAL, 5.4%.
Outcome
The primary outcome of this study was all-cause mortality. Data on vital status and date of death were obtained from medical records, providers, contacts, and the National Death Index. Deaths have been ascertained through May, 2011. Detailed methods have been previously published. (7, 21, 22)
Adjustment Variables
Covariates of interest included demographic characteristics, kidney disease risk factors, HIV-specific risk factors, and kidney disease measures. These were obtained as part of the WIHS semiannual exam. Candidate variables considered were age, race/ethnicity, menopause status, antihypertensive medication use, diabetes (defined as any of the following: fasting glucose ≥126mg/dL, self-reported diabetes, self-reported diabetes medication, or HbA1c ≥6.5), cigarette smoking (current, former, never); systolic and diastolic blood pressure, LDL and HDL cholesterol, triglycerides, body mass index, and waist circumference. GFR was estimated using the CKD Epi equation for cystatin C. (23) Cystatin C was measured by a particle-enhanced immunoturbidometric assay (Gentian, www.atlanticdiagnostics.com), which has been calibrated against the new World Standard Reference material ERM-DA471/IFCC). HIV-related factors included hepatitis C virus (HCV) infection (defined by detectable HCV RNA), current CD4 cell count, nadir CD4 cell count, history of AIDS diagnosis (including low CD4), heroin use, current HIV viral load, HAART use, NRTI use, NNRTI use at baseline, and current PI use. All covariates were measured at baseline, except CD4 count and HIV RNA which were time-updated. Multiple imputation with the Markov chain Monte Carlo method was used to impute missing data for other covariates, with 5 imputations to yield ~95% relative efficiency. The percentage of missing observations for each covariate ranged from less than 1% to 15%.
Statistical Analysis
We first present baseline characteristics of the 908 HIV-infected women included in this study. We estimated correlations of urinary biomarkers using Spearman correlation. Then, we evaluated the association of each injury marker with all-cause mortality using multivariable Cox proportional hazards regression. We first analyzed the urine injury biomarkers as continuous (log-transformed) predictors of mortality, but the assumption of linearity did not hold for all measures. We therefore present results with the biomarkers categorized into tertiles, as we have done in a prior study. (16) To determine whether each injury marker was independently associated with mortality, multivariable models were sequentially adjusted for: 1) demographics, and 2) traditional kidney disease risk factors, HIV-specific risk factors, eGFRcys and ACR. Factors forced in the full model included age, race/ethnicity, eGFRcys, ACR (for all the other biomarkers), hypertension and diabetes, current HIV viral load, current CD4 cell count, and HCV infection. Specifically, we included eGFRcys and ACR because these are established markers of kidney disease and they are strongly associated with mortality. We used a stepwise backward selection with a significance level of α=0.05 to remove candidate covariates that were not associated with the outcome. To allow each marker to compete as an independent predictor of mortality, we constructed a final model that concurrently adjusted for those markers that remained statistically significantly associated with mortality after full adjustment above.
In our initial phase of analyses, we observed that the association between urinary L-FABP and mortality appeared J-shaped. To understand joint associations of L-FABP and IL-18 as predictors of death, we compared the age-adjusted rates of mortality across L-FABP tertiles, stratified by IL-18 tertiles. Finally, to understand the utility of measuring several biomarkers concurrently, we evaluated the age-adjusted rates of mortality among persons who had 1, 2, 3 or more biomarkers in the “worst” tertile (i.e. indicating “worst” prognosis).
We conducted several sensitivity analyses. First, we repeated our analyses using the slope of eGFRcys as the adjustment variable. Decline in eGFRcys has been shown to be an independent predictor of adverse outcomes. Moreover, we believe that a useful urinary biomarker of subclinical kidney injury should be associated with adverse outcomes independently of change in kidney function over time. Second, we repeated our L-FABP analyses after exclusion of persons with Hepatitis C infection because L-FABP levels are known to vary in liver disease. We tested for an interaction by eGFRcys to examine whether associations were consistent across the eGFRcys spectrum. Since serum creatinine remains the clinical standard for eGFR estimation, we also conducted a sensitivity analysis accounting for eGFRcreat rather than eGFRcys, using the CKD Epi equation. (24) Finally, we used a complete case approach to model the association of urine biomarkers with mortality, excluding participants who were missing any of the covariates in the model. The largest fraction of missing data in our analysis was for Hepatitis C (11%), while 2–3% of subjects were missing CD4 or HIVRNA values. All other covariates were missing for less than 1% of subjects. All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Participant Characteristics
Among 908 HIV infected women, mean (SD) age was 41±8 years, and mean eGFRcys was 88±21 ml/min/1.73m2 at baseline. Thirty-one percent of participants had undetectable viral load, 31% had concomitant hepatitis C infection, and 24% had albumin to creatinine ratio ≥30 mg/g. (Table 1)
Table 1.
Baseline Characteristics of HIV-Infected Women in the WIHS Cystatin C Study
| Characteristic | All HIV+ Participants (N = 908) |
|---|---|
| Baseline Age,* years | 41 (36–46) |
| Race, N (%) | |
| African American | 524 (58) |
| Caucasian | 175 (19) |
| Other | 209 (23) |
| Post-Menopausal, N (%) | 185 (21) |
| Cigarette Smoking, N (%) | |
| Current | 464 (51) |
| Past | 224 (25) |
| Never | 220 (24) |
| Diabetes Mellitus, N (%) | 86 (9) |
| Systolic Blood Pressure,* mmHg | 118 (108–129) |
| Diastolic Blood Pressure,* mmHg | 72 (66–80) |
| Hypertension, N (%) | 228 (25) |
| Antihypertensive Use, N (%) | 98 (11) |
| LDL,* mg/dL | 103 (80–132) |
| HDL,* mg/dL | 44 (36–56) |
| Triglycerides,* mg/dL | 133 (93–196) |
| Body Mass Index,* kg/m2 | 26.5 (23.2–31.1) |
| Waist Circumference,* cm | 88 (80–99) |
| Current HAART Use, N (%) | 533 (59) |
| Current NRTI Use, N (%) | 606 (67) |
| Current NNRTI Use, N (%) | 246 (27) |
| Current PI Use, N (%) | 381 (42) |
| Current CD4 Count,* cells/mm3 | 397 (245–576) |
| Nadir CD4 Count,* cells/mm3 | 212 (109–326) |
| History of AIDS, N (%) | 445 (49) |
| Plasma HIV RNA, N (%) | |
| ≤80 copies/mL | 276 (31) |
| 81–1,999 | 204 (23) |
| 2,000–9,999 | 147 (16) |
| >10,000 | 275 (30) |
| Hepatitis C, N (%) | 281 (31) |
| Current heroin Use, N (%) | 43 (5) |
| Albuminuria,** N (%) | 183 (20) |
Data are presented as Median (IQR) where indicated; otherwise presented as N (%). **Defined as urine albumin-creatinine ratio >30 mg/g
Abbreviations: Interquartile range (IQR); Nucleoside reverse transcriptase inhibitor (NRTI); Non-nucleoside reverse transcriptase inhibitor (NNRTI); Protease inhibitor (PI)
Median (IQR) biomarker concentrations were: IL-18 122 (110–133) pg/mL, L-FABP 4.5 (1.8–9.5), KIM-1 483 (439–535) pg/mL, NGAL 36.4 (32.7–39.6) ng/mL, and ACR 10.0 (9.2–11.1) mg/g. Urinary tubular injury biomarkers were moderately intra-correlated, with most correlations ranging from 0.4 to 0.6. The correlation coefficients with ACR were: −0.018 for IL-18, 0.087 for L-FABP, 0.014 for KIM-1, and 0.119 for NGAL.
Overall, there were 201 deaths during 9,269 person-years of follow-up. Mean (SD) follow-up was 10.2 ± 2.4 years (median: 11.4, IQR: 10.3–11.5). In demographic-adjusted analyses, highest tertiles of IL-18, KIM-1, NGAL, and ACR were associated with increased ten-year mortality risk (Table 2), and the middle tertiles of IL-18 and ACR were also associated with increased risk.
Table 2.
Association of Urine Biomarkers With All-Cause Mortality in HIV-Infected WIHS Participants
| Hazard Ratio (95% Confidence Interval)
|
|||||
|---|---|---|---|---|---|
| Deaths/N | Demographic-Adjusted | P Value | Fully-Adjusted* | P Value | |
| IL-18 (pg/ml) | |||||
| Tertile 1 | 43/302 | reference | reference | ||
| Tertile 2 (80–196) | 62/303 | 1.53 (1.03, 2.28) | 0.035 | 1.23 (0.83, 1.84) | 0.31 |
| Tertile 3 (>196) | 96/303 | 2.54 (1.75, 3.68) | <0.0001 | 1.88 (1.29, 2.74) | 0.001 |
| L-FABP (ng/ml) | |||||
| Tertile 1 | 63/303 | reference | reference | ||
| Tertile 2 (2.7–7.3) | 51/303 | 0.73 (0.50, 1.06) | 0.095 | 0.67 (0.46, 0.98) | 0.04 |
| Tertile 3 (>7.3) | 87/302 | 1.22 (0.87, 1.71) | 0.24 | 0.92 (0.65, 1.31) | 0.65 |
| KIM-1 (pg/ml) | |||||
| Tertile 1 | 49/302 | reference | reference | ||
| Tertile 2 (318–721) | 55/303 | 1.00 (0.67, 1.48) | 0.99 | 0.93 (0.63, 1.38) | 0.72 |
| Tertile 3 (>721) | 97/303 | 2.04 (1.44, 2.89) | <0.0001 | 1.41 (0.99, 2.02) | 0.06 |
| NGAL (ng/ml) | |||||
| Tertile 1 | 55/302 | reference | reference | ||
| Tertile 2 (22–57) | 65/303 | 1.09 (0.76, 1.57) | 0.64 | 0.96 (0.66, 1.39) | 0.83 |
| Tertile 3 (>57) | 81/303 | 1.50 (1.05, 2.14) | 0.024 | 1.05 (0.74, 1.51) | 0.78 |
| ACR (mg/g) | |||||
| Tertile 1 | 48/302 | reference | reference | ||
| Tertile 2 (7.1–16) | 69/303 | 1.51 (1.04, 2.19) | 0.029 | 1.46 (1.00, 2.13) | 0.05 |
| Tertile 3 (>16) | 84/303 | 1.63 (1.13, 2.36) | 0.0084 | 1.46 (1.01, 2.12) | 0.045 |
Fully-adjusted Cox models control for age, ethnicity, traditional kidney risk factors, and HIV-related risk factors (all measured at baseline, except for CD4 and HIV RNA, which are time-updated). Traditional kidney risk factors include smoking, hypertension, diabetes, ACR, and eGFRcystatin. HIV-related risk factors include CD4 count, HIVRNA, and HCV. Biomarkers are included in the model individually
In fully adjusted models, persons in the highest tertile of IL-18 had over 80% higher risk for death compared with the lowest tertile, and this association remained significant even after adjustment for ACR and eGFRcys. For comparison, persons in the highest tertile of ACR had an almost 50% higher mortality risk, compared to the lowest tertile. Persons in the highest tertile of KIM-1 were at higher mortality risk, but this association was substantially attenuated after adjustment and no longer statistically significant. Levels of NGAL showed little association with mortality after adjustment. (Table 2) We observed a J-shaped association between L-FABP and mortality risk. Persons in the middle tertile of L-FABP had a 27% lower risk for death in demographic-adjusted analysis and a 33% lower risk after multivariable adjustment, compared with the lowest tertile. (Table 2)
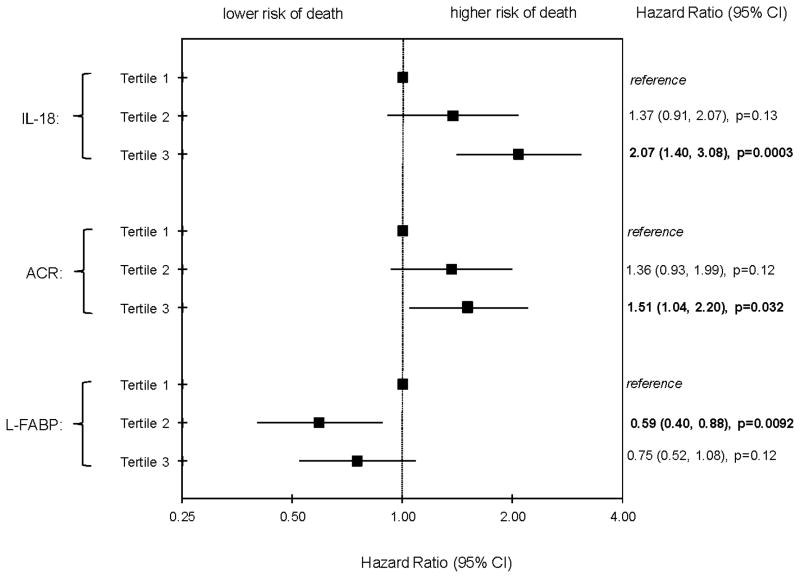
We next evaluated the joint associations of IL-18, L-FABP and ACR tertiles in the fully adjusted analyses. Persons in the highest tertile of IL-18 remained at a two-fold mortality risk compared to the lowest tertile. The middle tertile of L-FABP was associated with a 40% lower mortality risk, and the highest tertile appeared to have a moderately lower risk compared to the lowest tertile, but the latter finding was not statistically significant (p=0.12). The highest tertile of ACR remained associated with a 50% higher mortality risk. (Figure 1) When we also added KIM-1 to the joint model, IL-18 remained strongly associated with mortality: HR 1.95, 95%CI 1.29 to 2.93 for highest tertile compared to lowest. Findings were also similar for L-FABP, HR 0.60, 95%CI 0.41 to 0.89. In contrast, associations of KIM-1 with mortality were much weaker in the joint model: HR for highest tertile of KIM-1 compared to lowest was 1.23 (95%CI 0.81 to 1.87).
Figure 1.
Associations of Urine Biomarkers (Concurrently Adjusting for Each Other) with All-Cause Mortality in HIV-Infected WIHS Participants
Note: Fully-adjusted Cox models control for age, ethnicity, traditional kidney risk factors, and HIV-related risk factors (all measured at baseline, except for CD4 and HIVRNA, which are time-updated). Traditional kidney risk factors include smoking, hypertension, diabetes, ACR, and eGFRcystatin. HIV-related risk factors include CD4 count, HIVRNA, and HCV.
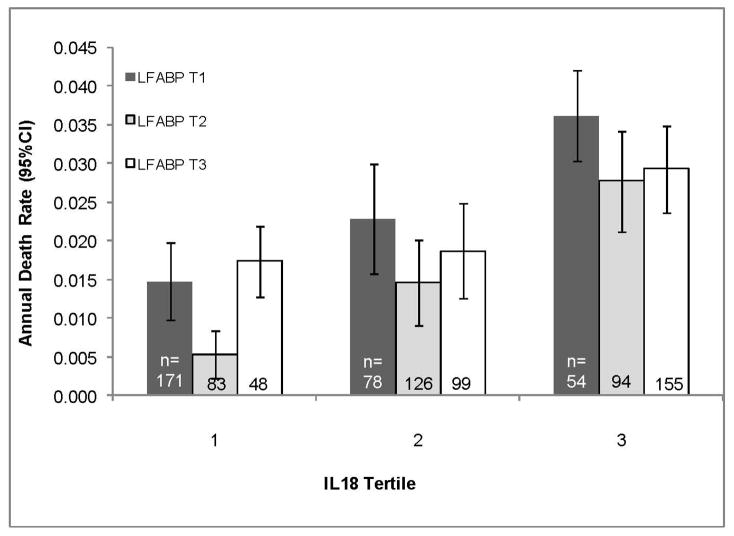
Due the unexpected direction of association between L-FABP and mortality, we further examined the joint associations of IL-18 and L-FABP with mortality. We found that similar association between L-FABP and mortality was observed within each tertile of IL-18, and that increasing IL-18 was associated with mortality, regardless of level of L-FABP (overall test for interaction from full model: p = 0.22). (Figure 2)
Figure 2.
Age-adjusted Mortality Rates by Tertile of IL-18 and L-FABP in HIV-Infected WIHS Participants
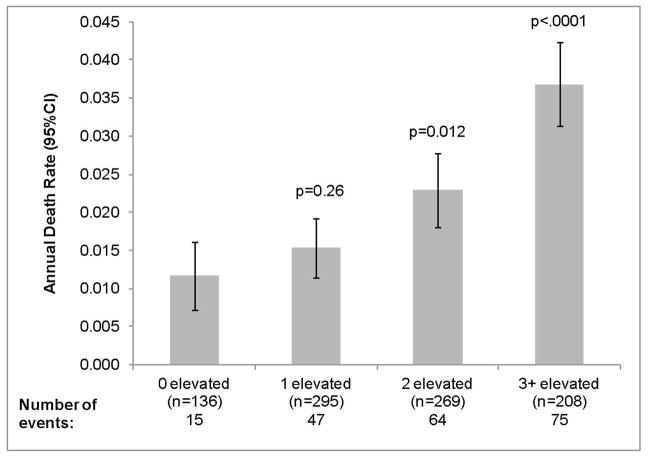
Finally, we evaluated additive associations of urine biomarkers with mortality risk by categorizing each participant as having an “abnormal” level if the biomarker was within the “worst” tertile (defined as the lowest tertile for L-FABP, highest tertile for all others). We found a progressive increase in mortality risk by number of elevated biomakers (Figure 3), whereby those with 3 or more abnormal markers had a three-fold higher death rate compared to those with no abnormal markers. (Figure 3)
Figure 3.
Association of Number* of Elevated Urine Biomarkers with All-Cause Mortality
*Number of the five biomarkers in the “worst” tertile (IL-18, NGAL, KIM-1, ACR, L-FABP)
Note: P-values denote comparison with 0 elevated category.
Rates above are age-adjusted.
Sensitivity Analyses
We found that adjustment for the slope rather than baseline eGFRcys did not change our findings. For example, persons in the highest tertile of IL-18 had a HR of 1.99 (95%CI 1.37 to 2.87) compared to the lowest. For L-FABP, HR for middle tertile was 0.68 (95%CI 0.47 to 0.99) compared with the lowest. Similar results were found in an additional sensitivity analysis in which we excluded persons with hepatitis C: HR=1.87 (95%CI 1.01 to 3.47) for tertile 3 vs. tertile 1 of IL-18; HR=0.55 (95%CI 0.30 to 0.99) for tertile 2 vs. tertile 1 of L-FABP, and HR=1.50 (95%CI 0.87 to 2.58) for tertile 3 vs. tertile 1 of ACR. We found no statistically significant interactions of eGFRcys with IL-18 (p=0.70), ACR (p=0.97), or L-FABP (p=0.57) on mortality. We also conducted a sensitivity analysis adjusting for eGFRcreat rather than eGFRcys. In fully adjusted models, concurrently adjusting for the other markers, the associations of each marker with death were not materially different. For example, compared to the lowest tertile of urinary IL-18, the HR for death for the highest tertile was 2.13 (1.44, 3.13). For ACR, HR was 1.56 (1.08, 2.26). The middle tertile of L-FABP had the lowest risk for death, with a HR of 0.65 (0.44, 0.95), compared with the lowest tertile. Finally, results using a complete case analysis showed that higher IL-18 (HR=1.66, p=0.02 for tertile 3 vs. tertile 1) and higher ACR (HR=1.75, p=0.007 for T3 vs. T1) remained independently associated with increased risk of mortality after adjustment, while L-FABP had a J-shaped association with mortality (HR=0.63, p=0.04 for T2 vs. T1).”
Discussion
In this cohort of ambulatory HIV infected women, we found that urinary biomarkers of tubular injury, representing subclinical kidney damage, are associated with all-cause mortality, independently of both eGFRcys and ACR. Specifically, we found that persons with urinary levels of IL-18 in the highest tertile were at double the mortality risk compared to persons in the lowest tertile, an effect size even larger than that observed for ACR in this cohort. While higher levels of KIM-1 were associated with doubling in mortality risk in demographic adjusted models, these findings were attenuated and non-significant after full adjustment, as were findings for NGAL. Interestingly, urinary levels of L-FABP appeared to have a J-shaped association with death. Our findings suggest that urinary biomarkers of tubular injury in the kidney are elevated even before the eGFR is reduced, are reflective of injury in parts of the nephron other than the glomerulus, and have strong and independent associations with mortality risk.
To our knowledge, this is the first study to show that urinary markers of tubular injury are associated with death in an ambulatory cohort. Previous studies in humans have shown that higher levels of urinary IL-18 and NGAL were associated with higher mortality risk in the setting of acute kidney injury in critically ill patients and after cardiac surgery. (13, 14) In a recent study, higher levels of KIM-1 were also associated with higher mortality risk or dialysis in persons with AKI presenting to an emergency room. (25) Mechanisms to explain why urinary IL-18 and, to a lesser extent, KIM-1 may be associated with higher mortality risk in ambulatory persons with HIV are not established. The most likely explanation is that these markers are able to capture subclinical kidney damage to sites other than the glomerulus, which are not captured by eGFR or ACR due to the kidney’s large reserve ability. In turn, this early injured kidney may have adverse effects on pathways such as inflammation, mineral metabolism regulation, acid buffering, hemodynamic regulation, and medication metabolism which may be associated with death. This hypothesis is supported by the strong and consistent associations of other kidney markers (cystatin C and albuminuria) with mortality in HIV-infected persons. (7, 8)
A second plausible hypothesis is that the subclinical kidney damage captured by these markers reflects parallel, ongoing injury in multiple organ systems. For example, in a Japanese population, persons with diabetic nephropathy had high urinary and plasma levels of IL-18. These persons also had high plasma levels of inflammatory markers, greater carotid intima thickness and increased arterial stiffness. (26) Future studies that measure urinary levels of kidney injury markers along with serum markers of cardiac, endothelial and inflammatory injury are needed to further elucidate observed associations.
The finding that the association of L-FABP appeared somewhat J-shaped and that the middle tertile had the lowest mortality risk is noteworthy. L-FABP is expressed in the renal proximal tubules, and high levels in the urine are associated with acute kidney injury in humans. In vitro and transgenic mice studies suggest that L-FABP is involved in metabolism of fatty acids and may have a protective role as an antioxidant. (27, 28) In fact, mice who cannot express L-FABP in renal tubular cells have the highest risk of renal injury after exposure to cisplatin. (27) Interestingly, a recent study showed that administration of fibrates to mice prior to exposure to cisplatin reduces renal injury, and that this protection may be due to increased expression of L-FABP. (29) It is possible that low levels of L-FABP in the urine may represent dysregulation of these protective mechanisms, but further research is necessary to understand these associations.
The finding that urinary NGAL was not a strong predictor of death in this cohort is also interesting. Urinary NGAL is a strong predictor of AKI and higher urine and serum levels have been associated with CKD progression. (30) Data show that a monomer of NGAL is associated with fibrosis in the kidney. (31) Future studies in other ambulatory cohorts using multiple NGAL assays are required to confirm our findings.
Our study expands on prior work from our group and others that showed early decrements in eGFR (higher than the threshold for CKD) to be associated with increased mortality risk in both HIV and non-HIV populations; (8, 32, 33) we now show associations at even earlier stages of kidney damage. In addition, we recently showed that higher urinary levels of IL-18 and KIM-1 are associated with kidney function decline in MESA and in WIHS. (15, 16) We believe that a particular strength of our report is that associations with death were independent of both eGFRcys and ACR. Our findings are supported by pathological studies demonstrating that HIV disease can be associated with injury to multiple sites of the nephron. (9, 34) In addition, we believe that any novel marker should be demonstrated to have associations independent of established markers before they can be considered for clinical use.
We must note several limitations. We do not have direct measures of GFR or kidney biopsies, but these are not possible in cohort studies. The assays for urinary biomarkers were performed from frozen samples and at only one point in time, and this may have resulted in protein degradation and cannot capture changes over time. However, this is more likely to bias results towards the null. We are aware that alternative assays are available to measure some of these urinary biomarkers. We believe differences in assays could result in different effect sizes, but would be less likely to affect the overall findings. We did not measure plasma levels of IL-18 or NGAL. Prior investigations from our group have shown that urine and plasma IL-18 are poorly correlated. Moreover, urinary, not plasma, levels of NGAL and IL-18 predict kidney dysfunction after transplantation. (35, 36) Therefore, although serum IL-18 may reflect systemic inflammation, we believe that urine IL-18 is specific to kidney damage, rather than a reflection of plasma levels. We are not able to discern cause of death. This study was conducted in HIV-infected women, and it remains unknown whether findings are applicable to other populations. However, our findings are robust even after accounting for time-dependent HIV related factors. Future research is required to further elucidate observed associations.
In summary, we found that levels of urinary biomarkers of tubular injury are associated with mortality risk in an ambulatory cohort of HIV-infected women. If replicated in other cohorts, these biomarkers may be useful in risk stratification of persons with and without HIV for both the onset of CKD and its adverse consequences. In addition, the observed pattern and direction of associations with each biomarker may provide clues toward understanding why kidney disease is associated with mortality.
Acknowledgments
Sources of Funding
Dr. Peralta is funded by the National Institutes of Health (NIDDK 23) and the Robert Wood Johnson Foundation Harold Amos Program. Dr. Parikh is a co-inventor on IL-18 patent issues to University of Colorado. Dr. Devarajan is a co-inventor on patents related to the use of NGAL as a marker of acute and chronic kidney injury. The WIHS Kidney Aging Study is funded by grant 1R01AG034853-01A2 (PI Shlipak), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Collaborative Study Group with centers (Principal Investigators) at New York City/Bronx Consortium (Kathryn Anastos); Brooklyn, NY (Howard Minkoff); Washington DC Metropolitan Consortium (Mary Young); The Connie Wofsy Study Consortium of Northern California (Ruth Greenblatt); Los Angeles County/Southern California Consortium (Alexandra Levine); Chicago Consortium (Mardge Cohen); Data Coordinating Center (Stephen Gange). The WIHS is funded by the National Institute of Allergy and Infectious Diseases (UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, UO1-AI-34989, UO1-AI-34993, and UO1-AI-42590) and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (UO1-HD-32632). The study is co-funded by the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute on Deafness and Other Communication Disorders. Funding is also provided by the National Center for Research Resources (UCSF-CTSI Grant Number UL1 RR024131). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Author Contributions
Drs. Peralta, Scherzer and Shlipak had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Transparency Declarations
There are no conflicts of interest to disclose. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis. 2010;56:872–882. doi: 10.1053/j.ajkd.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Volberding PA, O’Hare AM. The impact of HIV on chronic kidney disease outcomes. Kidney Int. 2007;72:1380–1387. doi: 10.1038/sj.ki.5002541. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 4.Cockerham L, Scherzer R, Zolopa A, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167:2213–2219. doi: 10.1001/archinte.167.20.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driver TH, Scherzer R, Peralta CA, et al. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS. 2013 doi: 10.1097/QAD.0b013e328362e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyatt CM, Hoover DR, Shi Q, et al. Pre-existing albuminuria predicts AIDS and non-AIDS mortality in women initiating antiretroviral therapy. Antivir Ther. 2011;16:591–596. doi: 10.3851/IMP1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121:651–658. doi: 10.1161/CIRCULATIONAHA.109.898585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyatt CM, Morgello S, Katz-Malamed R, et al. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2008 doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolignano D, Coppolino G, Campo S, et al. Neutrophil gelatinase-associated lipocalin in patients with autosomal-dominant polycystic kidney disease. Am J Nephrol. 2007;27:373–378. doi: 10.1159/000103912. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson MA, Vaidya VS, Waikar SS, et al. Urinary liver-type fatty acid-binding protein predicts adverse outcomes in acute kidney injury. Kidney Int. 2009 doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siew ED, Ikizler TA, Gebretsadik T, et al. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parikh CR, Devarajan P, Zappitelli M, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CA, Katz R, Bonventre JV, et al. Associations of Urinary Levels of Kidney Injury Molecule 1 (KIM-1) and Neutrophil Gelatinase-Associated Lipocalin (NGAL) With Kidney Function Decline in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2012 doi: 10.1053/j.ajkd.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shlipak MG, Scherzer R, Abraham A, et al. Urinary Markers of Kidney Injury and Kidney Function Decline in HIV-Infected Women: Biomarkers and Kidney Decline. J Acquir Immune Defic Syndr. 2012 doi: 10.1097/QAI.0b013e3182737706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkan SE, Melnick SL, Preston-Martin S, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology. 1998;9:117–125. [PubMed] [Google Scholar]
- 18.Bacon MC, von Wyl V, Alden C, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaturvedi S, Farmer T, Kapke GF. Assay validation for KIM-1: human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen MH, French AL, Benning L, et al. Causes of death among women with human immunodeficiency virus infection in the era of combination antiretroviral therapy. Am J Med. 2002;113:91–98. doi: 10.1016/s0002-9343(02)01169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French AL, Gawel SH, Hershow R, et al. Trends in mortality and causes of death among women with HIV in the United States: a 10-year study. J Acquir Immune Defic Syndr. 2009;51:399–406. doi: 10.1097/QAI.0b013e3181acb4e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J Am Coll Cardiol. 2012;59:246–255. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura A, Shikata K, Hiramatsu M, et al. Serum interleukin-18 levels are associated with nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Care. 2005;28:2890–2895. doi: 10.2337/diacare.28.12.2890. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto T, Noiri E, Ono Y, et al. Renal L-type fatty acid--binding protein in acute ischemic injury. J Am Soc Nephrol. 2007;18:2894–2902. doi: 10.1681/ASN.2007010097. [DOI] [PubMed] [Google Scholar]
- 28.Wang G, Gong Y, Anderson J, et al. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005;42:871–879. doi: 10.1002/hep.20857. [DOI] [PubMed] [Google Scholar]
- 29.Negishi K, Noiri E, Maeda R, Portilla D, Sugaya T, Fujita T. Renal L-type fatty acid-binding protein mediates the bezafibrate reduction of cisplatin-induced acute kidney injury. Kidney Int. 2008;73:1374–1384. doi: 10.1038/ki.2008.106. [DOI] [PubMed] [Google Scholar]
- 30.Bolignano D, Lacquaniti A, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nickolas TL, Forster CS, Sise ME, et al. NGAL (Lcn2) monomer is associated with tubulointerstitial damage in chronic kidney disease. Kidney Int. 2012;82:718–722. doi: 10.1038/ki.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 33.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–1552. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruggeman LA, Ross MD, Tanji N, et al. Renal epithelium is a previously unrecognized site of HIV-1 infection. J Am Soc Nephrol. 2000;11:2079–2087. doi: 10.1681/ASN.V11112079. [DOI] [PubMed] [Google Scholar]
- 35.Hall IE, Doshi MD, Poggio ED, Parikh CR. A comparison of alternative serum biomarkers with creatinine for predicting allograft function after kidney transplantation. Transplantation. 2011;91:48–56. doi: 10.1097/TP.0b013e3181fc4b3a. [DOI] [PubMed] [Google Scholar]
- 36.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21:189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]