Abstract
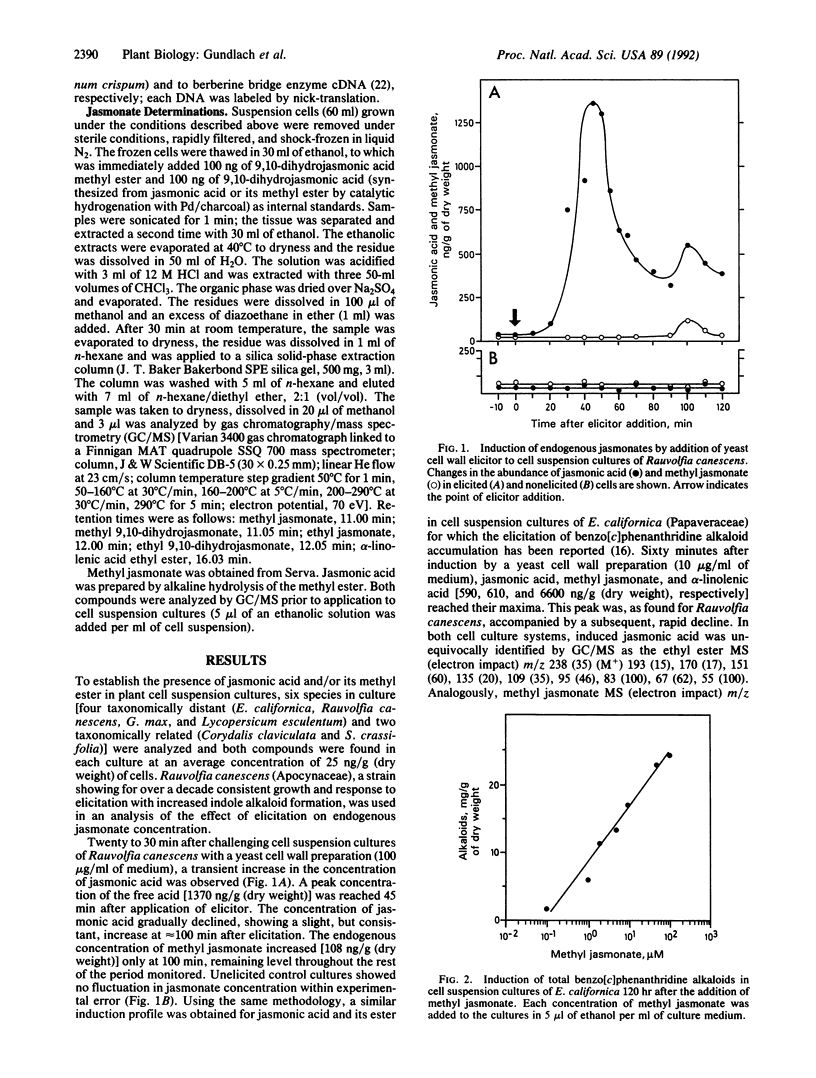
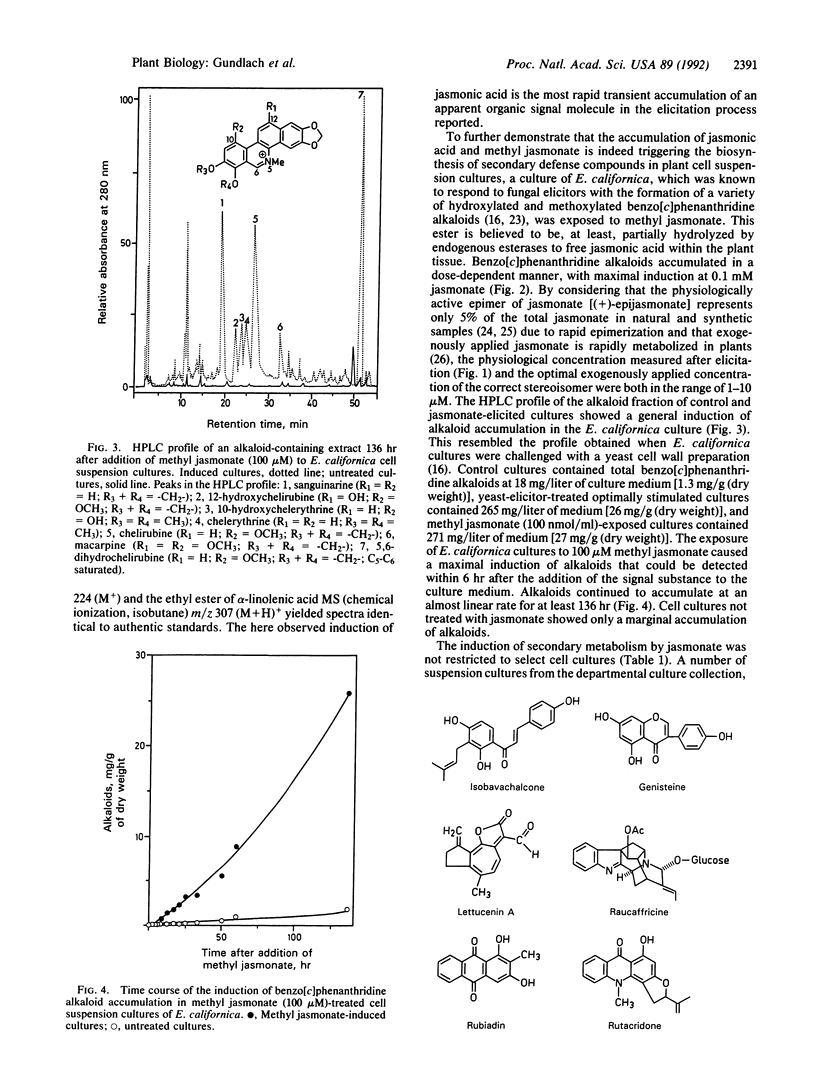
To deter pathogenic microorganisms and herbivores, plants have developed an inducible chemical defense system. It is known that the induced synthesis of low molecular weight compounds can be provoked by exposing cultured cells to fungal cell wall fragments. In this study we show that endogenous jasmonic acid and its methyl ester accumulate rapidly and transiently after treatment of plant cell suspension cultures of Rauvolfia canescens and Eschscholtzia californica with a yeast elicitor. Thirty-six plant species tested in cell suspension culture could be elicited with respect to the accumulation of secondary metabolites by exogenously supplied methyl jasmonate. Addition of methyl jasmonate initiates de novo transcription of genes, such as phenylalanine ammonia lyase, that are known to be involved in the chemical defense mechanisms of plants. These data demonstrate the integral role of jasmonic acid and its derivatives in the intracellular signal cascade that begins with interaction of an elicitor molecule with the plant cell surface and results, ultimately, in the accumulation of secondary compounds.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albersheim P., Valent B. S. Host-pathogen interactions in plants. Plants, when exposed to oligosaccharides of fungal origin, defend themselves by accumulating antibiotics. J Cell Biol. 1978 Sep;78(3):627–643. doi: 10.1083/jcb.78.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C. J., Watson D. G. Terpenoid phytoalexins. Nat Prod Rep. 1991 Aug;8(4):367–389. doi: 10.1039/np9910800367. [DOI] [PubMed] [Google Scholar]
- Cosio E. G., Frey T., Verduyn R., van Boom J., Ebel J. High-affinity binding of a synthetic heptaglucoside and fungal glucan phytoalexin elicitors to soybean membranes. FEBS Lett. 1990 Oct 1;271(1-2):223–226. doi: 10.1016/0014-5793(90)80411-b. [DOI] [PubMed] [Google Scholar]
- Dittrich H., Kutchan T. M. Molecular cloning, expression, and induction of berberine bridge enzyme, an enzyme essential to the formation of benzophenanthridine alkaloids in the response of plants to pathogenic attack. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9969–9973. doi: 10.1073/pnas.88.22.9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel J., Ayers A. R., Albersheim P. Host-Pathogen Interactions: XII. Response of Suspension-cultured Soybean Cells to the Elicitor Isolated from Phytophthora megasperma var. sojae, a Fungal Pathogen of Soybeans. Plant Physiol. 1976 May;57(5):775–779. doi: 10.1104/pp.57.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Ryan C. A. Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi V. R., Grimes H. D. Induction of soybean vegetative storage proteins and anthocyanins by low-level atmospheric methyl jasmonate. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6745–6749. doi: 10.1073/pnas.88.15.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M. G., Albersheim P. Host-Pathogen Interactions: XIV. Isolation and Partial Characterization of an Elicitor from Yeast Extract. Plant Physiol. 1978 Jul;62(1):107–111. doi: 10.1104/pp.62.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocourek J., Ballou C. E. Method for fingerprinting yeast cell wall mannans. J Bacteriol. 1969 Dec;100(3):1175–1181. doi: 10.1128/jb.100.3.1175-1181.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois R., Dietrich A., Hahlbrock K., Schulz W. A phenylalanine ammonia-lyase gene from parsley: structure, regulation and identification of elicitor and light responsive cis-acting elements. EMBO J. 1989 Jun;8(6):1641–1648. doi: 10.1002/j.1460-2075.1989.tb03554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G., Strydom D., Johnson S., Ryan C. A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science. 1991 Aug 23;253(5022):895–897. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Tanahashi T., Zenk M. H. New hydroxylated benzo[c]phenanthridine alkaloids from Eschscholtzia californica cell suspension cultures. J Nat Prod. 1990 May-Jun;53(3):579–586. doi: 10.1021/np50069a007. [DOI] [PubMed] [Google Scholar]
- Ueda J., Kato J. Isolation and Identification of a Senescence-promoting Substance from Wormwood (Artemisia absinthium L.). Plant Physiol. 1980 Aug;66(2):246–249. doi: 10.1104/pp.66.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. Biosynthesis of jasmonic Acid by several plant species. Plant Physiol. 1984 Jun;75(2):458–461. doi: 10.1104/pp.75.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick B. A., Zimmerman D. C. The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun. 1983 Mar 16;111(2):470–477. doi: 10.1016/0006-291x(83)90330-3. [DOI] [PubMed] [Google Scholar]


