Abstract
Recipients of kidney transplants (KTR) are at increased risk for cardiovascular events, graft failure, and death. It is unknown whether urine kidney injury biomarkers are associated with poor outcomes among KTRs. We conducted a post hoc analysis of the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial using a case-cohort study design, selecting participants with adjudicated cardiovascular events, graft failure, or death. Urine neutrophil gelatinase–associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, and liver–type fatty acid binding protein (L-FABP) were measured in spot urine samples and standardized to urine creatinine concentration. We adjusted for demographics, cardiovascular risk factors, eGFR, and urine albumin-to-creatinine ratio. Patients had 291 cardiovascular events, 257 graft failure events, and 359 deaths. Each log increase in urine NGAL/creatinine independently associated with a 24% greater risk of cardiovascular events (adjusted hazard ratio [aHR], 1.24; 95% confidence interval [95% CI], 1.06 to 1.45), a 40% greater risk of graft failure (aHR, 1.40; 95% CI, 1.16 to 1.68), and a 44% greater risk of death (aHR, 1.44; 95% CI, 1.26 to 1.65). Urine KIM-1/creatinine and IL-18/creatinine independently associated with greater risk of death (aHR, 1.29; 95% CI, 1.03 to 1.61 and aHR, 1.25; 95% CI, 1.04 to 1.49 per log increase, respectively) but not with risk of cardiovascular events or graft failure. Urine L-FABP did not associate with any study outcomes. In conclusion, among prevalent KTRs, higher urine NGAL, KIM-1, and IL-18 levels independently and differentially associated with greater risk of adverse outcomes.
Keywords: cardiovascular disease, kidney transplantation, mortality
Despite improvements in short–term patient and graft outcomes, long–term clinical outcomes of recipients of kidney transplants (KTRs) remain suboptimal.1,2 The leading cause of death in KTRs is cardiovascular disease (CVD), and KTRs also have significant risk of long–term graft failure.1 Identification of biomarkers that are independent risk factors for adverse outcomes may provide insight into underlying pathophysiologic mechanisms relevant to KTRs.
Urine biomarkers of kidney tubular injury have been studied in patients with native CKD3,4 and the general population.5–7 A number of these biomarkers, including neutrophil gelatinase–associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), IL-18, and liver–type fatty acid binding protein (L-FABP), were initially described in patients with acute tubular necrosis/AKI.8–11 NGAL is a 25-kD protein expressed in the kidney, liver, and epithelial cells in response to various pathologic states, such as inflammation, infection, intoxication, ischemia, AKI, and neoplastic transformation.12 NGAL is filtered in the glomerulus, and luminal NGAL is reabsorbed in the proximal tubule. In the setting of kidney injury, proximal tubule reabsorption of systemically produced NGAL is impaired, and NGAL production is increased in the distal nephron and shed into the urine, leading to higher urine NGAL levels.13,14 Urine KIM-1 is a phosphatidylserine receptor that recognizes apoptotic cells, directing them to lysosomes, and it also serves as a receptor for oxidized lipoproteins.15–17 KIM-1 transforms kidney proximal epithelial cells into phagocytes and may act to modulate immune response in kidney injury.16,17 IL-18 is a known mediator of inflammation and found in monocytes, fibroblasts, and proximal renal tubular epithelial cells.18,19 IL-18 mediates ischemic proximal tubule injury and proinflammatory responses through its actions on Toll-like receptor 4.20 L-FABP expression and urinary excretion increase under conditions of tubular stress. It is hypothesized that free fatty acids, which are, in part, albumin bound, exert oxidative stress on cells and that L-FABP inhibits the accumulation of intracellular fatty acids by promoting fatty acid metabolism.21–24
Among KTRs, previous studies have shown strong associations of serum creatinine and albuminuria with poor clinical outcomes, similar to patients with native CKD. Numerous studies in patients with CKD have recently suggested that, independent of serum creatinine, eGFR, and levels of albuminuria, tubular injury markers are risk factors for adverse outcomes, such as kidney failure, death, and CVD.3,4,6,25–28 It has been suggested that GFR and albuminuria mostly reflect glomerular disease, whereas these injury biomarkers reflect renal tubular disease. Published studies of urine injury biomarkers among KTRs largely have been limited to the peritransplant setting and focused on associations with short-term outcomes, such as delayed graft function or in-hospital mortality.29–33 It is plausible that these biomarkers of tubular injury or stress may signal earlier systemic disease or graft disease that contributes to adverse cardiovascular or renal outcomes among KTRs. We studied the associations of urine NGAL, KIM-1, IL-18, and L-FABP with long–term adverse outcomes, including cardiovascular events, graft failure, and all-cause mortality, among prevalent KTRs enrolled in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial, a randomized clinical trial of homocysteine lowering by B vitamins. We hypothesized that, similar to patients with native kidney disease,3,4 elevations in these urine injury biomarkers would be associated with poor clinical outcomes among KTRs, independent of traditional kidney measures.
Results
Participant Characteristics
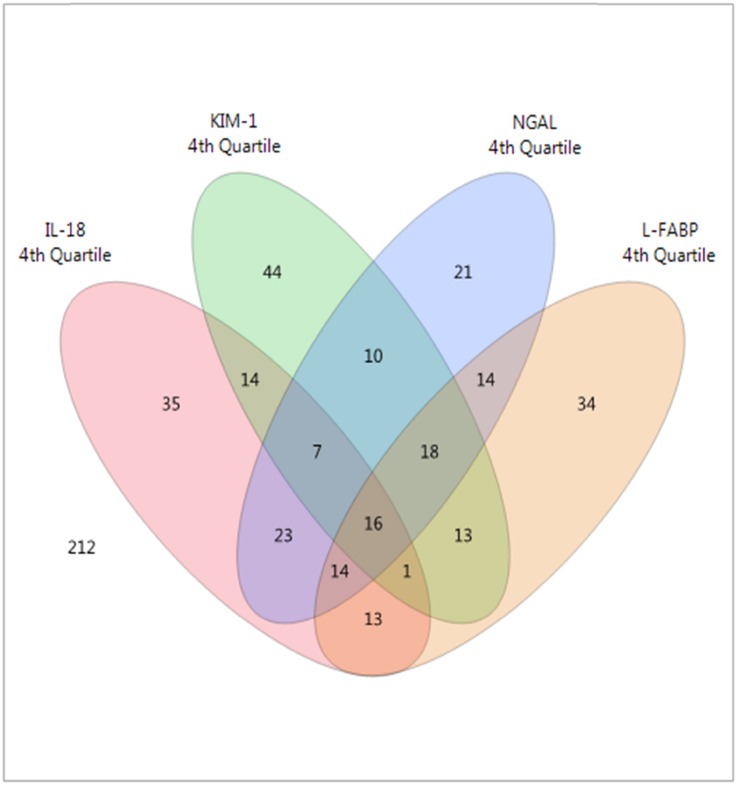
The mean age (±SD) of the random subcohort was 51 (±9) years old, and eGFR was 46 (±18) ml/min per 1.73 m2. Median (interquartile range) urine albumin-to-creatinine ratio (ACR) was 24.5 (9.5–104.7) μg/mg, urine NGAL was 20.2 (8.2–51.3) ng/ml, urine KIM-1 was 658 (319–1364) pg/ml, urine IL-18 was 29.1 (11.5–62.5) pg/ml, and urine L-FABP was 6.1 (3.0–17.6) ng/ml (Supplemental Table 1, column 1). Participants with the highest quartile of urine NGAL/creatinine were more likely to be women, have a lower eGFR, and have a higher urine ACR (Supplemental Table 1a). Participants with the highest quartile of urine KIM-1/creatinine were more likely to be women and have higher urine ACR and less likely to have received a living donor kidney (Supplemental Table 1b). Participants with the highest quartile of urine IL-18/creatinine were more likely to be women and have higher urine ACR. Graft vintage was longer among participants in the lowest quartile of urine IL-18/creatinine, and the biomarker distributions differed by country (Supplemental Table 1c). Participants with the highest quartile of urine L-FABP/creatinine were more likely to use sirolimus and have higher BP, lower eGFR, and higher urine ACR (Supplemental Table 1d). Overall, only 3% of KTRs (16 of 489 from the random subcohort) were in the highest quartile of all four urine biomarkers (as illustrated in Figure 1, with the Venn diagram showing overlap).
Figure 1.
Only 3% of KTRs were in the highest quartile of all four urine biomarkers. Venn diagram of the overlap of the top quartile of urine NGAL/creatinine, KIM-1/creatinine, IL-18/creatinine, and L-FABP/creatinine in the subcohort (n=489).
Participants who had a cardiovascular event or a graft failure or died were more likely to be from the United States, have older graft vintage, have higher systolic BP, have lower eGFR, and have higher urine ACR (Table 1).
Table 1.
Baseline participant characteristics by outcome in the case-cohort study
| Characteristics | Cardiovascular Events | Graft Failure | All-Cause Death | |||
|---|---|---|---|---|---|---|
| Nonevents (n=438) | Events (n=291) | Nonevents (n=439) | Events (n=257) | Nonevents (n=423) | Events (n=359) | |
| Age (yr) | 51.1±8.9 | 54.6±9.3 | 51.7±9.2 | 49.4±8.5 | 50.7±8.7 | 55.9±9.6 |
| Women | 174 (40%) | 100 (34%) | 174 (40%) | 90 (35%) | 161 (38%) | 133 (37%) |
| Race | ||||||
| White | 328 (75%) | 221 (76%) | 338 (77%) | 180 (70%) | 317 (75%) | 264 (74%) |
| Black | 81 (18%) | 54 (19%) | 75 (17%) | 60 (23%) | 78 (18%) | 72 (20%) |
| Other | 29 (7%) | 16 (5%) | 26 (6%) | 17 (7%) | 28 (7%) | 23 (6%) |
| Treatment group | ||||||
| High-dose vitamin | 216 (49%) | 147 (51%) | 211 (48%) | 135 (53%) | 211 (50%) | 180 (50%) |
| Low-dose vitamin | 222 (51%) | 144 (49%) | 228 (52%) | 122 (47%) | 212 (50%) | 179 (50%) |
| Location | ||||||
| United States | 298 (68%) | 233 (80%) | 302 (69%) | 217 (84%) | 283 (67%) | 287 (80%) |
| Canada | 52 (12%) | 35 (12%) | 50 (11%) | 26 (10%) | 55 (13%) | 36 (10%) |
| Brazil | 88 (20%) | 23 (8%) | 87 (20%) | 14 (5%) | 85 (20%) | 36 (10%) |
| Graft vintage (yr), median (25th, 75th) | 3.9 (1.8, 7.0) | 4.4 (1.9, 7.9) | 3.8 (1.7, 6.8) | 5.0 (2.3, 8.5) | 3.8 (1.8, 7.1) | 4.4 (1.8, 7.9) |
| Living donor kidney | 193 (44%) | 95 (33%) | 192 (44%) | 82 (32%) | 190 (45%) | 102 (28%) |
| History of CVD | 72 (16%) | 122 (42%) | 83 (19%) | 60 (23%) | 72 (17%) | 121 (34%) |
| History of diabetes mellitus | 147 (34%) | 189 (65%) | 162 (37%) | 116 (45%) | 141 (33%) | 211 (59%) |
| Smoking | ||||||
| Never | 214 (49%) | 131 (45%) | 218 (50%) | 118 (46%) | 218 (52%) | 139 (39%) |
| Current | 47 (11%) | 40 (14%) | 52 (12%) | 43 (17%) | 47 (11%) | 51 (14%) |
| Former | 177 (40%) | 120 (41%) | 169 (38%) | 96 (37%) | 158 (37%) | 169 (47%) |
| Calcineurin inhibitor use | 381 (87%) | 265 (91%) | 384 (87%) | 232 (90%) | 373 (88%) | 316 (88%) |
| Sirolimus use | 43 (10%) | 25 (9%) | 43 (10%) | 23 (9%) | 41 (10%) | 41 (11%) |
| Systolic BP (mmHg) | 134.7±19.4 | 143.5±20.8 | 134.8±19.9 | 141.3±19.1 | 135.4±19.8 | 140.8±19.9 |
| Diastolic BP (mmHg) | 79.5±12.4 | 77.6±12.6 | 78.9±12.6 | 79.6±11.7 | 79.8±12.5 | 76.6±12.5 |
| BMI (kg/m2) | 29.0±6.0 | 29.7±6.4 | 29.0±6.0 | 29.5±6.7 | 29.0±6.0 | 29.6±6.5 |
| HDL cholesterol (mg/dl) | 46.9±14.4 | 44.9±14.0 | 46.8±13.6 | 45.3±15.8 | 47.4±14.7 | 44.7±14.9 |
| LDL cholesterol (mg/dl) | 103.8±32.3 | 98.3±37.6 | 102.6±32.4 | 107.9±39.6 | 104.3±32.6 | 99.7±36.7 |
| Triglycerides (mg/dl) | 198.9±133.9 | 211.4±142.5 | 197.9±127.4 | 219.8±175.1 | 195.7±127.6 | 221.3±156.2 |
| eGFR (ml/min per 1.73 m2)a | 46.5±17.6 | 44.1±17.9 | 47.5±17.5 | 38.7±16.5 | 46.6±17.9 | 44.3±18.0 |
| ACR (μg/mg), median (25th, 75th) | 22.3 (8.6, 97.5) | 44.1 (13.6, 234.1) | 20.5 (8.4, 81.9) | 190.2 (42.6, 719.4) | 21.2 (8.4, 95.6) | 57.4 (14.8, 227.0) |
Estimated glomerular filtration rate as calculated by the CKD-EPI equation.
Correlations among Measures of Kidney Function and Urine Injury Biomarkers
Among participants in the subcohort, eGFR level was negatively correlated with urine NGAL and urine L-FABP (Supplemental Figure 1, Table 2). Urine albumin was positively correlated with all four biomarkers. There were moderately strong positive correlations among urine NGAL, KIM-1, IL-18, and L-FABP; the strongest correlation was between urine NGAL and L-FABP (correlation coefficient =0.47; P<0.001) (Supplemental Figure 1, Table 2).
Table 2.
Spearman correlations of eGFR and urine biomarkers in a random subcohort (n=489)
| Kidney/Urine Measure | Urine Albumin (mg/dl) | Urine NGAL (ng/ml) | Urine KIM-1 (pg/ml) | Urine IL-18 (pg/ml) | Urine L-FABP (ng/ml) |
|---|---|---|---|---|---|
| eGFR (ml/min per 1.73 m2) | |||||
| Rho | −0.17 | −0.21 | −0.02 | 0.02 | −0.21 |
| P value | <0.001 | <0.001 | 0.64 | 0.69 | <0.001 |
| Urine albumin (mg/dl) | |||||
| Rho | 0.39 | 0.42 | 0.29 | 0.62 | |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Urine NGAL (ng/ml) | |||||
| Rho | 0.36 | 0.42 | 0.47 | ||
| P value | <0.001 | <0.001 | <0.001 | ||
| Urine KIM-1 (pg/ml) | |||||
| Rho | 0.35 | 0.39 | |||
| P value | <0.001 | <0.001 | |||
| Urine IL-18 (pg/ml) | |||||
| Rho | 0.35 | ||||
| P value | <0.001 |
Urine Injury Biomarkers and Risk of Cardiovascular Events
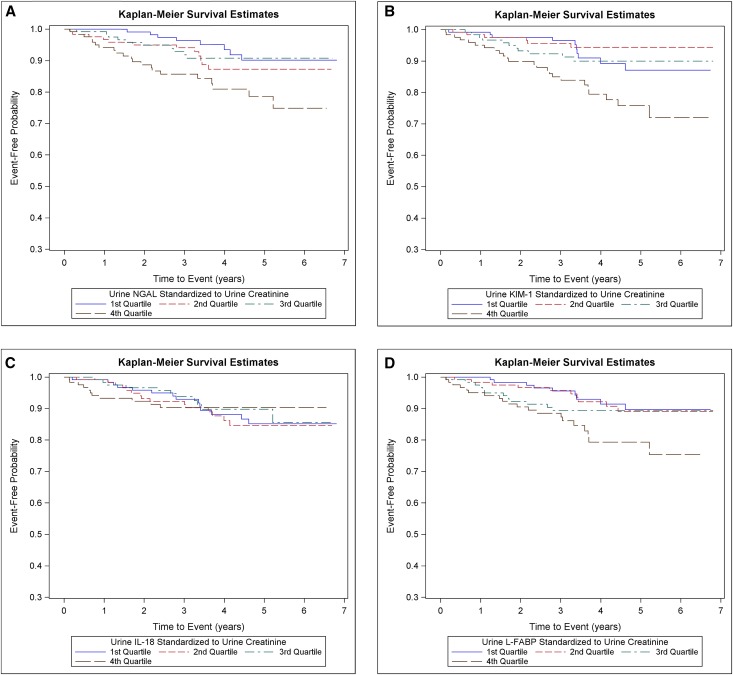
Overall mean follow-up time was 3.9 (±1.6) years. Among participants in the subcohort, the crude event rate was 2.7 per 100 person-years for cardiovascular events. The cumulative incidence of cardiovascular events was greater for participants in the highest quartile of urine NGAL/creatinine, KIM-1/creatinine, and L-FABP/creatinine but not IL-18/creatinine and seemed fairly constant over the follow-up period (Figure 2).
Figure 2.
Cumulative incidence of CVD events were higher among participants in the highest quartile of urine NGAL, KIM-1 and L-FABP. Cumulative incidence of cardiovascular events by quartiles of (A) urine NGAL/creatinine, (B) urine KIM-1/creatinine, (C) urine IL-18/creatinine, and (D) urine L-FABP/creatinine in a random subcohort (n=489).
In the full study population, in unadjusted analyses, there was a statistically significant association of urine NGAL/creatinine, urine KIM-1/creatinine, and urine L-FABP/creatinine (but not IL-18/creatinine) with risk of cardiovascular events (Table 3). These associations remained statistically significant after adjustment for participant demographics and other cardiovascular risk factors. With additional adjustment for urine ACR, only the association between urine NGAL/creatinine and cardiovascular events remained statistically significant. For each log higher urine NGAL/creatinine, the risk of cardiovascular events was 24% higher (adjusted hazard ratio [HR], 1.24; 95% confidence interval [95% CI], 1.06 to 1.45) (Table 3).
Table 3.
Unadjusted and adjusted HR estimates for risk of cardiovascular events according to urine biomarker (n=780 participants)
| Urine Biomarker | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|---|
| Urine NGAL/creatinine | |||
| Per log increase | 1.23 (1.11 to 1.37)a | 1.31 (1.13 to 1.52)a | 1.24 (1.06 to 1.45)a |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.41 (0.91 to 2.19) | 1.67 (0.97 to 2.88) | 1.52 (0.87 to 2. 63) |
| Quartile 3 | 1.48 (0.96 to 2.28) | 1.19 (0.64 to 2.20) | 1.10 (0.59 to 2.05) |
| Quartile 4 | 2.05 (1.34 to 3.14)a | 2.21 (1.21 to 4.05)a | 1.79 (0.95 to 3.34) |
| Urine KIM-1/creatinine | |||
| Per log increase | 1.33 (1.11 to 1.59)a | 1.28 (1.03 to 1.58)a | 1.14 (0.91 to 1.43) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.21 (0.78 to 1.87) | 1.40 (0.78 to 2.50) | 1.21 (0.68 to 2.17) |
| Quartile 3 | 1.43 (0.93 to 2.20) | 1.42 (0.80 to 2.52) | 1.22 (0.69 to 2.17) |
| Quartile 4 | 1.83 (1.21 to 2.79)a | 1.84 (1.06 to 3.18)a | 1.39 (0.79 to 2.44) |
| Urine IL-18/creatinine | |||
| Per log increase | 1.03 (0.91 to 1.16) | 1.11 (0.93 to 1.32) | 1.03 (0.86 to 1.23) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.06 (0.71 to 1.60) | 0.98 (0.58 to 1.65) | 0.90 (0.53 to 1.53) |
| Quartile 3 | 1.17 (0.78 to 1.76) | 1.61 (0.97 to 2.68) | 1.36 (0.80 to 2.31) |
| Quartile 4 | 1.06 (0.69 to 1.63) | 1.28 (0.68 to 2.43) | 1.05 (0.55 to 1.99) |
| Urine L-FABP/creatinine | |||
| Per log increase | 1.24 (1.10 to 1.39)a | 1.17 (1.01 to 1.36)a | 1.0 (0.83 to 1.20) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 0.96 (0.62 to 1.48) | 0.67 (0.36 to 1.24) | 0.59 (0.32 to 1.11) |
| Quartile 3 | 1.64 (1.08 to 2.50)a | 1.45 (0.85 to 2.48) | 1.16 (0.66 to 2.03) |
| Quartile 4 | 1.73 (1.14 to 2.64)a | 1.15 (0.67 to 1.98) | 0.69 (0.36 to 1.30) |
Model 1 was adjusted for demographics, treatment, country, history of CVD, diabetes, smoking, graft vintage, donor, BP, lipids, BMI, and eGFR. Model 2 was adjusted for demographics, treatment, country, history of CVD, diabetes, smoking, graft vintage, donor, BP, lipids, BMI, eGFR, and urine ACR.
Chi-squared test: P<0.05.
When participants with prevalent CVD at study entry were excluded, none of the urine biomarkers were associated with risk of cardiovascular events (although the 95% CIs were wider) (Supplemental Table 2).
Urine Injury Biomarkers and Risk of Graft Failure
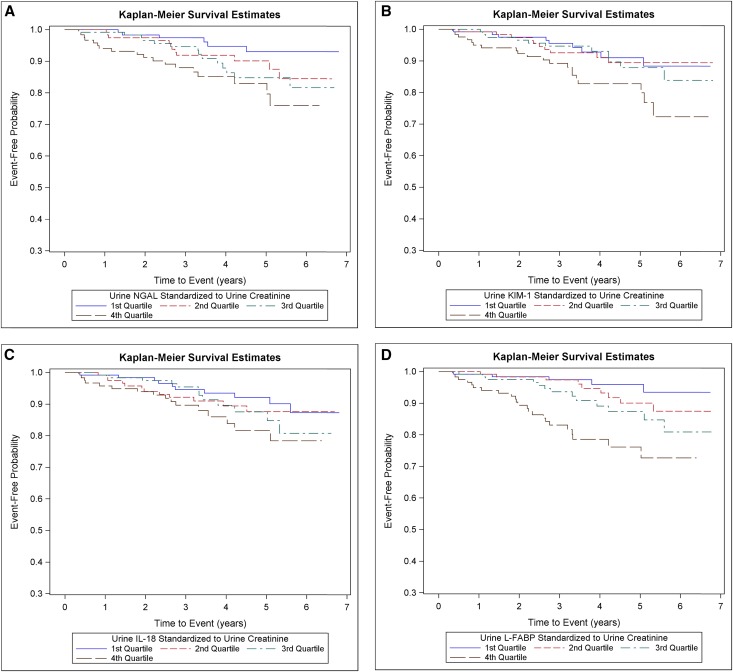
Within the subcohort, the crude graft failure event rate was 2.7 per 100 person-years. The cumulative incidence of graft failure was greatest among participants in the highest quartile of each urine biomarker/urine creatinine (Figure 3).
Figure 3.
Cumulative incidence of graft failure was higher among participants in the highest quartile of urine injury biomarkers. Cumulative incidence of graft failure by quartiles of (A) urine NGAL/creatinine, (B) urine KIM-1/creatinine, (C) urine IL-18/creatinine, and (D) urine L-FABP/creatinine in a random subcohort (n=489).
Among participants in the full study population, in unadjusted models, there was a statistically significant association of higher urine NGAL/creatinine, urine KIM-1/creatinine, urine IL-18/creatinine, and urine L-FABP/creatinine with increased risk of graft failure (Table 4). These associations remained robust after adjustment for participant demographics and other risk factors. After adjustment for urine ACR, only the association between urine NGAL/creatinine and graft failure remained statistically significant. For each log increase in urine NGAL/creatinine, there was a 40% (adjusted HR, 1.40; 95% CI, 1.16 to 1.68) higher risk of graft failure (Table 4).
Table 4.
Unadjusted and adjusted HR estimates for risk of graft failure according to urine biomarker (n=744 participants)
| Urine Biomarker | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|---|
| Urine NGAL/creatinine | |||
| Per log increase | 1.58 (1.40 to 1.79)a | 1.66 (1.42 to 1.94)a | 1.40 (1.16 to 1.68)a |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 3.39 (1.99 to 5.77)a | 3.48 (1.91 to 6.34)a | 2.45 (1.26 to 4.76)a |
| Quartile 3 | 2.37 (1.37 to 4.09)a | 2.22 (1.17 to 4.22)a | 1.27 (0.61 to 2.67) |
| Quartile 4 | 5.99 (3.55 to 10.13)a | 5.86 (3.03 to 11.36)a | 2.60 (1.17 to 5.78)a |
| Urine KIM-1/creatinine | |||
| Per log increase | 1.37 (1.12 to 1.69)a | 1.44 (1.17 to 1.78)a | 0.93 (0.71 to 1.22) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.14 (0.71 to 1.82) | 1.50 (0.83 to 2.70) | 0.75 (0.38 to 1.48) |
| Quartile 3 | 1.32 (0.84 to 2.09) | 1.44 (0.82 to 2.54) | 0.69 (0.37 to 1.29) |
| Quartile 4 | 2.05 (1.31 to 3.19)a | 2.22 (1.29 to 3.81)a | 0.72 (0.38 to 1.36) |
| Urine IL-18/creatinine | |||
| Per log increase | 1.22 (1.07 to 1.39)a | 1.37 (1.14 to 1.65)a | 1.15 (0.93–1.43) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.18 (0.75 to 1.87) | 1.22 (0.72 to 2.09) | 0.87 (0.48 to 1.59) |
| Quartile 3 | 1.47 (0.94 to 2.30) | 1.88 (1.08 to 3.28)a | 0.95 (0.49 to 1.84) |
| Quartile 4 | 1.90 (1.22 to 2.98)a | 2.85 (1.54 to 5.26)a | 1.42 (0.71 to 2.86) |
| L-FABP/creatinine | |||
| Per log increase | 1.77 (1.56 to 2.02)a | 1.67 (1.41 to 1.97)a | 1.07 (0.85 to 1.35) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.08 (0.63 to 1.86) | 1.00 (0.53 to 1.89) | 0.74 (0.36 to 1.52) |
| Quartile 3 | 2.61 (1.59 to 4.29)a | 2.50 (1.36 to 4.59)a | 1.17 (0.57 to 2.42) |
| Quartile 4 | 5.22 (3.21 to 8.51)a | 3.80 (2.07 to 6.96)a | 0.81 (0.36 to 1.86) |
Model 1 was adjusted for demographics, treatment, country, diabetes, smoking, graft vintage, donor, BP, lipids, BMI, and eGFR. Model 2 was adjusted for demographics, treatment, country, diabetes, smoking, graft vintage, donor, BP, lipids, BMI, eGFR, and urine ACR.
Chi-squared test: P<0.05.
Urine Injury Biomarkers and Risk of All-Cause Mortality
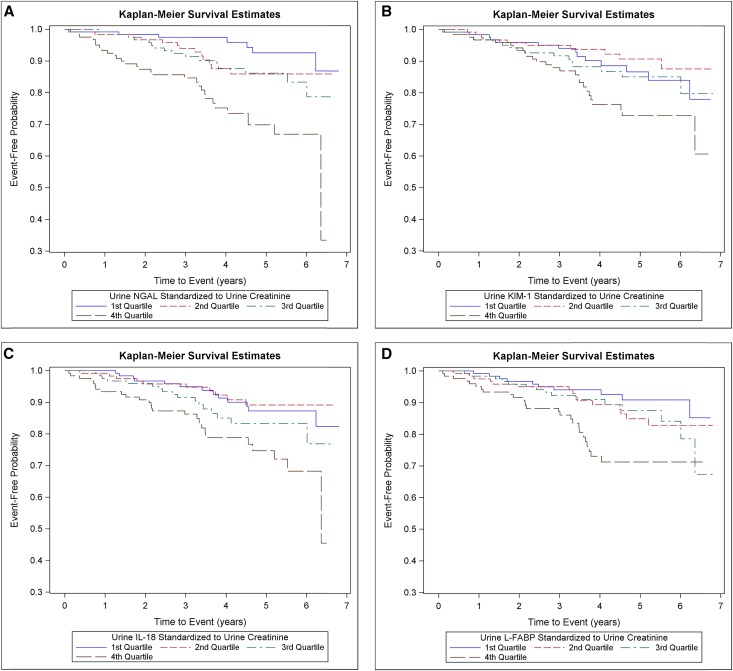
Within the subcohort, the crude mortality rate was 3.3 per 100 person-years. The cumulative incidence of all-cause mortality was greatest among participants in the highest quartile of each urine biomarker/creatinine (Figure 4).
Figure 4.
Cumulative incidence of all-cause mortality was greater among participants in the highest quartile of each urine injury biomarker. Cumulative incidence of all-cause mortality by quartiles of (A) urine NGAL/creatinine, (B) urine KIM-1/creatinine, (C) urine IL-18/creatinine, and (D) urine L-FABP/creatinine in a random subcohort (n=489).
Among participants in the full study population, in unadjusted analyses, all four urine injury biomarkers/creatinine, considered as continuous measures or quartiles, were associated with an increased risk of all-cause mortality (Table 5). These associations remained statistically significant after adjustment for demographic characteristics and other cardiovascular risk factors. After adjustment for urine ACR, the associations of urine NGAL/creatinine (HR, 1.44; 95% CI, 1.26 to 1.65 per log increase), urine KIM-1/creatinine (HR, 1.29; 95% CI, 1.03 to 1.61 per log increase), and urine IL-18/creatinine (HR, 1.25; 95% CI, 1.04 to 1.49 per log increase) remained statistically significant (Table 5).
Table 5.
Unadjusted and adjusted HR estimates for risk of all-cause death according to urine biomarker (n=848 participants)
| Urine Biomarker | Unadjusted HR (95% CI) | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|---|
| Urine NGAL/creatinine | |||
| Per log increase | 1.38 (1.25 to 1.53)a | 1.55 (1.36 to 1.76)a | 1.44 (1.26 to 1.65)a |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.93 (1.25 to 2.97)a | 2.19 (1.27 to 3.81)a | 1.98 (1.13 to 3.45)a |
| Quartile 3 | 1.78 (1.15 to 2.73)a | 1.73 (0.96 to 3.11) | 1.55 (0.85 to 2.80) |
| Quartile 4 | 3.26 (2.15 to 4.95)a | 4.08 (2.32 to 7.18)a | 3.12 (1.73 to 5.64)a |
| Urine KIM-1/creatinine | |||
| Per log increase | 1.53 (1.28 to 1.83)a | 1.44 (1.16 to 1.78)a | 1.29 (1.03 to 1.61)a |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 1.21 (0.79 to 1.86) | 1.19 (0.71 to 2.02) | 1.03 (0.60 to 1.74) |
| Quartile 3 | 1.64 (1.08 to 2.48)a | 1.56 (0.93 to 2.60) | 1.32 (0.79 to 2.21) |
| Quartile 4 | 2.51 (1.69 to 3.75)a | 2.33 (1.41 to 3.86)a | 1.76 (1.05 to 2.93)a |
| Urine IL-18/creatinine | |||
| Per log increase | 1.21 (1.07 to 1.36)a | 1.35 (1.14 to 1.61)a | 1.25 (1.04 to 1.49)a |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 0.98 (0.65 to 1.46) | 1.04 (0.62 to 1.74) | 0.93 (0.55 to 1.57) |
| Quartile 3 | 1.25 (0.84 to 1.85) | 1.85 (1.11 to 3.11)a | 1.55 (0.91 to 2.65) |
| Quartile 4 | 1.72 (1.17 to 2.53)a | 2.31 (1.30 to 4.08)a | 1.77 (0.98 to 3.17) |
| Urine L-FABP/creatinine | |||
| Per log increase | 1.33 (1.18 to 1.49)a | 1.31 (1.14 to 1.51)a | 1.12 (0.94 to 1.35) |
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 0.98 (0.65 to 1.49) | 0.78 (0.44 to 1.36) | 0.69 (0.39 to 1.21) |
| Quartile 3 | 1.47 (0.99 to 2.19) | 1.11 (0.66 to 1.86) | 0.84 (0.49 to 1.43) |
| Quartile 4 | 1.93 (1.29 to 2.88)a | 1.55 (0.93 to 2.57) | 0.85 (0.47 to 1.53) |
Model 1 was adjusted for demographics, treatment, country, history of CVD, diabetes, smoking, graft vintage, donor, BP, lipids, BMI, and eGFR. Model 2 was adjusted for demographics, treatment, country, history of CVD, diabetes, smoking, graft vintage, donor, BP, lipids, BMI, eGFR, and urine ACR.
Chi-squared test: P<0.05.
When participants with CVD at study entry were excluded, in multivariable analysis including adjustment for urine ACR, the association between NGAL/creatinine and all-cause mortality remained statistically significant but not for urine KIM-1/creatinine or urine IL-18/creatinine (although the 95% CIs were wider) (Supplemental Table 2).
Sensitivity Analyses: Analyses of Urine Biomarkers Not Standardized to Urine Creatinine
In the full study population, in multivariable models that examined raw urine biomarker concentrations (i.e., not standardizing them to urine creatinine concentration) and after adjusted for urine ACR, urine NGAL was associated with increased risk of cardiovascular events, graft failure, and all-cause mortality(Supplemental Table 3), similar to the main analysis (Tables 3–5). In multivariable models, urine IL-18 was significantly associated with increased risk of all-cause mortality (Supplemental Table 3), similar to the main analysis, whereas the association between urine KIM-1 and mortality was attenuated and no longer statistically significant (Supplemental Table 3).
Sensitivity Analyses: Composite Outcomes
To assess whether our conclusions regarding cardiovascular events or graft failure could be biased by competing risk of death, we repeated our multivariable models examining two composite outcomes: cardiovascular events or all-cause mortality and graft failure or all-cause mortality. Overall, reassuring results were obtained (Supplemental Table 4).
Post Hoc Exploratory Analyses: Interactions by Sex
Given the substantial differences in biomarker levels in men and women (Supplemental Table 1), we tested for interaction by sex in exploratory post hoc analyses. Across four biomarkers and three outcomes, we found a statistically significant interaction by sex only in the association of IL-18/creatinine with all-cause death (P value =0.01). In stratified analyses, the association was stronger in men.
Discussion
In prevalent KTRs, urine tubular injury biomarkers are associated with greater risk of adverse cardiovascular outcomes, graft failure, and all-cause mortality. Specifically, elevations in urine NGAL/creatinine were significantly associated with higher risk of cardiovascular events as well as increased risk of graft failure. Furthermore, elevations in urine NGAL/creatinine, KIM-1/creatinine, and IL-18/creatinine were associated with greater risk of all-cause mortality, with urine NGAL/creatinine being the most robust. The observed associations were independent of known cardiovascular risk factors as well as established markers of kidney disease, including eGFR and urine ACR. Our study suggests that urine injury markers may signal early biologic injury that may contribute to higher risk of adverse outcomes among prevalent KTRs.
Among the FAVORIT Trial participants that we studied (median graft vintage of 3.8 years), levels of urine injury biomarkers were moderately elevated, although overall, biomarker levels (not standardized to urine creatinine) were several magnitudes lower than those seen in patients with AKI after cardiac surgery10 or the immediate perikidney transplant setting.29 For example, in a study of KTRs, urine NGAL and IL-18 were markedly higher in the first 48 hours after kidney transplant in those who developed delayed or slow graft function compared with our patient population.29 Interestingly, among participants who had immediate graft function, urine NGAL on the second postoperation day was similar to levels seen among prevalent KTRs in our study.29 The levels of urine injury biomarkers seen in prevalent KTRs are similar to those reported in studies of the general CKD population4 or a community–based elderly study population.5 For example, urine NGAL levels at baseline among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study, an observational cohort study of persons with CKD (mean eGFR of 44 ml/min per 1.73 m2), were similar to levels that we observed among our study of prevalent KTRs (mean eGFR of subcohort of 46 ml/min per 1.73 m2).4
In multivariable models, we found that urine NGAL was significantly associated with higher risk of atherosclerotic cardiovascular events. A similar association was not observed for KIM-1, IL-18, or L-FABP when adjustment for urine ACR, in particular, attenuated associations between biomarkers and adverse outcomes. Consistent with our findings, a recent report from the CRIC Study showed a significant association of urine NGAL with atherosclerotic cardiovascular events.4 Another study of patients with CKD also noted a >3-fold higher risk of cardiovascular events among those with the highest urine NGAL levels.26 Also, similar to our findings, a study of older adults reported no association of urine KIM-1 or IL-18 with risk of CVD.5 Although we did not find an association between urine L-FABP and cardiovascular events, a prior study noted a strong association among patients with type 2 diabetes.34 Unlike the other urine injury biomarkers, urine NGAL is filtered through the glomerulus and thus, may reflect more systemic processes, including inflammation, which may possibly explain the association with atherosclerotic CVD.
Of four urine injury biomarkers that we investigated, only urine NGAL was significantly associated with higher risk of graft failure among prevalent KTRs. A prior study of prevalent KTRs also noted an association of urine NGAL with graft failure.35 Among incident KTRs in the peritransplant setting, multiple urine injury biomarkers have been associated with both delayed graft function29,30,36 and ESRD after 1 year.36 The associations of these urine injury biomarkers with kidney outcomes in the nontransplant CKD setting are conflicting.6,37–44 Similar to our findings, among patients with native CKD, elevated urine NGAL has been shown to be significantly associated with progression of CKD or incident ESRD.3,45,46 Some studies39 but not all6 have also noted associations of higher urine NGAL with incident CKD. For example, among high–risk American Indians with type 2 diabetes, elevations in urine NGAL were significantly associated with risk of ESRD.40 However, these findings were not observed among persons with type 1 diabetes.42,43 We did not find an association between urine KIM-1 and graft failure. Although some studies have concluded that urine KIM-1 was an independent risk factor for more rapid loss of renal function,6 others have not.39,40,42,43 With regard to IL-18, there have been relatively fewer studies of this biomarker. A prior study of HIV-infected women found a strong independent association between higher levels of urine IL-18 and risk of rapid decline in kidney function,25 which is not what we observed. Finally, we did not find an association between urine L-FABP and graft failure in our study, although prior studies of persons with diabetes have noted both inverse40 and linear associations between urine L-FABP and risk of progression of CKD.34,41,47,48 Because reasons for progressive loss of renal function after kidney transplant likely include both traditional kidney disease risk factors (such as elevated BP) as well as rejection/immunologic factors, it is perhaps not surprising that findings among prevalent KTRs would be different from nontransplant populations.
In our analysis of prevalent KTRs, we found a significant association of urine NGAL, KIM-1, and IL-18 with risk of all-cause mortality. Prior investigations have examined these associations in select populations, yielding conflicting results. For example, in the CRIC Study, urine NGAL was not associated with all-cause mortality after multivariable adjustment.4 Similarly, in a study of older men recruited from the community, urine NGAL was associated with higher risk of death in univariate analyses but not after multivariable adjustment.49 In a study of American Indians, higher urine NGAL but not KIM-1 was associated with increased risk of all-cause mortality.40 Community-based studies have also noted associations between elevated urine KIM-1 and higher risk of all-cause mortality.27,28 We did not find an association of urine L-FABP with all-cause mortality. Similar to other biomarkers, the data on L-FABP and risk of mortality also have been conflicting. In HIV-infected women, a J-shaped association was noted between urine L-FABP and risk of long-term mortality.50 In a study of persons with type 1 diabetes, a positive association was found between L-FABP and risk of death.41 Thus, data from our study and others suggest that urine injury biomarkers may have differential associations with risk of all-cause death on the basis of the study population and acuity of injury as well as the specific urine biomarker of interest.
It is interesting that, among the outcomes that we studied, the highest HRs were seen for all-cause mortality, despite the fact that the markers of kidney tubular injury are expressed from a transplanted kidney. There are severe possible explanations for these findings. First, these biomarkers may be indicators of levels or dimensions of kidney injury not captured by traditional markers of kidney function (e.g., serum creatinine and urine ACR). It is recognized that CKD is strongly associated with all-cause mortality51,52; therefore, it is possible that numerous different dimensions of kidney damage are risk factors for mortality. For example, many important physiologic pathways are regulated by the kidney tubules, including mineral metabolism, erythropoiesis, and acid-base regulation. Therefore, tubular dysfunction may lead to alterations in these important metabolic pathways, contributing to higher risk of death.53 Second, these biomarkers may reflect systemic injury from ischemia and other processes, which involve multiple organs in parallel, ultimately contributing to higher risk of death. For example, urine NGAL is thought to be a marker of both kidney damage and systemic inflammation.12 Additional studies are needed to elucidate the precise mechanisms to explain the observed associations.
Our study had several strengths. We studied a large, well characterized, international cohort of prevalent KTRs with rigorously adjudicated outcomes. We used a case-cohort study design, which efficiently allowed us to study multiple important clinical outcomes. Urine injury biomarkers were measured at a very experienced laboratory simultaneously from samples that had not undergone a previous freeze-thaw cycle. We were able to adjust for a substantial number of possible confounders. A few limitations should be noted as well. The FAVORIT Trial did not collect biospecimens starting from the time of transplant, and therefore, we could only study prevalent KTRs (rather than longitudinal measures starting at the time of surgery when injury is likely to be maximal). We only had single measures of each urine injury biomarker at study baseline. The urine samples were collected from 2002 to 2007. Although we used previously unthawed urine samples to measure our urine biomarkers, it is unclear how age of the sample may have affected the assays. Any nonspecific degradation would likely have biased our results toward the null. This was a clinical trial study population enrolling those with serum homocysteine level above a certain cutoff, and there was a relatively high proportion of living donor transplants; therefore, our results may not be generalizable to all KTRs. Finally, there needs to be some caution taken when comparing absolute biomarker concentrations across studies that used different platforms and assays.
In conclusion, elevations in baseline urine injury biomarkers NGAL, KIM-1, and IL-18 were significantly but differentially associated with a higher risk of long–term cardiovascular events, graft failure, and all-cause mortality, independent of known risk factors, including albuminuria. Elevations in these urine biomarkers may signal some dimension of kidney disease or systemic disease that contributes to adverse outcomes. Additional studies are needed to explore the possible mechanisms to explain these findings.
Concise Methods
Study Population
This report is a post hoc analysis of the FAVORIT Trial (clinicaltrials.gov: NCT00064753), a multicenter, double–blind, randomized, controlled clinical trial conducted to determine whether lowering homocysteine levels with vitamin therapy reduced the rate of pooled arteriosclerotic cardiovascular outcomes. The FAVORIT Study protocol was approved by the Institutional Review Boards at the participating institutions, and all participants provided written informed consent.
The design of the trial and the primary results have been described in detail elsewhere.54–56 In total, 4110 KTRs were enrolled from August of 2002 to January of 2007 and randomized to either a standard multivitamin with high doses of folic acid, vitamin B6, and vitamin B12 or a multivitamin containing low doses of vitamin B6 and vitamin B12 with no folic acid. Men and women ages 35–75 years old who were at least 6 months postkidney transplant were enrolled at 30 transplant centers in the United States, Canada, and Brazil. Entry criteria included elevated serum homocysteine level (≥11 μmol/L for women and ≥12 μmol/L for men) and stable kidney function defined by an estimated creatinine clearance ≥30 ml/min in men and ≥25 ml/min in women. Follow-up contacts occurred every 6 months through January 31, 2010 to obtain study-related outcomes through June 24, 2009. The primary outcome was pooled incident or recurrent CVD events. Graft failure and all-cause mortality were secondary outcomes. As reported previously, there was no significant difference between treatment groups for primary or secondary outcomes.57
Urine Injury Biomarkers
Urine NGAL, urine KIM-1, urine IL-18, and urine L-FABP were measured at a single laboratory at Cincinnati Children’s Hospital in previously unthawed urine samples obtained at baseline and stored at −80°C. All assays were performed in June of 2013. The urine NGAL ELISA was performed using a commercially available assay (NGAL ELISA Kit 036; Bioporto, Grusbakken, Denmark) that specifically detects human NGAL.58 The intra–assay coefficient of variation (CV) was 2.1%, and interassay CV was 9.1%. The urine KIM-1 ELISA was constructed using commercially available reagents (Duoset DY1750; R&D Systems, Inc., Minneapolis, MN) as described previously.59 Intra- and interassay CVs for KIM-1 were 2% and 7.8%, respectively. Urine IL-18 and L-FABP were measured using commercially available ELISA kits (Medical & Biologic Laboratories Co., Nagoya, Japan and CMIC Co., Tokyo, Japan, respectively). CVs for IL-18 and L-FABP were 7.3% and 6.1% (interassay) and 7.5% and 10.9% (intra-assay), respectively. There were 128 participants whose samples were below the lower limit of detection (LLD; 10.3 pg/ml) for IL-18 and 19 below the LLD (3 ng/ml) for L-FABP. For these participants, values halfway between zero and the LLD were assigned. Urine creatinine was assayed using the modified Jaffe method. Intra-assay CV was 2%, and interassay CV was 4%.
To correct for the effects of urine concentration and dilution, all biomarker levels were standardized by urine creatinine concentration in our main analysis. Because of the skewed distribution, urine biomarker/urine creatinine was log transformed. To avoid assumptions of linearity, biomarkers were modeled in quartiles as well as continuously.
Study Outcomes
Three outcomes were considered in our analysis: adjudicated CVD events, graft failure, and all-cause mortality. The CVD outcome was a composite of cardiovascular death, myocardial infarction, resuscitated sudden death, and stroke. Each of these outcomes was centrally reviewed and adjudicated by the FAVORIT Clinical Endpoints Committee.54–57 The same committee also reviewed medical records for occurrence of unstable angina and urgent coronary revascularization procedures to identify additional patients with myocardial infarction. Graft failure was defined as initiation of dialysis ascertained by local study staff. Study staff also identified deaths from review of medical records, regular participant contact, and contact with family. For each outcome, participants were censored at either the time of last follow-up visit or the end of study period. For the outcomes of CVD and graft failure, participants were also censored at time of death.
Covariates
At time of study enrollment (baseline), demographic characteristics (age, sex, race, and country of origin [United States, Canada, or Brazil]); smoking status (current, former, or never); past medical history by self-report (baseline CVD and diabetes mellitus); transplant characteristics (living donor kidney and time since transplant); physical examination findings (body mass index [BMI] and systolic and diastolic BPs); and laboratory measurements (total cholesterol, HDL cholesterol, and triglycerides) were obtained. Race was recorded as white, black, or other, including mixed race. LDL cholesterol was estimated using the Friedewald equation at triglyceride levels <400 and measured directly in 82 participants with triglyceride levels >400 mg/dl. Baseline BP was the average of two measurements. Diabetes was defined by the use of insulin or oral hypoglycemic medications or participant self-report. Prior history of CVD was determined by self-report at baseline and included prior myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and/or lower extremity arterial revascularization or amputation above the ankle. BMI was calculated using the formula weight (kg)/height (m)2. GFR was estimated from serum creatinine using the Chronic Kidney Disease Epidemiology Collaboration 2009 equation.60 Serum creatinine was measured using an alkaline picrate kinetic method on an Olympus AU 400e (Olympus America Inc., Center Valley, PA) instrument that was calibrated to an isotope dilution mass spectrometry traceable standard. Urine albumin and creatinine were measured in spot urine samples to calculate urine ACR. Urine albumin was measured using an immunoturbidimetric assay. Intra-assay CV was 2%, and interassay CV was 4%.
Study Design
To evaluate the association between urine kidney injury biomarkers and outcomes, we performed a case-cohort study. Both patients and nonpatients are drawn from the entire cohort, with patients identified at study completion. Participants can contribute to more than one type of case; for example, one participant can be included because of both CVD disease and death. Members of the subcohort are randomly selected from the entire cohort and can include individuals who are also selected as patients. After excluding 104 participants with missing urine samples at baseline (n=53) or missing key covariates (n=51), all patients with cardiovascular events (n=291), graft failure (n=257), and all-cause mortality (n=359) were included. We also sampled a random subcohort (n=489) of FAVORIT Trial participants, with a final study population of 1027 for this analysis.
Statistical Methods
Chi-squared test, ANOVA, and Kruskal–Wallis test were used as appropriate to compare baseline data across quartiles of each urine injury biomarker/urine creatinine in the random subcohort of 489 participants. We also reported characteristics of study participants who did and did not have an outcome of interest. We generated a Venn diagram to evaluate overlap among participants in the highest quartiles of each urine biomarker. Among the random subcohort participants, we then calculated the Spearman correlation coefficients and generated scatterplots between eGFR, urine albumin, urine NGAL, urine KIM-1, urine IL-18, and urine L-FABP.
Kaplan–Meier survival analysis was used to estimate the cumulative incidence of each outcome among subcohort study participants by quartile of each urine injury biomarker/urine creatinine. To take into account the case-cohort study design, weighted Cox proportional hazards regression61,62 was used to examine the association between baseline urine injury biomarkers and time to study outcomes. We examined the Schoenfeld residual plots from model 2 for each biomarker and outcome to check the proportional hazard assumption (which did not seem to be violated). Models were adjusted for baseline age, sex, race, randomly assigned treatment, country from which participants were recruited (United States, Canada, or Brazil), diabetes, smoking, graft vintage, donor type, diastolic BP, systolic BP, BMI, and eGFR. Models for the outcomes of CVD and death were additionally adjusted for history of CVD and lipids. In a final model, we further adjusted for urine ACR to test whether the association of each urine injury biomarker with our outcome of interest was independent of this established urine biomarker.
We performed three sensitivity analyses. First, we examined the association of each urine injury biomarker with risk of cardiovascular events and all-cause mortality, excluding participants with prevalent CVD at study entry. Second, we repeated our analyses without standardizing each urine biomarker to urine creatinine concentration. Third, to evaluate for possible competing risk, we repeated our multivariable models, examining the association of each urine biomarker with risk of (1) cardiovascular events or all-cause mortality and (2) graft failure or all-cause mortality.
All analyses were performed using SAS 9.3, SAS 9.4, and JMP.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by Grants K23 DK088865 (to N.B.) and K24 DK92291 (to C.-y.H.) from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). K.D.L. and C.-y.H. are also supported by Grant U01DK85649 as members of the CKD Biomarker Consortium. The Folic Acid for Vascular Outcome Reduction in Transplantation Study was supported by Cooperative Agreement U01 DK61700-01 from the NIDDK, and additional financial support was from the Office of Dietary Supplements, Department of Health and Human Services, the NIH. Vitamins were provided by Pamlab, LLC.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030292/-/DCSupplemental.
References
- 1.Available at: http://www.usrds.org/adr.htm. Accessed February 12, 2015
- 2.Djamali A, Samaniego M, Muth B, Muehrer R, Hofmann RM, Pirsch J, Howard A, Mourad G, Becker BN: Medical care of kidney transplant recipients after the first posttransplant year. Clin J Am Soc Nephrol 1: 623–640, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY Chronic Renal Insufficiency Cohort (CRIC) study investigators : Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney Int 83: 909–914, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu KD, Yang W, Go AS, Anderson AH, Feldman HI, Fischer MJ, He J, Kallem RR, Kusek JW, Master SR, Miller ER 3rd, Rosas SE, Steigerwalt S, Tao K, Weir MR, Hsu CY CRIC Study Investigators : Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 65: 267–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Katz R, Newman A, Harris T, Peralta CA, Devarajan P, Bennett MR, Fried L, Ix JH, Satterfield S, Simonsick EM, Parikh CR, Shlipak MG Health ABC Study : Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol 25: 1545–1553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driver TH, Katz R, Ix JH, Magnani JW, Peralta CA, Parikh CR, Fried L, Newman AB, Kritchevsky SB, Sarnak MJ, Shlipak MG Health ABC Study : Urinary kidney injury molecule 1 (KIM-1) and interleukin 18 (IL-18) as risk markers for heart failure in older adults: The Health, Aging, and Body Composition (Health ABC) Study. Am J Kidney Dis 64: 49–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han WK, Wagener G, Zhu Y, Wang S, Lee HT: Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol 4: 873–882, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyner JL, Vaidya VS, Bennett MR, Ma Q, Worcester E, Akhter SA, Raman J, Jeevanandam V, O’Connor MF, Devarajan P, Bonventre JV, Murray PT: Urinary biomarkers in the clinical prognosis and early detection of acute kidney injury. Clin J Am Soc Nephrol 5: 2154–2165, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX TRIBE-AKI Consortium : Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22: 1748–1757, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siew ED, Ware LB, Bian A, Shintani A, Eden SK, Wickersham N, Cripps B, Ikizler TA: Distinct injury markers for the early detection and prognosis of incident acute kidney injury in critically ill adults with preserved kidney function. Kidney Int 84: 786–794, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowland JB, Borregaard N: Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics 45: 17–23, 1997 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J: Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol 18: 407–413, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J: The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonventre JV, Yang L: Kidney injury molecule-1. Curr Opin Crit Care 16: 556–561, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV: Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem 277: 39739–39748, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV: Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest 118: 1657–1668, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, Peterson JF, Parikh CR, May AK, Ware LB: Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol 5: 1497–1505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gracie JA: Interleukin-18 as a potential target in inflammatory arthritis. Clin Exp Immunol 136: 402–404, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL: Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest 107: 1145–1152, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamijo-Ikemori A, Sugaya T, Kimura K: Urinary fatty acid binding protein in renal disease. Clin Chim Acta 374: 1–7, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Martin GG, Danneberg H, Kumar LS, Atshaves BP, Erol E, Bader M, Schroeder F, Binas B: Decreased liver fatty acid binding capacity and altered liver lipid distribution in mice lacking the liver fatty acid-binding protein gene. J Biol Chem 278: 21429–21438, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Atshaves BP, McIntosh AL, Payne HR, Mackie J, Kier AB, Schroeder F: Effect of branched-chain fatty acid on lipid dynamics in mice lacking liver fatty acid binding protein gene. Am J Physiol Cell Physiol 288: C543–C558, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Starodub O, McIntosh A, Atshaves BP, Woldegiorgis G, Kier AB, Schroeder F: Liver fatty acid-binding protein colocalizes with peroxisome proliferator activated receptor alpha and enhances ligand distribution to nuclei of living cells. Biochemistry 43: 2484–2500, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak MJ, Parikh CR: Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 61: 565–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa M, Ishii J, Kitagawa F, Takahashi K, Hayashi H, Koide S, Tomita M, Takahashi H, Ozaki Y, Yuzawa Y: Urinary neutrophil gelatinase-associated lipocalin as a predictor of cardiovascular events in patients with chronic kidney disease. Heart Vessels 30: 81–88, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, Bottai M, Sundström J, Ärnlöv J: Urinary kidney injury molecule-1 and the risk of cardiovascular mortality in elderly men. Clin J Am Soc Nephrol 9: 1393–1401, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Seaghdha CM, Hwang S-J, Larson MG, Meigs JB, Vasan RS, Fox CS: Analysis of a urinary biomarker panel for incident kidney disease and clinical outcomes. J Am Soc Nephrol 24: 1880–1888, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, Han WK, Marcus RJ, Parikh CR: IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 21: 189–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P: Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 6: 1639–1645, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Szeto CC, Kwan BC, Lai KB, Lai FM, Chow KM, Wang G, Luk CC, Li PK: Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol 5: 2329–2337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EY, Kim MS, Park Y, Kim HS: Serum neutrophil gelatinase-associated lipocalin and interleukin-18 as predictive biomarkers for delayed graft function after kidney transplantation. J Clin Lab Anal 26: 295–301, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollmen ME, Kyllönen LE, Inkinen KA, Lalla ML, Salmela KT: Urine neutrophil gelatinase-associated lipocalin is a marker of graft recovery after kidney transplantation. Kidney Int 79: 89–98, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Araki S, Haneda M, Koya D, Sugaya T, Isshiki K, Kume S, Kashiwagi A, Uzu T, Maegawa H: Predictive effects of urinary liver-type fatty acid-binding protein for deteriorating renal function and incidence of cardiovascular disease in type 2 diabetic patients without advanced nephropathy. Diabetes Care 36: 1248–1253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nauta FL, Bakker SJ, van Oeveren W, Navis G, van der Heide JJ, van Goor H, de Jong PE, Gansevoort RT: Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis 57: 733–743, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR: Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol 7: 1224–1233, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen SE, Reinhard H, Zdunek D, Hess G, Gutiérrez OM, Wolf M, Parving HH, Jacobsen PK, Rossing P: Tubular markers are associated with decline in kidney function in proteinuric type 2 diabetic patients. Diabetes Res Clin Pract 97: 71–76, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Conway BR, Manoharan D, Manoharan D, Jenks S, Dear JW, McLachlan S, Strachan MWJ, Price JF: Measuring urinary tubular biomarkers in type 2 diabetes does not add prognostic value beyond established risk factors. Kidney Int 82: 812–818, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Bhavsar NA, Köttgen A, Coresh J, Astor BC: Neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1) as predictors of incident CKD stage 3: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 60: 233–240, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Bonventre JV, Sabbisetti V, Waikar SS, Mifflin TE, Zhang X, Xie D, Hsu CY, Feldman HI, Coresh J, Vasan RS, Kimmel PL, Liu KD Chronic Kidney Disease Biomarkers Consortium Investigators : Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia 58: 188–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen SE, Sugaya T, Hovind P, Baba T, Parving HH, Rossing P: Urinary liver-type fatty acid-binding protein predicts progression to nephropathy in type 1 diabetic patients. Diabetes Care 33: 1320–1324, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P: Tubular markers do not predict the decline in glomerular filtration rate in type 1 diabetic patients with overt nephropathy. Kidney Int 79: 1113–1118, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Nielsen SE, Hansen HP, Jensen BR, Parving HH, Rossing P: Urinary neutrophil gelatinase-associated lipocalin and progression of diabetic nephropathy in type 1 diabetic patients in a four-year follow-up study. Nephron Clin Pract 118: c130–c135, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Chou K-M, Lee C-C, Chen C-H, Sun C-Y: Clinical value of NGAL, L-FABP and albuminuria in predicting GFR decline in type 2 diabetes mellitus patients. PLoS One 8: e54863, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith ER, Lee D, Cai MM, Tomlinson LA, Ford ML, McMahon LP, Holt SG: Urinary neutrophil gelatinase-associated lipocalin may aid prediction of renal decline in patients with non-proteinuric Stages 3 and 4 chronic kidney disease (CKD). Nephrol Dial Transplant 28: 1569–1579, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamijo-Ikemori A, Sugaya T, Yasuda T, Kawata T, Ota A, Tatsunami S, Kaise R, Ishimitsu T, Tanaka Y, Kimura K: Clinical significance of urinary liver-type fatty acid-binding protein in diabetic nephropathy of type 2 diabetic patients. Diabetes Care 34: 691–696, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panduru NM, Forsblom C, Saraheimo M, Thorn L, Bierhaus A, Humpert PM, Groop P-H FinnDiane Study Group : Urinary liver-type fatty acid-binding protein and progression of diabetic nephropathy in type 1 diabetes. Diabetes Care 36: 2077–2083, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helmersson-Karlqvist J, Larsson A, Carlsson AC, Venge P, Sundström J, Ingelsson E, Lind L, Arnlöv J: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with mortality in a community-based cohort of older Swedish men. Atherosclerosis 227: 408–413, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, Bennett M, Butch A, Anastos K, Cohen M, Nowicki M, Sharma A, Young M, Sarnak M, Parikh C, Shlipak M: Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med 15: 291–300, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, Kleefstra N, Naimark D, Roderick P, Tonelli M, Wetzels JF, Astor BC, Gansevoort RT, Levin A, Wen CP, Coresh J Chronic Kidney Disease Prognosis Consortium : Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Dominguez JR, Shlipak MG, Whooley MA, Ix JH: Fractional excretion of phosphorus modifies the association between fibroblast growth factor-23 and outcomes. J Am Soc Nephrol 24: 647–654, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, McKenney JL, Mercier RY, Pfeffer MA, Selhub J FAVORIT Study Investigators : Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis 53: 121–128, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, Jacques PF, McKenney J FAVORIT Investigators : Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J 152: 448.e1–448.e7, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, Kasiske BL, Kim SJ, Kusek JW, Bostom AG: Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: The FAVORIT trial. Am J Transplant 12: 2437–2445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, Selhub J, Jacques PF, Cole E, Gravens-Mueller L, House AA, Kew C, McKenney JL, Pacheco-Silva A, Pesavento T, Pirsch J, Smith S, Solomon S, Weir M: Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: Primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation 123: 1763–1770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P: Urine NGAL predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clin J Am Soc Nephrol 3: 665–673, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaturvedi S, Farmer T, Kapke GF: Assay validation for KIM-1: Human urinary renal dysfunction biomarker. Int J Biol Sci 5: 128–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barlow WE: Robust variance estimation for the case-cohort design. Biometrics 50: 1064–1072, 1994 [PubMed] [Google Scholar]
- 62.Barlow WE, Ichikawa L, Rosner D, Izumi S: Analysis of case-cohort designs. J Clin Epidemiol 52: 1165–1172, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.