Abstract
Depression is a significant public health problem for which currently available medications, if effective, require weeks to months of treatment before patients respond. Previous studies have shown that the G protein responsible for increasing cAMP (Gαs) is increasingly localized to lipid rafts in depressed subjects and that chronic antidepressant treatment translocates Gαs from lipid rafts. Translocation of Gαs, which shows delayed onset after chronic antidepressant treatment of rats or of C6 glioma cells, tracks with the delayed onset of therapeutic action of antidepressants. Because antidepressants appear to specifically modify Gαs localized to lipid rafts, we sought to determine whether structurally diverse antidepressants accumulate in lipid rafts. Sustained treatment of C6 glioma cells, which lack 5-hydroxytryptamine transporters, showed marked concentration of several antidepressants in raft fractions, as revealed by increased absorbance and by mass fingerprint. Closely related molecules without antidepressant activity did not concentrate in raft fractions. Thus, at least two classes of antidepressants accumulate in lipid rafts and effect translocation of Gαs to the non-raft membrane fraction, where it activates the cAMP-signaling cascade. Analysis of the structural determinants of raft localization may both help to explain the hysteresis of antidepressant action and lead to design and development of novel substrates for depression therapeutics.
Keywords: cyclic AMP (cAMP), depression, drug action, G protein, gas chromatography-mass spectrometry (GC-MS), glial cell, intracellular trafficking, lipid raft, monoamine transporter, selective serotonin reuptake inhibitor (SSRI), G protein-coupled receptor (GPCR), glia, heterotrimeric G protein, lipid, mass spectrometry (MS), membrane trafficking, plasma membrane, protein-drug interaction, protein translocation, protein-lipid interaction, serotonin, serotonin transporter
Introduction
Depression is the leading cause of long term disability in the industrialized world (1). Although depression is a significant health problem in the United States and antidepressants are heavily prescribed (2), the mechanism of action for these drugs is not understood. Further, nearly a third of those treated with these drugs do not achieve remission of their depression (3). Although most of these drugs do interfere with monoamine uptake or catabolism, they exert this effect within hours, even though most of the compounds require weeks before alleviation of symptoms is observed (4). Thus, other targets for antidepressant drugs may exist (4).
Chronic antidepressant treatment engages signaling pathways apart from increasing monoamine density in the synaptic cleft. One of these is an increased accumulation of cellular cAMP and sequelae thereof, such as increased cAMP-response element-binding protein (CREB) phosphorylation and increased transcription of cAMP-regulated genes (e.g. BDNF) (5). Moreover, positron emission tomography (PET) evidence suggests that cAMP is diminished throughout the brain of depressed human subjects (6). Thus, it is possible that some antidepressant effects are mediated through induction of the cAMP-generating system, including Gαs and adenylyl cyclase.
Previous studies demonstrated that chronic antidepressant treatment translocates Gαs from lipid rafts, whereupon it engages in a more facile activation of adenylyl cyclase (7, 8). Lipid rafts are regions of the plasma membrane rich in caveolin, cholesterol, sphingolipids, and cytoskeletal and glycosylphosphatidylinositol-anchored proteins (9, 10) that allow the clustering or sequestration of signaling molecules (11). The rigid structure afforded by increased cholesterol content tightly coordinates saturated membrane lipids and acylated proteins. As many GPCRs2 are lipid raft-localized and Gαs is palmitoylated, signaling through Gαs is impaired by lipid raft microdomains (12), presumably through inhibiting association(s) between raft- and non-raft-housed molecules (13, 14).
Dampened signaling, through Gαs and/or Gαs-coupled receptors, is consistent with the observed increase in Gαs association with rafts as well as lowered levels of cAMP seen in major depressive disorder (15). Accordingly, Gαs content in non-raft membrane domains increases after chronic treatment with fluoxetine, desipramine, and escitalopram (16) and cAMP is increased (17). Sustained activation of Gαs is also associated with increased microtubule dynamics and a resulting increase in neurite outgrowth (18, 19). Furthermore, lipid raft disruption displaces many raft proteins, but chronic antidepressant treatment displaces only Gαs (7). However, the precise biochemical mechanisms that account for the antidepressant-mediated translocation of Gαs from lipid rafts was not well defined and presented a significant knowledge gap in our understanding of the complex pharmacology of antidepressants.
We hypothesized that antidepressants accumulate gradually in lipid rafts and modulate the distribution of Gαs in the membrane. Results in this study are consistent with that hypothesis and reveal novel binding domains for antidepressants that may be consistent with delayed therapeutic response.
Results
Gradual Accumulation of Antidepressant Drugs in Plasma Membrane Microdomains Is Independent of SERT
Although there are many potential targets for monoamine-centric drugs, none offer an explanation for the hysteresis (6–8 weeks) between initiation of therapy and clinical efficacy. C6 glioma cells were used in these experiments because they do not express monoamine transport proteins (Fig. 1), yet sustained treatment with antidepressant drugs translocates Gαs from lipid rafts to non-raft regions of the plasma membrane (7, 8, 20). Furthermore, glia may contribute to both the etiology and the treatment of depression (21, 22).
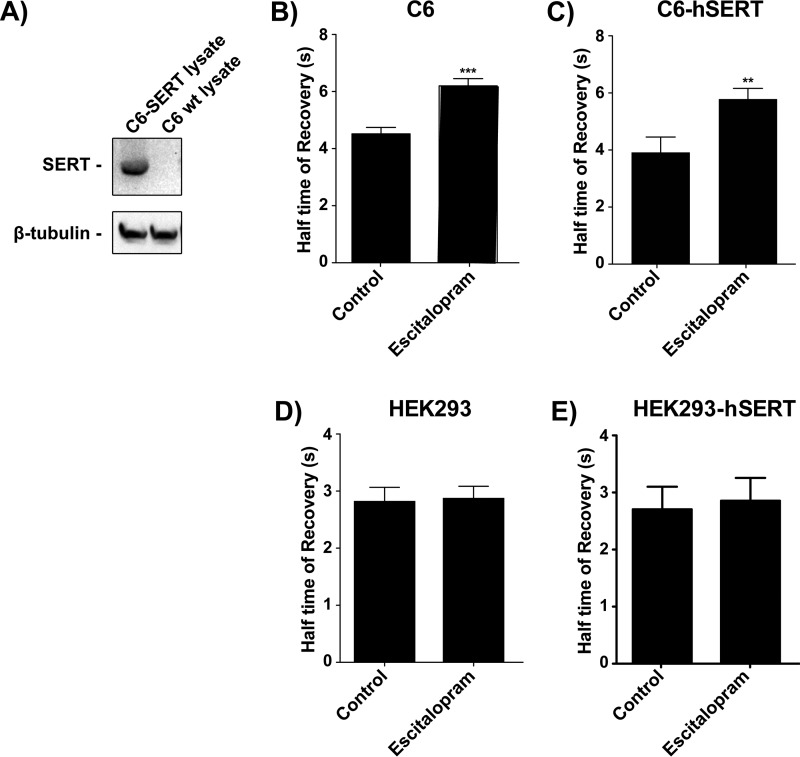
FIGURE 1.
Gαs-GFP FRAP is slowed by chronic antidepressant treatment and this is not changed by co-expression of hSERT. Gαs-GFP C6 cells were treated with 10 μm escitalopram for 72 h. A, whole cell homogenate from C6 cells stably transfected with hSERT (C6-hSERT), but not wild type C6 cells, display reactivity to an anti-SERT antibody by Western blotting (i.e. C6 glioma cells do not natively express SERT). B, half-time to recovery of Gαs-GFP is increased (mobility slowed) after chronic escitalopram treatment, suggesting increased coupling with adenylyl cyclase. C, C6-hSERT cells were transfected with Gαs-GFP, selected with G418, and treated with 10 μm escitalopram for 72 h. Half-time to recovery of Gαs-GFP is increased after chronic escitalopram treatment regardless of hSERT expression. D, half time of recovery of Gαs-GFP is not affected by 72 hrs treatment with escitalopram in HEK293 cells. E, transfection of Gαs-GFP expressing HEK293 cells with hSERT does not affect the half time of recovery of Gαs-GFP either. Sample size represents the number of cells assayed, with a minimum of 18 cells and a maximum of 77 cells assayed per experiment. Data were analyzed by Student's t test, and data are represented as mean ± S.E. (**, p < 0.001; ***, p < 0.0001).
One binding site for many of the antidepressant drugs (e.g. tricyclic antidepressants and SSRIs) is the serotonin reuptake transport protein (SERT). To determine whether SERT expression affects the redistribution of Gαs, C6 cells stably expressing Gαs-GFP were engineered to also express SERT.
Gαs-GFP is identical in its activation by GPCR and activation of adenylyl cyclase with wild type Gαs (23). When kept to a moderate level of expression (2–3× endogenous Gαs), Gαs-GFP is transparent to cellular physiology, allowing a window on its movements in response to treatment with antidepressants (Gαs moves out of lipid rafts) (24).
Gαs-GFP translocation from lipid rafts in response to escitalopram, as measured by fluorescence recovery after photobleaching (FRAP), does not improve upon expression of hSERT (Fig. 1, B and C, respectively). Moreover, HEK cells do not respond to chronic stimulation with escitalopram, nor when stably transfected with hSERT (Fig. 1, D and E, respectively). These data suggest that some additional cellular component is responsible for the redistribution of Gαs, and we hypothesized that the antidepressant drugs accumulate, gradually, in lipid rafts.
Gradual Accumulation of Antidepressant Drugs in Plasma Membrane Microdomains Correlates with Gαs Subcellular Redistribution
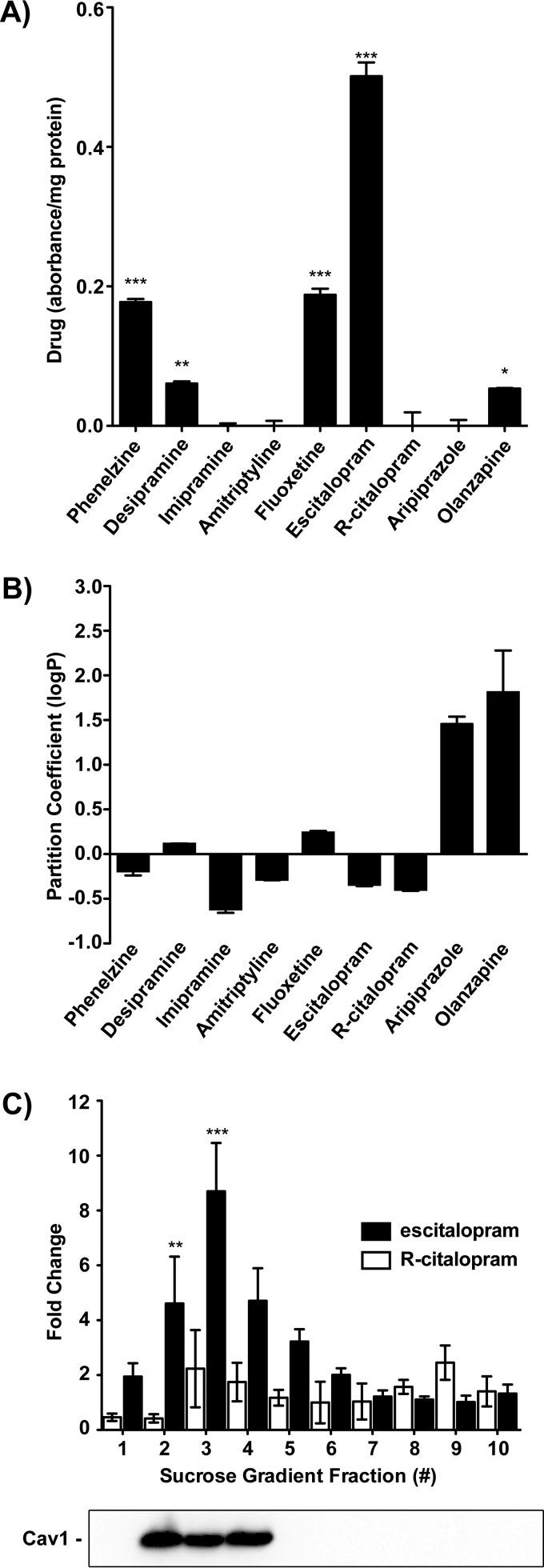
Due to our earlier observations that Gαs translocates from rafts after extended exposure to antidepressants (8), and the observations by Rupprecht and colleagues (25), we hypothesized that antidepressants preferentially associate with lipid rafts, and that all monoamine-centric antidepressants share this property. We assessed the accumulation of representative drugs from each antidepressant class: MAO inhibitor (phenelzine), tricyclic antidepressant (desipramine, imipramine, and amitriptyline), and SSRI (escitalopram/inactive stereoisomer R-citalopram and fluoxetine), as well as the atypical antipsychotic aripiprazole, which has some independent antidepressant properties, and the atypical antipsychotic, olanzapine, which does not (26). We expected that antidepressants would gradually accumulate in raft fractions of C6 cells over time to mediate the translocation of Gαs out of the lipid raft and that the non-antidepressant compounds tested would not have this property.
Lipid raft fractions were isolated via sucrose density gradient, and the UV-visible spectrum was taken for each fraction. Each antidepressant absorbs at a characteristic wavelength for which measurements were normalized to protein content in the sample and the -fold change was calculated relative to treatment-naive or untreated controls. The accumulation of drugs over time in lipid raft fractions of the plasma membrane appears to be a class-specific mechanism (e.g. SSRIs and MAO inhibitors) (Fig. 2A). Olanzapine, an antipsychotic lacking primary antidepressant properties (27), appears to accumulate as well, which may be due to its highly hydrophobic nature (Fig. 2B). However, analysis of raft fractions by GC-MS revealed that accumulation of drugs in rafts is independent of hydrophobicity, as olanzapine and aripiprazole do not accumulate.
FIGURE 2.
Treatment of C6 glioma cells with antidepressant drugs reveals their capacity to accumulate in plasma membrane microdomains. A, cells were treated for 3 days with the indicated compound (10 μm), and lipid raft fractions were prepared from membranes. Drug accumulation was determined by the characteristic absorbance of the drug. The -fold change in UV absorbance for phenelzine (256 nm), desipramine (252 nm), imipramine (295 nm), amitriptyline (262 nm), fluoxetine (226 nm), citalopram (238 nm), aripiprazole (298 nm), or olanzapine (270 nm) normalized to protein (280 nm) and the untreated sample suggests that accumulation in lipid rafts is a class-specific mechanism. B, UV absorbance was used to determine partition coefficient (logP) for psychoactive compounds. Partitioning between octanol and water reveals that each is relatively amphiphilic, with the exception of aripiprazole and olanzapine. C, sucrose density gradient fractions from treatment-naive or untreated C6 cells were incubated overnight with escitalopram or R-citalopram. Following methanol-chloroform precipitation of proteins, the -fold change in UV absorbance reveals that escitalopram, but not R-citalopram, is retained in the raft regions of the membrane as revealed by anti-caveolin 1 (Cav1) immunoreactivity (1:5000). Data were analyzed by Student's t test, and data are represented as mean ± S.E. (n = 3; *, p < 0.01; **, p < 0.001; ***, p < 0.0001).
Escitalopram, and its therapeutically inactive enantiomer, R-citalopram, were selected for further investigation. To parallel the experiments by Eisensamer et al. (25), escitalopram was added to sucrose density gradients prepared from membrane fractions. Escitalopram, but not R-citalopram, associated with lipid raft fractions of the plasma membrane (Fig. 2C). To minimize background measurements as much as possible for this method of detection, we normalized the readings to protein (280 nm) and subtracted the control absorbance. These measurements were then corroborated via mass spectrometry.
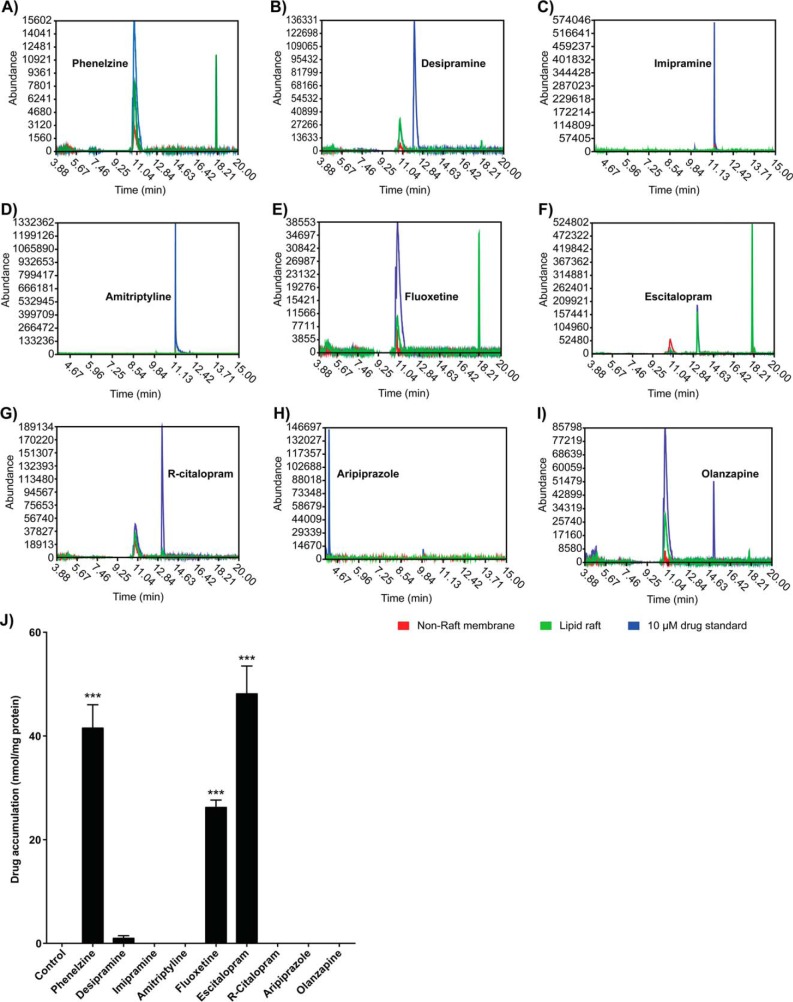
GC-MS is sensitive and selective, due in large part to the separation efficiency achieved in the analysis of small molecules. C6 cells were treated for 72 h with 10 μm of antidepressant, and the lipid raft fraction was extracted for determination of drug presence. Analysis of the total ion chromatograms (TICs) of lipid raft extractions showed that only phenelzine, fluoxetine, and escitalopram accumulated in lipid rafts after 72 h of treatment (Fig. 3); R-citalopram, imipramine, amitriptyline, aripiprazole, and olanzapine did not accumulate, although with the exception of R-citalopram, each of these drugs shows an “antidepressant signature” in translocating Gαs from lipid rafts. Base peaks of the molecular ion profile from each TIC elution profile were matched to the National Institute of Standards and Technology (NIST) database to identify the drug presence among membrane fractions.
FIGURE 3.
Representative GC-MS chromatograms and quantification of accumulated drug in C6 glioma cells treated for 3 days. Cells were treated for 3 days with the indicated compound (10 μm), and lipid raft fractions were prepared from membranes. TICs were pre-processed with denoising, Savitzky-Golay, and component detection algorithm (CODA) filters (42). A–I, representative TIC peaks for all drugs tested, confirming that antidepressant drugs accumulate in lipid rafts of C6 cells. Red, lipid raft; green, lipid raft + accumulated drug; blue, 10 μm drug standard. J, drug accumulation was determined and quantified by comparing peak intensities from the TICs of GC-MS analyses on accumulated drug with standard curves generated from methanol standards. Calculated moles of drug were normalized to protein content and reported as μmol mg−1 of protein. Phenelzine (41.51 ± 4.52), fluoxetine (26.24 ± 1.41), escitalopram (48.13 ± 5.35), and to a lesser extent desipramine (0.98 ± 0.51) were observed to accumulate in lipid rafts, whereas the inactive stereoisomer R-citalopram and the antipsychotic olanzapine did not. Data were analyzed by Student's t test, and data are represented as mean ± S.E. (n = 3; ***, p < 0.0001).
The accumulation of escitalopram, fluoxetine, and phenelzine, but not R-citalopram or olanzapine, parallels their capacity to mediate the movement of Gαs from lipid rafts (20). Because neither the tricyclic antidepressants (desipramine, imipramine, and amitriptyline) nor aripiprazole accumulate in rafts, this phenomenon may be class-specific for antidepressants. Furthermore, accumulation is independent of the lipophilicity of the compound (Fig. 2B).
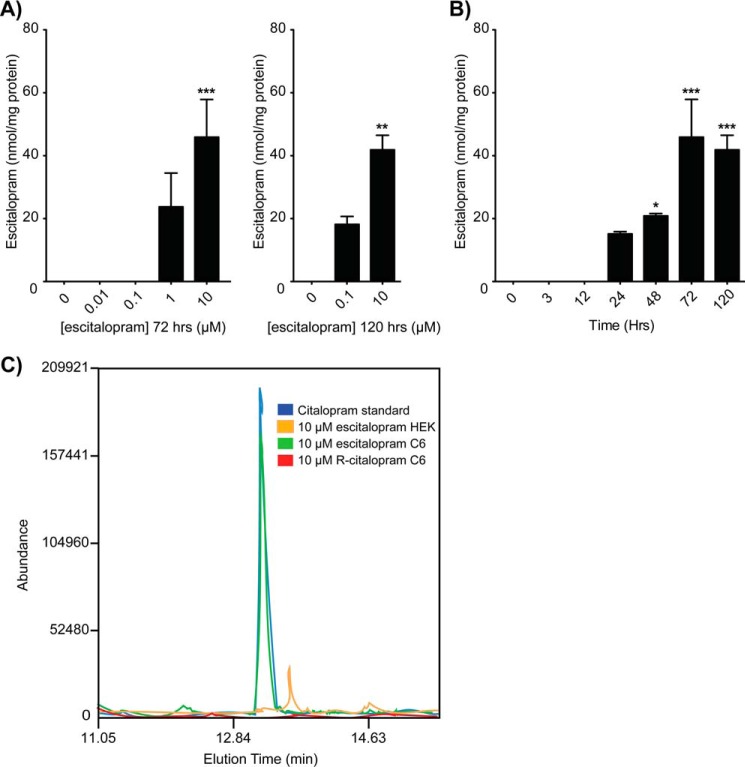
Gradual Accumulation of Escitalopram in Plasma Membrane Microdomains Is Time- and Concentration-dependent
The finding that antidepressants, but not other related drugs, demonstrated gradual accumulation, along with the stereospecificity of antidepressant accumulation, led us to select escitalopram for closer analysis. Tracking the accumulation of escitalopram in the lipid raft fraction derived from C6 cells with GC-MS analysis revealed that escitalopram accumulates in a concentration- and time-dependent manner. Detectable accumulation occurred following 1 μm treatments for 72 h or 100 nm treatments for 120 h and at 24-, 48-, and 72-h treatments with 10 μm escitalopram (Fig. 4). Treatment with 10 μm antidepressant for 72 h is a standard assay condition (8) and parallels doses used in rat studies (7, 28). However, these drugs translocate Gαs at concentrations as low as 50 nm over the same period (29).
FIGURE 4.
GC-MS analysis of escitalopram-treated C6 glioma cells confirms accumulation in plasma membrane microdomains. A, lipid rafts isolated from C6 cells treated for 3 days with escitalopram from 10 nm to 10 μm revealed that escitalopram accumulates in a concentration-dependent manner beginning with 1 μm for 72 h or 100 nm for 120 h and achieving maximal accumulation with 10 μm at 72 h. B, lipid rafts isolated from C6 cells treated with 10 μm escitalopram revealed that escitalopram accumulates over time in lipid rafts beginning at 24 h and plateauing at 72 h. C, TIC chromatogram overlay of drug accumulation in lipid rafts of C6 cells treated with 10 μm escitalopram versus R-citalopram as compared with lipid rafts isolated from escitalopram-treated HEK cells. Data were analyzed by Student's t test, and data are represented as mean ± S.E. (n = 3; *, p < 0.01; **, p < 0.001; ***, p < 0.0001).
These measurements are consistent with the drug time and dose required for Gαs translocation from rafts, measured either directly (8) or by FRAP (20). Moreover, stereo specificity and the fact that escitalopram does not accumulate over time in HEK cells indicates a specific target (Fig. 4C). Furthermore, antidepressant-mediated redistribution of Gαs independent of SERT suggests a molecular drug-binding target that is distinct from the monoamine transport system. Only a protein target(s) could account for both the enantioselectivity as well as the lack of hydrophobic contribution toward the gradual accumulation of SSRIs in lipid rafts.
Discussion
Gαs is a membrane-associated protein that inhabits cholesterol-rich lipid raft microdomains (15). Data presented above suggest that sustained treatment of cells with several antidepressants results in the accumulation of those compounds in lipid rafts and correlates with the membrane redistribution of Gαs. Because the antidepressant drugs used in this study were not strongly hydrophobic, and escitalopram, but not R-citalopram (equal lipophilicity), accumulates in lipid rafts, it is likely that an unidentified lipid raft protein is the binding site for antidepressants.
Lipid rafts contain many of the anchoring cytoskeleton-associated membrane structures and facilitate molecular association(s) of a vast array of different membrane-embedded and -associated proteins to initiate intracellular signaling. Although lipid rafts can facilitate this clustering of signaling molecules, the rigid structure afforded by increased cholesterol content appears to have a globally dampening effect on Gαs signaling by inhibiting association(s) between raft- and non-raft-based molecules (13). Dampened signaling, through Gαs and/or Gαs-coupled receptors, is consistent with the observed increase in Gαs association with rafts as well as the increased lipid raft localization of Gαs seen in postmortem major depressive disorder brain (15).
Consistent with these observations, chronic treatment with antidepressant drugs results in Gαs translocation from lipid rafts to non-raft regions of the plasma membrane, and the extent of translocation is dependent upon both drug dose and treatment duration. This has been observed in both rats (7) and cell culture (8, 16, 30). Moreover, lipid raft disruption through cholesterol depletion or cytoskeletal disruption displaces many raft proteins, but GPCR activation or antidepressant treatment displaces only Gαs (7). Binding to the reuptake transport machinery and inhibition of neurotransmitter reuptake are rapid, but require several weeks of treatment to achieve clinical efficacy, and this is duplicated (albeit in a more rapid timescale) in C6 glioma cells.
Estimates of the ratio of glia to neurons vary considerably, but evidence suggests that glia control the number and stability of neuronal synapses formed (31, 32). Furthermore, chronic antidepressant treatment has been shown to increase the expression and release of glial cell-derived neurotrophic factor (GDNF) (33–35). Curiously, the molecular entities most commonly associated with antidepressants, serotonin and norepinephrine transporters, are not endogenously expressed in C6 cells (36), which still respond to antidepressants by mediating lipid raft translocation of Gαs.
All antidepressants examined thus far move Gαs from lipid rafts. This does not imply a single mechanism of action, but does suggest that antidepressants have a similar molecular footprint to exploit for the purposes of diagnostics and therapeutics. It is possible that the active sites for some antidepressants are downstream from their membrane-binding sites. However, we predicted that antidepressants would accumulate in the lipid raft regions of the plasma membrane unless the drug translocates across the membrane to bind an intracellular target. A seemingly simplistic explanation for the antidepressant-mediated translocation of Gαs from the lipid raft is the accumulation of drugs in lipid raft regions of the plasma membrane as Gαs moves out.
Previous studies on the concentration of psychoactive drugs in the lipid raft do not correlate exactly with our findings (25). Any discrepancies may well be due to the method of detection. Furthermore, these earlier studies used HEK cells transfected with 5-hydroxytryptamine type 3 (5-HT3) receptors, which were suggested to bind the drugs used in the study. Gαs is not translocated from native HEK cells after antidepressant treatment, and membranes prepared from kidney do not show augmentation of Gαs-activated adenylyl cyclase in antidepressant-treated rats (37). Finally, in the studies by Eisensamer et al. (25), drug accumulation was determined by absorbance after exogenous addition to membrane fractions, similar to experiments shown in Fig. 2C of this study. As revealed in this study, results from acute drug addition to membranes are quite different from the time-dependent accumulation of antidepressants in lipid raft fractions (Fig. 3). Future studies to refine these observations might employ moclobemide, an antidepressant that did not concentrate in rafts in the Eisensamer studies (21).
Different/multiple mechanisms are likely to exist for the actions of different antidepressants, but all drugs examined, thus far, translocate Gαs from lipid rafts. The observed behavior of the antipsychotic, olanzapine, was expected, as it does not move Gαs out of rafts (20). However, further support for distinct molecular drug targets comes from the enantiomer-selective accumulation of escitalopram, but not the therapeutically inactive enantiomer R-citalopram, and from the finding that that accumulation appears restricted to the SSRIs and MAO inhibitors.
Thus, it appears that at least one action of antidepressants is to accumulate in lipid rafts and mediate the movement of Gαs out of lipid rafts. This may represent a novel biochemical hallmark for antidepressant action. Furthermore, identification of the antidepressant-sensitive molecular anchor for Gαs in lipid rafts may lead to the development of more targeted therapies for depression, including compounds that may have a much more rapid course of action.
Materials and Methods
Chemicals
DMEM, fetal bovine serum, trypsin, and penicillin/streptomycin were purchased from Sigma-Aldrich. Cell culture flasks were from NUNC (VWR International, West Chester, PA). Escitalopram and R-citalopram were kindly provided from H. Lundbeck A/S, Copenhagen, Denmark. Desipramine hydrochloride and olanzapine were purchased from Tocris Bioscience, Ellisville, MO. Phenelzine sulfate, fluoxetine hydrochloride, amitriptyline hydrochloride, imipramine hydrochloride, and aripiprazole were purchased from Sigma-Aldrich.
Drug Treatments
C6 cells were cultured in DMEM, 4.5 g of glucose/liter, 10% newborn calf serum (HyClone Laboratories, Logan, UT), 100 mg ml−1 bacteriostatic penicillin-streptomycin at 37 °C in humidified 5% CO2 atmosphere to a confluence of ∼40% before drug treatments were begun. Treatment with 10 μm for 72 h is a standard assay condition (8); however, the effects of these drugs in this cellular system can be observed at concentrations as low as 50 nm after 72 h (20). Culture media and drug were changed daily, and no apparent change in cell morphology was observed during treatment. Before assay, cells were rinsed twice with pre-warmed 1× PBS to remove debris and wash away unbound drugs.
Lipid Raft Isolation
Cells were washed and harvested in ice-cold 1× PBS. Lipid raft fractions were prepared as described previously (38). Briefly, C6 cells were extracted in 1 ml of ice-cold lysis buffer (10 mm HEPES, pH 7.4; 150 mm NaCl; 1 mm DTT; 0.25 m sucrose; 1% Triton X-100; protease inhibitor cocktail). Following a 30-min incubation on ice, the lysates were homogenized, and 1 ml was gently mixed (v/v) with ice-cold 80% (w/v) sucrose in TME (10 mm Tris-HCl; 1 mm MgCl2; 1 mm EDTA, pH 7.5; 1 mm DTT; protease inhibitors), and then loaded in the bottom of a centrifuge tube. Samples were overlaid by syringe and fine needle with 1 ml each of 30% sucrose, 15% sucrose, and finally 5% sucrose. Sucrose gradients were spun at 40,000 rpm in an SW55-Ti rotor in a Beckman ultracentrifuge at 4 °C overnight for 16–18 h. Lipid rafts exist between 5 and 15% sucrose layers as opaque white clusters. Raft fractions were collected and sucrose was removed via sequential mixing (v/v) in wash buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, 1 mm DTT) and centrifugation at 21,000 rpm at 4 °C for 20 min (∼4–5 times) until a pellet emerged. Lipid raft pellets were reconstituted in 0.5 ml of TME buffer. Protein content was determined by absorbance at 280 nm on a NanoDrop UV-visible spectrophotometer.
Accumulation of Antidepressants Measured by UV-visible Spectrophotometer
UV absorbance of antidepressants was used to determine their association with membrane fractions similar to before (25), but in the absence of HPLC purification. C6 cells treated (72 h) with 10 μm escitalopram, R-citalopram, fluoxetine, desipramine, phenelzine, or olanzapine were extracted by Triton X-100/114, and the cytosolic, non-raft membrane, and lipid raft fractions were analyzed by UV absorbance and normalized to protein content (λ = 280 nm).
Furthermore, 500-μl sucrose density gradient fractions were incubated with a final concentration of 10 μm escitalopram or R-citalopram. S- and R-citalopram absorbance (λ = 238 nm) in each fraction was assessed before and after incubation, measurements were normalized to protein, and the -fold change was reported.
Drug Hydrophobicity
Partition coefficients of drugs were determined as described previously (39) in a 1:1 v/v octanol to double-distilled H2O, and the UV-visible spectrum was recorded for each phase. Absorbances are as follows: phenelzine (256 nm), desipramine (252 nm), imipramine (295 nm), amitriptyline (262 nm), fluoxetine (226 nm), citalopram (238 nm), aripiprazole (298 nm), or olanzapine (270 nm).
Gas Chromatography Mass Spectrometry (GC-MS)
Analyses were performed using an Agilent HP-6890 gas chromatograph, equipped with an Agilent 19091S-602 HP-1MS capillary column (25 m, 0.20 mm, 0.33 μm, 7-inch cage), and interfaced with an Agilent HP-5973 mass-selective detection spectrometer equipped with a Single Flame Ionization Detector, single 100-psi EPC Split/Splitless Injection Ports, 7673C-6890 Auto Sampler: 6890 Control Electronics, 6890 Injector, 100 Position Tray, and 6890 Mounting Bracket. Helium was used as the carrier gas at 1.0 ml min−1 in corrected constant flow mode. Primary oven temperature was programmed at 70 °C for 2 min and increased at 20 °C for min−1 to 230 °C where it was held for 10 min. The front inlet thermal modulator was set to 20 °C higher relative to the primary oven and 18.91 psi. Constant flow injection of 1 μl was used, and inject split mode was changed to Splitless. The injector, transfer line, and ion source temperatures were maintained at 250, 280, and 230 °C, respectively, throughout each analysis. Data acquisition was performed in the full scan mode from m/z 50 to 550 with an acquisition rate of 20 Hz. Molecular ion profiles were matched against the standard mass spectral database of the NIST.
Accumulation of Antidepressants Measured by GC-MS
The accumulation of antidepressants in lipid rafts and non-raft membranes of C6 glioma cells was measured via GC/MS to accompany results obtained via increases in the UV absorbance spectrum for escitalopram as opposed to R-citalopram. C6 cells were treated (72 h) with 10 μm escitalopram, R-citalopram, fluoxetine, desipramine, imipramine, amitriptyline, aripiprazole, phenelzine, or olanzapine. More elaborate concentration and temporal measurements were restricted to escitalopram. The accumulation of increasing concentrations, from 10 nm to 10 μm, of escitalopram over 72 h, as well as temporally from 3 to 120 h with 10 μm escitalopram was measured in lipid raft and non-raft membrane; R-citalopram served as the control.
Cells were collected, and membranes were fractionated into Triton X-100-soluble and Triton X-114-soluble fractions. Extraction of accumulated antidepressant drugs in lipid rafts (Triton X-114 fraction) may be assessed on large volume samples as described previously (40), but is not appropriate for the small volumes used here. Membrane fractions were chloroform-methanol precipitated as described previously (41), and the water, chloroform, and methanol phases vacuum were centrifuged to recover accumulated drug. Desiccant was dissolved into 1 ml of methanol, and then injected directly onto a GC Capillary Column (Agilent J&W HP-1ms), interfaced with an Agilent HP-5973 mass-selective detection spectrometer. Finally, quantitation of accumulated drug was performed by the internal standard method. Peak-area ratios were determined for the controls. Drug concentrations were calculated from the standard curve values. All data were acquired and analyzed by Agilent software, Enhanced G1701BA ChemStation version B.00.00. Molecular ion profiles were matched against the standard mass spectral database of the NIST.
FRAP
C6 glioma cells stably expressing hSERT were a kind gift from Dr. Kim Neve, Oregon Health & Science University (OHSU). These cells were further transfected with GFP-Gαs, and cells expressing the fluorescent construct were selected with G418. Cells were plated on glass microscopy dishes and treated with 10 μm escitalopram or desipramine for 3 days. For imaging, drug was washed out for 1 h prior, and medium was replaced with low serum (2.5% newborn calf serum) phenol red-free DMEM to limit fluorescent background. Temperature was maintained at 37 °C using a PeCon temperature-controlled stage during imaging. Imaging utilized a Zeiss LSM 710 confocal microscope at 512 × 512 resolution with an open pinhole to maximize signal but minimize photobleaching. One hundred fifty data points, ∼300 ms apart (including 10 pre-bleach values), were measured for each cell. Zeiss Zen software was used to calculate FRAP recovery half-time utilizing a one-phase association fit, correcting for total photobleaching of the analyzed regions.
Western Blotting
Western blots were conducted according to standard protocols with a rabbit polyclonal anti-SERT (1:1,000; EMD Millipore, Billerica, MA, catalogue number AB9726), rabbit polyclonal anti-caveolin 1 (1:10,000; BD Biosciences, catalogue number 610059), and rabbit monoclonal anti-β-tubulin (1:5,000; made in-house).
Statistical Analysis
All measurements are presented as the mean (a minimum of n = 3) ± S.E. Calculation error was propagated throughout each calculation. We further subjected each data set to statistical analyses using GraphPad Prism (version 5.0), using a one-way analysis of variance followed by a post hoc Student's t test (two groups) or Dunnett's t test (multiple groups) (95% confidence interval).
Author Contributions
S. J. E. designed, coordinated, performed, and analyzed the experiments in Figures 2–4 and wrote the paper. J. M. S. designed, performed, and analyzed experiments shown in Figure 1. M. M. R. designed the experiments, analyzed data, and wrote the paper. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
We thank Drs. Karl Larsen, at the University of Illinois at Chicago, and Thomas Hoye, at the University of Minnesota, who kindly provided access to their Agilent GC-MS instruments. We also thank Dr. Kim Neve for C6 SERT cells and Lundbeck Inc. for escitalopram and R-citalopram.
This work was supported by Veterans Administration Merit Award BX001149 and National Institutes of Health Grants R01AT009169 and T32 MH067631. M. M. R. has received research support from Eli Lilly and Lundbeck, Inc. and is a consultant to Otsuka Pharmaceuticals. He also has ownership in Pax Neuroscience. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article was selected as a Paper of the Week.
- GPCR
- G-protein-coupled receptor
- SERT
- serotonin reuptake transport protein
- hSERT
- human SERT
- SSRI
- selective serotonin reuptake inhibitor
- MAO
- monoamine oxidase
- FRAP
- fluorescence recovery after photobleaching
- TIC
- total ion chromatogram.
References
- 1. Chen G., Twyman R., and Manji H. K. (2010) p11 and gene therapy for severe psychiatric disorders: a practical goal? Sci. Transl. Med. 2, 54ps51. [DOI] [PubMed] [Google Scholar]
- 2. Olfson M., and Marcus S. C. (2009) National patterns in antidepressant medication treatment. Arch. Gen. Psychiatry 66, 848–856 [DOI] [PubMed] [Google Scholar]
- 3. Rush A. J., Trivedi M. H., Wisniewski S. R., Nierenberg A. A., Stewart J. W., Warden D., Niederehe G., Thase M. E., Lavori P. W., Lebowitz B. D., McGrath P. J., Rosenbaum J. F., Sackeim H. A., Kupfer D. J., Luther J., and Fava M. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917 [DOI] [PubMed] [Google Scholar]
- 4. Maes M., Yirmyia R., Noraberg J., Brene S., Hibbeln J., Perini G., Kubera M., Bob P., Lerer B., and Maj M. (2009) The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 24, 27–53 [DOI] [PubMed] [Google Scholar]
- 5. Duman R. S., Heninger G. R., and Nestler E. J. (1997) A molecular and cellular theory of depression. Arch. Gen. Psychiatry 54, 597–606 [DOI] [PubMed] [Google Scholar]
- 6. Fujita M., Hines C. S., Zoghbi S. S., Mallinger A. G., Dickstein L. P., Liow J. S., Zhang Y., Pike V. W., Drevets W. C., Innis R. B., and Zarate C. A. Jr. (2012) Downregulation of brain phosphodiesterase type IV measured with 11C-(R)-rolipram positron emission tomography in major depressive disorder. Biol. Psychiatry 72, 548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toki S., Donati R. J., and Rasenick M. M. (1999) Treatment of C6 glioma cells and rats with antidepressant drugs increases the detergent extraction of Gsα from plasma membrane. J. Neurochem. 73, 1114–1120 [DOI] [PubMed] [Google Scholar]
- 8. Zhang L., and Rasenick M. M. (2010) Chronic treatment with escitalopram but not R-citalopram translocates Gαs from lipid raft domains and potentiates adenylyl cyclase: a 5-hydroxytryptamine transporter-independent action of this antidepressant compound. J. Pharmacol. Exp. Ther. 332, 977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown D. A., and London E. (1998) Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14, 111–136 [DOI] [PubMed] [Google Scholar]
- 10. Kalinowska M., Castillo C., and Francesconi A. (2015) Quantitative profiling of brain lipid raft proteome in a mouse model of fragile X syndrome. PLoS ONE 10, e0121464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kasahara K., and Sanai Y. (2000) Functional roles of glycosphingolipids in signal transduction via lipid rafts. Glycoconj. J. 17, 153–162 [DOI] [PubMed] [Google Scholar]
- 12. Insel P. A., Head B. P., Patel H. H., Roth D. M., Bundey R. A., and Swaney J. S. (2005) Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem. Soc. Trans. 33, 1131–1134 [DOI] [PubMed] [Google Scholar]
- 13. Allen J. A., Halverson-Tamboli R. A., and Rasenick M. M. (2007) Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 8, 128–140 [DOI] [PubMed] [Google Scholar]
- 14. Allen J. A., Yu J. Z., Dave R. H., Bhatnagar A., Roth B. L., and Rasenick M. M. (2009) Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol. Pharmacol. 76, 1082–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donati R. J., Dwivedi Y., Roberts R. C., Conley R. R., Pandey G. N., and Rasenick M. M. (2008) Postmortem brain tissue of depressed suicides reveals increased Gsα localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J. Neurosci. 28, 3042–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Donati R. J., and Rasenick M. M. (2005) Chronic antidepressant treatment prevents accumulation of Gsα in cholesterol-rich, cytoskeletal-associated, plasma membrane domains (lipid rafts). Neuropsychopharmacology 30, 1238–1245 [DOI] [PubMed] [Google Scholar]
- 17. Ozawa H., and Rasenick M. M. (1991) Chronic electroconvulsive treatment augments coupling of the GTP-binding protein Gs to the catalytic moiety of adenylyl cyclase in a manner similar to that seen with chronic antidepressant drugs. J. Neurochem. 56, 330–338 [DOI] [PubMed] [Google Scholar]
- 18. Yu J. Z., Dave R. H., Allen J. A., Sarma T., and Rasenick M. M. (2009) Cytosolic Gαs acts as an intracellular messenger to increase microtubule dynamics and promote neurite outgrowth. J. Biol. Chem. 284, 10462–10472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sarma T., Koutsouris A., Yu J. Z., Krbanjevic A., Hope T. J., and Rasenick M. M. (2015) Activation of microtubule dynamics increases neuronal growth via the nerve growth factor (NGF)- and Gαs-mediated signaling pathways. J. Biol. Chem. 290, 10045–10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Czysz A. H., Schappi J. M., and Rasenick M. M. (2015) Lateral diffusion of Gαs in the plasma membrane is decreased after chronic but not acute antidepressant treatment: role of lipid raft and non-raft membrane microdomains. Neuropsychopharmacology 40, 766–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanacora G., Treccani G., and Popoli M. (2012) Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 62, 63–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Benedetto B., Rupprecht R., and Czéh B. (2013) Talking to the synapse: how antidepressants can target glial cells to reshape brain circuits. Curr. Drug Targets 14, 1329–1335 [DOI] [PubMed] [Google Scholar]
- 23. Yu J. Z., and Rasenick M. M. (2002) Real-time visualization of a fluorescent Gαs: dissociation of the activated G protein from plasma membrane. Mol. Pharmacol. 61, 352–359 [DOI] [PubMed] [Google Scholar]
- 24. Allen J. A., Yu J. Z., Donati R. J., and Rasenick M. M. (2005) β-Adrenergic receptor stimulation promotes Gαs internalization through lipid rafts: a study in living cells. Mol. Pharmacol. 67, 1493–1504 [DOI] [PubMed] [Google Scholar]
- 25. Eisensamer B., Uhr M., Meyr S., Gimpl G., Deiml T., Rammes G., Lambert J. J., Zieglgänsberger W., Holsboer F., and Rupprecht R. (2005) Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. J. Neurosci. 25, 10198–10206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson J. C., and Papakostas G. I. (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am. J. Psychiatry 166, 980–991 [DOI] [PubMed] [Google Scholar]
- 27. Rothschild A. J., Bates K. S., Boehringer K. L., and Syed A. (1999) Olanzapine response in psychotic depression. J. Clin. Psychiatry 60, 116–118 [DOI] [PubMed] [Google Scholar]
- 28. Vetulani J., and Sulser F. (1975) Action of various antidepressant treatments reduces reactivity of noradrenergic cyclic AMP-generating system in limbic forebrain. Nature 257, 495–496 [DOI] [PubMed] [Google Scholar]
- 29. Chen J., and Rasenick M. M. (1995) Chronic treatment of C6 glioma cells with antidepressant drugs increases functional coupling between a G protein (Gs) and adenylyl cyclase. J. Neurochem. 64, 724–732 [DOI] [PubMed] [Google Scholar]
- 30. Donati R. J., Thukral C., and Rasenick M. M. (2001) Chronic treatment of C6 glioma cells with antidepressant drugs results in a redistribution of Gsα. Mol. Pharmacol. 59, 1426–1432 [DOI] [PubMed] [Google Scholar]
- 31. Ullian E. M., Christopherson K. S., and Barres B. A. (2004) Role for glia in synaptogenesis. Glia 47, 209–216 [DOI] [PubMed] [Google Scholar]
- 32. Yan K., Popova J. S., Moss A., Shah B., and Rasenick M. M. (2001) Tubulin stimulates adenylyl cyclase activity in C6 glioma cells by bypassing the β-adrenergic receptor: a potential mechanism of G protein activation. J. Neurochem. 76, 182–190 [DOI] [PubMed] [Google Scholar]
- 33. Hisaoka K., Maeda N., Tsuchioka M., and Takebayashi M. (2008) Antidepressants induce acute CREB phosphorylation and CRE-mediated gene expression in glial cells: a possible contribution to GDNF production. Brain Res. 1196, 53–58 [DOI] [PubMed] [Google Scholar]
- 34. Hisaoka K., Tsuchioka M., Yano R., Maeda N., Kajitani N., Morioka N., Nakata Y., and Takebayashi M. (2011) Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J. Biol. Chem. 286, 21118–21128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Golan M., Schreiber G., and Avissar S. (2011) Antidepressants elevate GDNF expression and release from C6 glioma cells in a β-arrestin1-dependent, CREB interactive pathway. Int. J. Neuropsychopharmacol. 14, 1289–1300 [DOI] [PubMed] [Google Scholar]
- 36. Eshleman A. J., Stewart E., Evenson A. K., Mason J. N., Blakely R. D., Janowsky A., and Neve K. A. (1997) Metabolism of catecholamines by catechol-O-methyltransferase in cells expressing recombinant catecholamine transporters. J. Neurochem. 69, 1459–1466 [DOI] [PubMed] [Google Scholar]
- 37. Menkes D. B., Rasenick M. M., Wheeler M. A., and Bitensky M. W. (1983) Guanosine triphosphate activation of brain adenylate cyclase: enhancement by long-term antidepressant treatment. Science 219, 65–67 [DOI] [PubMed] [Google Scholar]
- 38. Li S., Okamoto T., Chun M., Sargiacomo M., Casanova J. E., Hansen S. H., Nishimoto I., and Lisanti M. P. (1995) Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J. Biol. Chem. 270, 15693–15701 [DOI] [PubMed] [Google Scholar]
- 39. Chiou C. T., Schmedding D. W., and Block J. H. (1981) Correlation of water solubility with octanol-water partition coefficient. J. Pharm. Sci. 70, 1176–1177 [DOI] [PubMed] [Google Scholar]
- 40. Winecker R. E. (2010) Quantification of antidepressants using gas chromatography-mass spectrometry. Methods Mol. Biol. 603, 45–56 [DOI] [PubMed] [Google Scholar]
- 41. Wessel D., and Flügge U. I. (1984) A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 138, 141–143 [DOI] [PubMed] [Google Scholar]
- 42. Wenig P., and Odermatt J. (2010) OpenChrom: a cross-platform open source software for the mass spectrometric analysis of chromatographic data. BMC Bioinformatics 11, 405. [DOI] [PMC free article] [PubMed] [Google Scholar]