Summary
Background
Whole brain radiotherapy (WBRT) is the standard of care to improve intracranial control following resection of brain metastasis. However, stereotactic radiosurgery (SRS) to the surgical cavity is widely used in an attempt to reduce cognitive toxicity, despite the absence of high-level comparative data substantiating efficacy in the postoperative setting. We aimed to establish the effect of SRS on survival and cognitive outcomes compared with WBRT in patients with resected brain metastasis.
Methods
In this randomised, controlled, phase 3 trial, adult patients (aged 18 years or older) from 48 institutions in the USA and Canada with one resected brain metastasis and a resection cavity less than 5·0 cm in maximal extent were randomly assigned (1:1) to either postoperative SRS (12–20 Gy single fraction with dose determined by surgical cavity volume) or WBRT (30 Gy in ten daily fractions or 37·5 Gy in 15 daily fractions of 2·5 Gy; fractionation schedule predetermined for all patients at treating centre). We randomised patients using a dynamic allocation strategy with stratification factors of age, duration of extracranial disease control, number of brain metastases, histology, maximal resection cavity diameter, and treatment centre. Patients and investigators were not masked to treatment allocation. The co-primary endpoints were cognitive-deterioration-free survival and overall survival, and analyses were done by intention to treat. We report the final analysis. This trial is registered with ClinicalTrials.gov, number NCT01372774.
Findings
Between Nov 10, 2011, and Nov 16, 2015, 194 patients were enrolled and randomly assigned to SRS (98 patients) or WBRT (96 patients). Median follow-up was 11·1 months (IQR 5·1–18·0). Cognitive-deterioration-free survival was longer in patients assigned to SRS (median 3·7 months [95% CI 3·45–5·06], 93 events) than in patients assigned to WBRT (median 3·0 months [2·86–3·25], 93 events; hazard ratio [HR] 0·47 [95% CI 0·35–0·63]; p<0·0001), and cognitive deterioration at 6 months was less frequent in patients who received SRS than those who received WBRT (28 [52%] of 54 evaluable patients assigned to SRS vs 41 [85%] of 48 evaluable patients assigned to WBRT; difference −33·6% [95% CI −45·3 to −21·8], p<0·00031). Median overall survival was 12·2 months (95% CI 9·7–16·0, 69 deaths) for SRS and 11·6 months (9·9–18·0, 67 deaths) for WBRT (HR 1·07 [95% CI 0·76–1·50]; p=0·70). The most common grade 3 or 4 adverse events reported with a relative frequency greater than 4% were hearing impairment (three [3%] of 93 patients in the SRS group vs eight [9%] of 92 patients in the WBRT group) and cognitive disturbance (three [3%] vs five [5%]). There were no treatment-related deaths.
Interpretation
Decline in cognitive function was more frequent with WBRT than with SRS and there was no difference in overall survival between the treatment groups. After resection of a brain metastasis, SRS radiosurgery should be considered one of the standards of care as a less toxic alternative to WBRT for this patient population.
Introduction
20–40% of patients with cancer develop brain metastases, and therefore management of this condition has a tremendous impact on health-care systems, patients, and their families.1 Brain metastases are often surgically resected, especially for large lesions with mass effect (ie, a tumour can cause damage by pushing or shifting brain tissue), improving survival in some patient populations.2 However, recurrence in the surgical bed is common following resection alone.
Findings from prospective trials have shown that postoperative, adjuvant, whole brain radiotherapy (WBRT) reduces the risk of recurrence in the surgical bed and also reduces the incidence of new metastases.3,4 Although adjuvant WBRT improves intracranial control, it has no substantiated survival benefit and has detrimental effects on quality of life and cognitive function.5 To avoid the toxic effects of WBRT, there is a growing practice to treat the surgical bed with stereotactic radiosurgery (SRS): precise delivery of large, highly focused doses of radiation, which is an established and effective treatment for brain metastases although its efficacy compared with WBRT in the postoperative setting is unproven.6 Findings from the only study to directly address comparative efficacy for neurological or cognitive failure did not show non-inferiority of SRS compared with WBRT in the postoperative setting, and showed worse survival in the SRS treatment group than in the WBRT group.7
Research in context.
Evidence before this study
Whole brain radiotherapy (WBRT) is the standard of care to improve intracranial control after resection of brain metastasis. However, stereotactic radiosurgery (SRS) to the surgical cavity is widely used in an attempt to reduce cognitive toxicity, despite the lack of high-level comparative data substantiating efficacy in the postoperative setting. We searched PubMed and the abstracts of major conferences (such as the American Society of Clinical Oncology and American Society for Radiation Oncology) using the terms “brain metastases”, “irradiation (or radiotherapy)”, “radiosurgery”, and “surgery (or resection)”, with no language restrictions, and with no constraints imposed on the timeframe for the search, for randomised evidence to support this practice. We found only one relevant randomised clinical trial. The trial was underpowered and did not demonstrate non-inferiority of SRS compared with WBRT for neurological or cognitive failure in the postoperative setting.
Added value of this study
To our knowledge, this study is the only adequately powered randomised clinical trial directly comparing SRS with WBRT, the standard of care in the postoperative setting. Additionally, this trial assesses both quality of life and cognitive function, which are especially important endpoints in this patient population given the absence of a substantiated given the absence of a substantiated survival advantage with adjuvant radiotherapy.
Implications of all the available evidence
The combined evidence suggests that SRS to the surgical cavity results in no significant difference in survival and improved preservation of quality of life and cognitive outcomes compared with WBRT. Taken in context with other phase 3 trials assessing SRS to the surgical bed, the implication for clinical care is that SRS in the postoperative setting is a viable treatment option to improve surgical bed control and should be considered a standard of care and a less toxic alternative than WBRT. The implication for future research is that continued refinement of the SRS technique, such as fractionated or preoperative radiosurgery, is needed to further improve outcomes such as surgical bed control.
To address these knowledge gaps, we investigated the role of adjuvant SRS compared with WBRT in patients with one resected brain metastasis.
Methods
Study design and participants
N107C/CEC·3 was a randomised, controlled, phase 3 trial enrolling patients from 48 institutions in the USA and Canada (appendix p 15). Adult patients (18 years of age or older) with one resected metastatic brain lesion and a resection cavity measuring less than 5·0 cm in maximal extent were eligible for the trial. Up to three unresected metastases (each <3 cm in maximal extent) were allowed. Eligibility criteria included Eastern Cooperative Oncology Group performance status 0–2 and pathology from the resected brain metastasis consistent with a non-CNS primary site. The estimated median overall survival of eligible patients was 9–11 months.1,3,4 Exclusion criteria included pregnant or nursing women, men or women of childbearing potential unwilling to use adequate contraception, inability to complete an MRI scan with contrast, planned chemotherapy during the radiation, previous cranial radiotherapy, leptomeningeal metastases, lesion located within 5 mm of the optic chiasm or within the brainstem, or metastases from primary germ-cell tumours, small-cell carcinoma, or lymphoma. Previous treatment with systemic therapies (eg, chemotherapy) was permitted. Cytotoxic chemotherapy was not allowed during SRS or WBRT, but could start immediately afterwards. The full inclusion and exclusion criteria are given in the protocol (appendix pp 30–122).
Before patient enrolment, each participating institution provided approval from institutional review boards and each patient provided written informed consent. The North Central Cancer Treatment Group (now part of the Alliance for Clinical Trials in Oncology) led the trial in collaboration with other cooperative groups, including the Canadian Cancer Trials Group and NRG Oncology Group. The trial was closed to enrolment on Dec 18, 2015, after meeting accrual goals.
Randomisation and masking
Patients were randomly assigned (1:1) to either SRS to the surgical cavity or WBRT. We used a dynamic allocation strategy with stratification according to age (<60 years vs ≥60 years), duration of extracranial disease control (≤3 months vs >3 months), number of brain metastases (one vs two to four), histology (lung vs radioresistant [defined as sarcoma, melanoma, or renal-cell carcinoma] vs other), maximal diameter of the resection cavity (≤3 cm vs >3 cm), and treatment centre. Randomisation group assignment was done electronically via a web-based system. Due to electronic assignment and the use of a dynamic allocation algorithm, users could not deduce the next assignment in the sequence. Neither patients, clinicians, nor study statisticians were masked to treatment assignment, although the neuropsychologists grading the cognitive assessments were masked to treatment assignment.
Procedures
For patients randomly assigned to SRS, the prescribed dose was determined by surgical cavity volume: 20 Gy if cavity volume was less than 4·2 mL, 18 Gy if 4·2–7·9 mL, 17 Gy if 8·0–14·3 mL, 15 Gy if 14·4–19·9 mL, 14 Gy if 20·0–29·9 mL, and 12 Gy if 30·0 mL or more up to the maximal surgical cavity extent of 5 cm.8 The surgical cavity was treated with a 2 mm margin.6 Patients randomly assigned to WBRT were treated with either 30 Gy in ten fractions of 3·0 Gy, or 37·5 Gy in 15 fractions of 2·5 Gy, delivered 5 days a week. Sites predetermined the fractionation schedule, based on institutional preference, that would be used for all patients randomised at the site.
For patients randomly assigned to receive SRS to the surgical cavity, any unresected metastases were treated with SRS with 24 Gy in a single fraction if lesions were less than 1·0 cm, 22 Gy if 1·0–2·0 cm, and 20 Gy if lesions were 2·1–2·9 cm in maximal diameter.5 For patients randomly assigned to receive WBRT, any unresected metastases were treated with SRS with 22 Gy in a single fraction if lesions were less than 1·0 cm, 20 Gy if 1·0–2·0 cm, and 18 Gy if lesions were 2·1–2·9 cm in maximal diameter.5 For both study groups, the SRS dose was prescribed to the highest isodose line encompassing the target. To participate in the trial, all centres were required to have their radiosurgery treatment device credentialled and approved by the Imaging and Radiation Oncology Core Houston Quality Assurance Center.
Before registration and randomisation, each patient had a baseline assessment consisting of medical history and physical examination, neurological examination, MRI (volumetric MRI scans and specific types of contrast were not required), assessment of cognitive function, quality of life, performance status, and functional independence status. All baseline evaluations along with assessment of adverse events were repeated at week 12, and months 6, 9, 12, 16, and 24. Progression (local, distant, and leptomeningeal disease) was assessed by the treating physician using MRI scans. Future analyses of patterns of failure will use central review and dosimetric data. We defined surgical bed recurrence as the development of new nodular contrast enhancement in the surgical bed compared with the baseline postoperative MRI. Local failure for unresected metastases was defined as an increase of more than 25% in the size of the treated lesion. Distant brain failure was defined as the development of new, non-contiguous lesions. Leptomeningeal disease was diagnosed by imaging results consistent with this condition (either local or diffuse leptomeningeal disease) or by examination of spinal fluid positive for malignant cells.9
Quality of life was assessed by the Functional Assessment of Cancer Therapy-Brain (FACT-Br), Fatigue/Uniscale Assessment, and the Linear Analog Self-Assessment (LASA).10,11 Functional independence was assessed by the Barthel Index of Activities of Daily Living (ADL).12 We used well established cognitive tests to assess learning and immediate memory (Hopkins Verbal Learning Test-Revised [HVLT-R] Immediate Recall), verbal fluency (Controlled Oral Word Association Test [COWAT]), processing speed (Trail Making Test part A [TMT-A]), executive function (Trail Making Test part B [TMT-B]), delayed memory (HVLT-R Delayed Recall), and recognition (HVLT-R Recognition).13,14 The cognitive testing was administered by a trained, certified member of the site study team. All treatment-related toxicities and adverse events were recorded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Outcomes
The co-primary endpoints were overall survival and cognitive-deterioration-free survival. We defined overall survival as the time from randomisation to death from any cause and cognitive-deterioration-free survival as the time from randomisation to a drop of greater than 1 SD from baseline in at least one of the six cognitive tests (all tests are standardised on the basis of published norms). For cognitive-deterioration-free survival, patients who died before 6 months or were alive and did not complete all the neurocognitive tests were judged as having cognitive deterioration at the time of death or at the time they missed their first cognitive evaluation (if there were no subsequent cognitive evaluations).
Secondary endpoints were quality of life (change from baseline to 6 months in FACT-Br and LASA), functional independence (assessed by the Barthel ADL index), local surgical bed recurrence, local recurrence of unresected metastases, distant brain recurrence, development of leptomeningeal disease, intracranial progression (time from randomisation to recurrence in the local surgical bed, progression of unresected metastases, distant brain recurrence, or development of leptomeningeal disease), long-term cognitive status, and toxicity. Cause-specific survival was not assessed due to concerns regarding accuracy of assignment of cause of death.15,16
Statistical analysis
The primary goals of the study were to detect whether there was less cognitive deterioration after randomisation in patients who received SRS than in patients who received WBRT, and whether overall survival with post-surgical SRS was marginally superior to WBRT. On the basis of previously reported studies, we assumed the proportion of patients with cognitive-deterioration-free survival at 6 months after randomisation would be 65% for patients undergoing WBRT.17 Under a 1:1 randomisation, a sample size of 174 (87 patients in each group) yields at least 85% power to detect a 20% (absolute) difference in cognitive-deterioration-free survival at 6 months between the two treatment groups, assuming a one-sided type I error rate of 0·05. We used a one-sided test because we assumed that WRBT would result in greater cognitive deterioration than SRS, on the basis of evidence in other patient populations.18,19 There was a planned over-accrual of 18 patients (target accrual of 192 patients) to account for cancellations, withdrawal before treatment, or ineligible patients.
The null hypothesis for overall survival was that SRS is inferior to WBRT in terms of overall survival, versus the alternative hypothesis that SRS is marginally superior to WBRT. The estimated median overall survival in patients with adjuvant WBRT was 9 months and the median overall survival for patients with post-surgical SRS is approximately 11 months.1,3,4 With the design proposed by Freidlin and colleagues20 a sample size of 174 patients yields at least 90% power at a 0·05 one-sided significance level for targeting a hazard ratio of 1·3 (in favour of WBRT) versus 0·8 (in favour of SRS), assuming an accrual period of 2·8 years and a minimum follow-up of 10 months. In other words, the trial will have 95% probability of rejecting the alternative hypothesis of marginal superiority of SRS with respect to overall survival if the true hazard ratio is 1·3 (in favour of WBRT), and 90% probability of concluding the marginal superiority of SRS if the true hazard ratio is 0·8 (in favour of SRS).
The trial had one planned interim analysis (which was accounted for in the sample size calculations using O’Brien-Fleming boundaries). Interim analyses for cognitive-deterioration-free survival were done after 50% of evaluable patients had been followed up for 6 months, and for overall survival when 50% of deaths had been observed. Each of the two co-primary endpoints was tested independently at the interim analysis, and for each co-primary endpoint, early rejection of the null hypothesis was considered.
Efficacy analyses were done on the intention-to-treat principle with all patients analysed on the basis of the treatment group to which they were randomised. The safety population was defined as all patients that began treatment and had at least one adverse event evaluation. The alpha was not split for the analysis of the co-primary endpoints. Cognitive-deterioration-free survival and overall survival for the treatment groups were summarised with the Kaplan-Meier estimator and compared with a log-rank test. We also used stratified Cox proportional hazards models, using trial stratification variables, to assess whether the distributions of cognitive-deterioration-free survival and overall survival differed between treatment groups. Proportional hazards were assessed with Schoenfeld residuals and visually (appendix pp 16–29). We computed median follow-up using the patients who were censored at the time of the analysis.
We analysed time until intracranial progression (including surgical bed recurrence, local recurrence, development of leptomeningeal disease, and distant brain failure) using competing risks with death counted as a competing risk.21,22 The quality-of-life scores (both FACT-Br and LASA) were transformed to a 0–100 scale (with 100 being most favourable). A 10-point change on this scale was considered clinically significant.23 We compared the intergroup proportion of patients with significant deterioration in quality of life using an exact binomial test, and intergroup changes in quality-of-life scores using a two-sample t test. In addition to point estimates, we provide 95% CIs. The analysis of quality-of-life (FACT-Br and LASA) outcomes at 3 months was a post-hoc analysis. We defined categorical decline in functional independence, as assessed by the Barthel ADL index (ADL), as a score that fell by at least 10% below the baseline level. Patients with baseline values paired with 3-month or 6-month Barthel ADL index values were included in the 3-month and 6-month analysis, respectively. The duration of stable or better function independence was also analysed using Kaplan-Meier plots and log-rank tests for the time a patient’s Barthel ADL index stayed at or above their baseline value. To assess long-term cognitive status, we did a subset analysis for long-term survivors (defined as a patient who had a cognitive evaluation 12 or more months from time of randomisation).5 All secondary analyses used a two-sided 0·05 level of significance. Post-hoc and sensitivity analyses use the same statistical methods as the primary and secondary analyses unless otherwise noted. A post-hoc sensitivity analysis of cognitive-deterioration-free survival was done removing patients who had WBRT before cognitive deterioration. A post-hoc analysis of cognitive deterioration was done using different definitions of cognitive deterioration. A post-hoc analysis of the frequency of local salvage therapy was also done. There was no adjustment for multiple comparisons for the secondary endpoint analyses, so these results should be interpreted as exploratory. Missing data were handled with complete case analysis unless otherwise specified (eg, patients missing a neurocognitive assessment were considered to have had an event as described). All analyses were done in either SAS version 9.3 or R version 3.2.3. The data for this analysis were frozen on Feb 18, 2017.
This trial is registered with ClinicalTrials.gov, number NCT01372774.
Role of the funding source
The funder provided peer-reviewed approval of the trial but had no other role in study design, data collection, data analysis, data interpretation, or writing of the report. The Alliance Statistics and Data Center was responsible for the collection, maintenance, and analyses of the data. Data confidentiality was governed by National Institutes of Health policy. The corresponding author and principal investigator of the study (PDB) had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
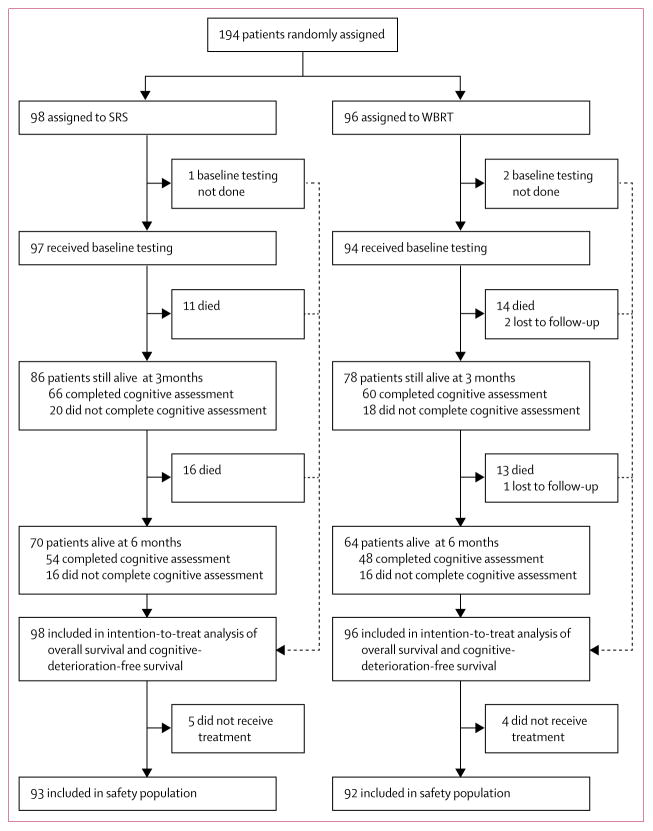
Between Nov 10, 2011, and Nov 16, 2015, 194 patients were enrolled and randomly assigned to SRS to the surgical bed (98 patients; five patients did not receive treatment) or WBRT (96 patients; 49 patients received 30 Gy in 10 fractions, 43 received 37·5 Gy in 15 fractions, and four patients did not receive treatment; figure 1). There was one major protocol violation (one patient randomly assigned to the SRS group, whose treatment was switched by the site, received WBRT). Median follow-up was 11·1 months (IQR 5·1–18·0) for all patients and 22·6 months (13·8–34·6) for patients who had not died. Baseline characteristics were well balanced between the study groups (table 1). Mean cognitive scores at baseline were below population norms and ranged from mild to severe impairment. 66 (77%) of 86 patients in the SRS group and 60 (77%) of 78 patients in the WBRT group completed cognitive testing at 3 months, and 54 (77%) of 70 patients in the SRS group and 48 (75%) of 64 patients in the WBRT group did so at 6 months, including only patients that were alive at the timepoint and who had the baseline cognitive evaluation. No patients were on memantine at enrolment; at week 12, no patients in the SRS group and four patients in the WBRT group were receiving memantine.
Figure 1. Study profile.
WBRT=whole brain radiotherapy. SRS=stereotactic radiosurgery.
Table 1.
Baseline characteristics
| SRS (n=98) | WBRT (n=96) | |
|---|---|---|
| Age (median [IQR]) | 61 (54–66) | 62 (54–68) |
| 18–59 years | 39 (40%) | 37 (39%) |
| ≥60 years | 59 (60%) | 59 (61%) |
|
| ||
| Sex | ||
| Male | 46 (47%) | 50 (52%) |
| Female | 52 (53%) | 46 (48%) |
|
| ||
| Period of systemic disease control | ||
| ≤3 months | 54 (55%) | 54 (56%) |
| >3 months | 44 (45%) | 42 (44%) |
|
| ||
| Resection cavity diameter | ||
| ≤3 cm | 59 (60%) | 58 (60%) |
| >3 cm | 39 (40%) | 38 (40%) |
|
| ||
| Extent of resection | ||
| Subtotal | 8 (8%) | 13 (14%) |
| Total (gross) | 90 (92%) | 83 (86%) |
|
| ||
| Surgical approach | ||
| En bloc | 54 (55%) | 61 (64%) |
| Piecemeal | 43 (44%) | 35 (36%) |
| Data missing | 1 (1%) | 0 |
|
| ||
| Number of brain metastases | ||
| One | 75 (77%) | 74 (77%) |
| Two to four | 23 (23%) | 22 (23%) |
|
| ||
| ECOG performance status | ||
| 0 | 39 (40%) | 33 (34%) |
| 1 | 49 (50%) | 56 (58%) |
| 2 | 10 (10%) | 7 (7%) |
|
| ||
| Primary tumour site | ||
| Lung | 58 (59%) | 56 (58%) |
| Other | 29 (30%) | 30 (31%) |
| Radioresistant | 11 (11%) | 10 (10%) |
|
| ||
| Cranial nerves | ||
| Normal | 92 (94%) | 91 (95%) |
| Abnormal | 5 (5%) | 4 (4%) |
| Data missing | 1 (1%) | 1 (1%) |
|
| ||
| Sensation | ||
| Normal | 93 (95%) | 94 (98%) |
| Abnormal | 3 (3%) | 1 (1%) |
|
| ||
| Motor | ||
| Normal | 89 (91%) | 81 (84%) |
| Abnormal | 8 (8%) | 14 (15%) |
| Data missing | 1 (1%) | 1 (1%) |
|
| ||
| Cerebellar | ||
| Normal | 90 (92%) | 84 (88%) |
| Abnormal | 5 (5%) | 10 (10%) |
| Data missing | 3 (3%) | 2 (2%) |
|
| ||
| Baseline Barthel ADL index | 95·8 (9·1) | 98·2 (6·4) |
|
| ||
| Baseline total score FACT-Br (range 0–100) | 72·2 (14·5) | 71·8 (13·2) |
|
| ||
| Baseline cognitive tests | ||
| HVLT-R Immediate Recall | −1·5 (1·3) | −1·4 (1·2) |
| HVLT-R Delayed Recall | −1·5 (1·5) | −1·6 (1·5) |
| HVLT-R Recognition | −0·7 (1·4) | −0·6 (1·5) |
| TMT-A time to complete | −2·2 (3·0) | −1·7 (3·1) |
| TMT-B time to complete | −3·0 (3·4) | −2·8 (3·3) |
| COWAT Total | −1·1 (1·2) | −1·2 (1·0) |
Data are n (%) or mean (SD) unless otherwise stated. Cognitive tests reported as standardized scores (Z scores). SRS=stereotactic radiosurgery. WBRT=whole brain radiotherapy. ECOG=Eastern Cooperative Oncology Group. ADL=Activities of Daily Living. FACT-Br=Functional Assessment of Cancer Therapy—Brain Cognitive. HVLT-R=Hopkins Verbal Learning Test—Revised. TMT-A=Trail Making Test part A. TMT-B=Trail Making Test part B. COWAT=Controlled Oral Word Association Test.
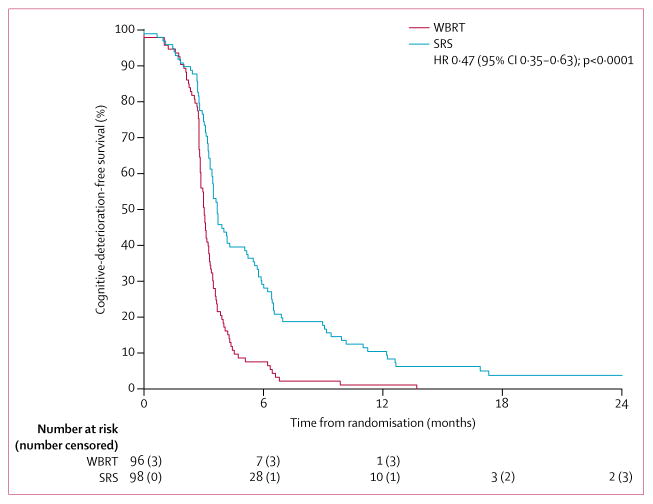
Median cognitive-deterioration-free survival was longer after SRS to the surgical cavity (3·7 months [95% CI 3·45–5·06]) than after WBRT (3·0 months [2·86–3·25]; HR 0·47 [95% CI 0·35–0·63], p<0·0001; figure 2). Of the 93 cognitive-deterioration-free survival events in the SRS group, 49 patients (53%) had confirmed cognitive deterioration, 25 (27%) died before 6 months without confirmed cognitive deterioration, and 19 (20%) were presumed cognitive deterioration. Of the 93 cognitive-deterioration-free survival events in the WBRT group, 63 patients (68%) had confirmed cognitive deterioration, 22 (24%) died before 6 months without confirmed cognitive deterioration, and eight (9%) were presumed cognitive deterioration. Stratified analysis (with the stratification factors of age, extracranial disease control status, number of brain metastases, histology, and size resection cavity) showed a similar difference in cognitive-deterioration-free survival between the treatment groups (adjusted median 3·7 months for the SRS group and 3·1 months for the WBRT group; adjusted HR 0·47 [95% CI 0·35–0·64], p<0·0001). 20 patients in the SRS group received salvage WBRT, of whom 13 had cognitive deterioration before WBRT and seven had cognitive deterioration after WBRT. A post-hoc sensitivity analysis removing the seven patients who had cognitive deterioration after WBRT showed a similar difference in median cognitive-deterioration-free survival compared with the primary analysis: 3·7 months (95% CI 3·45–5·16) in the SRS group (86 events) compared with 3·0 months (2·86–3·25) in the WBRT group (93 events; HR 0·46 [95% CI 0·34–0·63], p<0·0001). At 6 months, for patients with a cognitive evaluation, cognitive deterioration was less frequent in the SRS group than in the WBRT group (28 [52%] of 54 patients vs 41 [85%] of 48 patients, difference −33·6% [95% CI −45·3 to −21·8], p=0·00031) and was documented for all cognitive domains evaluated (table 2). A post-hoc analysis with different definitions of cognitive deterioration (ie, a 1·5 SD drop in at least two tests, 2 SD drop in one test, or 3 SD drop in one test), and evaluation of cognitive function at 3 months, consistently revealed similar findings as the primary analysis (appendix pp 2–5).14,17
Figure 2. Cognitive-deterioration-free survival.
WBRT=whole brain radiotherapy. SRS=stereotactic radiosurgery.
Table 2.
Cognitive deterioration at 6 months
| SRS (n=54) | WBRT (n=48) | Mean difference (95% CI) | p value | |
|---|---|---|---|---|
| HVLT-R Immediate Recall | ||||
|
| ||||
| Deterioration | 9 (17%) | 23 (49%) | ·· | ·· |
| Non-deterioration | 45 (83%) | 24 (51%) | 32·3 (20 to 44·5) | 0·00062 |
| Test not attempted | 0 | 1 | ·· | ·· |
|
| ||||
| HVLT-R Delayed Recall | ||||
|
| ||||
| Deterioration | 14 (26%) | 29 (62%) | ·· | ·· |
| Non-deterioration | 39 (74%) | 18 (38%) | 35·3 (22·5 to 48·1) | 0·00054 |
| Test not attempted | 1 | 1 | ·· | ·· |
|
| ||||
| HVLT-R Recognition | ||||
|
| ||||
| Deterioration | 10 (19%) | 16 (36%) | ·· | ·· |
| Non-deterioration | 43 (81%) | 29 (64%) | 16·7 (4·6 to 28·8) | 0·0707 |
| Test not attempted | 1 | 3 | ·· | ·· |
|
| ||||
| TMT-A time to complete | ||||
|
| ||||
| Deterioration | 9 (17%) | 18 (38%) | ·· | ·· |
| Non-deterioration | 45 (83%) | 29 (62%) | 21·6 (9·6 to 33·6) | 0·0233 |
| Test not attempted | 0 | 1 | ·· | ·· |
|
| ||||
| TMT-B time to complete | ||||
|
| ||||
| Deterioration | 10 (19%) | 19 (42%) | ·· | ·· |
| Non-deterioration | 43 (81%) | 26 (58%) | 23·4 (11 to 35·7) | 0·0149 |
| Test not attempted | 1 | 3 | ·· | ·· |
|
| ||||
| COWAT total | ||||
|
| ||||
| Deterioration | 4 (7%) | 7 (15%) | ·· | ·· |
| Non-deterioration | 50 (93%) | 39 (85%) | 7·8 (−0·9 to 16·5) | 0·3368 |
| Test not attempted | 0 | 2 | ·· | ·· |
|
| ||||
| Overall outcome for cognitive deterioration | ||||
|
| ||||
| Deterioration | 28 (52%) | 41 (85%) | −33·6 (−45·3 to −21·8) | ·· |
| Non-deterioration | 26 (48%) | 7 (15%) | ·· | 0·00031 |
We defined cognitive deterioration as a drop of 1 SD in score from baseline. There are missing values for some cognitive tests, as reflected by total number of a particular test being less than the total number of patients. p values were calculated with Fisher’s exact test. WBRT=whole brain radiotherapy. SRS=stereotactic radiosurgery. HVLT-R=Hopkins Verbal Learning Test—Revised. TMT-A=Trail Making Test part A. TMT-B=Trail Making Test part B. COWAT=Controlled Oral Word Association Test.
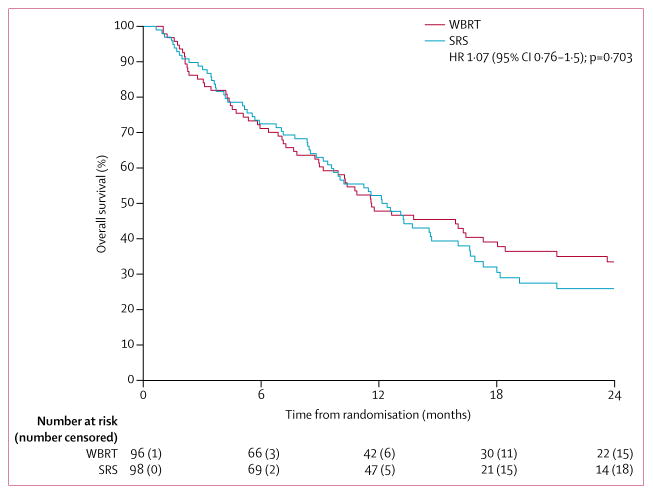
After 69 deaths in the SRS group and 67 deaths in the WBRT group, median overall survival was 12·2 months (95% CI 9·7–16·0) versus 11·6 months (9·9–18·0; HR 1·07 [95% CI 0·76–1·50]; p=0·70; figure 3). There was no evidence at the 0·05 one-sided significance level that the HR was greater than 1·3 (in favour of WBRT) or 0·8 (in favour of SRS). SRS was not found to be inferior to WBRT but neither was SRS marginally superior to WBRT.
Figure 3. Overall survival.
WBRT=whole brain radiotherapy. SRS=stereotactic radiosurgery.
SRS was associated with a shorter time to intracranial tumour progression than was WBRT (median 6·4 months [95% CI 5·16–8·90], 66 events vs 27·5 months [14·85–not reached], 34 events; HR 2·45 [95% CI 1·62–3·72]; p<0·0001; appendix p 6). 6-month surgical bed control was 80·4% (95% CI 72·8–88·7) in the SRS group compared with 87·1% (80·5–94·2) in the WBRT group (p=0·00068); 3-month and 12-month estimates are shown in the appendix (p 6). Local control and distant brain control were worse in the SRS group than the WBRT group, but there was no difference in the development of leptomeningeal disease between treatment groups (appendix p 6). There was no difference in frequency of local salvage therapy after SRS compared with after WBRT (31 [32%] of 98 vs 20 [21%] of 96; difference 10·8% [95% CI −2·5 to 24·1]; p=0·12). In the SRS group, 20 (20%) of the 98 patients received WBRT as a component of their salvage therapy.
65 patients in the SRS group and 64 patients in the WBRT group completed quality-of-life questionnaires at baseline and had at least one subsequent assessment (appendix p 9). For FACT-Br scores at 6 months compared with baseline, a clinically significant improvement was noted more frequently in the SRS group compared with the WBRT group for physical well being, whereas there were no significant differences between treatment groups in social, emotional, or functional wellbeing, brain-specific concerns, or overall FACT-Br (appendix p 8). Analyses of LASA at 3 and 6 months are shown in the appendix (pp 10–11).
Functional independence change from baseline values were available for 70 patients at 3 months and 60 patients at 6 months in the SRS group and for 66 patients at 3 months and 48 patients at 6 months in the WBRT group. Functional independence at 3 months as assessed with the Barthel ADL index was higher after SRS than after WBRT (of 70 patients receiving SRS, four [6%] showed decline and eight [11%] showed improvement vs eight [12%] and one [2%] of 66 patients receiving WBRT; p=0·036). At 6 months, we noted no significant difference in functional independence between patients who received SRS compared with those who received WBRT (three [5%] of 60 patients in the SRS group showed decline and five [8%] showed improvement vs seven [15%] and one [2%] of 48 patients in the WBRT group; p=0·10). Median duration of stable or better functional independence was longer after SRS (median not yet reached [95% CI 17·6 to not yet reached]) than after WBRT (14·0 months [8·4–27·0]; HR 0·56 [95% CI 0·32–0·96], p=0·034).
There were 54 (28%) long-term survivors (patients who had a cognitive evaluation 12 or more months from time of randomisation) out of 194 patients enrolled in our study (27 assigned to SRS and 27 assigned to WBRT). In these patients, cognitive deterioration was less frequent after SRS than after WBRT (ten [37%] of 27 patients in the SRS group vs 24 [89%] of 27 patients in the WBRT group who had assessments at 3 months, p=0·00016; 12 [46%] of 26 vs 23 [88%] of 26 at 6 months, p=0·0025; 12 [48%] of 25 vs 21 [81%] of 26 at 9 months, p=0·020; and 15 [60%] of 25 vs 21 [91%] of 23 at 12 months, p=0·0188). In these patients, intracranial tumour control was 70·4% (95% CI 55·1–89·9; eight events) at 6 months and 40·7% (25·9–64·2; 16 events) at 12 months in the SRS group compared with 92·6% (83·2–1·00; two events) at 6 months and 81·5% (68·1–97·5; five events) at 12 months in the WBRT group (overall HR 3·12 [95% CI 1·40–6·94], p=0·0033).
93 patients in the SRS group and 92 patients in the WBRT group were evaluable for treatment toxic effects. 220 individual toxicities of any grade (irrespective of attribution) were reported by patients receiving SRS (71 [76%] patients reported at least one toxicity), compared with 362 individual toxicities reported by patients receiving WBRT (79 [86%] patients reported at least one toxicity). Of these, grade 3 or worse toxic effects were reported in 36 (39%) patients in the SRS group and 37 (40%) patients in the WBRT group (table 3). The most common grade 3 or 4 adverse events reported with a relative frequency greater than 4% were hearing impairment (three [3%] patients in the SRS group vs eight [9%] in the WBRT group) and cognitive disturbance (three [3%] vs five [5%]).
Table 3.
Adverse events
| SRS (n=93) | WBRT (n=92) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 | |
| Alopecia | 30 (32%) | 0 | 0 | 0 | 48 (52%) | 0 | 0 | 0 |
|
| ||||||||
| Nausea | 22 (24%) | 0 | 0 | 0 | 34 (37%) | 4 (4%) | 0 | 0 |
|
| ||||||||
| Cognitive disturbance | 15 (16%) | 3 (3%) | 0 | 0 | 21 (23%) | 5 (5%) | 0 | 0 |
|
| ||||||||
| Fatigue | 0 | 4 (4%) | 0 | 0 | 20 (22%) | 1 (1%) | 0 | 0 |
|
| ||||||||
| Vomiting | 0 | 0 | 0 | 0 | 20 (22%) | 2 (2%) | 0 | 0 |
|
| ||||||||
| Dermatitis radiation | 0 | 0 | 0 | 0 | 14 (15%) | 0 | 0 | 0 |
|
| ||||||||
| Hearing impaired | 12 (13%) | 3 (3%) | 0 | 0 | 13 (14%) | 8 (9%) | 0 | 0 |
|
| ||||||||
| Dehydration | 0 | 0 | 0 | 0 | 0 | 6 (7%) | 0 | 0 |
|
| ||||||||
| Anorexia | 0 | 0 | 0 | 0 | 0 | 4 (4%) | 0 | 0 |
|
| ||||||||
| Hyperglycaemia | 0 | 0 | 0 | 0 | 0 | 3 (3%) | 0 | 0 |
|
| ||||||||
| Hypoalbuminaemia | 0 | 1 (1%) | 0 | 0 | 0 | 2 (2%) | 0 | 0 |
|
| ||||||||
| Hypophosphataemia | 0 | 0 | 0 | 0 | 0 | 2 (2%) | 0 | 0 |
|
| ||||||||
| Lymphocyte count decreased | 0 | 2 (2%) | 0 | 0 | 0 | 2 (2%) | 1 (1%) | 0 |
|
| ||||||||
| Seizure | 0 | 3 (3%) | 1 (1%) | 0 | 0 | 2 (2%) | 1 (1%) | 0 |
|
| ||||||||
| Thromboembolic event | 0 | 3 (3%) | 1 (1%) | 0 | 0 | 2 (2%) | 0 | 1 (1%) |
|
| ||||||||
| Weight loss | 0 | 1 (1%) | 0 | 0 | 0 | 2 (2%) | 0 | 0 |
|
| ||||||||
| Generalised muscle weakness | 0 | 1 (1%) | 0 | 0 | 0 | 2 (2%) | 0 | 0 |
|
| ||||||||
| Alanine aminotransferase increased | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Alkaline phosphatase increased | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Anaemia | 0 | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
|
| ||||||||
| Aspartate aminotransferase increased | 0 | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Aspiration | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
|
| ||||||||
| Blood bilirubin increased | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Dental caries | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Depressed level of consciousness | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Dysphagia | 0 | 2 (2%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Fall | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| General disorders and administration site conditions—other | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Hypokalaemia | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Hyponatraemia | 0 | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 1 (1%) | 0 |
|
| ||||||||
| Hypotension | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Meningitis | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Mucositis oral | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Myalgia | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Oral pain | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Pain | 0 | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Pain in extremity | 0 | 1 (1%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Peripheral motor neuropathy | 14 (15%) | 2 (2%) | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Proteinuria | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Sore throat | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Wound dehiscence | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Tooth infection | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Haemoglobin increased | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Oesophageal infection | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Mucosal infection | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 | 0 |
|
| ||||||||
| Respiratory failure | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 2 (2%) | 2 (2%) |
|
| ||||||||
| Sepsis | 0 | 0 | 0 | 0 | 0 | 0 | 2 (2%) | 0 |
|
| ||||||||
| Agitation | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Blood and lymphatic system disorders—other | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Creatinine increased | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Depression | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Hypocalcaemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Lipase increased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Multiorgan failure | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Nervous system disorders—other | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Platelet count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Pneumonitis | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Serum amylase increased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| White blood cell decreased | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Localised oedema | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Acute kidney injury | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 1 (1%) | 0 |
|
| ||||||||
| Death NOS | 0 | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 2 (2%) |
|
| ||||||||
| Neoplasms benign, malignant, and unspecified | 0 | 0 | 0 | 4 (4%) | 0 | 0 | 0 | 2 (2%) |
|
| ||||||||
| Stroke | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
|
| ||||||||
| Sudden death NOS | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
|
| ||||||||
| Pancreas infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (1%) |
|
| ||||||||
| Dyspnoea | 0 | 3 (3%) | 3 (3%) | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Back pain | 0 | 2 (2%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Headache | 0 | 2 (2%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Bone pain | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Colitis | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Heart failure | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Hypoxia | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Intracranial haemorrhage | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Neutrophil count decreased | 0 | 1 (1%) | 1 (1%) | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Otitis media | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Sinus tachycardia | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Wound infection | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Ejection fraction decreased | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Reversible posterior leukoencephalopathy syndrome | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Chronic kidney disease | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Bronchopulmonary haemorrhage | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Muscle weakness lower limb | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Central nervous system necrosis | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Pulmonary oedema | 0 | 0 | 2 (2%) | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
| Wound complication | 0 | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 |
Table shows any grade 1–2 adverse events occurring in ≥10% of patients, and all grade 3–5 adverse events. There were no treatment-related deaths. WBRT=whole brain radiotherapy. SRS=stereotactic radiosurgery. NOS=not otherwise specified.
Individual toxic effects of any grade that were at least possibly related to treatment were reported as 78 toxic effects by 47 (51%) of 93 patients in the SRS group compared with 199 toxic effects reported by 65 (71%) or 92 patients in the WBRT group. Grade 3 or worse toxic effects were reported by 11 (12%) patients in the SRS group compared with 17 (18%) patients in the WBRT group. The most frequent grade 3 or worse toxic effects in the SRS group were fatigue (two [2%] patients) and dyspnoea (two [2%] patients); and in the WBRT group they were cognitive disturbance (five [5%] patients), hearing impairment (four [4%] patients), dehydration (three [3%] patients), and nausea (two [2%] patients). Four (4%) of 93 patients in the SRS group developed grade 2 or worse CNS necrosis compared with no patients in the WBRT group. Additional adverse event data are provided in the appendix pp 12–14. Seven (7%) of the 93 patients in the SRS group and ten (11%) of 92 patients in the WBRT group were reported to have died from adverse events (all deemed unrelated or unlikely related to treatment after review). Specifically for the SRS group, the grade 5 events were: four (4%) neoplasms benign, malignant, and unspecified; one (1%) multiorgan failure, one (1%) respiratory failure, and one (1%) death not otherwise specified. For the WBRT group the grade 5 events were: two (2%) neoplasms benign, malignant, and unspecified; two (2%) respiratory failures, two (2%) deaths not otherwise specified, one (1%) thromboembolic event, one (1%) stroke, one (1%) pancreas infection, and one (1%) sudden death not otherwise specified.
Discussion
In this multicentre, randomised, controlled, phase 3 study, patients receiving SRS to the surgical cavity had improved cognitive function and quality of life compared with patients receiving WBRT, with no difference in overall survival; intracranial tumour control was better in patients receiving WBRT than SRS. To our knowledge, no large, randomised, controlled trial has compared SRS with WBRT, the standard of care after resection of brain metastases, and simultaneously assessed both quality of life and cognitive function.3 Findings from a previous randomised trial of 59 patients with resected brain metastases did not show non-inferiority of SRS compared with WBRT for neurological or cognitive failure, although the investigators stated that their trial was underpowered and that larger randomised trials were needed.7 Additionally, the trial did not assess quality of life and measured cognitive function with an insensitive tool, the Mini–Mental State Examination.13,24 Because adjuvant WBRT has not been shown to improve survival,3,4,25 it is essential to adequately assess both quality of life and cognitive function to thoroughly understand the risks and benefits of the therapies to guide treatment decisions.
Potential limitations of the current trial include the fact that surgical bed control after SRS was worse than reported in previous studies, including a single-institution clinical trial of SRS to the surgical cavity compared with observation after complete resection of brain metastases.26 In this single-institution trial, surgical bed control at 12 months was achieved in 43% of patients for resection alone compared with 72% of patients for SRS. The surgical bed control achieved with SRS in this single-institution trial was similar to that after WBRT (81%) in our multicentre trial, and higher than that after SRS (61%) in our trial despite lower SRS doses and smaller treatment margins in the single-institution study. The differences in surgical bed control might have resulted from the inclusion of patients with subtotal resection in our study. Additionally, surgical bed control was determined differently; in our study, local control was determined by the treating physician rather than by central review. Considering the higher SRS doses in our study, especially in patients with small surgical cavity volumes, some of the declared surgical bed recurrences might simply have been post-radiosurgery changes (ie, pseudoprogression), which could have falsely elevated the frequency of recurrence in our trial.27 Finally, the patient populations differed substantially between the trials, including primary tumour histology (eg, lung primary 59% in the current multi-institutional trial vs 20% in the single institutional trial), making comparisons very difficult. However, taking these two randomised, controlled trials in context provides evidence that more than half of patients will have recurrences in the surgical bed even after gross total resection, that postoperative SRS substantially improves surgical bed control compared with resection alone, and SRS did not result in a difference in overall survival, suggesting resection cavity SRS is an effective strategy to delay WBRT and the associated cognitive, functional, and quality of life decreases.
Another potential limitation is that memantine was not mandated for the patients receiving WBRT on the current trial. Findings from a placebo-controlled, double-blind, phase 3 trial for patients with brain metastases treated with WBRT did not show a significant improvement in the primary endpoint, delayed memory, with memantine compared with placebo (HVLT-R Delayed Recall at 24 weeks, median decline of 0 with WBRT vs 0·9 with placebo; p=0·059).28 However, the memantine group had improvement on several cognitive outcomes, including a significantly increased time to cognitive decline. The results of the memantine trial were not reported at the time we designed the current study, and although memantine might have improved cognitive outcomes for patients in the WBRT group of the current trial, it probably would not have sufficiently improved cognitive outcomes to match those achieved with SRS. Additionally, there is no evidence memantine would have improved quality of life in these patients, arguably one of the more important outcomes in this report. Although memantine is commonly used in clinical practice, its use with WBRT is still not supported in the National Comprehensive Cancer Network guidelines so there is no expert agreement on its efficacy based on randomised trial findings.29 Additionally, findings from a phase 2 trial of hippocampal-avoidance WBRT showed favourable cognitive outcomes compared with historical controls; decreased radiation dose to the hippocampal region could therefore improve the therapeutic ratio.30 On the basis of these results, an ongoing phase 3 trial of hippocampal-avoidance WBRT for patients with brain metastases was launched (NRG CC001; NCT02360215). Until further evidence supports other approaches, we believe that SRS is an effective treatment to reduce the risk of cognitive decline and maintain quality of life in patients with brain metastases.
To better assess if the long-term improvement in surgical bed control with WBRT translated into better patient outcomes, we analysed patients surviving at least 12 months from study entry. In these long-term survivors, although intracranial control was better with WBRT than with SRS, the negative cognitive effects of WBRT remained persistent over time. These results—improved intracranial control yet worse cognitive outcomes even in long-term survivors—are consistent with those from other phase 3 trials assessing the effects of WBRT in patients with brain metastases.5
The frequency of radiation necrosis after SRS to the surgical cavity in the current trial (4%) was similar to that reported in other studies.31,32 Future research to further refine the SRS technique, such as fractionated SRS for large cavities or preoperative SRS, could potentially improve outcomes such as surgical bed control while possibly maintaining or decreasing SRS-related complications.33
In conclusion, SRS to the surgical cavity results in improved cognitive outcomes compared with those for WBRT. Despite worse intracranial control, including surgical bed control, we noted no significant difference in survival and better preservation of quality of life and functional independence compared with WBRT. Therefore, SRS in the postoperative setting is a viable treatment option to improve surgical bed control and should be considered one of the standards of care as a less toxic alternative to WBRT.
Supplementary Material
Acknowledgments
Funding National Cancer Institute.
Research reported in this publication was fully supported by the National Cancer Institute of the National Institutes of Health under the Award Numbers U10CA180821 and U10CA180882 (Alliance for Clinical Trials in Oncology NCTN grants), UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA011789, U10CA025224, U10CA032291, U10CA076001, U10CA007968, U10CA180790, U10CA180858; and in collaboration with other cooperative groups including Canadian Cancer Trials Group (CCTG) supported by U10CA180863 and CCSRI grant 021039, and NRG Oncology Group, supported by RTOG U10CA21661, NRG U10CA180868, and U10CA180822 from the National Cancer Institute. None of the authors are employed by the National Institutes of Health. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. We thank the patients and their families for participation in the study. For the complete list of acknowledgements see appendix p 15.
Footnotes
Contributors
PDB, KVB, JHC, SKA, XWC, ACW, JG, IFP, NNIL, JBA, J-PB, CGH, JJU, FGB, EF, DK, CG, JCB, EG, and DR contributed to the literature search, study design, data collection, analysis, and interpretation, and writing. All authors approved the final version of the report.
Declaration of interests
DR has received honoraria and research support from BrainLab, Varian Medical Systems, Elekta, and Accuray. DK is Senior Vice President and Chief Medical Officer of Varian Medical Systems. The other authors declare no competing interests.
References
- 1.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–91. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 3.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–89. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 4.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–41. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–09. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soltys SG, Adler JR, Lipani JD, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187–93. doi: 10.1016/j.ijrobp.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 7.Kepka L, Tyc-Szczepaniak D, Bujko K, et al. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: results from a randomized trial. Radiother Oncol. 2016;121:217–24. doi: 10.1016/j.radonc.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–98. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MD, Avkshtol V, Baschnagel AM, et al. Surgical resection of brain metastases and the risk of leptomeningeal recurrence in patients treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2016;94:537–43. doi: 10.1016/j.ijrobp.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 10.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–61. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Locke DE, Decker PA, Sloan JA, et al. Validation of single-item linear analog scale assessment of quality of life in neuro-oncology patients. J Pain Symptom Manage. 2007;34:628–38. doi: 10.1016/j.jpainsymman.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wade DT. Measurement in neurological rehabilitation. Curr Opin Neurol Neurosurg. 1992;5:682–86. [PubMed] [Google Scholar]
- 13.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–09. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 14.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157–65. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 15.Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst. 2002;94:1044–5. doi: 10.1093/jnci/94.14.1044. [DOI] [PubMed] [Google Scholar]
- 16.Bailar JC, 3rd, Smith EM. Progress against cancer? N Engl J Med. 1986;314:1226–32. doi: 10.1056/NEJM198605083141905. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–66. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 18.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–44. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 19.Roberge D, Parney I, Brown PD. Radiosurgery to the postoperative surgical cavity: who needs evidence? Int J Radiat Oncol Biol Phys. 2012;83:486–93. doi: 10.1016/j.ijrobp.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Freidlin B, Korn EL, George SL, Gray R. Randomized clinical trial design for assessing noninferiority when superiority is expected. J Clin Oncol. 2007;25:5019–23. doi: 10.1200/JCO.2007.11.8711. [DOI] [PubMed] [Google Scholar]
- 21.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–54. [Google Scholar]
- 22.Eisele SC, Wen PY, Lee EQ. Assessment of brain tumor response: RANO and its offspring. Curr Treat Options Oncol. 2016;17:35. doi: 10.1007/s11864-016-0413-5. [DOI] [PubMed] [Google Scholar]
- 23.Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncology. 2006;76:283–91. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 24.Brown PD, Buckner JC, O’Fallon JR, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination examination. J Clin Oncol. 2003;21:2519–24. doi: 10.1200/JCO.2003.04.172. [DOI] [PubMed] [Google Scholar]
- 25.Roos DE, Wirth A, Burmeister BH, et al. Whole brain irradiation following surgery or radiosurgery for solitary brain metastases: mature results of a prematurely closed randomized Trans-Tasman Radiation Oncology Group trial (TROG 98. 05) Radiother Oncol. 2006;80:318–22. doi: 10.1016/j.radonc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Mahajan A, Ahmed S, McAleer MF, et al. Postoperative stereotactic radiosurgery versus observation for completely resected brain metastases: results of a prospective randomized study. Int J Radiat Oncol Biol Phys. 2016;96(2 suppl):S2. [Google Scholar]
- 27.Ruzevick J, Kleinberg L, Rigamonti D. Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg Rev. 2014;37:193–201. doi: 10.1007/s10143-013-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–37. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabors LB, Portnow J, Ammirati M, et al. Central nervous system cancers, version 1. 2015. J Natl Compr Canc Netw. 2015;13:1191–202. doi: 10.6004/jnccn.2015.0148. [DOI] [PubMed] [Google Scholar]
- 30.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–16. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhu R, Shu HK, Hadjipanayis C, et al. Current dosing paradigm for stereotactic radiosurgery alone after surgical resection of brain metastases needs to be optimized for improved local control. Int J Radiat Oncol Biol Phys. 2012;83:e61–66. doi: 10.1016/j.ijrobp.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Robbins JR, Ryu S, Kalkanis S, et al. Radiosurgery to the surgical cavity as adjuvant therapy for resected brain metastasis. Neurosurgery. 2012;71:937–43. doi: 10.1227/NEU.0b013e31826909f2. [DOI] [PubMed] [Google Scholar]
- 33.Asher AL, Burri SH, Wiggins WF, et al. A new treatment paradigm: neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. doi: 10.1016/j.ijrobp.2013.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.