ABSTRACT
The objective of this analysis was to compare the anti-HPV GMTs and their distribution after a 6-month or a 3–8 -y interval between two HPV vaccine doses. The results from two clinical trials, conducted by the same team in the same region, with serological assays performed at the same laboratory using the same ELISA methodology were compared. In the first study, 173 9–10-y-old girls and boys received two doses of 9vHPV vaccine at a 6-month interval; in the second study, 31 girls vaccinated with one dose of 4vHPV at the age of 9–14 y received a dose of 9vHPV 3–8 y later (mean 5.4 y). In both studies, blood samples were collected before and 1 month post second dose. Despite large differences in the time since the first dose, all subjects (100%) were seropositive to the common 4 HPV types (6, 11, 16 and 18) to both vaccines, with comparable GMTs and titer distributions before the second dose. One month post second dose, the GMTs increased 40–91-fold for those with a 6-month interval between doses and 60–82-fold for those with a 3–8-y interval. Titer distributions after the booster dose were comparable in the two studies. These results indicate that 2-dose HPV vaccination schedules with an interval of several years could be used for pre-adolescents. Intervals longer than 6 months may facilitate logistics for immunization programs and could be useful during periods of vaccine shortage or as a transition while the effectiveness of a one-dose schedule is being evaluated.
KEYWORDS: HPV Vaccine, One Dose, Two Doses, Long Intervals
Introduction
Limited data are available about the impact of dosing intervals and the magnitude of the immune response to HPV vaccination. For the 2-dose HPV vaccination schedule, applicable to girls and boys less than 15 y of age, the product monographs of the quadrivalent (4vHPV) and the nonavalent (9vHPV) vaccines specify administration with a 0, 6–12-months schedule and consideration of a third dose of vaccine if the interval between doses was less than 5 or 6 months.1, 2 Slightly differently, the product monograph of the bivalent vaccine (2vHPV) specifies administration with a 6-month interval, allowing flexibility for a 5–7-month interval between doses.3 Generally, in 2-dose schedules, a short interval between the two doses can reduce the immune response to the second dose that is intended to be a booster inducing long-lasting immunity.4 As an example, dosing intervals shorter than 4 months for 2 doses of HPV vaccine induce lower antibody titers compared to a 6-month interval.5,6 With a 3-dose HPV vaccine schedule, delayed administration of the second (≥4 months after dose 1; mean 472 d) or third doses (≥8 months after dose 2; mean 501 d) resulted in higher antibody titers compared to the recommended 0-, 2- and 6-month schedule.7 While current recommendations indicate an interrupted vaccination schedule should be completed without re-initiation of the series,5,8,9 there are no data regarding the immune response to 2 doses of HPV vaccines administered with an interval of several years versus an interval of 6 months.
The objective of this post hoc analysis was to compare the anti-HPV geometrical mean IgG antibody titers (GMTs) and their distribution with a 6-month or a 3–8-y interval between the two HPV vaccine doses.
Results
All subjects in both studies were seropositive to HPV6, 11, 16 and 18 prior to receiving their second dose of vaccine. Anti-HPV6, 11 and 16 GMTs were comparable among subjects tested 1–6 months or 3–8 y post first-dose administration (Table 1). The anti-HPV18 GMTs were higher in subjects who received 9vHPV vaccine as the first dose and were tested 1–6 months later (P = .005) (Table 2). The titer distribution was comparable in two studies (Figure 1), as well as among subjects in Study B who received the first dose either 3–4, 5–6 or 7–8 y before testing (Figure 2).
Table 1.
Antibody geometrical mean titers pre and post second HPV dose and GMT-fold increase post/pre second dose.
| Post first dose |
One month post second dose of HPV9 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study A 1–6 months post first dose of HPV9 n = 173 |
Study B 3–8 y post first dose of HPV4 n = 31* |
Study A n = 173 |
Study B n = 31* |
GMT-fold increase post second dose (95% CI) |
||||||
| HPV type | % sero+ | GMT [IU-AU/ml] (95% CI) |
% sero + | GMT [IU-AU/ml] (95% CI) |
% sero + | GMT [IU-AU/ml] (95% CI) |
% sero + | GMT [IU-AU/ml] (95% CI) |
Study A n = 173 |
Study B n = 31 |
| HPV6 | 100 | 5.3 (4.6–6.1) | 100 | 6.1 (3.0–10.6) | 100 | 375.9 (334.6–422.2) | 100 | 405.5 (271.6–605.3) | 71.1 (59.0–85.5) | 66.8 (34.1–130.9) |
| HPV11 | 100 | 5.8 (5.1–6.6) | 100 | 7.7 (4.5–13.1) | 100 | 525.2 (470.1–586.8) | 100 | 552.9 (348.5–877.2) | 90.9 (76.6–108.0) | 71.7 (36.0–143.1) |
| HPV16 | 100 | 29.7 (26.2–33.7) | 100 | 20.1 (12.0–33.7) | 100 | 1174.5 (1049.0–1315.3) | 100 | 1640.5 (1094.7–2458.3) | 39.5 (33.3–46.9) | 81.5 (42.9–154.8) |
| HPV18 | 100 | 11.0 (9.5–12.7) | 100 | 6.3 (3.8–10.2) | 100 | 593.9 (527.7–668.3) | 100 | 374.7 (246.7–569.1) | 53.9 (44.8–64.8) | 59.8 (31.8–112.5) |
*Received the 4vHPV vaccine as the first dose
Table 2.
Studies designs and participants characteristics.
| Study | A | B |
|---|---|---|
| Vaccination | Two doses of 9vHPV | One dose of 4vHPV + one dose of 9vHPV |
| Vaccines administration | Both doses of 9vHPV given at the study site | 4vHPV given through regular school-based immunization program 9vHPV dose given at the study site |
| Gender of subjects | Girls and boys | Girls |
| Number of subjects | 173 | 31 |
| Age at the first dose administration | 9–11 y (mean 9.6 y) | 9–14 y (mean 10.1 y) |
| Age at the second dose administration | 9.5–11 y (mean 10.1 y) |
13–18 y (Mean 15.5 y) |
| Interval between the first and the second dose administration | 6 months (mean 6.0 months) |
36–96 months (mean 65.3 months) |
| Interval between the last dose administration and blood collection | 1 month (range: 21–41 d) |
1 month (range: 28–35 d) |
| Serological assay | IgG ELISA (M9ELISA) CDC, Atlanta |
IgG ELISA (M9ELISA) CDC, Atlanta |
Figure 1.
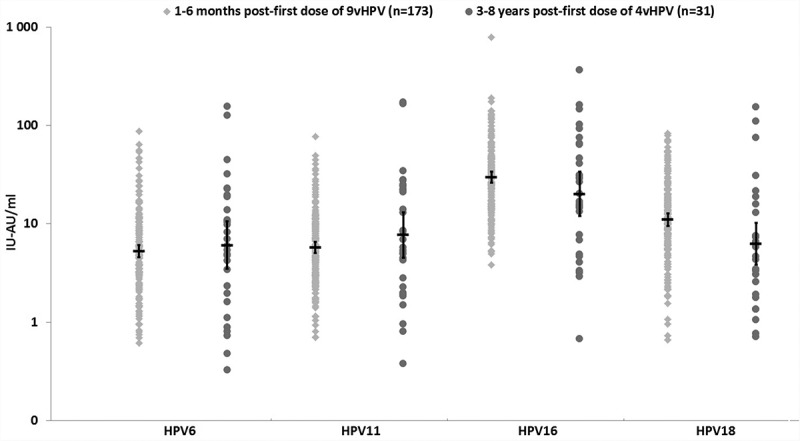
Antibody distribution and GMTs (95%CI) post first-dose administration.
Figure 2.
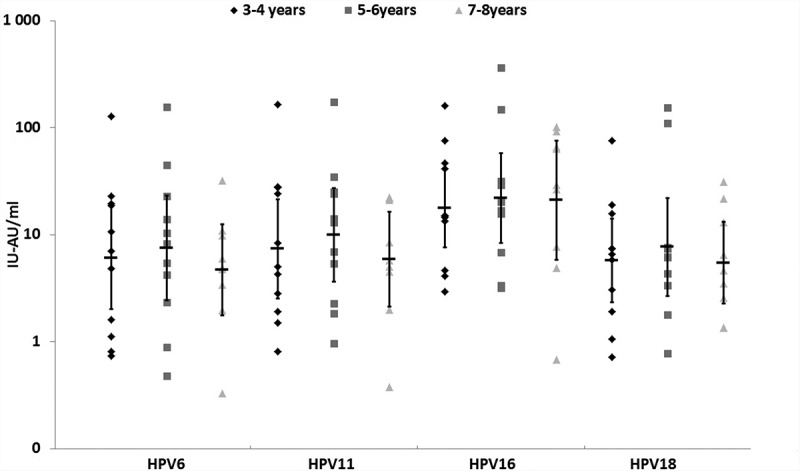
Antibody distribution by the time since the first dose of 4vHPV vaccine administration (n = 31).
After the second dose, all subjects were seropositive to HPV6, 11, 16 and 18 types. Subjects who received their second dose with a 6 month interval (study A) showed a 40–91-fold increase in GMTs, and those who received their second dose with a 3–8 y interval (study B) showed a 60–82-fold increase in GMTs. A 4-fold or greater increase in antibodies titers was observed in 93–100% of subjects. Six subjects who had a less than a 4-fold increase already had high antibody titers (above the GMTs in their group) to a given HPV type before their second dose administration (data not presented). One month post second dose, the anti-HPV6, 11, 16 GMTs were comparable in subjects participating in study A and study B (Table 2). The anti-HPV18 GMTs were 1.6-fold higher in subjects who received two doses of 9vHPV vaccine (p = .003) but the fold increase after the second dose of 9vHPV were similar (54–60-fold increase; p = .68) (Table 2). Titer distributions in the two studies were comparable (Figure 3).
Figure 3.
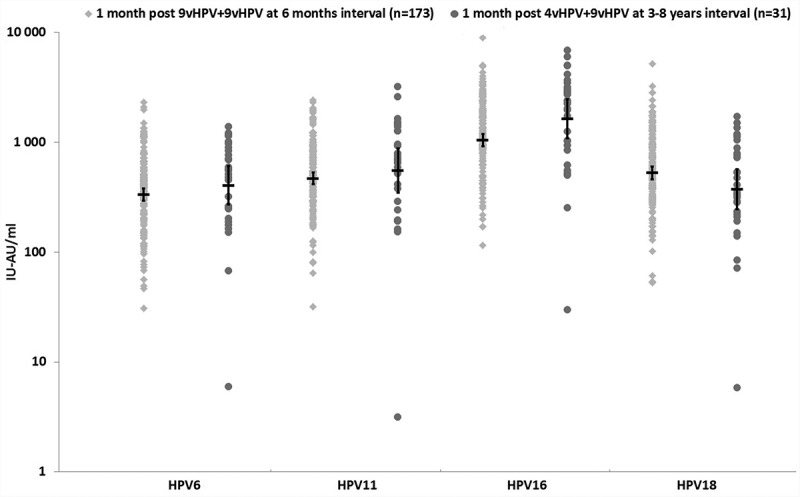
Antibody distribution and GMTs (95%CI) one month post second-dose administration.
Discussion
To our knowledge, this is the first analysis comparing the anti-HPV distribution and GMTs at different time points post one dose of 4vHPV or 9vHPV vaccine. The 100% seropositivity to HPV6, 11, 16 and 18 1–6 months and 3–8 y after a single dose of either vaccine is reassuring and consistent with previous reports.5,10,11 In the present analysis, no important difference in antibody GMTs and titer distributions were observed when measured 1–6 months or 3–8 y post first-dose administration. This observation is congruent with previous reports showing little variation in antibody titers with time since vaccination with one dose of vaccine.5,12,13 The slightly higher anti-HPV18 GMTs in subjects who received the 9vHPV vaccine as the first and second dose is most probably explained by the double quantity of antigens in this vaccine when compared to 4vHPV vaccine (40 µg/dose versus 20 µg/dose).1,2 These findings are in line with those reported by a recent analysis of immunogenicity of 4vHPV and 9vHPV vaccine in Latin American young females, which show higher anti-HPV18 GMTs in subjects who received the 9vHPV when compared to 4vHPV vaccine.14 The higher antibody titers after 9vHPV vaccine but similar fold increase post second dose in our studies suggest a stronger priming to HPV18 is induced by the first dose of this vaccine when compared to 4vHPV. The anti-HPV16 GMTs were slightly higher in subjects who received two different vaccines (all p > .05). An exploratory sub-analysis by periods of time post first-dose administration (3–4 y, 5–6 y or 7–8 y) shows comparable titer distributions and suggests little or no change occurs in antibody titers between year 3 and 8 post first-dose administration. This observation is consistent with previous reports which indicate that very little fluctuations in GMTs occurs after 18–24 months post-HPV vaccination with 1, 2 or 3 doses of the 4vHPV or 2vHPV vaccine.5,12
The similarities in anti-HPV GMTs and titer distributions post second dose and their multi-fold increase independent of the time interval between the two doses indicate that the first dose induced an excellent immune memory lasting for at least several years. These findings are in agreement with those reported for other vaccines which showed a non-inferior or even a more robust immune response with intervals between vaccine doses up to 7–11 y longer than those recommended by product monographs.15-21 These observations do not correlate with higher numbers of memory B-cells at early time points post first-dose administration. However, memory cells are not all equal in their proliferative potential, and the memory of the system matures over time, as cells with high proliferative capacity are generated and/or maintained.22
Our analysis has some limitations. First, the data come from two different clinical trials with different designs, different first HPV vaccines and slightly different populations. Namely, the mean age at the time of first dose administration was 9.6 ± 0.3 y (range 9–10 y) in study A in which vaccines were administered 6 months apart and 10.1 ± 1.2 y (range 9–14 y) in study B with a 3–8 year interval between doses. A recent analysis of five phase III immunogenicity clinical trials with 9vHPV shows that GMTs decrease with the age at first vaccination, being the highest in the youngest age cohort which received the vaccine (9–10-year-olds) and the lowest in older age cohorts included in the analysis (16–26-year-olds).23 We did not observe lower GMTs in study B with slightly older subjects, except for HPV18. The possibility that the equivalency of titers after delaying boosting for several years might be in part driven by environmental exposure to HPV cannot be excluded. Non-completers of HPV vaccination course may have more risk factors for HPV exposure as a previous study reported they were younger at first sexual exposure and of lower socioeconomic status.24,25 However, these findings may not be easily extrapolated to our source studies in which vaccination was initiated at a younger age, in a free-of-charge school-based program that has been shown to minimize socioeconomic inequalities on vaccination course completion.26
The relative titers across types are the same for the 9–10 year old pre-adolescents not yet sexually active and 13–18-years-old adolescents, an age group in which we cannot rule out sexual activity.27,28 This observation suggests that adolescents vaccinated with one dose of vaccine are not getting significant boosting from viruses shed by their sex partners. A study conducted in the same province five years post vaccination program implementation showed a 1% combined prevalence of HPV6/11/16/18 DNA in 17–19 year-old sexually active girls.26 Such a low circulation of the 4 HPV types included in the 4vHPV vaccine support the hypothesis that environmental exposure had very little, if any, impact on antibody persistence.
Second, study A included boys and girls whereas study B only had girls. Immunogenicity studies have shown higher GMTs in boys compared to girls.23 In our analysis, we did not observe higher GMTs in boys either post first or second dose. A potential explanation of this discrepancy might be the age difference between subjects included in the two analyses. In our analysis the great majority of subjects were 9–10 y old at the time of first dose administration and in the previous analysis 9–15-y-old subjects were included.23 Third, we measured antibody GMTs only at the peak of their level (1 month post second dose). Ongoing 36 month follow-up of participants in these source studies will allow comparison of antibody persistence. And finally, the small sample size in study B (31 girls) could limit the statistical power to detect a difference. However, the literature on prolonged intervals between the prime and the boost doses of several other vaccines15-21 support our findings, and 95% CI in our study are narrow.
In summary, these results suggest that a 2-dose vaccination schedule with an interval of several years does not reduce the response to the second dose. As such, delayed delivery of the booster dose might be used when vaccinating pre-adolescents and adolescents against HPV. A less rigid immunization schedule might (I) facilitate the co-administration of HPV vaccine with other vaccines (i.e. meningococcal, TdaP) recommended to the same age groups and reduce the number of vaccination visits; (II) support the decision to offer only one dose in case of vaccine shortage with the possibility of giving the second dose years later when the shortage is resolved, and (III) offer a safety net with the possibility of giving the second dose several years post first-dose (if judged necessary) during a potential transition period to one dose vaccination, until ongoing studies deliver more robust information regarding the level of protection ensured by one dose of vaccine.6,29
Methods
Data were obtained from two previously reported clinical trials conducted by our team, in Quebec City, Quebec, Canada.27,28 The serological assays for both studies were performed using a 9-plex VLP IgG ELISA (M9ELISA) at the Centers for Disease Control and Prevention (CDC, Atlanta, USA).30 Antibody titers were measured in International Units (IU/ml) for HPV16 and 18. In the absence of international standards for the other 2 HPV types, Arbitrary Units (AU/ml) were used. Test samples were considered positive if they passed parallel line method (PLL) conditions as well as were above Median+2 Standard Deviations of the PLL/titer generated from the children sera. Cut off value for HPV6, 11, 16, 18 were 0.1 AU/ml, 0.1 AU/ml, 0.5 IU/ml, 0.4 IU/ml, respectively.
This post hoc analysis included all 173 participants from study A who received two doses of 9vHPV vaccine and had blood samples collected at both study time points. All 31 participants from study B were included (Table 2).
In study A, 88 girls and 85 boys, age 9–10 y, received two doses of 9vHPV vaccine at a 6-month interval (range 5.7–6.9 months). The results were pooled together as the immune responses post-first and post-second dose were similar in boys and girls (GMTs in boys were 5.0–28.9 IU-AU/ml and girls 5.6–30.5 IU-AU/ml post-first dose, and respectively 391.6–1220.9 IU-AU/ml and 361.3–1131.4 IU-AU/ml post second dose depending on HPV type (95% CI were as follows: HPV6: 304–430 vs. 334-459AU/ml; HPV11: 411–567 vs. 492-669AU/ml; HPV16: 970–1320 vs. 1031-1446IU/ml; and HPV18: 483–682 vs. 522-725IU/ml, in girls and boys, respectively).
In study B, 31 girls vaccinated with one dose of 4vHPV at the age of 9–14 y were administered a dose of 9vHPV 3–8 y later (mean 5.4 y). The dose of 4vHPV was given in the regular school-based immunization program. The immunization status pre-second dose was assessed using regional vaccination registry data, reviewing individual vaccination cards, and confirming receipt of only single dose with the subjects and their parents. The pre-second dose blood samples included in this analysis were collected 1 or 6 months following the first dose in study A,28 and 3–8 y after the first dose in study B.27 In both studies, the post second-dose blood sample was collected after 1 month (range 21–41 d) (Table 2).
We compared the proportion of anti-HPV6, 11, 16 and 18 seropositive subjects using Fisher’s exact test and, anti-HPV titer distributions and GMTs with Wilcoxon’s test. All statistical tests were 2-tailed. P values of 0.05 or less were considered significant. SAS Institute software version 9.4 (Cary, NC, USA) was used for statistical analysis.
Both clinical trials were approved by Laval University Research Hospital Center Research Ethics Committee and are registered with ClinicalTrials.gov: NCT03431246 and NCT02567955.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or of the Quebec Public Health Institute.
References
- 1.Merck Frosst Canada ltée GARDASIL - Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) Recombinant Vaccine. Product Monograph Kirkland: Merck Canada Inc; February 2015. p. 62 accessed on February13, 2019 [En ligne]. Disponible: https://www.merck.ca/static/pdf/GARDASIL-PM_E.pdf [Google Scholar]
- 2.Merck Canada Inc Highlights of prescribing information [On line] [accessed 2019 Feb 13] https://www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf
- 3.GlaxoSmithKline CERVARIX® human papillomavirus vaccine types 16 and 18 (recombinant, AS04 adjuvanted) [On line]; 2008. [accessed 2019 Feb 13] https://ca.gsk.com/media/589880/cervarix.pdf
- 4.Plotkin SA, Orenstein WA, Offit PA.. Vaccines. 6th edn ed. Philadelphia, USA: Saunders Elsevier; 2012. p. 1550. [Google Scholar]
- 5.Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, Nene BM, Esmy PO, Joshi S, Poli URR, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. Jan. 2016;17(1):67–77. doi: 10.1016/S1470-2045(15)00414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sankaranarayanan R, Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, Bhatla N, Nene BM, Shaw J, Poli URR, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018. June;36(32Pt A):4783–91. doi: 10.1016/j.vaccine.2018.02.087. [DOI] [PubMed] [Google Scholar]
- 7.Widdice LE, Unger ER, Panicker G, Hoagland R, Callahan ST, Jackson LA, Berry AA, Kotloff K, Frey SE, Harrison CJ, et al. Antibody responses among adolescent females receiving two or three quadrivalent human papillomavirus vaccine doses at standard and prolonged intervals. Vaccine. 2018;36(6):881–89. doi: 10.1016/j.vaccine.2017.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Timing and spacing of immunobiologics [One line]. [accessed 2019 Feb 13] https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/timing.html
- 9.World Health Organisation Table 3: recommendations* for interrupted or delayed routine immunization - summary of WHO position papers [On line]. [accessed 2019 Feb 13] https://www.who.int/immunization/policy/Immunization_routine_table3.pdf
- 10.Zhu F-C, Chen W, Hu Y-M, Hong Y, Li J, Zhang X, Zhang Y-J, Pan Q-J, Zhao F-H, Yu J-X, et al. Efficacy, immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese women aged 18–25 years: results from a randomized controlled trial. Int J Cancer. 2014. December 1;135(11):2612–22. doi: 10.1002/ijc.v135.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilca V, Sauvageau C, Boulianne N, De Serres G, Couillard M, Krajden M, Ouakki M, Murphy D, Trevisan A, Dionne M. Immunogenicity of quadrivalent HPV and combined hepatitis A and B vaccine when co-administered or administered one month apart to 9-10 year-old girls according to 0-6 month schedule. Hum Vaccines Immunother. 2014;10(8):2438–45. doi: 10.4161/hv.29617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, Lowy DR, Wacholder S, Schiffman M, Rodriguez AC, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica vaccine trial. Cancer Prev Res (Phila Pa). 2013. November;6(11):1242–50. doi: 10.1158/1940-6207.CAPR-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreimer AR, Herrero R, Sampson JN, Porras C, Lowy DR, Schiller JT, Schiffman M, Rodriguez AC, Chanock S, Jimenez S, et al. Evidence for single-dose protection by the bivalent HPV vaccine-review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018. January 20;36:4774–82. doi: 10.1016/j.vaccine.2017.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ám R-S, Moreira ED, Restrepo JA, Lazcano-Ponce E, Cabello R, Silva A, Andrade R, Revollo F, Uscanga S, Victoria A, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine in Latin American girls, boys, and young women. Papillomavirus Res Amst Neth. 2018;5:63–74. doi: 10.1016/j.pvr.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittman PR, Cavicchia MA, Kingsbury JL, Johnson NA, Barrera-Oro JG, Schmader T, Korman L, Quinn X, Ranadive M. Anthrax vaccine adsorbed: further evidence supporting continuing the vaccination series rather than restarting the series when doses are delayed. Vaccine. 2014. September 3;32(39):5131–39. doi: 10.1016/j.vaccine.2014.03.076. [DOI] [PubMed] [Google Scholar]
- 16.Bai C, He J, Niu H, Hu L, Luo Y, Liu X, Peng L, Zhu B. Prolonged intervals during Mycobacterium tuberculosis subunit vaccine boosting contributes to eliciting immunity mediated by central memory-like T cells. Tuberc Edinb Scotl. 2018;110:104–11. doi: 10.1016/j.tube.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Albas A, Fontolan OL, Pardo PE, Bremer Neto H, Sartori A. Interval between first dose and booster affected antibody production in cattle vaccinated against rabies. J Venom Anim Toxins Incl Trop Dis. 2006. September 26;12(3):476–86. doi: 10.1590/S1678-91992006000300010. [DOI] [Google Scholar]
- 18.Schöndorf I, Schönfeld C, Nicolay U, Zent O, Banzhoff A. Response to tick-borne encephalitis (TBE) booster vaccination after prolonged time intervals to primary immunization with the rapid schedule. Int J Med Microbiol IJMM. 2006. May;296(Suppl 40):208–12. doi: 10.1016/j.ijmm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Askling HH, Vene S, Rombo L, Lindquist L. Immunogenicity of delayed TBE-vaccine booster. Vaccine. 2012. January 11;30(3):499–502. doi: 10.1016/j.vaccine.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 20.Ott JJ, Wiersma ST. Single-dose administration of inactivated hepatitis A vaccination in the context of hepatitis A vaccine recommendations. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2013. November;17(11):e939–e944. [DOI] [PubMed] [Google Scholar]
- 21.Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine. 2007. April 30;25(17):3482–84. doi: 10.1016/j.vaccine.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Castiglione F, Mantile F, De Berardinis P, Prisco A. How the interval between prime and boost injection affects the immune response in a computational model of the immune system. Comput Math Methods Med. 2012;2012:842329. doi: 10.1155/2012/842329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen LK, Restrepo J, Moreira ED, Iversen O-E, Pitisuttithum P, Van Damme P, Joura EA, Olsson S-E, Ferris D, Block S, et al. Impact of baseline covariates on the immunogenicity of the 9-valent HPV vaccine - A combined analysis of five phase III clinical trials. Papillomavirus Res Amst Neth. 2017. June;3:105–15. doi: 10.1016/j.pvr.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith LM, Brassard P, Kwong JC, Deeks SL, Ellis AK, Lévesque LE. Factors associated with initiation and completion of the quadrivalent human papillomavirus vaccine series in an Ontario cohort of grade 8 girls. BMC Public Health. 2011. August 13;11:645. doi: 10.1186/1471-2458-11-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman AJH, Gamboa C, Darbinian JA, Littell RD, Torrente S. Disparities in human papillomavirus vaccine completion rates among females in an integrated health care system. Obstet Gynecol. 2018;132(3):717–23. doi: 10.1097/AOG.0000000000002802. [DOI] [PubMed] [Google Scholar]
- 26.Lefevere E, Theeten H, Hens N, De Smet F, Top G, Van Damme P. From non school-based, co-payment to school-based, free human papillomavirus vaccination in Flanders (Belgium): a retrospective cohort study describing vaccination coverage, age-specific coverage and socio-economic inequalities. Vaccine. 2015. September 22;33(39):5188–95. doi: 10.1016/j.vaccine.2015.07.088. [DOI] [PubMed] [Google Scholar]
- 27.Gilca V, Sauvageau C, Panicker G, De Serres G, Ouakki M, Unger ER. Antibody persistence after a single dose of quadrivalent HPV vaccine and the effect of a dose of nonavalent vaccine given 3-8 years later – an exploratory study. Hum Vaccines Immunother. 2018. September 25:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilca V, Sauvageau C, Panicker G, De Serres G, Ouakki M, Unger ER. Immunogenicity and safety of a mixed vaccination schedule with one dose of nonavalent and one dose of bivalent HPV vaccine versus two doses of nonavalent vaccine - A randomized clinical trial Vaccine. 2018. October 9. Hum Vaccin Immunother. 2019;15(2):503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trial C. Scientific evaluation of one or two doses of the bivalent or nonavalent prophylactic HPV vaccines [On line]. [accessed 2019 Feb 13] https://clinicaltrials.gov/ct2/show/NCT03180034?term=Kreimer&cond=HPV+Infection&rank=1. Vaccine. 2018 Nov 12;36(46):7017–7024. [DOI] [PMC free article] [PubMed]
- 30.Panicker G, Rajbhandari I, Gurbaxani BM, Querec TD, Unger ER. Development and evaluation of multiplexed immunoassay for detection of antibodies to HPV vaccine types. J Immunol Methods. 2015. February;417:107–14. doi: 10.1016/j.jim.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Merck Canada Inc Highlights of prescribing information [On line] [accessed 2019 Feb 13] https://www.merck.com/product/usa/pi_circulars/g/gardasil_9/gardasil_9_pi.pdf


