Abstract
Background
Early biomarkers for acute kidney injury after kidney transplantation have been studied because delayed graft function (DGF) is associated with increased risk of acute rejection and graft loss. We investigated the usefulness of serum neutrophil gelatinase‐associated lipocalin (NGAL) and interleukin‐18 (IL‐18) for the prediction of DGF after kidney transplantation.
Materials and Methods
Fifty‐nine kidney transplant recipients were included and they were separated into DGF and immediate graft function (IGF) groups. Serum samples were collected on the preoperative day as well as days 1, 5, and 14 after the transplantation, and assayed for NGAL and IL‐18.
Results
After transplantation, serum levels of NGAL were significantly higher at any time in patients with DGF compared to those with IGF. Serum concentrations of IL‐18 were not different between both groups. The receiver operating characteristics(ROC)‐area under the curve (AUC) values of NGAL, IL‐18, and creatinine on day 1 for the discrimination of DGF from IGF were 0.86, 0.63, and 0.65. On POD1, the sensitivities of NGAL and creatinine were respectively 78.6%, and 50.0% at 77.8% specificity, and the AUC values for any combinations including NGAL and that for NGAL alone were higher than that of creatinine.
Conclusion
Serum NGAL is an early and sensitive marker of graft dysfunction in kidney transplantation, while serum IL‐18 showed limited values. J. Clin. Lab. Anal. 26:286‐294, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: neutrophil gelatinase‐associated lipocalin (NGAL), interleukin‐18 (IL‐18), creatinine, kidney transplantation, delayed graft function
INTRODUCTION
Kidney transplantation is the renal replacement therapy of choice for most patients with end‐stage renal disease. Although kidney transplantation may be successful, impaired kidney function could arise. The frequency of delayed graft function (DGF) varies from 4 to 10% in living donor kidney transplants and 5 to 50% in deceased donor transplants 1. DGF can be usually caused from ischemic injuries before and during kidney transplantations 2. It may also be further aggravated by the reperfusion syndrome 3, and is associated with the increased risk of graft loss and acute rejection 4. Therefore, early assessment of kidney function following renal transplantation is crucial for predicting graft survival and early decision making. Unfortunately, serum creatinine concentration is not a reliable parameter for describing acute kidney injury (AKI) 5. Creatinine can be influenced by non‐renal factors including muscle mass of the subject, and its serum levels may not be increased until 50% of renal functions are lost. Moreover, creatinine does not reflect the degree of kidney function until a steady state has been reached, which may take several days 6. Accordingly, some translational studies have evaluated serum and urine biomarkers for the assessment of kidney function 7.
Among those biomarkers, neutrophil gelatinase‐associated lipocalin (NGAL), a member of the lipocalin family 8, is expressed by neutrophil as well as tissues of kidneys, prostate, and the epithelia of the respiratory and alimentary tracts 9. In recent publications, NGAL was detected in the blood and urine after AKI. Interleukin‐18 (IL‐18), a member of cytokines, has been also known as a urinary marker of kidney injuries 10. However, usefulness of serum IL‐18 as a marker for detecting AKI is still controversial 11, 12, 13.
In this study, we aimed to assess whether DGF after kidney transplantation can be predicted by NGAL and IL‐18 levels in the serum samples collected serially from the recipients before and after transplantation.
MATERIALS AND METHODS
Patients
Of the 333 patients who underwent kidney transplantation from March 2006 to December 2008 in the Transplantation Center of Severance Hospital, 59 recipients were retrospectively included in this study after subjects with the following characteristics were excluded to eliminate various confounding factors; recipients with a human leukocyte anitgen (HLA)‐identical in living related donor, mismatches of six HLA antigens in living related or deceased donor, recipients younger than 20 years of age or older than 55 years of age, donors younger than 20 years of age or older than 55 years of age, negative conversion of lymphocyte cross‐matching report, pre‐/post‐transplant hepatitis B or C positive recipients, diabetic recipients, and follow‐up loss recipients. All the living donors of our study had normal blood pressure. Marginal or non‐heart beating donors were not included in the deceased donors of our study. Marginal donor was defined as donor age of more than 60 years, serum creatinine >3.0 mg/dl, creatinine clearance <60 ml/min, or urine protein ≥2+. Causes of death of deceased donors were divided into trauma (n = 10) and non‐trauma (n = 21).
Fifty‐nine patients were separated into two groups: a DGF group (n = 14) and immediate graft function (IGF) group (n = 45). The patients with DGF had experience of dialysis within 1 week after transplantation or pathologic findings such as acute tubular necrosis of kidney graft 14. The allograft biopsy was done when graft failure was clinically suspected in the recipient, who had symptoms such as decrease of urine output, increase of serum creatinine within 2 weeks after transplantation, and the biopsied specimens were examined by an anatomic pathologist specialized in nephrology. All the patients with DGF experienced dialysis after transplantation, and three patients among them showed pathologic findings of acute tubular necrosis in allograft kidney.
Serum samples of the subjects were collected on the preoperative day, the first postoperative day (POD 1), the fifth postoperative day (POD 5), and the 14th postoperative day (POD 14). Venous blood from all subjects was drawn in the morning after an overnight fast, and collected into Vacuette serum separate tube (Greiner‐Bio‐One, Kremsműnster, Austria). After sera were separated, all the samples were aliquotted and stored at –80°C until assayed. This study was approved by the Institutional Review Board of Severance Hospital.
Assays for NGAL, IL‐18, and Creatinine
Serum NGAL levels were measured using enzyme‐linked immunosorbent assay (ELISA) kit (KIT 037, BioPorto Diagnostics, Gentofte, Denmark) according to the manufacturer's instructions. The ELISA reader was set at 450 nm to quantify color intensity. The NGAL concentrations in the samples were calculated from the calibration curve generated by the NGAL‐calibrators and its corresponding optical density (OD).
Serum IL‐18 levels were assayed by human IL‐18 ELISA kit (Medical and Biologic Laboratories, Nagoya, Japan) in accordance with the instructions given by the manufacturer. Resultant ODs were measured at a wavelength of 450 nm using a microplate reader. The concentration of human IL‐18 was calculated from a dose response curve based on reference standards.
Serum creatinine concentrations were determined with a Hitachi 7600–210 clinical analyzer (Hitachi High‐Technologies Co., Tokyo, Japan).
Statistical Analysis
All statistical analyses were performed by PASW statistics 18.0 (formerly SPSS statistics, SPSS Inc., Chicago, IL) and Analyse‐it software Method Evaluation Edition version 2.22 (Analyse‐it Software Ltd., City West Business Park, Leeds, UK). Clinical characteristics and levels of markers between the study groups were compared using the Mann–Whitney U test for continuous variables and Chi‐square test for categorical variables. The association of the levels of markers after transplantation with cold ischemia time (CIT) of transplantation cases from deceased donors was assessed by Spearman's correlation test. Receiver operating characteristics (ROC) curves were generated to compare the performances of NGAL, IL‐18, and creatinine for predicting DGF. In order to evaluate the performances of the markers with same evaluation basis, cut‐off levels with a fixed specificity were determined from the results of the ROC analysis. Logistic regression analysis was performed to calculate the predicted probability values for the combinations of the markers with the presence of DGF as the binary dependent variable and the levels of the markers as the predictor variable. These values were used to estimate the ROC‐area under the curve (AUC) for the respective markers’ combinations. Multivariate analysis using logistic regression with backward stepwise model was also performed with the presence of DGF as the binary dependent variable and subjects’ characteristics (sex and age of donors and recipients, CIT, and types of donor) and serum levels of the biomarkers at POD 1 as the predictor variables. A p‐value of less than 0.05 was considered as statistically significant.
RESULTS
Characteristics and Levels of NGAL, IL‐18, and Creatinine in the Patient Groups
Table 1 demonstrates clinical characteristics in recipients with DGF or IGF. Clinical parameters excluding age of recipients were not significantly different between the two groups. At all times measured after transplantation, serum NGAL levels were significantly higher in patients with DGF compared to those with IGF. The median serum IL‐18 levels were also higher in the DGF group, although statistically significant differences were not found (Table 2). Serum creatinine was not different between the two groups on the POD 1 (p = 0.1030), but was higher in DGF group after 5 days from transplantation (p = 0.0002). In the recipients transplanted from deceased donors, the levels of markers except creatinine at POD 5 were not significantly different according to the causes of death of the donors (trauma and non‐trauma). The median of creatinine levels at POD 5 in the patients received allografts from deceased donors with traumatic causes of death was 1.6 mg/dl (first to third quartiles = 1.2–2.0 mg/dl), and that in the recipients transplanted with kidney from the donors who had died from non‐traumatic causes was 3.0 mg/dl (first to third quartiles = 2.0–6.8 mg/dl) (p = 0.0264 for the difference).
Table 1.
Summary of Clinical Characteristics According to the Study Groups
| IGF | (n = 45) | DGF | (n = 14) | P‐value | |
|---|---|---|---|---|---|
| Age of recipients, mean (SD) | 39.4 | (9.8) | 46.8 | (6.7) | 0.0097 |
| Male recipient, n (%) | 29 | (64.4) | 6 | (42.9) | 0.1510 |
| Cause of ESRD | |||||
| Hypertension, n (%) | 6 | (13.3) | 5 | (35.7) | 0.0604 |
| Chronic glomerulonephritis, n (%) | 10 | (22.2) | 1 | (7.1) | 0.2058 |
| IgA nephropathy, n (%) | 7 | (15.6) | 0 | (0.0) | 0.1160 |
| Polycystic kidney, n (%) | 2 | (4.4) | 1 | (7.1) | 0.6881 |
| FSGS, n (%) | 4 | (8.9) | 0 | (0.0) | 0.2479 |
| Other, n (%) | 16 | (35.6) | 7 | (50.0) | 0.3331 |
| Age of donor, mean (SD) | 41 | (10.5) | 44.7 | (10.6) | 0.1724 |
| Male donor, n (%) | 28 | (62.2) | 8 | (57.1) | 0.7336 |
| Deceased donor, n (%) | 21 | (46.7) | 10 | (71.4) | 0.1051 |
| CIT of transplantation from deceased donora, mean (SD) | 310.8 | (107.2) | 337 | (132.2) | 0.5970 |
Units are in minutes and data were calculated only from a deceased donor group. All the transplantation with graft from living donors presented CIT of less than 20 min.
IGF, immediate graft function; DGF, delayed graft function; SD, standard deviation; ESRD, end stage renal disease; FSGS, focal segmental glomerulosclerosis; CIT, cold ischemic time.
Table 2.
Median Serum NGAL, IL‐18, and Creatinine Levels According to the Graft Functions After Transplantation
| Reagent | Time | IGF (n = 45)a | DGF (n = 14)a | p‐value |
|---|---|---|---|---|
| Serum NGAL (ng/mL) | Preoperative | 837.7 (574.7‐1141.3) | 952.1 (647.1‐1118.9) | 0.3184 |
| POD 1 | 183.6 (145.2‐232.5) | 490.4 (238.3‐722.6) | <0.0001 | |
| POD 5 | 123.7 (93.4‐178.9) | 362.0 (184.5‐502.1) | 0.0001 | |
| POD 14 | 108.1 (238.7‐497.1) | 194.6 (115.2‐421.0) | 0.0019 | |
| Serum IL‐18 (pg/mL) | Preoperative | 358.9 (238.7‐497.1) | 421.6 (362.0‐467.5) | 0.4436 |
| POD 1 | 265.4 (162.5‐376.0) | 343.1 (229.6‐570.9) | 0.1416 | |
| POD 5 | 285.3 (143.6‐367.5) | 344.5 (242.4‐511.4) | 0.0823 | |
| POD 14 | 249.0 (143.7‐338.8) | 320.1 (204.7‐388.2) | 0.2616 | |
| Serum Cr (mg/dL) | Preoperative | 9.8 (7.4‐12.7) | 11.1 (9.3‐14.1) | 0.2090 |
| POD 1 | 4.9 (3.2‐7.5) | 7.6 (4.2‐9.6) | 0.1030 | |
| POD 5 | 1.4 (1.0‐2.0) | 6.1 (4.0‐8.5) | 0.0002 | |
| POD 14 | 1.2 (1.0‐1.5) | 2.3 (1.6‐5.6) | <0.0001 |
Data are shown as ‘median (1st to 3rd quartiles)’.
Abbreviations: IGF, immediate graft function; DGF, delayed graft function; POD, postoperative day; Cr, creatinine
Correlation Coefficients Between CIT in the Cases Transplanted From Deceased Donors and Levels of Biomarkers
There was a positive relationship between CIT and the levels of other markers: NGAL at POD 5 (r = 0.19, p = 0.2989), IL‐18 at POD 5 (r = 0.10, p = 0.5760), IL‐18 at POD 14 (r = 0.22, p = 0.2437), creatinine at POD 5 (r = 0.16, p = 0.3780), and creatinine at POD 14 (r = 0.32, p = 0.0825). There was also a negative correlation between CIT and creatinine levels at POD 1 (r = −0.13, p = 0.4846). No correlation was found between CIT and NGAL concentrations at POD 1 (r = 0.07, p = 0.7089), at POD 14 (r = −0.00, p = 0.9845), IL‐18 at POD 1 (r = −0.05, p = 0.7875). However, all the correlation coefficients were not statistically significant.
ROC Analysis, Cut‐off Determination, and Comparison of Diagnostic Performances
The ROC curves at POD 1 are shown in Figure 1, and the ROC–AUC values of NGAL, IL‐18, and creatinine for predicting DGF on the respective collection days are summarized in Table 3. The AUC value of NGAL at POD 1 was higher than those of IL‐18 and creatinine (p = 0.0414 and p = 0.0213, respectively). The AUC value of NGAL was not significantly different from those of IL‐18 and creatinine at POD 5 (p = 0.0714 for IL‐18 and p = 0.9746 for creatinine) and POD 14 (p = 0.0761 for IL‐18 and p = 0.1941 for creatinine).
Figure 1.
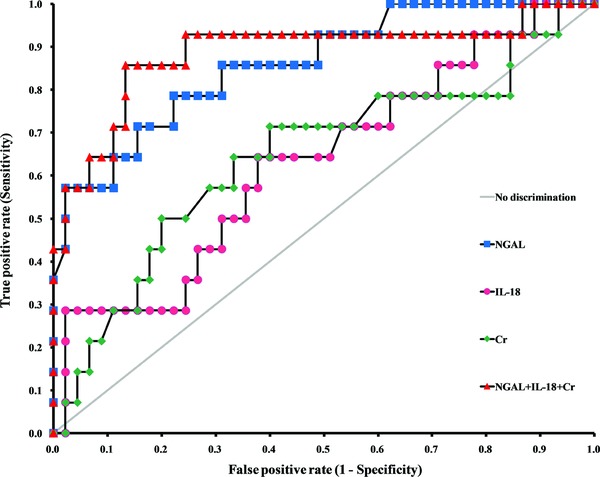
Receiver operating characteristics curves for serum biomarkers on the first postoperative day for predicting delayed graft function.
Table 3.
Comparisons Between AUC Value of Creatinine and Those of the Rest Markers and Their Combinations
| Combination panel | POD 1 | POD 5 | POD 14 | |||
|---|---|---|---|---|---|---|
| AUC (95% CI) | P‐valuea | AUC (95% CI) | P‐valuea | AUC (95% CI) | P‐valuea | |
| Cr | 0.65 (0.46–0.83) | – | 0.84 (0.66–1.00) | – | 0.86 (0.74–0.98) | – |
| NGAL | 0.86 (0.75–0.98) | 0.0213 | 0.84 (0.70–0.98) | 0.9746 | 0.78 (0.64–0.91) | 0.1941 |
| IL‐18 | 0.63 (0.46–0.81) | 0.8762 | 0.65 (0.48–0.83) | 0.0481 | 0.60 (0.42–0.78) | 0.0055 |
| NGAL + IL‐18 | 0.88 (0.79–0.98) | 0.0027 | 0.86 (0.72–1.00) | 0.6551 | 0.77 (0.63–0.92) | 0.1649 |
| NGAL + Cr | 0.86 (0.71–1.00) | 0.0888 | 0.89 (0.76–1.00) | 0.1405 | 0.87 (0.75–0.98) | 0.6120 |
| IL‐18 + Cr | 0.64 (0.47–0.82) | 0.9521 | 0.84 (0.67–1.00) | 0.4465 | 0.87 (0.75–0.98) | 0.2472 |
| NGAL + IL‐18 + Cr | 0.89 (0.76–1.00) | 0.0377 | 0.89 (0.75–1.00) | 0.1622 | 0.87 (0.75–0.98) | 0.8277 |
vs. the AUC values for creatinine.
POD, postoperative day; CI, confidence interval; Cr, creatinine.
The sensitivities for DGF prediction and corresponding cut‐off levels of NGAL, and creatinine according to specificities are shown in Table 4. For instance, the sensitivities of NGAL, and creatinine at 77.8% specificity were 78.6%, and 50.0%; the cut‐off levels at these sensitivities and specificities were 233.3 ng/ml, and 7.5 mg/dl at POD 1.
Table 4.
Sensitivities and Cut‐off Values of Serum NGAL, IL‐18, and Creatinine According to the Specificities
| Specificity (95% CI) | POD 1 | POD 5 | ||||||
|---|---|---|---|---|---|---|---|---|
| NGAL | Cr | NGAL | Cr | |||||
| Sensitivity | Sensitivity | Sensitivity | ||||||
| Cut‐offa | (95% CI)b Sensitivity | Cut‐offa | (95% CI)b Sensitivity | Cut‐offa | (95% CI)b Sensitivity | Cut‐offa | (95% CI)b Sensitivity | |
| 97.8 | 411.4 | 57.1 | 11.1 | 7.1 | 261.4 | 57.1 | 4.0 | 78.6 |
| (88.2–99.9) | (28.9–82.3) | (0.2–33.9) | (28.9–82.3) | (49.2–95.3) | ||||
| 88.9 | 348.5 | 64.3 | 9.5 | 28.6 | 230.8 | 57.1 | 2.6 | 78.6 |
| (75.9–96.3) | (35.1–87.2) | (8.4–58.1) | (28.9–82.3) | (49.2–95.3) | ||||
| 77.8 | 233.3 | 78.6 | 7.5 | 50.0 | 186.5 | 74.1 | 2.0 | 78.6 |
| (62.9–88.8) | (49.2–95.3) | (23.0–77.0) | (41.9–91.6) | (49.2–95.3) | ||||
| 68.9 | 222.1 | 85.7 | 7.2 | 57.1 | 144.4 | 85.7 | 1.7 | 85.7 |
| (53.4–81.8) | (57.2–98.2) | (28.9–82.3) | (57.2–98.2) | (57.2–98.2) | ||||
Units are in ng/ml for NGAL, and in mg/dl for Cr.
Units are in percent.
POD, postoperative day; Cr, creatinine; CI, confidence interval.
ROC Analysis of Combinations of NGAL, IL‐18, and Creatinine
Table 3 shows the AUC values of the individual markers or their combinations, and those were compared to the AUC value of creatinine. The markers with p‐value of less than 0.05 were considered as having statistically different AUC values from that of creatinine. On POD 1, the AUC values for any combinations including NGAL and NGAL alone were higher than that of creatinine. On POD 5 and POD 14, the AUC values of any makers or combinations were not significantly higher than that of creatinine, and the AUC values of IL‐18 was even lower than that of creatinine.
Multivariate Analysis
Multiple logistic regression was performed with the presence of DGF as the binary dependent variable and the levels of the three markers on POD 1 and donors and recipients factors as the independent variables. As a result, only serum NGAL levels on POD 1 were associated with DGF (odds ratio for NGAL = 1.02, p = 0.0048), while IL‐18 and creatinine concentrations on POD 1 and other factors were not significantly related to DGF.
DISCUSSION
This study evaluated serum NGAL and IL‐18 as new biomarkers for renal injury after kidney transplantation. NGAL have been evaluated in many studies; however, there are few studies on NGAL in serum samples. Furthermore, the usefulness of serum IL‐18 for renal injury has been controversial. In our results, NGAL, IL‐18, and creatinine were not significantly different between the DGF and IGF groups before kidney transplantation. These findings suggest that the kidney functions of the recipients before transplantation were not different between the two groups. On the POD 1, NGAL concentration was significantly higher in DGF group than in IGF group, while serum IL‐18 and creatinine levels were not different between the two groups. DGF was able to be discriminated from IGF by creatinine levels after POD 5. These findings suggest that serum NGAL measured after transplantation could predict DGF earlier than IL‐18 and creatinine. NGAL levels also showed large differences between patients with DGF and IGF on POD 1. Therefore, adding serum NGAL for follow‐up laboratory tests not only would facilitate early diagnosis of DGF but also could aid prompt decision making.
ROC analyses also showed that NGAL performed better than serum creatinine at POD 1. The AUC value of NGAL at POD 1 was 0.86 (95% confidence interval [CI]: 0.75–0.98); however, that of creatinine at POD 1 was 0.65 (95% CI: 0.46–0.83), and those were statistically different (p = 0.0213). Similar to our results, Hall et al. reported that the AUC value of urine NGAL at POD 1 for predicting dialysis events was 0.82 (95% CI: 0.72–0.92) 15. Irrespective of specimen type, the AUC value of NGAL seems to be similar; however, the diagnostic performances could be variable between studies due to the patients’ characteristics, numbers of subjects enrolled, and the assays utilized. Therefore, further investigations with larger numbers of subjects might be necessary.
In our study, 14 patients with DGF and 45 with IGF were enrolled. Since the number of patients was small, the results of ROC analyses might have limited external validity. The optimal cut‐off might be decided according to sensitivity and specificity; thus, we compared marker's sensitivities with fixed specificity from ROC curve. The optimal cut‐off for serum NGAL was 233.3 ng/ml with a sensitivity of 76.6% and a specificity of 77.8% at POD 1. The relatively low sensitivity and specificity of NGAL may result from the small number of subjects. For urine NGAL, Hall et al. reported a 77% sensitivity and 74% specificity with a cut‐off of 350 ng/ml 15. In the same study, 34 patients with DGF were included, which was larger than our study. Therefore, sensitivity and specificity at a specific cut‐off might be evaluated with a larger number of subjects.
On POD 1, the markers having AUC value higher than creatinine were NGAL alone and the combinations including NGAL. The AUC value for the combination of NGAL, IL‐18, and creatinine was highest among all of the combinations and individual markers. However, the AUC values for any combinations including NGAL and NGAL alone were not different from that of all three markers’ combination (data were not shown). This means that NGAL alone and NGAL combined with IL‐18 or creatinine would be as equally useful as the combination of all three markers, and more useful than creatinine. On POD 5 and POD 14, the AUC values of any markers or combinations were not significantly higher than that of creatinine. Since NGAL and creatinine were able to predict renal dysfunction at POD 5 in our study, further research with samples collected on POD 2, 3, and 4 may be necessary to estimate the predictive values for DGF of both markers on the respective days. With these values, physicians would be able to determine which test is more efficient to predict DGF on the respective days, because NGAL assays are still expensive for routine use.
IL‐18 is a multifunctional cytokine that increases both innate and acquired immune responses and potentiates Th1 and Th2 reactions 16. Urinary IL‐18 is an early and accurate predictor of the need for dialysis in renal transplantation 15, 17. However, usefulness of serum IL‐18 is still controversial 11, 12, 13. In our study, serum IL‐18 levels were higher in the DGF group than in IGF group after transplantation; however, statistically significant differences were not found.
In the patients transplanted from deceased donors, the levels of creatinine at POD 5 were higher when the donor's cause of death was nontraumatic, while those of NGAL and IL‐18 were not different according to the groups classified by donors’ causes of death. This might imply that an allograft from the donors who had died of an illness has a decreased function. However, our result may not have external validity because of the small sample size. In addition, the correlation test showed a weak positive relationship between CIT and other biomarkers except NGAL at POD 1 and POD 14, IL‐18 at POD 5, and creatinine at POD 1. However, statistical significance was not found. This might be also due to the small sample size of our data. Therefore, further large‐scaled study would be required to identify whether the CIT affects the levels of biomarkers or not.
We found that serum NGAL levels could predict kidney dysfunctions after transplantation. Most of the previous research evaluated the usefulness of NGAL in urine samples, and only few studies performed with serum samples. Furthermore, in these studies, serum samples were collected only on a certain single day after the transplantation. In addition, they estimated the correlation between serum levels of NGAL and other marker (creatinine, glomerular filtration rate, high‐sensitivity C‐reactive protein, etc.) in kidney transplantation recipients, but did not compare the predictive performances of NGAL in the renal insufficiency group to those in the group without renal insufficiency 18, 19. In our study, we analyzed serum NGAL levels in the two groups according to graft function after kidney transplantation. Moreover, serum samples were sequentially obtained on the preoperative day as well as POD1, POD 5, and POD 14. Serum samples can be collected more easily than urine, because patients with renal dysfunctions would manifest decreased urine output. Besides, results with urinary NGAL could be unreliable in patients with hematuria after kidney transplantation due to assay interferences by hemoglobin. According to the previous study, NGAL concentrations were highly skewed toward the higher values in the presence of hemoglobin 20.
In conclusion, serum NGAL exhibited to be an early, sensitive marker for kidney dysfunctions in renal transplantation, while serum IL‐18 and creatinine showed limited value for the prediction of renal dysfunctions.
REFERENCES
- 1. Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol Dial Transplant 2008;23:2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pratschke J, Weiss S, Neuhaus P, Pascher A. Review of nonimmunological causes for deteriorated graft function and graft loss after transplantation. Transpl Int 2008;21:512–522. [DOI] [PubMed] [Google Scholar]
- 3. Snoeijs MG, van Heurn LW, Buurman WA. Biological modulation of renal ischemia‐reperfusion injury. Curr Opin Organ Transplant 2010;15:190–199. [DOI] [PubMed] [Google Scholar]
- 4. Yarlagadda SG, Coca SG, Formica RN Jr, Poggio ED, Parikh Cr. Association between delayed graft function and allograft and patient survival: A systematic review and meta‐analysis. Nephrol Dial Transplant 2009;24:1039–1047. [DOI] [PubMed] [Google Scholar]
- 5. Ronco C, Grammaticopoulos S, Rosner M, et al. Oliguria, creatinine and other biomarkers of acute kidney injury. Contrib Nephrol 2010;164:118–127. [DOI] [PubMed] [Google Scholar]
- 6. Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: Physiological principles. Intensive Care Med 2004;30:33–37. [DOI] [PubMed] [Google Scholar]
- 7. Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: A systematic review. Kidney Int 2008;73:1008–1016. [DOI] [PubMed] [Google Scholar]
- 8. Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem 1993;268:10425–10432. [PubMed] [Google Scholar]
- 9. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase‐associated lipocalin from humans. Genomics 1997;45:17–23. [DOI] [PubMed] [Google Scholar]
- 10. Szeto CC, Kwan BC, Lai KB, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol 2010;5:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hu RH, Tsai MK, Lee PH. Serum profiles of IL‐12, ‐15, and ‐18 in renal allograft dysfunction in man. Transplant Proc 2003;35:246–248. [DOI] [PubMed] [Google Scholar]
- 12. Liu KD, Altmann C, Smits G, et al. Serum interleukin‐6 and interleukin‐8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: A case‐control study. Crit Care 2009;13:R104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Striz I, Krasna E, Honsova E, et al. Interleukin 18 (IL‐18) upregulation in acute rejection of kidney allograft. Immunol Lett 2005;99:30–35. [DOI] [PubMed] [Google Scholar]
- 14. Perico N, Cattaneo D, Sayegh MH, Remuzzi G. Delayed graft function in kidney transplantation. Lancet 2004;364:1814–1827. [DOI] [PubMed] [Google Scholar]
- 15. Hall IE, Yarlagadda SG, Coca SG, et al. IL‐18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 2010;21:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gracie JA, Robertson SE, McInnes IB. Interleukin‐18. J Leukoc Biol 2003;73:213–224. [DOI] [PubMed] [Google Scholar]
- 17. Parikh CR, Jani A, Mishra J, et al. Urine NGAL and IL‐18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant 2006;6:1639–1645. [DOI] [PubMed] [Google Scholar]
- 18. Malyszko J, Malyszko JS, Mysliwiec M. Serum neutrophil gelatinase‐associated lipocalin correlates with kidney function in renal allograft recipients. Clin Transplant 2009;23:681–686. [DOI] [PubMed] [Google Scholar]
- 19. Przybylowski P, Malyszko J, Malyszko JS. Serum neutrophil gelatinase‐associated lipocalin correlates with kidney function in heart allograft recipients. Transplant Proc 2010;42:1797–1802. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen KR, Ravn HB, Hjortdal VE, Norregaard R, Povlsen JV. Neutrophil gelatinase‐associated lipocalin (NGAL): Validation of commercially available ELISA . Scand J Clin Lab Invest 2010;70:374–382. [DOI] [PubMed] [Google Scholar]


