Key Points
Question
Is the outcome of first-line treatment of idiopathic sudden sensorineural hearing loss associated with how the corticosteroid is administered?
Findings
This systematic review and meta-analysis of 7 randomized clinical trials did not find evidence that recovery from moderate to severe hearing loss was different in patients for whom corticosteroids were administered intratympanically vs systemically. It also found no evidence that combining the 2 administration routes improved hearing recovery.
Meaning
Future research and guidelines should determine which patient groups should be allocated to receive what administration (eg, systemic corticosteroids in elderly patients may be associated with increased risk of adverse effects, and so these patients should be treated intratympanically).
Abstract
Importance
To our knowledge, evidence-based recommendations on the intratympanic vs systemic administration of corticosteroids for the treatment of idiopathic sudden sensorineural hearing loss remain unestablished, and contradictory conclusions have been reported in previous meta-analyses.
Objective
To compare recovery from idiopathic sudden sensorineural hearing loss based on systemic, intratympanic, or a combined treatment with corticosteroids as first-line treatment.
Data Sources
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. We searched PubMed, Embase, OvidSP, CINAHL, and Cochrane Library from January 1, 1966, to July 1, 2018. This study was registered in the International Prospective Register of Systematic Reviews (CRD42018109314).
Study Selection
We included randomized studies. Included studies must have excluded identifiable causes. Corticosteroids must have been administered solitarily. We excluded studies that did not define hearing loss as a minimum 30 dB within 72 hours.
Data Extraction and Synthesis
We identified 170 titles, of which 56 (32.9%) were eligible for full-text screening. We independently extracted data. We applied a fixed-effects model to investigate our objectives.
Main Outcomes and Measure
We aimed to (1) estimate the difference in mean pure tone average (PTA) gain in decibels from intratympanic treatment vs systemic treatment and (2) investigate odds ratios for recovery between the different treatment groups.
Results
We included 7 eligible studies. A total of 710 patients were allocated to receive either intratympanic treatment (IT group, 235 [33%]), systemic treatment (ST group; 325 [46%]) or combined intratympanic and systemic treatment (CB group; 150 [21%]). The PTA was measured by taking the mean of 4 frequencies: 4 studies measured at 500, 1000, 2000, and 3000 Hz and 3 studies measured at 500, 1000, 2000, and 4000 Hz. The ST group had a 2.01-dB higher PTA gain (95% CI, −5.61 dB to 1.59 dB; P = .96; I2 = 0%) compared with the IT group and the odds for achieving complete recovery was not significantly different at an odds ratio of 0.94 (95% CI, 0.61 to 1.44; P = .19; I2 = 34.5%). For the CB group vs the ST group, the odds ratio was 1.11 (95% CI, 0.68 to 1.82; P = .75; I2 = 0%). The analysis of the CB group vs IT group comprised only 2 studies.
Conclusions and Relevance
This study does not suggest that corticosteroid delivered intratympanically is more beneficial than systemic treatment in the case of moderate to severe idiopathic sudden sensorineural hearing loss. There were no indications that combined treatment was associated with improved hearing outcomes compared with either systemic or intratympanic treatment.
This systematic review and meta-analysis examines hearing outcomes for intratympanic vs systemic corticosteroids as a first-line treatment of idiopathic sudden sensorineural hearing loss.
Introduction
A sudden sensorineural hearing loss has an identifiable cause in approximately 10% to 15% of cases. Retrocochlear disorders, such as vestibular schwannoma, comprise approximately 1%, whereas the remaining identifiable cases are mostly associated with causes such as Meniére disease, autoimmune disease, or perilymphatic fistula.1
Idiopathic sudden sensorineural hearing loss (ISSNHL) constitutes up to 90% of cases and is defined by acute hearing loss developed within 72 hours with a decrease in hearing of at least 30 dB at 3 consecutive frequencies on pure-tone audiometry.2 Idiopathic sudden sensorineural hearing loss is almost exclusively unilateral and typically occurs between ages 43 to 53 years with equal sex distribution. The incidence rate has been estimated between 5 and 20 per 100 000 person-years but is most likely underestimated because many who recover quickly and spontaneously never seek medical attention.1,3,4
A widely used standard treatment of ISSNHL is a tapering course of oral corticosteroid. This practice is primarily based on a randomized, double-blind placebo-controlled study from 1980 in which Wilson et al5 demonstrated significant hearing improvement in 61% of patients treated with oral corticosteroid compared with 32% in the placebo group. Intratympanic treatment (ie, corticosteroid injected directly into the middle ear) has gained wide popularity because of the theoretical advantage of an increased drug concentration at the targeted organ and the potential benefit of reduced systemic corticosteroid exposure and associated systemic adverse effects.
We aimed to systematically review the current literature on corticosteroids as first-line treatment of ISSNHL. We identified randomized trials and sought to meta-analytically (1) estimate the difference in mean pure tone average (PTA) gain in dB from intratympanic treatment vs systemic treatment and (2) investigate odds ratios for recovery between intratympanic treatment, systemic treatment, or the combination of the 2 in first-line treatment of ISSNHL.
Methods
This systematic review and meta-analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.6 This study was registered in the International Prospective Register of Systematic Reviews (CRD42018109314). This systematic review and meta-analysis of aggregated, published data did not require approval from the institutional review board nor the ethical committee according to Danish law because it used deidentified patient data.
Study Inclusion
Included studies were randomized studies that must have excluded identifiable causes of sensorineural hearing loss, such as vestibular schwannoma or Meniére disease. Corticosteroids must have been administered exclusively and not as combination therapy (such as with antiviral medicine or hyperbaric oxygen therapy). We excluded 1 study that did not apply the diagnostic criteria of a hearing loss of a minimum 30 dB within 72 hours.7
Search Strategy and Identification of Eligible Studies
PubMed, Embase, OvidSP, CINAHL, and Cochrane Library were searched from January 1, 1966, to July 1, 2018, as follows: “sudden deafness OR ISSNHL OR sudden sensorineural hearing loss OR acute hearing loss” AND “steroid OR corticosteroid OR dexamethasone OR methylprednisolone” AND “randomised OR randomized.” We restricted the search to randomized clinical trials (either placebo controlled or not), systematic reviews, and meta-analyses. We imposed no language restrictions.
The preliminary search identified 170 publications, which were screened by title or abstract by both authors (C.M. and T.O.). A total of 56 publications (32.9%) were eligible for full-text assessment. Subsequently, the abstract of these articles were jointly read by both authors: in total, 51 studies (91.1%) were excluded because they violated the inclusion criteria, including nonrandomized studies (eg, retrospective reviews and case reports) and studies investigating combination therapy with other drugs (eg, antiviral medicine). We screened the reference lists of systematic reviews and meta-analyses. Two studies were identified and added (eFigure in the Supplement).
In total, 7 eligible articles were identified and reviewed independently by both authors. Data of interest were subsequently extracted in consensus and compiled, of which the reported PTA gain in dB from baseline to follow-up was included first. The PTA was measured by taking the mean of 4 frequencies: 4 studies measured at 500, 1000, 2000, and 3000 Hz8,9,10,11 and 3 studies measured at 500, 1000, 2000, and 4000 Hz.12,13,14 The second type of data extracted was the severity of the mean hearing loss. Based on the baseline mean PTA, data from the individual studies were allocated accordingly to the Goodman criteria of hearing loss: mild (26 dB-40 dB), moderate (41 dB-55 dB), moderate to severe (56 dB-70 dB), severe (71 dB-90 dB), and profound (≥91 dB). Third, data were included on the proportion of patients achieving complete recovery as defined within each study and in the different treatment arms.
Data Management
Data were aggregated and allocated according to the different treatment arms: patients receiving the intratympanic corticosteroid (IT group), systemic treatment (ST group); and a combination of intratympanic and systemic treatment (CB group). The odds for achieving complete recovery were computed for the IT, ST, and CB groups. Odds ratios were subsequently calculated between the groups. An assessment of risk of bias was performed in accordance with previously established Cochrane Collaboration tools.15
Statistical Analysis
The fixed-effect model, assuming that 1 true effect size underlies all included studies in the analysis, was used for effect size calculation. Heterogeneity was quantified accordingly to Higgins et al,16 with low, moderate, and high corresponding to I2 values of 25%, 50%, and 75%.16 We performed the statistical analyses in R-Studio by using the metafor package.17 Statistical significance was set at P < .05.
Results
We included 7 randomized studies that investigated the efficacy of corticosteroids as first-line treatment of ISSNHL.8,9,10,11,12,13,14 In total, 710 patients were allocated to receive either intratympanic treatment (IT group; 235 [33%]), systemic treatment (ST group; 325 [46%]), or combined intratympanic and systemic treatment (CB group; 150 [21%]). The follow-up time ranged from 17 days to 3 months but was not reported in 1 study.11 The mean time from symptom presentation to treatment initiation ranged between 3.1 to 10.1 days but was not reported in 1 study.13 The mean PTA at the time of diagnosis was equivalent to a severe hearings loss according to Goodman criteria in five studies9,10,11,12,14 and moderate to severe in the remaining two.8,13 The mean age ranged between 45.9 to 56.9 years (eTable 1 in the Supplement).
Risk of Bias Assessment
We assessed the risk of bias in each of the included studies. We present the detailed assessment in eTables 2 and 3 in the Supplement. The randomization and treatment allocation were known in all 7 studies (to the key staff and patient), yielding a high risk of bias in all 7 studies. Further associations with a high risk of bias were observed in the included studies: first, the allocation of patients was not concealed in 2 studies9,10; second, 1 study allocated patients based on a principle of rotation and alternation8; and finally, 1 study did not report the length of follow-up.11 Hence, insufficient concealment of randomization and nonmasked personnel and patients are predominant in the current literature, with the latter affecting all current publications. Similarly, there were numerous unknown risks of bias because of insufficient reporting (eTables 2 and 3 in the Supplement).
Intratympanic Treatment vs Systemic Treatment
Five studies investigated the IT group compared with the ST group,8,10,12,13,14 composed of a total of 464 patients, of whom 235 (51%) were allocated to the IT group and 229 (49%) to the ST group. According to the Goodman criteria, 3 studies had a baseline PTA equivalent to a severe hearing loss,10,12,14 whereas the remaining 2 were classified as moderate to severe hearing loss8,13 (Table 18,9,10,11,12,13,14). The mean days until treatment initiation ranged from 3.4 to 10.1 days in the IT group vs 3.1 to 7.1 days in the ST group. One study did not report days until treatment initiation.13 Follow-up ranged from 17 days to 3 months (eTable 1 in the Supplement).
Table 1. Patient Characteristics of Intratympanic Treatment vs Systemic Treatment vs Combined Treatment.
| Study | Pre-IT PTA,a Mean (SD) | IT Dosage | Hearing Gain IT, Mean (SD) | Pre-ST PTA, Mean (SD) | ST Dosage | Hearing Gain ST, Mean (SD) | Pre-CB PTA, Mean (SD) | CB Dosage | Hearing Gain CB, Mean (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Rauch et al14 | 86.4 (NA) | 40 mg × 4b | 28.7 (21.6) | 86.7 (NA) | Prednisolonec | 30.2 (21.6) | NA | NA | NA |
| Hong et al10 | 77.5 (27.6) | 1.5-2 mg × 8 | NA | 79.9 (23.5) | Prednisoloned | NA | NA | NA | NA |
| Swachia et al13 | 66.1 (24.2) | 40 mg × 4b | 18.2 (8.7) | 61.0 (22.0) | Prednisolonee | 14.7 (12.9) | NA | NA | NA |
| Gundogan et al9 | NA | 25 mg × 4b | NA | 76.3 (272) | Prednisolonef | 25.7 (19.8) | 80.7 (22.8) | + IT dosagef | 44.0 (21.5) |
| Tsounis et al12 | 81.4 (23.3) | 25-37.5 mg × 4b | 27.0 (24.3) | 81.1 (28.8) | Prednisoloneg | 29.0 (21.1) | 79.1 (25.1) | + IT dosageg | 29.8 (17.8) |
| Ahn et al11 | NA | 1.5-2 mg × 3 | NA | 70.3 (21.3) | Prednisoloneh | NA | 74.3 (27.8) | + IT dosageh | NA |
| Lim et al8 | 58.9 (31.2) | 1.5-2 mg × 4 | 12.1 (14.6) | 57.8 (28.5) | Prednisolonei | 12.8 (15.4) | 56.8 (28.3) | + IT dosagei | 21.9 (26.2) |
Abbreviations: CB, combined treatment; IT, intratympanic treatment group; NA, not available; PTA, pure tone average; ST, systemic treatment group.
PTA is calculated as the arithmetic mean of hearing thresholds in decibels at the stated audiometry frequencies in kilohertz.
Methylprednisolone and not dexamethasone.
19-day taper: 60 mg × 14, 50 mg × 1, 40 mg × 1, 30 mg × 1, 20 mg × 1, and 10 mg × 1.
8-day taper: 60 mg × 4, 40 mg × 2, and 20 mg × 2.
14-day taper: 1 mg/kg × 10, 0.5 mg/kg × 2, and 0.25 mg/kg × 2.
14-day taper: participants received 1 mg/kg and a 10 mg taper every 3 days.
17-day taper: 1 mg/kg × 7, 0.5 mg/kg × 3, 32 mg × 4, and 16 mg × 3.
14-day taper: participants received 48 mg prednisolone for 9 days, followed by tapering for 5 days.
10-day taper: 60 mg × 5, 40 mg × 2, 20 mg × 2, and 10 mg × 1.
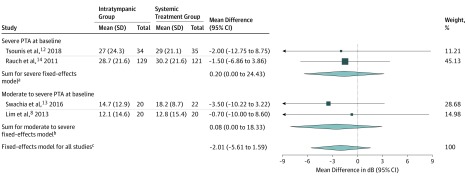
Four studies were eligible for analysis of differences in mean PTA gain.8,12,13,14 The fixed-effects model estimated no significant difference in mean PTA gain between the 2 groups (−2.01 dB; 95% CI, −5.61 dB to 1.59 dB; P = .96; I2 = 0%; χ2 P = .62). The model indicated no significant heterogeneity (Figure 18,12,13,14).
Figure 1. Mean Hearing Gain in Decibels (dB) From Baseline (Pretreatment) Pure Tone Average (PTA) Compared With PTA at Follow-up (Between 1 to 3 Months).
The forest plot illustrates the mean difference, which is the PTA mean gain in dB of intratympanic therapy subtracted from the PTA mean gain in dB of systemic therapy. Total indicates the number of patients.
aQ1 = 0.01; P = .93; I2 = 0%.
bQ1 = 0.23; P = .63; I2 = 0%.
cQ3 = 0.30; P = .96; I2 = 0%.
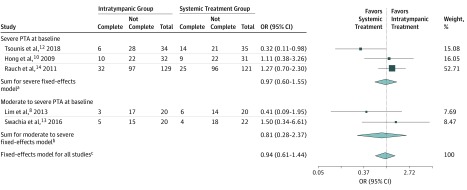
For the IT group, complete recovery was achieved in 56 of 235 patients (24%), and for the ST group, complete recovery was achieved in 58 of 229 patients (25%) (Table 22,8,9,10,11,12,13,14,18). Figure 28,10,12,13,14 comprises odds for recovery stratified for baseline PTA; first, moderate to severe hearing loss, which indicated no significant difference, and second, severe hearing loss, which indicated no significant difference. The heterogeneity was moderate but not significant.
Table 2. Definitions of Recovery Used in the Different Studies and Recovery Rates of Intratympanic vs Systemic vs Combined Treatment.
| Source | Definition of Recovery | Goodman Criteria | No. of Patients | Complete Recovery, Events/Total Group Size, No./Total No. (%) | ||||
|---|---|---|---|---|---|---|---|---|
| IT | ST | CB | IT Group | ST Group | CB Group | |||
| Rauch et al14 | PTA <30 dB: hearing recovery to normal 30 dB to 90 dB: hearing aid range |
Severe | 129 | 121 | NA | 32/129 (25) | 25/121 (21) | NA |
| Swachia et al13 | From Furuhashi et al18 complete recovery: PTA ≤25dB Marked recovery: PTA improvement >30 dB Slight recovery: 10 dB< PTA improvement <30 dB No recovery: PTA improvement <10 dB |
Moderate to severe | 20 | 22 | NA | 5/20 (25) | 4/22 (18) | NA |
| Hong et al10 | Siegel criteria Complete recovery: final hearing better than 25 dB Partial recovery: >15 dB gain and final hearing 25-45 dB Slight improvement: >15 dB gain and final hearing poorer than 45 dB No improvement: <15 dB gain and final hearing poorer than 75 dB |
Severe | 32 | 31 | NA | 10/32 (31) | 9/31 (29) | NA |
| Gundogan et al9 | Siegel criteria Complete recovery: final hearing better than 25 dB Partial recovery: >15 dB gain and final hearing 25-45 dB Slight improvement: >15 dB gain and final hearing poorer than 45 dB No improvement: <15 dB gain and final hearing poorer than 75 dB |
Severe | 36 | 37 | NA | 10/36 (28) | 14/37 (38) | |
| Tsounis et al12 | Siegel criteria | Severe | 34 | 35 | 33 | 6/34 (18) | 14/35 (40) | 12/33 (36) |
| Ahn et al11 | Siegel criteria | Severe | 60 | 60 | NA | 16/60 (27) | 15/60 (25) | |
| Lim et al8 | Defined accordingly to the AAO-HNS2 Complete recovery: Within 10 dB of the unaffected PTA |
Moderate to severe | 20 | 20 | 20 | 3/20 (15) | 6/20 (30) | 8/20 (40) |
Abbreviations: AAO-HNS, American Academy of Otolaryngology–Head and Neck Surgery; CB, combined treatment; IT, intratympanic treatment group; NA, not available; PTA, pure tone average; ST, systemic treatment group.
Figure 2. Odds for Complete Recovery When Treated With Intratympanic Corticosteroids vs Systemic Corticosteroids.
Complete indicates the proportion of patients achieving complete recovery as defined within the individual study. Not complete indicates the proportion of patients not achieving complete recovery. Total indicates number of patients in the study. OR indicates odds ratio; PTA, pure tone average.
aQ2 = 4.63; P = .10; I2 = 56.8%.
bQ1 = 1.39; P = .24; I2 = 28.0%.
cQ4 = 6.11; P = .19; I2 = 34.5%.
For the entire cohort comprising all 5 studies, the fixed-effects model estimate indicated no significant difference between the 2 groups, with an odds ratio of 0.94 (95% CI, 0.61 to 1.44). The heterogeneity was low and not significant (I2 = 34.5%; χ2 P = .19; Figure 2).
Combined Treatment vs Systemic Treatment
Four studies investigated the CB vs ST group.8,9,11,12 In total, the studies comprised 301 patients, of whom 150 (49.8%) were randomly allocated to the CB group and the remaining 151 patients (50.2%) randomized to the ST group. The definition of complete recovery varied between studies and is listed in Table 2. All studies had a mean PTA at baseline corresponding to severe hearing loss according to Goodman criteria except for 1 study that corresponded to moderate to severe hearings loss8 (Table 1). The mean days until treatment initiation ranged between 4.0 to 9.6 days in the CB group and 3.1 and 7.1 days in the ST group. Follow-up ranged from 17 days to 3 months. One study did not report the duration of follow-up11 (eTable 1 in the Supplement).
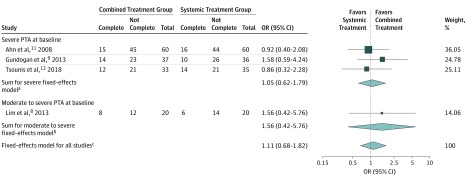
For the CB group, complete recovery was achieved in 49 of 150 patients (33%), and for the ST group, complete recovery was achieved in 46 of 151 patients (30%) (Table 2). The fixed-effects model estimated no significant difference. The odds ratio was 1.11 (95% CI, 0.68 to 1.82). The heterogeneity was low and not significant (I2 = 0%; χ2 P = 0.75) (Figure 3).
Figure 3. Odds for Complete Recovery Between Combined Intratympanics (Dexamethasone or Methylprednisolone) and Systemic Corticosteroids vs Systemic Corticosteroids.
Complete indicates the proportion of patients achieving complete recovery as defined within the individual study. Not complete indicates the proportion of patients not achieving complete recovery. Total indicates number of patients in the study. OR indicates odds ratio; PTA, pure tone average.
aQ2 = 0.93; P = .63; I2 = 0%.
bQ0 = 0; P > .99; I2 = 0%.
cQ3 = 1.23; P = .75; I2 = 0%.
Combined Treatment vs Intratympanic Treatment
Two studies investigated the CB group compared with the IT group,8,12 comprising a total of 107 eligible patients. The CB group consisted of 53 patients (50%), whereas the IT group consisted of 54 patients (50%). Neither of the 2 studies solitarily indicated any significant difference between the treatments; for severe hearing loss, the odds ratio was 2.7 (95% CI, 0.86 to 8.27), and for the moderate to severe hearing loss, the odds ratio was 3.78 (95% CI, 0.83 to 17.25) (eFigure in the Supplement).
Discussion
This meta-analysis did not indicate any differences in hearing outcomes for intratympanic vs systemic corticosteroids in a first-line treatment of ISSNHL equivalent of a moderate to severe hearing loss. This was derived from several observations: first, there was no mean difference in average PTA gain between the IT and ST groups based on 4 studies, 8,12,13,14 and second, there was no difference in the odds for recovery between the IT and ST groups based on 5 studies.8,10,12,13,14
Comparison With Existing Literature
In the investigation of the IT group vs the ST group, a meta-analysis applied a random-effects model to estimate a nonsignificant mean difference between the 2 groups.19 We were surprised to learn that specific data apparently used from 2 of the included studies did not appear in the original articles.7,20 Further, the meta-analysis included Kosyakov et al,21 who report baseline PTA characteristics as a mean (SD) 41.0 (12.87) dB and 37.1(16.67) dB, respectively. This indicates that an unknown proportion of the included patients are not confined within the ISSNHL-definitory criteria of a minimum 30-dB hearing loss.21
Another meta-analysis applied a fixed-effects model to estimate the effect sizes of the IT and ST group.22 The mean difference in dB was reported significantly as 3.42 dB (95% CI, 0.17-6.67 dB; I2 = 37%; P = .04) in favor for the IT group. However, we are concerned of the validity of these results. We observed that data from the Kosyakov et al19 were included and used equivalently to the beforementioned meta-analysis. Similarly, the meta-analysis included results from the Battaglia et al7 study; however, this specific study defined ISSNHL as a hearing loss of a minimum of 20 dB and not 30 dB. The meta-analysis stated to have included randomized trials exclusively. Therefore, we were surprised to learn that the included study of Filipo et al23 was clearly stated as nonrandomized. These observations might highlight some perspectives as to why findings remain contradictory and evidence-based recommendations have not yet been established.
One randomized study concluded that patients in the CB group had a significantly higher likelihood of hearing recovery compared with the ST group.7 However, in addition to defining ISSNHL as a hearing loss of a minimum 20 dB, the mean days to treatment was 4 days in the CB group vs 11 days in in the ST group. Acknowledging time-to-treatment initiation as an important prognostic parameter, this baseline discrepancy is highly associated with treatment outcomes and could explain the results.24
Five randomized trials met the inclusion criteria but were not included for analysis.7,20,25,26,27 First, the previously mentioned study from Battaglia et al7 defined ISSNHL as a hearing loss of a minimum 20 dB. This conflicts with the international consensus that ISSNHL is defined as a hearing loss of minimum 30 dB. Hence, this implies heterogeneity and we consequently excluded the article.
Further, none of the remaining 4 studies reported the PTA mean gain in dB ± the standard deviation. Three of the four studies defined complete recovery as a gain of a minimum 10 dB in PTA,20,25,26 whereas the remaining defined complete recovery as a gain of a minimum 15 dB.27 Thus, recovery rates were not graduated but dichotomous in association with whether complete recovery was achieved. Consequently, if we included the 4 studies in this meta-analysis, we would have had to define a gain of 10 dB as criteria for achieving complete recovery. Therefore, we included studies with graduated recovery exclusively.
Association With Future Clinical Practice and Research
The International Consensus (ICON) on treating sudden sensorineural hearing loss is a product of a panel discussion at the International Federation of Otorhinolaryngological Societies 2017 ENT World Congress. The ICON Study agreed that the main limitations present in the current literature are associated with the wide heterogeneity, which characterizes the initial hearing deficit and the amount of hearing recovery. Systemic steroids are considered as the current standard care; however, the evidence is debatable. The ICON Study proposes that the number of participants for future studies should be restricted to moderate to profound levels of hearing loss to improve the comparability.28 This meta-analysis qualitatively adds to this consensus.
Strengths and Limitations
One major strength of meta-analyses of randomized studies is the reduced risk of bias. However, in general, the included studies did not provide a placebo: patients allocated to receive oral treatment should concomitantly be given intratympanic placebo (and vice versa). When a placebo was not administered, randomization was not masked to the personnel nor the patient. Thus, we associated all included studies with a high risk of bias on masking and it cannot be denied that the presented results were biased. We encountered other limitations too: first, the individual studies applied different guidelines in evaluating hearing recovery. Complete recovery was defined as a final PTA of at least 25 dB in 3 studies,10,12,13 at least 30 dB in 1 study14 and, finally, as a PTA within 10 dB from the unaffected ear in 1 study.8 The definitions of complete recovery were fairly comparable and similar; however, we could not adjust for the heterogeneity implied within the use of different assessment schemes, nor the difference in baseline prognostic factors,29 including (1) time to treatment onset, (2) age at baseline, and (3) PTA at baseline. We observed that the mean time to treatment onset varied no more than 7 days between the studies (ST group range, 3.1-7.1 days; IT group, 3.4-10.1 days; CB group, 4.0-9.6 days). We could not quantify the association of this difference with the results, but it has been estimated that the treatment window is between 2 to 4 weeks from the onset of symptoms.24,30 As the maximum duration between the presentation of symptoms to treatment was 10.1 days, we do not consider this aspect a limitation of significance. We observed that the mean age range at the time of symptom presentation ranged between 45.9 to 56.9 years. Older age is often described as a prognostic factor,24,31 although older age is not defined. Typically, the age described is presented as categories in the literature (eg, 20-29, 30-39) exclusively and is not treated as a continuous covariate. Thus, it is difficult to assess whether the presented mean ages of the included studies has inherent bias; however, we have observed that the older ages described in literature comprise the age categories 70 to 79 years and 80 to 89years.24,31 Therefore, we do not consider the varying mean ages as a limitation of significance, as we do not consider the age interval of 45.9 to 56.9 years to be older ages nor is presbycusis a relevant parameter for patients in this age interval. Finally, we do not consider the different PTA at baseline a limitation of significance, as we allocated studies accordingly to mean PTA at baseline and performed our analyses based on the stratification according to Goodman criteria.32 We observed that follow-up in the included studies ranged from 17 days to 3 months. We cannot adjust for the inherent heterogeneity of varying follow-up and this must be considered a limitation for arriving at concise recommendations. Finally, there is the effect of applying different corticosteroids. There is also an emerging question on whether differences in hearing outcomes exist between dexamethasone vs methylprednisolone. The literature is sparse concerning this question, but it has been reported that no difference exists between the 2 drugs in association with intratympanic administration.33 Based on this, we do not intuitively consider the application of different corticosteroids to be a significant limitation; however, future randomized or noninferiority trials should settle this question.
Conclusions
We evaluated by meta-analysis the association between different administration routes of corticosteroids as first-line treatment of 710 patients with a diagnosis of ISSNHL. There was no difference in therapeutic outcomes between the IT, ST, and CB groups for patients with moderate to severe hearing loss.
eTable 1. Study and patient characteristics
eTable 2. Risk of bias in the included studies investigating the effect of systemic or intratympanic treatment with corticosteroids as first-line therapy of ISSNHL
eTable 3. Risk of bias in the included studies investigating the effect of systemic or intratympanic or combined treatment with corticosteroids as first-line therapy of ISSNHL
eFigure. Study inclusion parameters
References
- 1.Rauch SD. Clinical practice: idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359(8):833-840. doi: 10.1056/NEJMcp0802129 [DOI] [PubMed] [Google Scholar]
- 2.Stachler RJ, Chandrasekhar SS, Archer SM, et al. ; American Academy of Otolaryngology-Head and Neck Surgery . Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146(3)(suppl):S1-S35. doi: 10.1177/0194599812436449 [DOI] [PubMed] [Google Scholar]
- 3.Byl FMJ., Jr Sudden hearing loss: eight years’ experience and suggested prognostic table. Laryngoscope. 1984;94(5 Pt 1):647-661. doi: 10.1288/00005537-198405000-00014 [DOI] [PubMed] [Google Scholar]
- 4.Simmons FB. Sudden idiopathic sensori-neural hearing loss: some observations. Laryngoscope. 1973;83(8):1221-1227. doi: 10.1288/00005537-197308000-00005 [DOI] [PubMed] [Google Scholar]
- 5.Wilson WR, Byl FM, Laird N. The efficacy of steroids in the treatment of idiopathic sudden hearing loss: a double-blind clinical study. Arch Otolaryngol. 1980;106(12):772-776. doi: 10.1001/archotol.1980.00790360050013 [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battaglia A, Burchette R, Cueva R. Combination therapy (intratympanic dexamethasone + high-dose prednisone taper) for the treatment of idiopathic sudden sensorineural hearing loss. Otol Neurotol. 2008;29(4):453-460. doi: 10.1097/MAO.0b013e318168da7a [DOI] [PubMed] [Google Scholar]
- 8.Lim HJ, Kim YT, Choi SJ, et al. . Efficacy of 3 different steroid treatments for sudden sensorineural hearing loss: a prospective, randomized trial. Otolaryngol Head Neck Surg. 2013;148(1):121-127. doi: 10.1177/0194599812464475 [DOI] [PubMed] [Google Scholar]
- 9.Gundogan O, Pinar E, Imre A, Ozturkcan S, Cokmez O, Yigiter AC. Therapeutic efficacy of the combination of intratympanic methylprednisolone and oral steroid for idiopathic sudden deafness. Otolaryngol Head Neck Surg. 2013;149(5):753-758. doi: 10.1177/0194599813500754 [DOI] [PubMed] [Google Scholar]
- 10.Hong SM, Park CH, Lee JH. Hearing outcomes of daily intratympanic dexamethasone alone as a primary treatment modality for ISSHL. Otolaryngol Head Neck Surg. 2009;141(5):579-583. doi: 10.1016/j.otohns.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 11.Ahn JH, Yoo MH, Yoon TH, Chung JW. Can intratympanic dexamethasone added to systemic steroids improve hearing outcome in patients with sudden deafness? Laryngoscope. 2008;118(2):279-282. doi: 10.1097/MLG.0b013e3181585428 [DOI] [PubMed] [Google Scholar]
- 12.Tsounis M, Psillas G, Tsalighopoulos M, Vital V, Maroudias N, Markou K. Systemic, intratympanic and combined administration of steroids for sudden hearing loss: a prospective randomized multicenter trial. Eur Arch Otorhinolaryngol. 2018;275(1):103-110. doi: 10.1007/s00405-017-4803-5 [DOI] [PubMed] [Google Scholar]
- 13.Swachia K, Sharma D, Singh J. Efficacy of oral vs. intratympanic corticosteroids in sudden sensorineural hearing loss. J Basic Clin Physiol Pharmacol. 2016;27(4):371-377. doi: 10.1515/jbcpp-2015-0112 [DOI] [PubMed] [Google Scholar]
- 14.Rauch SD, Halpin CF, Antonelli PJ, et al. . Oral vs intratympanic corticosteroid therapy for idiopathic sudden sensorineural hearing loss: a randomized trial. JAMA. 2011;305(20):2071-2079. doi: 10.1001/jama.2011.679 [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 18.Furuhashi A, Matsuda K, Asahi K, Nakashima T. Sudden deafness: long-term follow-up and recurrence. Clin Otolaryngol Allied Sci. 2002;27(6):458-463. doi: 10.1046/j.1365-2273.2002.00612.x [DOI] [PubMed] [Google Scholar]
- 19.Lai D, Zhao F, Jalal N, Zheng Y. Intratympanic glucocorticosteroid therapy for idiopathic sudden hearing loss: meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96(50):e8955. doi: 10.1097/MD.0000000000008955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dispenza F, Amodio E, De Stefano A, et al. . Treatment of sudden sensorineural hearing loss with transtympanic injection of steroids as single therapy: a randomized clinical study. Eur Arch Otorhinolaryngol. 2011;268(9):1273-1278. doi: 10.1007/s00405-011-1523-0 [DOI] [PubMed] [Google Scholar]
- 21.Kosyakov S, Atanesyan A, Gunenkov A, Ashkhatunyan E, Kurlova A. Intratympanic steroids for sudden sensorineural hearing loss. J Int Adv Otol. 2011;7:323-332. https://www.advancedotology.org/en/intratympanic-steroids-for-sudden-sensorineural-hearing-loss-161247. Accessed August 1, 2018. [Google Scholar]
- 22.Qiang Q, Wu X, Yang T, Yang C, Sun H. A comparison between systemic and intratympanic steroid therapies as initial therapy for idiopathic sudden sensorineural hearing loss: a meta-analysis. Acta Otolaryngol. 2017;137(6):598-605. doi: 10.1080/00016489.2016.1260157 [DOI] [PubMed] [Google Scholar]
- 23.Filipo R, Attanasio G, Russo FY, et al. . Oral versus short-term intratympanic prednisolone therapy for idiopathic sudden hearing loss. Audiol Neurootol. 2014;19(4):225-233. doi: 10.1159/000360069 [DOI] [PubMed] [Google Scholar]
- 24.Hara JH, Zhang JA, Gandhi KR, et al. . Oral and intratympanic steroid therapy for idiopathic sudden sensorineural hearing loss. Laryngoscope Investig Otolaryngol. 2018;3(2):73-77. doi: 10.1002/lio2.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arslan N, Oğuz H, Demirci M, et al. . Combined intratympanic and systemic use of steroids for idiopathic sudden sensorineural hearing loss.Otol Neurotol. 2011;32(3):393-397. doi: 10.1097/MAO.0b013e318206fdfa [DOI] [PubMed] [Google Scholar]
- 26.Koltsidopoulos P, Bibas A, Sismanis A, Tzonou A, Seggas I. Intratympanic and systemic steroids for sudden hearing loss.Otol Neurotol. 2013;34(4):771-776. doi: 10.1097/MAO.0b013e31828bb567 [DOI] [PubMed] [Google Scholar]
- 27.Arastou S, Tajedini A, Borghei P. Combined intratympanic and systemic steroid therapy for poor-prognosis sudden sensorineural hearing loss. Iran J Otorhinolaryngol. 2013;25(70):23-28. [PMC free article] [PubMed] [Google Scholar]
- 28.Marx M, Younes E, Chandrasekhar SS, et al. . International consensus (ICON) on treatment of sudden sensorineural hearing loss. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(1S):S23-S28. doi: 10.1016/j.anorl.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 29.Kang WS, Yang CJ, Shim M, et al. . Prognostic factors for recovery from sudden sensorineural hearing loss: a retrospective study. J Audiol Otol. 2017;21(1):9-15. doi: 10.7874/jao.2017.21.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mattox DE, Simmons FB. Natural history of sudden sensorineural hearing loss. Ann Otol Rhinol Laryngol. 1977;86(4 Pt 1):463-480. doi: 10.1177/000348947708600406 [DOI] [PubMed] [Google Scholar]
- 31.Nosrati-Zarenoe R, Arlinger S, Hultcrantz E. Idiopathic sudden sensorineural hearing loss: results drawn from the Swedish national database. Acta Otolaryngol. 2007;127(11):1168-1175. doi: 10.1080/00016480701242477 [DOI] [PubMed] [Google Scholar]
- 32.Anyah A, Mistry D, Kevern E, Markiewicz K. Idiopathic sudden sensorineural hearing loss: average time elapsed before presentation to the otolaryngologist and effectiveness of oral and/or intratympanic steroids in late presentations. Cureus. 2017;9(12):e1945-e1945. doi: 10.7759/cureus.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarkan Ö, Dağkıran M, Sürmelioğlu Ö, et al. . Intratympanic methylprednisolone versus dexamethasone for the primary treatment of idiopathic sudden sensorineural hearing loss. J Int Adv Otol. 2018;14(3):451-455. doi: 10.5152/iao.2018.4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Study and patient characteristics
eTable 2. Risk of bias in the included studies investigating the effect of systemic or intratympanic treatment with corticosteroids as first-line therapy of ISSNHL
eTable 3. Risk of bias in the included studies investigating the effect of systemic or intratympanic or combined treatment with corticosteroids as first-line therapy of ISSNHL
eFigure. Study inclusion parameters