Abstract
Background
Monitoring allograft function during the early stages is crucial, and therefore requires biomarkers more sensitive than serum creatinine (Scr). Kidney injury molecular-1 (KIM-1) is a potent biomarker; however, disparities exist in the literature concerning its predictive value in allograft function. Therefore, this study aimed to evaluate its predictive value for the long-term prognosis of kidney transplantation patients.
Methods
A prospective study with a cohort comprising 160 patients scheduled for kidney transplantation was conducted to evaluate the predictive power of urinary KIM-1 (uKIM-1) and other renal ischemia-reperfusion biomarkers including urinary L-type fatty acid binding protein (uL-FABP), urinary N-acetyl-β-D glucosaminidase (uNAG), and urinary neutrophil gelatinase-related lipoprotein (uNGAL) for allograft prognosis.
Results
One hundred and forty kidney recipients who were admitted to our hospital between September 2014 and December 2017 with a median follow-up of 30.3 months were included. Thirty-seven recipients had functional delayed graft function (fDGF) in the first week post transplantation, and 42 recipients had progressed to allograft dysfunction [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] by the end of the study, while nine recipients deteriorated into allograft loss (defined by the initiation of dialysis). The levels of uKIM-1 in the fDGF group were higher than those in the immediate graft function (IGF) recipients (P<0.05) at 0 hour post transplantation [5.885 (4.420–7.913) vs. 4.605 (3.417–5.653) ng/mmol], and on the first day post transplantation [5.569 (4.181–6.722) vs. 4.002 (3.222–6.488) ng/mmol]. The levels of uL-FABP in the fDGF group were also higher than those in the IGF group at 0 hour post transplantation (89.818±39.332 vs. 69.187±37.926 µg/mmol) and on the third day post transplantation [77.835 (60.368–100.678) vs. 66.841 (28.815–89.783) µg/mmol]. Multivariate Cox regression analysis demonstrated that recipients with higher uKIM-1 levels on the first day post transplantation had a 23.5% increase in the risk of developing fDGF and a 27.3% increase in the risk of prolonged renal allograft dysfunction.
Conclusions
uKIM-1 on the first day post transplantation can predict short-term graft function and is a potent biomarker for the long-term prognosis of graft function.
Keywords: Biomarker, kidney transplantation, delayed graft function (DGF), predictive value, graft survival
Introduction
Despite surgical techniques and immunotherapy having progressed rapidly in recent years, the incidence of delayed graft function (DGF) and the occurrence of long-term adverse events after kidney transplantation are still increasing (1,2). One of the reasons for this is the expansion of the donor pool through donation after cardiac death (DCD) in the last few decades, accompanied by the increased incidences of DGF and long-term graft loss caused by ischemia-reperfusion injury (3,4). Elderly donors, prolonged cold ischemia time, and recipient characteristics [including age, human leukocyte antigen (HLA) matches, panel-reactive antibody (PRA) percentages] have also been reported to be factors affecting the incidences of DGF and long-term graft survival (2,5). Thus, monitoring allograft function at an early stage is of paramount importance. The two clinical indicators that currently exist are not adequate for this purpose (6); serum creatinine (Scr) shows inadequate sensitivity, while obtaining surveillance allograft biopsy is invasive. Therefore, there is growing interest in exploring blood and urine biomarkers for predicting the prognosis of kidney transplantation patients.
To predict both short- and long-term prognosis of patients with renal transplantation, non-invasive and diagnostic urinary or serum biomarkers are needed for early detection. The existing method for assessing allograft function requires invasive renal puncture, which can cause hemorrhage and other complications. Studies focused on kidney tubular injury-related proteins, namely neutrophil gelatinase-related lipoprotein (NGAL), kidney injury molecule-1 (KIM-1), and interleukin-18 (IL-18), as potential markers for monitoring DGF are under investigation (6-8). Nevertheless, these biomarker candidates still lack clinical validation or positive predictive value for long-term allograft function (7). Moreover, NGAL and IL-18 are also produced by immune cells during urinary tract infections and sepsis. The confounding effect of infections or sepsis on the predictive value of NGAL and IL-18 for allograft prognosis has not been clarified in those studies.
KIM-1 is a transmembrane immunoglobulin that is not detectable in healthy kidneys. It is dramatically upregulated in damaged tubular epithelial cells in multiple types of kidney injury. Disparities exist within the literature on the predictive value of KIM-1 in allograft function. For instance, in a cohort study of 145 renal transplantation recipients, KIM-1 was reported to be a potent predictor of transplantation failure (9); however, another study found that KIM-1 could not effectively predict DGF (10).
Therefore, to determine the predictive value of KIM-1 for both the short-term and long-term prognosis of kidney transplantation, we conducted a cohort study of renal transplantation recipients. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-2215a).
Methods
Study subjects
This study was a prospective, single-center cohort study from September 2014 to December 2017. The inclusion criteria were as follows: (I) end-stage renal disease (ESRD) patients aged 18–65 years old who were scheduled to receive kidney transplantation through DCD in the hospital within 3 days who (II) had an HLA mismatch (ABDR) at no more than 3 sites, and (III) had received anti-thymocyte globulin (ATG) as induction therapy, and tacrolimus with mycophenolate mofetil as the maintenance immunosuppression regimen. The exclusion criteria were as follows: (I) recipients who had received transplantation before or who had been infected with human immunodeficiency virus (HIV) or the hepatitis C virus; (II) recipients who were infected or had sepsis during follow-up.
The patients were followed up every week within the first month post transplantation, every month within the first year post transplantation, and every 3 months afterwards. Each enrolled patient had been followed up for at least 1 year. With reference to Boom et al. (11), functional delayed graft function (fDGF) was defined as a recovery of Scr of <70% in the first week after transplantation, regardless of dialysis. Conversely, immediate graft function (IGF) was characterized by a recovery of Scr of >70% in the first week after transplantation (10,12). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (14140903200). All patients provided informed consent.
Endpoint definitions
The primary endpoint for the analysis was allograft loss (marked by the initiation of dialysis). Time to event was calculated from enrollment to either allograft loss or the end of the study (13). The secondary endpoint was graft dysfunction [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2].
Sampling
Fresh blood samples were collected from all patients within the 1 week before transplantation; specifically, immediately after the perfusion of the donor kidney (0 hour), and at 24 and 72 hours after transplantation. Urine samples were obtained before transplantation if the patient was able to urinate, and collected from the bag and catheter of each patient after the perfusion of the donor kidney during transplantation, and at 24 and 72 hours after transplantation. All samples were submitted to the biochemical laboratory for analysis shortly after collection. The rate of centrifugation was 3,500 ×g for 15 min for fresh urine (10 mL) and serum (500 µL). The supernatant was then transferred to Eppendorf tubes and stored at −80 °C until further analysis.
Biomarker measurement
The levels of KIM-1, L-type fatty acid binding protein (L-FABP), NGAL, and N-acetyl-β-D glucosaminidase (NAG) in the patients’ serum and urine were examined using enzyme-linked immunosorbent assay (ELISA) kits from American R&D Corporation (Minneapolis, MN, USA). A standard curve was generated to calculate the concentration of the samples. The results were corrected through synchronous normalization to urinary creatinine (uCr), which was measured by sarcosine oxidase method (14,15).
Other measurements
Patient characteristics assessed at enrollment included demographic features (age and sex), physical examination findings [body mass index (BMI)], medical history (hypertension and diabetes mellitus), and transplantation features (duration of dialysis and mode of dialysis before transplantation). Routine pathology tests were also performed 1 day before transplantation, and included the following markers: Scr, hemoglobin, serum albumin, serum globulin, white blood cells, serum potassium, serum sodium, serum bicarbonate, pre-albumin, glutamate pyruvic transaminase, aspartate aminotransferase, γ-glutamyl transpeptidase, alkaline phosphatase, serum total cholesterol, serum triglyceride, serum high density lipoprotein, serum low-density lipoprotein, serum calcium ion, serum phosphorus, and immunoreactive serum parathyroid hormone.
Scr was measured by sarcosine oxidase method pre-transplantation, and at 0 hour, 1, 3, 5, 7, 10, 14, 21 days, every 1 month within the first year post transplantation, and every 3 months thereafter (4). eGFR was calculated using the modification of diet in renal disease (MDRD) formula: eGFR =186 × (Scr/88.4)−1.154 × age−0.203 (×0.742, female) (16,17). BMI was calculated by weight (kg)/height (m)2. Medical history was self-reported at admission.
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics (version 25, SPSS Inc., Chicago, IL, USA), GraphPad Prism (version 8.0, GraphPad Software, San Diego, CA, USA), and survival package in R, version 6.0.1. Before analysis, all data were tested for normal distribution. Student’s t-test was applied to parametric continuous variables, the Mann-Whitney U test was performed for non-parametric data, and a chi-squared test was used for categorical variables. Comparisons between multiple groups were performed using analysis of variance (ANOVA) for parametric variables and the Kruskal-Wallis test for nonparametric variables. Kaplan-Meier survival analysis was carried out to compare allograft survival between the fDGF and IGF groups, together with the log-rank test for calculation. Receiver operating characteristic (ROC) analysis was performed to determine the biomarker thresholds that were best associated with fDGF development, and Spearman’s correlation analysis was used to measure the degree of association between variables. Univariate and multivariate linear regression analyses were performed to examine the relationships between fDGF and independent variables. Univariate and multivariate Cox regression analyses were performed to assess the hazard ratios (HRs) and 95% confidence intervals (CIs). For all tests, statistical significance was considered as a two-sided P value of ≤0.05. Normally distributed data were expressed as mean ± standard deviation (SD), non-normally distributed data were expressed as median (interquartile range), and categorical data were expressed as frequencies (percentages).
Results
Patient characteristics
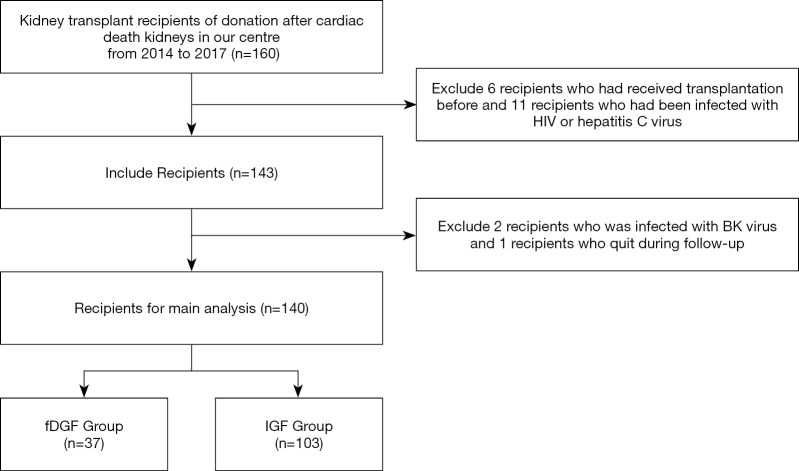
A total of 160 kidney transplantation recipients through DCD were included in this study between September 2014 to December 2017. A total of 17 transplant recipients who had HIV or hepatitis C virus infection were excluded. Subsequently, 143 patients were followed up for a median duration of 30.3 months (IQR, 9.18–44.25 months) after transplantation. Three recipients were excluded during follow-up due to BK virus infection or loss to follow-up. Finally, a total of 140 patients were enrolled for the final analysis (Figure 1).
Figure 1.
Flow diagram of the study design. fDGF, functional delayed graft function; IGF, immediate graft function.
At baseline, the median age of the 140 enrolled recipients was 41.0 years old (32.0–50.0 years old). A total of 37 recipients progressed to fDGF during the first week after transplantation; 7 of these fDGF cases were elicited by allograft rejection and another 3 by surgical reasons. A total of 3 recipients in the IGF group had allograft rejection during the follow-up after the first week post transplantation. There were no statistically significant differences between fDGF and IGF recipients in terms of HLA mismatches, hot and cold ischemia time, creatinine clearance rate, donor age, or medical history before transplantation (Table 1 & Tables S1-S3).
Table 1. Characteristics of transplant recipients at baseline.
| Characteristics | Total (n=140) | fDGF (n=37) | IGF (n=103) | P value |
|---|---|---|---|---|
| Age (years) | 41.0 (32.0–50.0) | 40.0 (31.5–48.5) | 42.0 (32.0–50.0) | 0.607 |
| Sex male, n (%) | 85 (60.7) | 18 (48.6) | 67 (65.0) | 0.080 |
| BMI (kg/m2) | 21.35 (19.24–23.91) | 22.86 (19.29–25.40) | 21.88 (19.89–25.31) | 0.978 |
| Pre-Scr (μmol/L) | 880.20 (690–1172.25) | 904.00 (739.15–1,165.00) | 874.00 (685.00–1,175.00) | 0.789 |
| Pre-eGFR (mL/min/1.73 m2) preoperative | 5.207 (4.025–7.154) | 4.797 (3.737–6.885) | 5.349 (4.112–7.272) | 0.345 |
| Hb (g/L) | 108.00 (96.50–120.00) | 113.00 (99.50–126.00) | 110.00 (99.00–125.00) | 0.355 |
| Alb (g/L) | 46.00 (42.00–48.80) | 44.75 (41.73–48.28) | 47.90 (44.80–51.00) | 0.077 |
| TC (mmol/L) | 1.54 (1.09–3.06) | 1.54 (1.00–3.21) | 1.62 (1.30–2.93) | 0.494 |
| TG (mmol/L) | 4.55 (3.55–5.28) | 4.62 (3.95–5.08) | 4.70 (4.00–5.48) | 0.637 |
| HDL (mmol/L) | 1.13 (0.87–1.55) | 1.07 (0.90–1.78) | 1.05 (0.82–1.31) | 0.485 |
| LDL (mmol/L) | 2.37 (1.93–3.14) | 2.35 (1.93–2.75) | 2.47 (2.02–3.22) | 0.332 |
| Ca2+ (mmol/L) | 2.36 (2.23–2.51) | 2.36 (2.26–2.50) | 2.36 (2.22–2.51) | 0.650 |
| P (mmol/L) | 1.79 (1.31–2.27) | 2.09 (1.41–2.55) | 1.74 (1.27–2.35) | 0.779 |
| iPTH (pg/L) | 206.45 (90.10–412.43) | 165.75 (110.88–320.83) | 228.70 (105.10–552.00) | 0.716 |
| Duration time of dialysis (m) | 10.00 (3.00–24.00) | 12.00 (5.50–31.00) | 10.00 (2.00–24.00) | 0.155 |
| Mode of dialysis, n (%) | 0.069 | |||
| HD | 73 (52.1) | 19 (51.4) | 57 (52.4) | |
| PD | 42 (30.0) | 16 (43.2) | 29 (28.2) | |
| Hypertension, n (%) | 106 (75.7) | 32 (86.5) | 74 (71.8) | 0.075 |
| Diabetes, n (%) | 7 (5.0) | 1 (2.7) | 6 (5.8) | 0.455 |
| Neutrophil (%) | 68.80 (63.65–73.40) | 69.20 (67.00–74.30) | 67.60 (63.40–73.50) | 0.121 |
| Lymphocyte (%) | 21.40 (17.85–26.05) | 20.50 (15.00–24.50) | 23.00 (18.20–27.10) | 0.079 |
| WBC (×109/L) | 7.29 (6.09–8.24) | 7.56 (5.91–8.76) | 7.18 (6.00–8.61) | 0.972 |
| K (mmol/L) | 4.10 (3.55–4.70) | 4.10 (3.40–4.70) | 4.10 (3.60–4.60) | 0.397 |
| Na+ (mmol/L) | 139.00 (137.00–141.00) | 139.00 (137.00–140.00) | 139.00 (137.00–141.00) | 0.944 |
| HCO3− (mmol/L) | 23.25 (21.80–25.63) | 24.50 (21.80–28.20) | 23.20 (21.80–25.60) | 0.290 |
| Globulin (g/L) | 29.322±5.673 | 28.017±4.705 | 29.767±5.927 | 0.152 |
| Prealbumin (mg/L) | 408.475±84.416 | 399.207±96.139 | 411.877±80.087 | 0.492 |
| ALT (U/L) | 13.10 (11.00–19.35) | 13.00 (11.00–19.00) | 14.00 (10.00–20.00) | 0.982 |
| AST (U/L) | 14.00 (12.00–17.70) | 13.90 (11.00–18.00) | 14.90 (11.95–18.05) | 0.716 |
| γ-GT (U/L) | 20.00 (14.00–31.50) | 19.00 (13.00–28.00) | 20.00 (13.70–31.40) | 0.637 |
| HLA mismatches (ABDR), n (%) | 0.357 | |||
| 0 | 135 (96.4) | 35 (94.6) | 100 (97.1) | |
| 1–2 | 4 (2.9) | 1 (2.7) | 3 (2.9) | |
| 3 | 1 (0.7) | 1 (2.7) | 0 (0.0) | |
| Hot ischemia time (min) | 5.00 (4.73–5.70) | 4.90 (4.70–5.20) | 5.10 (4.80–5.60) | 0.057 |
| Cold ischemia time (hours) | 4.60 (2.50–5.10) | 4.30 (2.25–4.95) | 4.70 (3.40–5.10) | 0.067 |
Data that conform to a normal distribution are represented by the mean ± standard deviation (SD), and non-normally distributed data are represented by the median (interquartile range). Frequency of occurrence of the event is expressed as the frequency (percentage). fDGF, functional delayed graft function; IGF, immediate graft function; BMI, body mass index; pre-Scr, pre-operative serum creatinine; pre-eGFR, pre-operative estimated glomerular filtration rate; Hb, hemoglobin; Alb, serum albumin; TC, serum total cholesterol; TG, serum triglyceride; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; Ca2+, serum total calcium; P, serum phosphorus; iPTH, immunoreactive serum parathyroid hormone; HD, hemodialysis; PD, peritoneal dialysis; WBC, white blood cells; K, serum potassium; Na+, serum sodium; HCO3−, serum bicarbonate; ALT, glutamate pyruvic transaminase; AST, aspartate aminotransferase; γ-GT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; HLA, human leukocyte antigen.
Increased long-term graft dysfunction in patients with fDGF
A total of 42 recipients exhibited impaired graft function (eGFR ≤60 mL/min/1.73 m2) (17, 45.90% in fDGF group vs. 25, 24.20% in IGF group, P=0.004) during the last follow-up visit (Table S3). Nine recipients deteriorated into allograft loss by the end of this study (Table S4). The fDGF group included 7 (18.9%) allograft loss recipients, which was more than the 2 (1.9%) in the IGF group (P=0.000). Among the 9 recipients who deteriorated into allograft loss, 3 died during dialysis, due to allograft rejection, infection, and cardiac arrest, respectively, although this was not statistically significant.
Comparison of biomarkers between recipients with and without fDGF
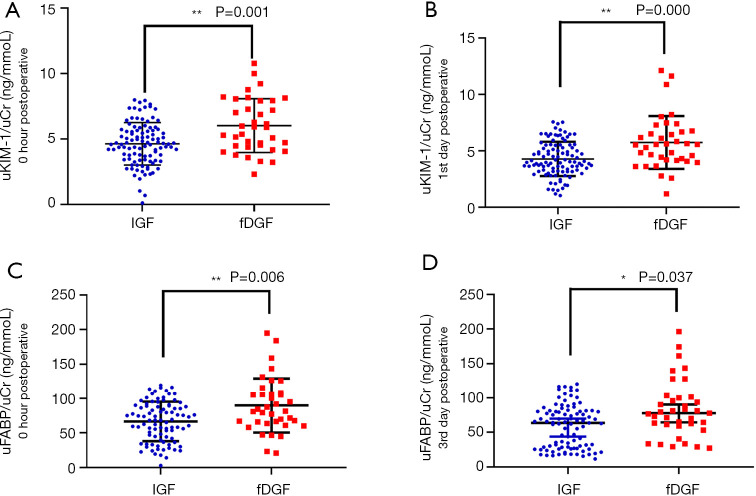
Both urinary KIM-1 (uKIM-1)/uCr and urinary L-FABP (uL-FABP)/uCr were higher in the fDGF group than in the IGF group immediately after transplantation, at 0 hour [uKIM-1/uCr: 5.885 (4.420–7.913) vs. 4.605 (3.417–5.653) ng/mmol, P<0.05; uL-FABP/uCr: 89.818±39.332 vs. 69.187±37.926 µg/mmol, P<0.05; Figure 2, Table 2]. On the first day after transplantation, only uKIM-1/uCr was significantly different between the two groups [5.569 (4.181–6.722) vs. 4.002 (3.222–6.488) ng/mmol, P<0.05]. However, on the third day after transplantation, only uL-FABP/uCr was significantly different between the two groups [77.835 (60.368–100.678) vs. 66.841 (28.815–89.783) µg/mmol, P<0.05]. Other biomarkers did not show significant differences between the two groups. The levels of serum biomarkers were also tested, but no differences were observed (Table S5).
Figure 2.
Levels of uKIM-1/uCr (at 0 hour and day 1 post transplantation) and uL-FABP/uCr (at 0 hour and day 3 post-transplantation) in the fDGF group were both significantly higher than those in the IGF group after kidney transplantation. (A) uKIM-1/uCr expression at 0 hour post-surgery in the fDGF and the IGF group; (B) uKIM-1/uCr expression on day 1 post-surgery in the fDGF and the IGF group; (C) uL-FABP/uCr expression at 0 hour post-surgery in the fDGF and the IGF group; (D) uL-FABP/uCr expression on day 3 post-surgery in the fDGF and the IGF group. *, P<0.05; **, P<0.01. fDGF, functional delayed graft function; IGF, immediate graft function; uKIM-1, urinary kidney injury molecular 1; uL-FABP, urinary L-type fatty acid binding protein; uCr, urinary creatinine; uKIM-1/uCr, the ratio of uKIM-1 to uCr; uL-FABP/uCr, the ratio of uL-FABP to uCr.
Table 2. Comparison of urinary biomarker levels between fDGF group and IGF group.
| Biomarker | Time after transplant | Total (n=140) | fDGF (n=37) | IGF (n=103) | P value |
|---|---|---|---|---|---|
| uKIM-1/uCr (ng/mmol) | 0 hour postoperative | 4.741 (3.770–6.011) | 5.885 (4.420–7.913) | 4.605 (3.417–5.653) | 0.001** |
| 1st day postoperative | 4.452 (3.602–5.705) | 5.569 (4.181–6.722) | 4.002 (3.222–6.488) | 0.000** | |
| 3rd day postoperative | 4.099 (2.241–6.5913) | 4.362 (3.501–6.950) | 3.696 (1.879–6.547) | 0.175 | |
| uL-FABP/uCr (μg/mmol) | 0 hour postoperative | 74.639±39.237 | 89.818±39.332 | 69.187±37.926 | 0.006** |
| 1st day postoperative | 74.630±43.795 | 82.197±44.773 | 71.912±43.336 | 0.222 | |
| 3rd day postoperative | 69.705 (32.723–92.533) | 77.835 (60.368–100.678) | 66.841 (28.815–89.783) | 0.037* | |
| uNGAL/uCr (ng/mmol) | 0 hour postoperative | 0.720 (0.500–1.483) | 0.640 (0.440–0.915) | 0.760 (0.550–1.540) | 0.060 |
| 1st day postoperative | 0.740 (0.513–1.268) | 0.700 (0.500–0.895) | 0.820 (0.510–1.390) | 0.147 | |
| 3rd day postoperative | 0.775 (0.500–1.275) | 0.610 (0.510–1.140) | 0.810 (0.480–1.350) | 0.357 | |
| uNAG/uCr (ng/mmol) | 0 hour postoperative | 12.435 (8.650–17.525) | 13.697 (10.262–19.575) | 11.671 (8.136–17.317) | 0.109 |
| 1st day postoperative | 12.395 (8.040–16.535) | 12.542 (10.280–17.933) | 12.254 (7.379–16.031) | 0.220 | |
| 3rd day postoperative | 12.125 (7.248–1.155) | 13.341 (11.142–17.158) | 11.554 (6.453–17.237) | 0.083 |
Data that conform to a normal distribution are represented by the mean ± standard deviation (SD), and non-normally distributed data are represented by the median (interquartile range). *, P<0.05; **, P<0.01. fDGF, functional delayed graft function; IGF, immediate graft function; uKIM-1, urinary kidney injury molecular 1; uL-FABP, urinary fatty acid-binding protein; uCr, urinary creatinine; uNGAL, urinary neutrophil gelatinase-related lipoprotein; uNAG, N-acetyl-β-D glucosaminidase; uKIM-1/uCr, the ratio of uKIM-1 to uCr; uL-FABP/uCr, the ratio of uL-FABP to uCr; uNGAL/uCr, the ratio of uNGAL to uCr; uNAG/uCr, the ratio of uNAG to uCr.
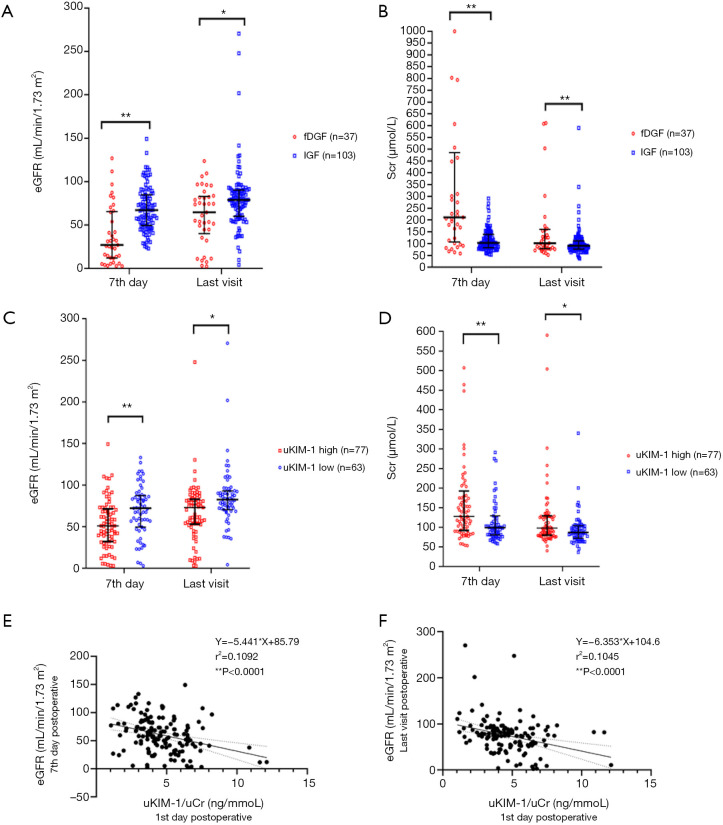
Correlation between uKIM-1 and fDGF
Correlation between uKIM-1 and fDGF was shown in Figure 3. On both the seventh day post transplantation and at the last follow-up visit, Scr was significantly higher and eGFR significantly lower in the fDGF group than in the IGF group (Figure 3A,B). Spearman’s correlation analysis indicated that uKIM-1 on the first day post-surgery was positively correlated with eGFR on the seventh day post-surgery (r2=0.1092, P<0.0001) and at the last visit (r2=0.1045, P<0.0001), respectively (Figure 3E,F). The correlation between uKIM-1 (first day post-surgery) and Scr started from the third day post-surgery (r=0.256, P<0.05, Table S6). Univariate and multivariate cox regression analyses demonstrated a 23.5% increase in the risk for fDGF for each 1 ng/mmol increase in uKIM-1 on the first day post-surgery while uL-FABP demonstrate no statistical significance in the effect for fDGF in the multivariate cox regression analyses (Figure S1).
Figure 3.
Association between uKIM-1/uCr and graft function at different time points post transplantation. (A,B,C,D) Medians and interquartile ranges for Scr and eGFR after kidney transplantation on day 7 post-surgery and at the last follow-up visit. (E,F) Spearman’s correlation analysis between uKIM-1/uCr (1st day post-operation) and Scr (days 3 and 7 post-surgery). *, P<0.05; **, P<0.01. fDGF, functional delayed graft function; IGF, immediate graft function; uKIM-1, urinary kidney injury molecular 1; uCr, urinary creatinine; uKIM-1/uCr, the ratio of uKIM-1 to uCr; Scr, serum creatinine; uKIM-1/uCr low, uKIM-1/uCr <4.08 (µg/mmol); uKIM-1/uCr high, uKIM-1/uCr ≥4.08 (µg/mmol); r, regression coefficient.
Assessment of short-term and long-term graft function on the basis of uKIM-1 levels on the first day post transplantation
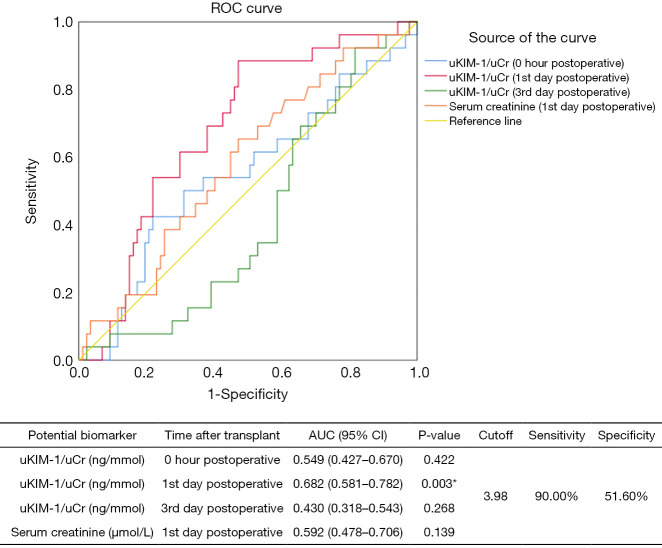
Figure S2 displays the ROC curves of uKIM-1/uCr at multiple timepoints for predicting the diagnosis of fDGF. On the first day post-surgery, uKIM-1/uCr exhibited a sensitivity of 81.10%, which was the highest among all biomarkers, together with a specificity of 52.40% for fDGF. The combination of uKIM-/uCr and Scr on the first day post-surgery had the second highest area under curve (AUC) for fDGF at 0.761 (95% CI, 0.664–0.857).
By reclassifying recipients into the KIM-1-high group (uKIM-1/uCr ≥ cutoff value for fDGF, 4.08 µg/mmol) or the KIM-1-low group (uKIM-1/uCr < cutoff value for fDGF, 4.08 µg/mmol), the median Scr were higher, and eGFR were lower in the uKIM-1-high group than in the uKIM-1-low group on the seventh day post transplantation and during the last follow-up visit (Figure 3C,D).
uKIM-1 can predict long-term graft prognosis
To determine whether uKIM-1 is related to long-term allograft prognosis, recipients who once presented with increased Scr due to surgical reasons, allograft rejection, infection, or cardiac arrest during the follow-up period were excluded. Ultimately, 125 recipients were included in the Kaplan-Meier analysis. The Kaplan-Meier plots indicated that recipients in the KIM-1-low group had a higher graft function survival rate (eGFR >60 mL/min/1.73m2) than those in the KIM-1-high group (log-rank test, P<0.0001; Figure 4).
Figure 4.
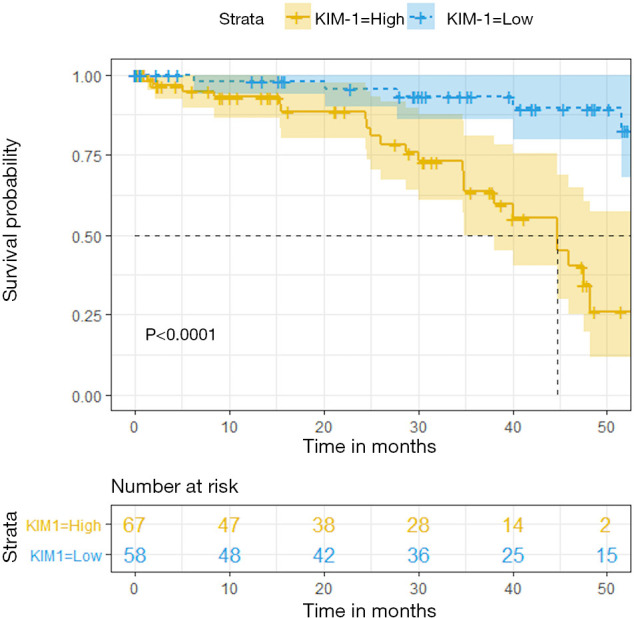
Kaplan-Meier plot of long-term graft dysfunction. Graft dysfunction: eGFR <60 (mL/min/1.73 m2); KIM-1 = low: uKIM-1/uCr <4.08 µg/mmol (n=58); KIM-1 = high: uKIM-1/uCr ≥4.08 µg/mmol (n=67). eGFR, estimated glomerular filtration rate; uKIM-1, urinary kidney injury molecular 1; uCr, urinary creatinine.
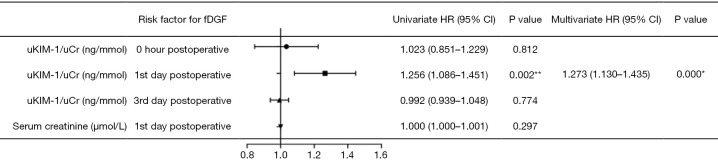
ROC curves revealed that uKIM-1/uCr on the first day post-surgery had a sensitivity of 90.00% and a specificity of 51.60% for diagnosing long-term graft dysfunction (eGFR <60 mL/min/1.73m2, Figure 5). Univariate and multivariate Cox regression analyses demonstrated that uKIM-1 on the first day post-surgery increased the risk of developing allograft dysfunction by 27.3% in for each 1 ng/mmol increase (Figure 6).
Figure 5.
Receiver operating characteristic (ROC) curves and areas under the curves for predicting long-term graft dysfunction. *, P<0.05. AUC, area under the curve; 95% CI, 95% confidence interval; uKIM-1/uCr, the ratio of uKIM-1 to uCr; uKIM-1, urinary kidney injury molecular 1; uCr, urinary creatinine.
Figure 6.
Forest plot of Cox regression analysis in long-term graft function among recipients. *, P<0.05; **, P<0.01. HR, hazard ratio; 95% CI, 95% confidence interval; fDGF, functional delayed graft function.
Discussion
DGF is a critical risk factor for long-term allograft function (11,18). Despite controversy over its definition, DGF fundamentally refers to the absence of allograft function following kidney transplantation (11,19,20). An increasing number of studies have demonstrated that 30-day re-admission, poorer graft function, acute rejection, allograft loss, and death occur more frequently in patients with DGF compared to non-DGF patients (21-23). Acute tubular necrosis is an important marker of the deterioration stage towards DGF (24); however, its prognostic significance for long-term graft outcome remains unclear. The broadening criteria for DCD kidney donors accounts for the growing incidence of DGF (21). Hence, biomarkers for the early detection of DGF are urgently needed. In this study, to consider the impact of slow graft function, we applied the definition of fDGF as a decrease of Scr of <70% in the first week after transplantation, regardless of dialysis, with reference to Boom et al. and Hall et al. (6,11).
During a median follow-up of 30.3 months, we discovered that high concentrations of uKIM-1 were strongly associated with fDGF and future allograft loss, while other acute kidney injury (AKI) biomarkers including L-FABP, NGAL, and NAG were not. These associations were independent of uCr, Scr, and acute allograft rejection situations where immunity might be a confounding factor. These results provide insights for graft function monitoring of patients after transplantation, as well as a foundation for future studies in this field.
In our previous work, we studied the diagnostic value of novel and non-invasive markers for AKI (25-27); in the current study, we extended our investigation to renal transplantation. KIM-1 has diverse characteristics that make it an ideal biomarker for the prediction of graft function. Specifically, as a transmembrane protein, KIM-1 is significantly upregulated in urine by injured proximal tubular cells after various types of kidney injury, but it remains undetectable in healthy kidneys (28). Serum KIM-1 level testing revealed no difference between the fDGF and IGF groups, supporting KIM-1 as an independent urinary tubular injury marker. Also, the existence of an immunoglobulin and mucin domain allows KIM-1 to participate in innate immunity downregulation by mediating epithelial cell phagocytosis of apoptotic cells, which subsequently reduces inflammation and innate immunity (29,30). Furthermore, KIM-1 is also essential in maladaptive repair between AKI and progressive chronic kidney disease (CKD) by promoting monocyte chemotactic protein-1 (MCP-1)-dependent kidney fibrosis (31). Additionally, KIM-1 exhibits sensitivity-enhanced responses to AKI compared to the traditional marker Scr (32,33), making it an ideal candidate as an early biomarker of graft function.
Our previous work examined the relationship between AKI and CKD. In a case-control study of 201 participants with AKI, we observed that 98 patients did not recover renal function during 1 year of follow-up, whereas 50% of patients presented with renal function deterioration. A higher level of uKIM-1 was associated with an elevated risk of renal function deterioration (HR, 1.018; 95% CI, 1.004–1.031) (16). Our studies in early and obstructive nephropathy demonstrated similar results (26,34).
Few prior studies have evaluated the association between uKIM-1 and the long-term outcomes of kidney transplantation patients. While Hall et al. (6) proposed that uKIM-1 did not predict allograft failure in 91 patients, another 1-year follow-up cohort study led by Szeto et al. (35) showed that uKIM-1 predicted the rate of graft function decline through the detection of messenger RNA (mRNA), which is in accordance with our conclusions. van Timmeren et al. (9) also reported recipients graded by uKIM-1 tertiles as 0.72 (interquartile range, 0.49–1.09) or 1.69 (interquartile range, 1.15–10.04) ng/24 h had a HR of 3.6 (95% CI, 0.9–13.5) or 5.1 (95% CI, 1.5–17.8) for predicting graft loss, respectively. Discrepancies in findings among different studies may be the consequence of diverse cohort population sizes, complication conditions, and the possibility of residual confounding, necessitating further investigation.
Based on the aforementioned literature, our study further detected uKIM-1 at 3 time points after transplantation in a larger cohort of 140 recipients. We demonstrated that on the first day post-surgery, uKIM-1 predicted a 23.5% increase in the risk of fDGF and a 27.3% increase in the risk of long-term allograft dysfunction.
Recent studies focusing on DGF have uncovered several promising biomarkers, including NGAL, IL-18, KIM-1, and NAG in particular (22,24). The present study also included L-FABP, a fatty acid-binding protein excreted from the cytoplasm of proximal renal tubular cells in response to acute injury (36,37), NGAL, a siderophore binding protein expressed by distal epithelial cells which is sensitive to AKI (38,39), and NAG, a lysosomal enzyme of proximal tubular cells that is sensitive to proximal tubular injury caused by drugs, environmental toxicants, contrast-induced toxicity, and ischemia (40,41). We found no statistically significant differences between the fDGF group and the IGF group in terms of either NGAL or NAG in the first 3 days post transplantation. Further regression analysis reinforced this result. These findings support those of an American study which indicated that urine NGAL/Cr was independently associated with cardiovascular events rather than fDGF or long-term allograft outcomes (42). Additionally, another report from Nauta et al. (15) confirmed that NAG could not predict graft loss after adjustment with albuminuria, which further validates our conclusion.
Multivariate regression analysis showed that uL-FABP/Cr on the first day post transplantation was also not related to fDGF. Research by Bansal et al. (42) produced comparable results. Despite being strongly associated with AKI, urinary biomarkers for tubular injury may not be applicable for fDGF or long-term allograft outcomes. A probable explanation may be the slower responses of these biomarkers compared to KIM-1, or there may be a unique mechanism underlying ischemia tubular injury and long-term allograft outcomes that involves KIM-1 but not other markers. Our findings suggest that proximal tubular injury, which is more closely related to fibrosis, contributes to long-term allograft loss, regardless of kidney filtration.
There were also some limitations to this study. Firstly, it is a single-center study. Bias in patient selection might therefore have been introduced. Moreover, extracorporeal membrane oxygenation (ECMO) was not performed during the acquirement of all DCD kidneys in our center. The relationships between uKIM-1, fDGF, allograft loss, and all-cause mortality may be further explored with longer follow-ups in the future. Secondly, our findings require validation in separate cohorts, due to our cohort only covering recipients with a deceased donor kidney. Thirdly, we only tested biomarker levels during the first 3 days after transplantation. Whether uKIM-1 can be restored to a normal level, or if there are other biomarkers of AKI related to allograft outcomes at later time points post-surgery remains uncertain.
Conclusions
In this cohort of 140 renal transplantation recipients, we found that high uKIM-1 expression on the first day post-surgery predicts short-term fDGF and is a potential biomarker for long-term graft function. This study therefore proposes a potent biomarker for early monitoring and graft deterioration interventions due to graft ischemia-reperfusion injury, and a feasible therapeutic target for chronic allograft nephropathy.
Supplementary
The article’s supplementary files as
Acknowledgments
We are very grateful to all the medical, nursing, and supportive staff of Department of Nephrology and Transplantation Center of Renji Hospital for their dedication in looking after the patients.
Funding: This study was supported in part by Science & Technology Cooperation Program of China (2017YFE0110500). The study was also sponsored by the National Natural Science Foundation of China (81373865, 81573748, 81770668) as well as by a grant (14140903200) from the Science and Technology Commission of Shanghai Municipality, China. The study was also sponsored by the Program of Shanghai Academic Research Leader (16XD1401900), a grant {[2017]485} from the Shanghai Leadership Training Program, and a grant (18zxy001) from Shanghai Jiao Tong University School of Medicine.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University (14140903200). All participants had signed informed consent.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-2215a
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-2215a
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-2215a). The authors have no conflicts of interest to declare.
References
- 1.Summers DM, Johnson RJ, Hudson A, et al. Effect of donor age and cold storage time on outcome in recipients of kidneys donated after circulatory death in the UK: a cohort study. Lancet 2013;381:727-34. 10.1016/S0140-6736(12)61685-7 [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 2015;15 Suppl 2:1-34. 10.1111/ajt.13195 [DOI] [PubMed] [Google Scholar]
- 3.Summers DM, Johnson RJ, Allen J, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 2010;376:1303-11. 10.1016/S0140-6736(10)60827-6 [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Wang C, Ko DS, et al. Comparison of outcomes of kidney transplantation from donation after brain death, donation after circulatory death, and donation after brain death followed by circulatory death donors. Clin Transplant 2017;31. doi: . 10.1111/ctr.13110 [DOI] [PubMed] [Google Scholar]
- 5.Coemans M, Susal C, Dohler B, et al. Analyses of the short- and long-term graft survival after kidney transplantation in Europe between 1986 and 2015. Kidney Int 2018;94:964-73. 10.1016/j.kint.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 6.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 2010;21:189-97. 10.1681/ASN.2009030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naesens M, Anglicheau D. Precision Transplant Medicine: Biomarkers to the Rescue. J Am Soc Nephrol 2018;29:24-34. 10.1681/ASN.2017010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anglicheau D, Naesens M, Essig M, et al. Establishing Biomarkers in Transplant Medicine: A Critical Review of Current Approaches. Transplantation 2016;100:2024-38. 10.1097/TP.0000000000001321 [DOI] [PubMed] [Google Scholar]
- 9.van Timmeren MM, Vaidya VS, van Ree RM, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 2007;84:1625-30. 10.1097/01.tp.0000295982.78039.ef [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnuelle P, Gottmann U, Hoeger S, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA 2009;302:1067-75. 10.1001/jama.2009.1310 [DOI] [PubMed] [Google Scholar]
- 11.Boom H, Mallat MJ, de Fijter JW, et al. Delayed graft function influences renal function, but not survival. Kidney Int 2000;58:859-66. 10.1046/j.1523-1755.2000.00235.x [DOI] [PubMed] [Google Scholar]
- 12.Perico N, Cattaneo D, Sayegh MH, et al. Delayed graft function in kidney transplantation. Lancet 2004;364:1814-27. 10.1016/S0140-6736(04)17406-0 [DOI] [PubMed] [Google Scholar]
- 13.Pianta TJ, Peake PW, Pickering JW, et al. Clusterin in kidney transplantation: novel biomarkers versus serum creatinine for early prediction of delayed graft function. Transplantation 2015;99:171-9. 10.1097/TP.0000000000000256 [DOI] [PubMed] [Google Scholar]
- 14.Weiner DE, Park M, Tighiouart H, et al. Albuminuria and Allograft Failure, Cardiovascular Disease Events, and All-Cause Death in Stable Kidney Transplant Recipients: A Cohort Analysis of the FAVORIT Trial. Am J Kidney Dis 2019;73:51-61. 10.1053/j.ajkd.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nauta FL, Bakker SJ, van Oeveren W, et al. Albuminuria, proteinuria, and novel urine biomarkers as predictors of long-term allograft outcomes in kidney transplant recipients. Am J Kidney Dis 2011;57:733-43. 10.1053/j.ajkd.2010.12.022 [DOI] [PubMed] [Google Scholar]
- 16.Xie Y, Wang Q, Wang C, et al. High urinary excretion of kidney injury molecule-1 predicts adverse outcomes in acute kidney injury: a case control study. Crit Care 2016;20:286. 10.1186/s13054-016-1455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766-72. 10.1373/clinchem.2006.077180 [DOI] [PubMed] [Google Scholar]
- 18.Marek C, Thomson B, Shoker A, et al. The prognostic value of time needed on dialysis in patients with delayed graft function. Nephrol Dial Transplant 2014;29:203-8. 10.1093/ndt/gft412 [DOI] [PubMed] [Google Scholar]
- 19.Gonwa TA, Mai ML, Smith LB, et al. Immunosuppression for delayed or slow graft function in primary cadaveric renal transplantation: use of low dose tacrolimus therapy with post-operative administration of anti-CD25 monoclonal antibody. Clin Transplant 2002;16:144-9. 10.1034/j.1399-0012.2002.1o078.x [DOI] [PubMed] [Google Scholar]
- 20.Govani MV, Kwon O, Batiuk TD, et al. Creatinine reduction ratio and 24-hour creatinine excretion on posttransplant day two: simple and objective tools to define graft function. J Am Soc Nephrol 2002;13:1645-9. 10.1097/01.ASN.0000014253.40506.F6 [DOI] [PubMed] [Google Scholar]
- 21.Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant 2011;11:2279-96. 10.1111/j.1600-6143.2011.03754.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol 2014;25:1842-8. 10.1681/ASN.2013070784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapiawala SN, Tinckam KJ, Cardella CJ, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 2010;21:153-61. 10.1681/ASN.2009040412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schröppel B, Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney Int 2014;86:251-8. 10.1038/ki.2014.18 [DOI] [PubMed] [Google Scholar]
- 25.Shao X, Tian L, Xu W, et al. Diagnostic value of urinary kidney injury molecule 1 for acute kidney injury: a meta-analysis. PLoS One 2014;9:e84131. 10.1371/journal.pone.0084131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Xue W, Shao X, et al. Analysis of a urinary biomarker panel for obstructive nephropathy and clinical outcomes. PLoS One 2014;9:e112865. 10.1371/journal.pone.0112865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Xie Y, Shao X, et al. L-FABP: A novel biomarker of kidney disease. Clin Chim Acta 2015;445:85-90. 10.1016/j.cca.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 28.Han WK, Bailly V, Abichandani R, et al. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 2002;62:237-44. 10.1046/j.1523-1755.2002.00433.x [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Brooks CR, Xiao S, et al. KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest 2015;125:1620-36. 10.1172/JCI75417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant 2009;24:3265-8. 10.1093/ndt/gfp010 [DOI] [PubMed] [Google Scholar]
- 31.Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest 2013;123:4023-35. 10.1172/JCI45361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desanti De Oliveira B, Xu K, Shen TH, et al. Molecular nephrology: types of acute tubular injury. Nat Rev Nephrol 2019;15:599-612. 10.1038/s41581-019-0184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol 2008;48:463-93. 10.1146/annurev.pharmtox.48.113006.094615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue W, Xie Y, Wang Q, et al. Diagnostic performance of urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin for acute kidney injury in an obstructive nephropathy patient. Nephrology (Carlton) 2014;19:186-94. 10.1111/nep.12173 [DOI] [PubMed] [Google Scholar]
- 35.Szeto CC, Kwan BC, Lai KB, et al. Urinary expression of kidney injury markers in renal transplant recipients. Clin J Am Soc Nephrol 2010;5:2329-37. 10.2215/CJN.01910310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noiri E, Doi K, Negishi K, et al. Urinary fatty acid-binding protein 1: an early predictive biomarker of kidney injury. Am J Physiol Renal Physiol 2009;296:F669-79. 10.1152/ajprenal.90513.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamijo A, Kimura K, Sugaya T, et al. Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med 2004;143:23-30. 10.1016/j.lab.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 38.Paragas N, Qiu A, Zhang Q, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 2011;17:216-22. 10.1038/nm.2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haase M, Bellomo R, Devarajan P, et al. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012-24. 10.1053/j.ajkd.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 40.Westhuyzen J, Endre ZH, Reece G, et al. Measurement of tubular enzymuria facilitates early detection of acute renal impairment in the intensive care unit. Nephrol Dial Transplant 2003;18:543-51. 10.1093/ndt/18.3.543 [DOI] [PubMed] [Google Scholar]
- 41.Kuźniar J, Marchewka Z, Krasnowski R, et al. Enzymuria and low molecular weight protein excretion as the differentiating marker of complications in the early post kidney transplantation period. Int Urol Nephrol 2006;38:753-8. 10.1007/s11255-006-0052-z [DOI] [PubMed] [Google Scholar]
- 42.Bansal N, Carpenter MA, Weiner DE, et al. Urine Injury Biomarkers and Risk of Adverse Outcomes in Recipients of Prevalent Kidney Transplants: The Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J Am Soc Nephrol 2016;27:2109-21. 10.1681/ASN.2015030292 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as