To the Editor:
SARS-CoV-2 vaccine is considered the primary health strategy able to end the current COVID-19 pandemic. This viral infection impacts more severely solid organ transplant recipients (SOTRs) than general population, but the effect of vaccination in this subgroup of immunosuppressed patients is not known due to their exclusion from vaccination trials. Preliminary reports suggest a lower antibody production after BNT162b2 Pfizer/BioNTech mRNA-vaccine,1, 2, 3, 4 but no data are currently available on the elicited virus-specific T cell responses.
To better characterize the effect of vaccination on the immune response in SOTRs, we assessed the B-cell and T cell responses in 16 SOTRs and in 23 immunocompetent subjects (ICs), after the second dose of BNT162b2 vaccine (subjects’ characteristics are showed in Table 1). All subjects included in this study were vaccinated between December 2020 and March 2021. The study was approved by the IRCCS-ISMETT Institutional Research Review Board (IRRB 00/21). All participants signed a written informed consent. None of SOTRs had a history of rejection in the last 12 months. Blood samples of ICs and SOTRs were taken at least 15 days after the administration of the second dose. The samples were tested to detect anti-Spike protein IgG using LIAISON SARS-CoV-2 S1/S2-IgG chemiluminescent assay, (DiaSorin), whereas ex vivo IFN-γ-ELISpot assay (Mabtech) was used for evaluating the number of T cells specific to Spike protein among the peripheral blood mononuclear cells (PBMCs) of the vaccinated people (JPT Peptide Technologies GmbH).
TABLE 1.
Characteristics of vaccinated solid organ transplant recipients and control group
| SOTRs (n = 16) | ICs (n = 23) | p value | |
|---|---|---|---|
| Age, mean year (SD) | 57 (15.9) | 44 (7.2) | p < .05 |
| Gender, M (%) | 13 (81.2) | 10 (43.5) | |
| Days after second dose of vaccine, median (range) | 20 (15–76) | 15 (15–20) | p < .05 |
| Time from transplant, median year (SD) | 9 (7.5) | — | — |
| Type of transplant | — | — | |
| Kidney | 5 | ||
| Lung | 5 | ||
| Liver | 4 | ||
| Heart | 2 | ||
| Immunosuppressive treatment (%) | |||
| Tacrolimus | 93.7 | — | — |
| Everolimus | 6.3 | — | — |
| Mycophenolate-mofetil | 62.5 | — | — |
| Corticosteroids | 56.3 | — | — |
Note: SOTRs were the first 16 consecutive vaccinated patients recruited in our institute, while ICs were the first 23 healthy volunteers enrolled among ISMETT staff. Welch’s t test was used to compare age between two groups. Wilcoxon’s test was used for days after second dose of vaccine. p < .05 was considered statistically significant for both analysis.
Abbreviations: ICs, immunocompetent subjects; M, male; SD, standard deviation; SOTRs, solid organ transplant recipients.
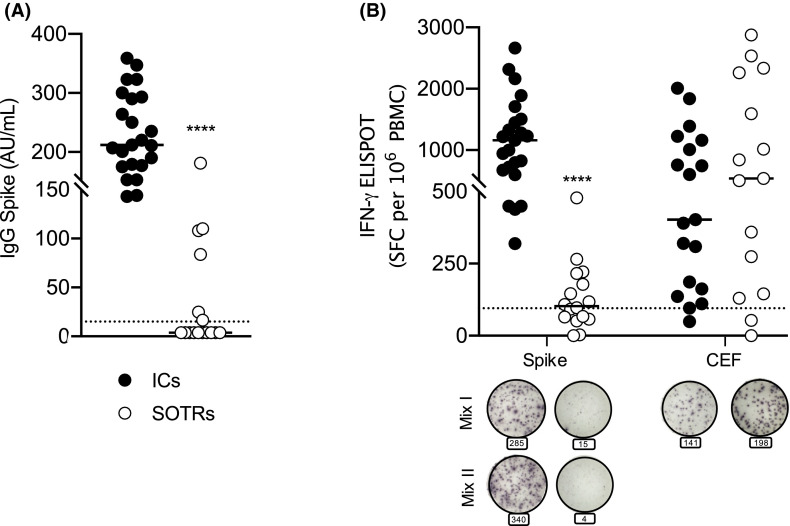
Humoral response was significantly lower in SOTRs than in ICs (p < .0001) ( Figure 1A). Whereas all ICs resulted positive for anti-SARS-CoV2 IgG, only 6/16 SOTRs (37%) had positive serology showing besides a much lower mean titer of antiviral antibodies of 87.32 UA/ml versus 233 UA/ml in ICs. Likewise, the ELISpot results (threshold 95 SFC/106 PBMC) indicate that BNT162b2 vaccine induced lower specific T cell responses in SOTRs than ICs (p < .0001). The percentage of SOTRs with detectable cellular response was 56.25%. Remarkably, the stimulation of PBMC with Cytomegalovirus, Epstein-Barr virus, and Influenza virus (CEF) immuno-dominant peptides pool led instead to a similar response in both groups (Figure 1B). Moreover, in 21 SOTRs recovered from SARS-CoV-2 infection, we detected B and T responses similar to those demonstrated in vaccinated ICs (manuscript in preparation). Our preliminary results suggest that current vaccination Pfizer-BioNTech approach against SARS-CoV-2 might be less effective than expected in SOTRs. Limitations of our study are the small number of enrolled subjects, the differences in age and in the time of analysis after vaccination in the two groups (Table 1). To overcome these limitations, we are going to increase the number of enrolled subjects to render both groups homogeneous. Including SOTRs in future clinical trials would confirm the accuracy of our results supporting novel strategies to better stimulate specific immunogenicity, such as the use of high-dose vaccines, repeated booster-dose, or use of adjuvants, as suggested elsewhere for influenza vaccination in SOTRs.5 Finally, assessing only humoral response may underestimate the immunogenicity of the vaccine, so that additional evaluation of cell-mediated immunity is essential to estimate the response to the vaccine.
FIGURE 1.
(A) Anti-SARS-CoV-2 S1/S2 IgG concentration in solid organ transplant recipients (SOTRs) (n = 16) compared to ICs (n = 23). The horizontal bars correspond to the median value, respectively, 3.8 AU/ml in SOTRs and 212 AU/ml in immunocompetent subjects (ICs). Samples with anti-SARS-CoV-2 S1/S2 IgG concentration >15 AU/ml were considered positive. The dotted line corresponds to threshold. (B) T cell responses (IFN-γ ELISpot SFC per 106 PBMC) to Spike (Mix I and II, respectively, of 158 and 157 peptides derived from a peptide scan, 15mers with 11 aa overlap) (p < .0001), or CEF (p = .08), were compared between ICs (n = 23) and SOTRs (n = 16). Each dot plot represents normalized mean spot count from duplicate wells (2.5 ± 0.5 × 105 PBMC/well) for each subject, after subtraction of spots count of unstimulated cells (the medium with peptides pool diluent-only). Circles under the graphics are representative of ELISpot from each group against Spike and CEF peptide pools. Bold black line represents the median and dotted line the experimental threshold (95 SFC per 106 PBMC). The significance was determined using a Mann-Whitney U-test (two-sides), ****p < .0001
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Blumberg EA, Manuel O, Sester M, Ison MG. The future of SARS-CoV-2 vaccines in transplant recipients: to be determined [published online ahead of print 2021]. Am J Transplant. 2021. 10.1111/ajt.16598. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 2.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325:1784–1786. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients [published online ahead of print 2021]. Am J Transplant. 2021. 10.1111/ajt.16607. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 4.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus [published online ahead of print 2021]. Am J Transplant. 2021. 10.1111/ajt.16615. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 5.Haddadin Z, Krueger K, Thomas LD, Overton ET, Ison M, Halasa N. Alternative strategies of posttransplant influenza vaccination in adult solid organ transplant recipients. Am J Transplant. 2021;21(3):938–949. doi: 10.1111/ajt.16295. [DOI] [PubMed] [Google Scholar]