To the Editor: A weak immune response to two doses of vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been observed in recipients of solid-organ transplants.1,2 Severe cases of coronavirus disease 2019 (Covid-19) have also been reported in transplant recipients who had received two doses of vaccine.3 These reports prompted the French National Authority for Health to recommend the use of a third dose in immunosuppressed patients.4 Here, we report the humoral response in a group of 101 consecutive solid-organ transplant recipients (mean [±SD] age, 58±2 years; 69% were men) who were given three doses of the messenger RNA vaccine BNT162b2 (Pfizer–BioNTech). The group included 78 kidney-transplant recipients, 12 liver-transplant recipients, 8 lung-transplant or heart-transplant recipients, and 3 pancreas-transplant recipients. The first two doses were given 1 month apart, and the third dose was administered 61±1 days after the second dose. The time between transplantation and the initiation of vaccination was 97±8 months. Immunosuppression was due to the use of glucocorticoids (in 87% of patients), calcineurin inhibitors (in 79% of patients), mycophenolic acid (in 63% of patients), mammalian target of rapamycin inhibitors (in 30% of patients), and belatacept (in 12% of patients). The levels of antibodies to SARS-CoV-2 spike protein were assessed in all the patients with the use of the Wantai enzyme-linked immunosorbent assay (Beijing Wantai Biological Pharmacy Enterprise).5 Antibody titers are expressed as the ratio of the sample signal to a calibrator-assigned cutoff signal (the signal-to-cutoff ratio). According to French law, because this was an anonymous retrospective study, institutional review board approval was not required.
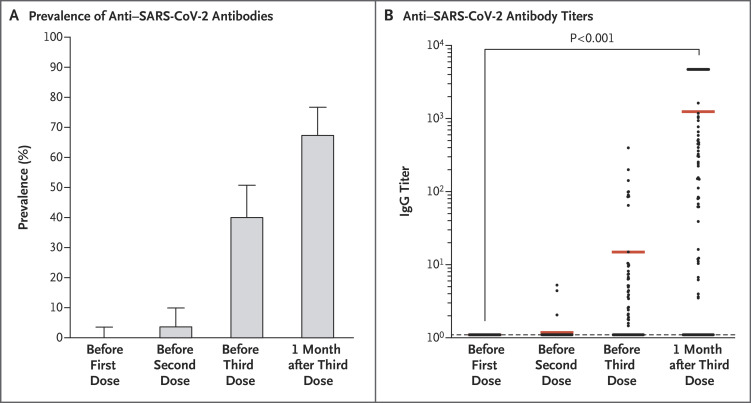
The prevalence of anti–SARS-CoV-2 antibodies was 0% (95% confidence interval [CI], 0 to 4; 0 of 101 patients) before the first dose, 4% (95% CI, 1 to 10; 4 of 101 patients) before the second dose, 40% (95% CI, 31 to 51; 40 of 99 patients) before the third dose, and 68% (95% CI, 58 to 77; 67 of 99 patients) 4 weeks after the third dose (Figure 1). Among the 59 patients who had been seronegative before the third dose, 26 (44%) were seropositive at 4 weeks after the third dose (mean [±SD] signal-to-cutoff ratio, 690±293). All 40 patients who had been seropositive before the third dose were still seropositive 4 weeks later; their antibody titers increased from 36±12 before the third dose to 2676±350 1 month after the third dose (P<0.001). Patients who did not have an antibody response were older, had a higher degree of immunosuppression, and had a lower estimated glomerular filtration rate than patients who had an antibody response (see the Supplementary Appendix, available with the full text of this letter at NEJM.org). As of this writing, Covid-19 had not developed in any of the patients after they received the three vaccine doses. No serious adverse events were reported after the administration of the third dose, and no acute rejection episodes occurred.
Figure 1. Immunogenicity.
Panel A shows the prevalence of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies before and after vaccination in the study population. Panel B shows anti–SARS-CoV-2 antibody titers before and after vaccination in the study population.
This study showed that administration of a third dose of the BNT162b2 vaccine to solid-organ transplant recipients significantly improved the immunogenicity of the vaccine, with no cases of Covid-19 reported in any of the patients. However, a large proportion of the patients remain at risk for Covid-19. Barrier measures should be maintained, and vaccination of the relatives of these patients should be encouraged.
Supplementary Appendix
Disclosure Forms
This letter was published on June 23, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA 2021;325:2204-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marion O, Del Bello A, Abravanel F, et al. Safety and immunogenicity of anti-SARS-CoV-2 messenger RNA vaccines in recipients of solid organ transplants. Ann Intern Med 2021. May 25 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wadei HM, Gonwa TA, Leoni JC, Shah SZ, Aslam N, Speicher LL. COVID-19 infection in solid organ transplant recipients after SARS-CoV-2 vaccination. Am J Transplant 2021. April 23 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DGS-Urgent. Vaccins contre la Covid-19: modalites d’administration des rappels. 2021. (https://www.mesvaccins.net/textes/dgs_urgent_n43_vaccination_modalites_d_administration_des_rappels.pdf).
- 5.Abravanel F, Miédouge M, Chapuy-Regaud S, Mansuy J-M, Izopet J. Clinical performance of a rapid test compared to a microplate test to detect total anti SARS-CoV-2 antibodies directed to the spike protein. J Clin Virol 2020;130:104528-104528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.