Graphical Abstract
Key Words: cardiotoxicity, coronary vasospasm, fluoropyrimidine
Abbreviations and Acronyms: 5-FU, 5-fluorouracil; CAD, coronary artery disease; CTA, computed tomography angiogram; CV, cardiovascular; FLOX, bolus dosing 5 fluorouracil, leucovirin, oxaliplatin; FOLFOX, continuous infusion 5 fluorouracil, leucovorin, oxaliplatin
The fluoropyrimidines (5-fluorouracil [5-FU] and oral pro-drug capecitabine) are the central components of gastrointestinal malignancy chemotherapy, with efficacy for brain, head and neck, bladder, and breast cancers. These agents are also used as radio-sensitizing agents in conjunction with therapeutic radiation. Their toxicity has been extensively reviewed (1), and their use is limited by noncardiac (cytopenias, mucositis, diarrhea, palmar-plantar erythdysthesia syndrome) and cardiac toxicities (electrocardiographic [ECG] changes, atrial fibrillation, and other arrhythmias; ischemia; pericarditis; stress cardiomyopathy; myocarditis; and death). Cardiac toxicity typically presents as chest pain that may be atypical or typical, or consistent with an acute coronary syndrome. Asymptomatic ECG changes have also been reported. Vasospasm is the predominant mechanism of chest pain (1, 2, 3, 4), and varies between 1% and 13%, dependent on the drug, route of administration, co-administered chemotherapy (e.g., platinum-based drugs), and reporting criteria (2, 3, 4, 5).
The precise role of age, cardiovascular (CV) risk factors, and underlying CV disease on the risk of coronary vasospasm remains uncertain. Jensen et al. (6) found no significant differences in the distribution of pre-treatment risk characteristics and subsequent cardiac symptoms due to fluoropyrimidine treatment. Nine of 106 patients had chest pain, and only 1 had underlying coronary artery disease (CAD), whereas 7 patients with known CAD did not have treatment-induced symptoms. Therefore, underlying CAD or CV disease and/or CV risk factors should not be a reason to withhold fluoropyrimidine therapy. These patients should be optimized medically before initiating therapy, often with cardio-oncology involvement. Universal prophylactic pre-treatment with coronary vasodilators has not reduced the risk of coronary vasospasm and is not currently recommended (7). We describe our institutional experience in the management of fluoropyrimidine-induced coronary vasospasm. In our experience, there can be 2 distinct clinical presentations, as demonstrated in the following cases.
Case 1: Early Presentation of Cardiac Toxicity
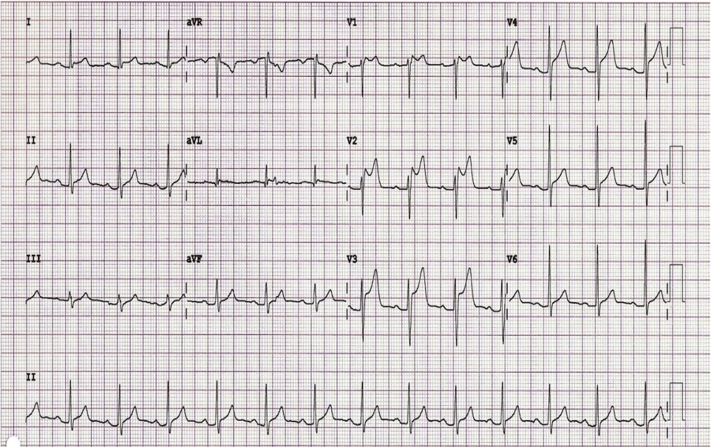
A 42-year-old woman with rectal carcinoma with no pre-existing CV disease or risk factors developed crushing chest pain across her precordium during the first infusional bolus dosing of 5-FU. ECG showed diffuse ST-segment elevation (Figure 1). She was treated with sublingual nitroglycerin, and her pain resolved. Troponin was negative. Urgent coronary angiography showed no epicardial CAD.
Figure 1.
Diffuse ST-Segment Elevation From Coronary Vasospasm
Electrocardiogram showing diffuse ST-segment elevation during bolus 5-fluorouracil administration attributed to coronary artery vasospasm.
Coronary vasospasm from bolus dosing of 5-FU most often presents as classic sudden-onset chest pain during the first exposure with associated ECG changes that range from ST-segment elevation suggestive of ST-segment elevation myocardial infarction to nonspecific ST-T changes. Circulating troponin levels may or may not be elevated, and echocardiography may or may not show regional wall motion abnormalities. Because this event often occurs in an infusion suite, an appropriate diagnostic work-up is often executed in an expeditious manner (Figure 2).
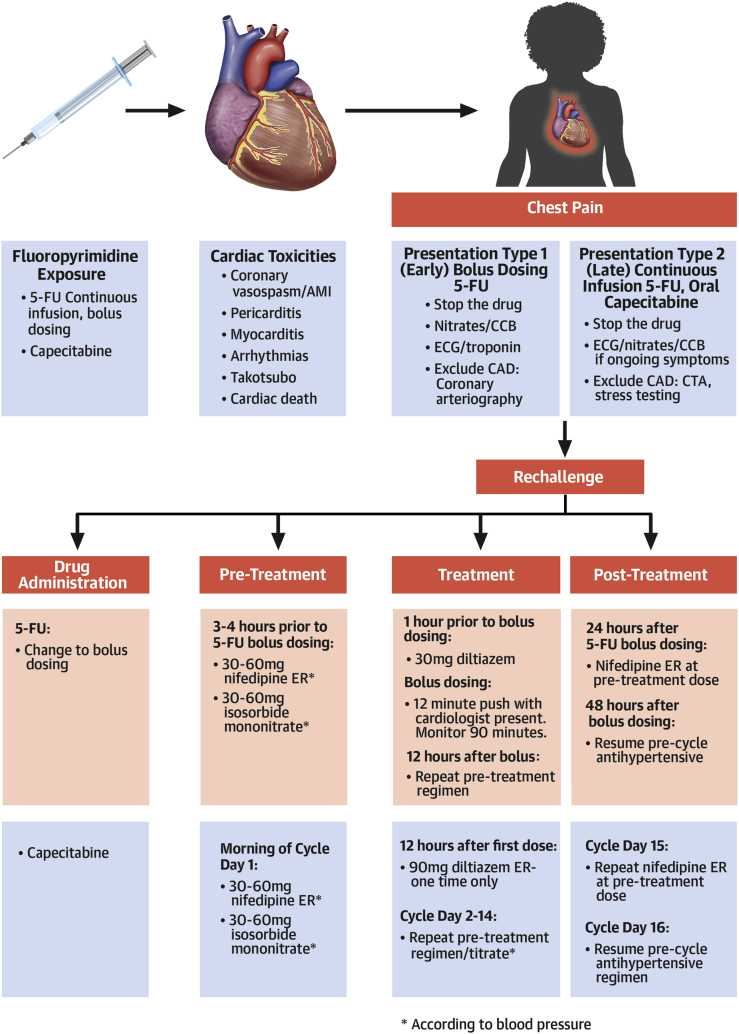
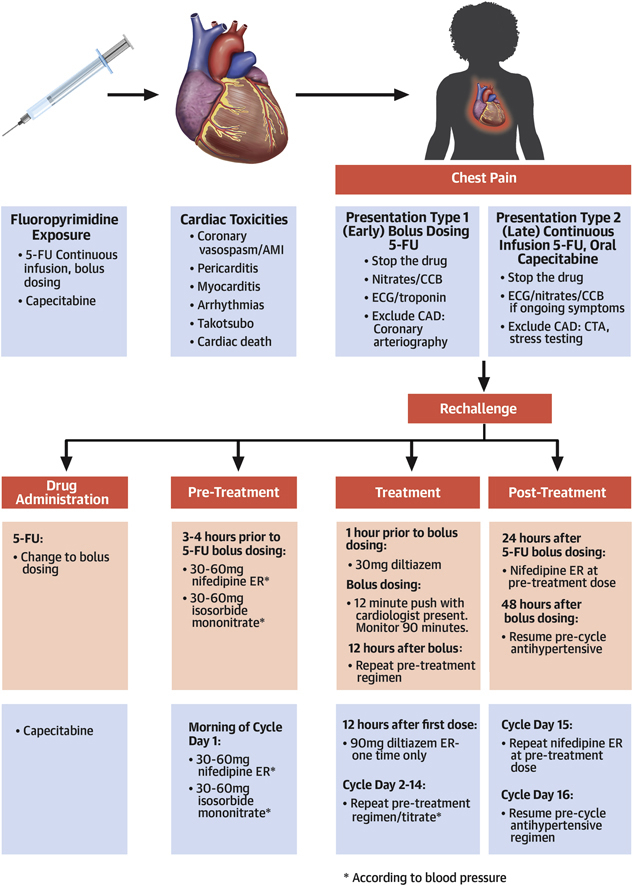
Figure 2.
Fluoropyrimidine Cardiac Toxicity and Rechallenge
Various cardiac toxicities from fluoropyrimidines, distinct chest pain syndromes attributable to specific agents, and appropriate treatment and/or diagnostic steps. We outline an algorithm using anti-anginal administration for safe fluoropyrimidine rechallenge. ∗According to blood pressure. AMI = acute myocardial infarction; CAD = coronary artery disease CCB = calcium channel blocker; CTA = computed tomography angiography; ECG = electrocardiogram; ER = extended release; 5-FU = 5-fluorouracil.
Case 2: Late Presentation of Cardiac Toxicity
A 54-year-old man with colorectal adenocarcinoma began adjuvant 48-h continuous infusion 5-FU, oxaliplatin, and leucovorin (FOLFOX). At hour 40 of cycle 1, he experienced 10 min of chest discomfort at rest but did not seek medical attention. During cycle 2, he ignored the same chest symptoms, the longest lasting 30 min at hours 18, 32, and 44. Before cycle 3, he reported the chest pain to his oncologist.
Fluoropyrimidine-induced vasospasm is also observed with continuous infusion of 5-FU or oral capecitabine, with intermittent chest discomfort at rest that occurs during treatment. In our experience, with subsequent cycles, symptoms typically occur earlier, are more intense, last longer, and recur if chemotherapy continues. Because this occurred at home and reporting was inconsistent, the oncologist only learned about this at the visit before the next cycle. Treatment was initially held, and the patient was referred to cardio-oncology.
The clinical impression was 5-FU−induced vasospasm. A subsequent ECG was normal, and troponin was <0.01 ng/ml. He was referred to cardio-oncology.
When chest pain is recognized during treatment, treatment is often stopped (by discontinuing the infusion pump or stopping oral dosing) with prompt emergency department evaluation and management as a presumed acute coronary syndrome. When recognized after chest pain resolution, this typically prompts a cardiovascular evaluation. Because of the time course of presentation, which may be after the resolution of pain, it is plausible that ischemic ECG changes and troponin elevations are undetectable.
We then excluded underlying CAD through a noninvasive strategy, and we considered using coronary computed tomography angiography (CTA) or stress testing. In this patient, coronary CTA was performed with no evidence of epicardial CAD.
After discussion between the cardio-oncologist and oncologist, outpatient rechallenge with a 5-FU−based regimen was planned.
A historical recurrence rate of at least 90% and lack of proven effective antispasm prophylaxis has discouraged rechallenge, placing the risk of future cardiac events against the risk of withholding effective anticancer therapy. Alternative approaches (reduced dosing, alternate 5-FU drugs, antidote therapy [uridine triacetate], and alternate non-fluoropyrimidine regimens) have either less cancer efficacy or are not available in the United States (1,4,5).
Collaboration with oncology in these cases is paramount. If fluoropyrimidine-based treatment is the best cancer therapy, and the manifested cardiac toxicity is chest pain, we typically attempt a rechallenge (Figure 2). We have previously reported our early successful experience with fluoropyrimidine rechallenge in 11 patients (8). To date, we have rechallenged an additional 16 of 16 patients safely, completing planned outpatient fluoropyridimine-based chemotherapy with no recurrent cardiac toxicity.
The patient subsequently completed 12 5-FU based cycles with a combination of nifedipine, diltiazem, and isosorbide mononitrate prophylaxis without recurrence of any cardiac toxicity.
Because of the concerns regarding recurrent vasospasm with continuous 5-FU and the oncological equipoise between bolus dosing and continuous infusion regimens, we typically switch patients with suspected coronary vasospasm from FOLFOX to bolus dosing 5-FU, leucovorin, oxaliplatin (FLOX) regimens for subsequent outpatient treatments (9,10). With capecitabine rechallenge, we continue with oral dosing. For patients with hypertension, we switch antihypertensive medications to a calcium blocker and continue this at a tolerated and effective dose throughout chemotherapy. We pre-treat all patients with 30 to 60 mg of extended-release nifedipine and 30 to 60 mg of isosorbide mononitrate 3 to 4 h before chemotherapy, with dosing dictated by blood pressure. Because 5-FU is preceded by an infusion of leucovorin, we add a second calcium blocker (30 mg of short-acting oral diltiazem) at the start of infusion. The 5-FU bolus is delivered over 12 min. For the first 2 rechallenges, we maintained a presence during bolus and for 30 min subsequently to monitor status. Monitoring continued for 45 to 90 min, and patients were reassessed by cardiology before discharge home. We also kept sublingual nitroglycerin and short-acting nifedipine (10 mg used sublingual) available at the “chairside” for suspected coronary spasm. At home following treatment, we re-dose extended-release nifedipine at a previous dosage after dinner and the following morning (day +1). We routinely call to check patient status the evening after the first cycle, and patients are instructed to call with any symptom in the 36 h following treatment.
The pharmacokinetics for oral twice daily capecitabine are similar to continuous infusion 5-FU. Oncologists typically treat 2 weeks on and 1 week off (a cycle). We have modified the previous steps for capecitabine (Figure 2). During days 2 to 14 of the cycle, we repeat the pre-treatment nifedipine and isosorbide mononitrate dosing in the morning. We also use 90 mg of extended-release diltiazem in the evening, 12 h after the first dose. All patients have nitroglycerin and are instructed on its use. Patients monitor their blood pressure daily and call us each morning before taking any medication. In the absence of symptoms, capecitabine is continued, and because metabolites accumulate over the 2 weeks, nifedipine and isosorbide mononitrate doses are up-titrated according to systolic blood pressure to maintain a systolic blood pressure of >100 mm Hg. On day 15 after cycle completion, we re-dose nifedipine at the day 14 dose as monotherapy for all patients, and only continue subsequent dosing for patients with hypertension. We repeat this regimen with each cycle (Figure 2).
In our experience, we have not encountered any patient with limiting pre-treatment blood pressure. However, low blood pressure (systolic <100 mm Hg) may limit the ability to up-titrate antispasm medication on days 2 to 14 of the capecitabine cycle. Baseline hypotension and/or inability to tolerate the 3-drug regimen precludes outpatient rechallenge. An alternative strategy, if there is no other chemotherapy choice, is inpatient FLOX with intravenous nitroglycerin and monitoring.
At our center, patients routinely receive cardiac toxicity symptom education before treatment, with reinforcement with every cycle. Every patient has a baseline ECG. Patients are instructed to stop therapy and to call with any chest discomfort. If they call with active symptoms, they go to the emergency department for formal evaluation. If they call after cessation of symptoms, chemotherapy is temporarily interrupted, and they proceed with prompt outpatient cardio-oncology evaluation. Before treatment, patients who may be at presumed higher risk (e.g., known CAD) are routinely referred to cardio-oncology for medication optimization.
The cooperation and commitment to patient care between oncologists and cardio-oncologists represents the fundamental essence and raison d’etre for cardio-oncology and demonstrates the achievement of the underlying goals of both specialties: providing the most effective oncology care while minimizing cardiovascular toxicity. We achieved these goals through a jointly developed pathway unique to the Abramson Cancer Center that has successfully and safely allowed re-challenge for patients with suspected fluropyrimidine-induced chest pain. We present this as one possible approach to allow patients to continue potentially life-saving cancer treatment.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Jaskanwal D.S., Kaur J., Khodadadi R. 5-fluorourcil and cardiotoxicity: a review. Therap Adv Med Oncol. 2018;10:1–18. doi: 10.1177/1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Focaccetti C., Bruno A., Magnani E. Effects of 5-fluorouracil on morphology, cell cycle, proliferation, apoptosis, autophagy and ROS production in endothelial cells and cardiomyocytes. PLoS One. 2015;10 doi: 10.1371/journal.pone.0115686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polk A., Vistisen K., Vaage-Nilsen M., Nielsen D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol. 2014;15:47. doi: 10.1186/2050-6511-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan C., Parekh H., Allegra C. 5-FU induced cardiotoxicity: case series and review of the literature. Cardiooncology. 2019;5:3. doi: 10.1186/s40959-019-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polk A., Shahmarvandis N.N., Vistisen K. Incidence and risk-factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen S.A., Hasbak P., Mortensen J. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J Clin Oncol. 2010;28:5280–5286. doi: 10.1200/JCO.2009.27.3953. [DOI] [PubMed] [Google Scholar]
- 7.Eskilsson J., Albertsson M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncol. 1990;29:1001–1003. doi: 10.3109/02841869009091790. [DOI] [PubMed] [Google Scholar]
- 8.Clasen S.C., Ky B., O’Quinn R. Fluoropyrimidine-induced cardiac toxicity: challenging the current paradigm. J Gastrointest Oncol. 2017;8:970–979. doi: 10.21037/jgo.2017.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakrabarti S., Sara J., Lobo R. Bolus 5-fluorouracil (5-FU) in combination with oxaliplatin is safe and well tolerated in patients who experienced coronary vasospasm with infusional 5-FU or capecitabine. Clin Colorectal Cancer. 2019;18:52–57. doi: 10.1016/j.clcc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Sharif S., O’Connell M.J., Yothers G. FOLFOX and FLOX regimens for the adjuvant treatment of resected stage II and III colon cancer. Cancer Invest. 2008;25:956–963. doi: 10.1080/07357900802132550. [DOI] [PMC free article] [PubMed] [Google Scholar]