Abstract
Barriers posed by the COVID-19 pandemic have led to reduced access to Human Immunodeficiency virus (HIV) care, leaving untreated patients at risk for various superimposed infections and malignancies such as Kaposi sarcoma (KS). We recently encountered a 37-year-old African-American male with a past medical history of HIV who tested positive for SARS-CoV-2 and was diagnosed with AIDS-related disseminated KS, representing the first reported case of COVID-19 infection with a newly diagnosed concomitant KS. The patient experienced multi-organ failure requiring tracheostomy, renal replacement therapy, and a prolonged intensive care unit (ICU) stay. Goals of care were changed to comfort measures and the patient passed away shortly afterwards. He was made comfort measures and passed away shortly afterwards. AIDS-related KS is a vascular tumor seen in association with Human Herpes Virus-8 (HHV-8). Management of limited AIDS-related KS typically includes combined antiretroviral therapy (ART) while multi-organ KS disease demands systemic chemotherapy. Immunosuppression should be avoided in patients with AIDS-related KS as it can lead to progression of KS. This recommendation is in conflict with the usual standard of care for patients with COVID-19 pneumonia, requiring clinical judgment and a customized approach based on the stage and severity of both the KS and the COVID-related disease.
We briefly review HIV-COVID-19 coinfection, AIDS related KS and challenges associated with their management.
Keywords: Kaposi sarcoma, COVID-19, HIV, Social inequities, HHV-8
Highlights
-
•
HIV increases susceptibility to COVID-19 infection and impart a poor prognosis in COVID-19 patients older than 50 years with underlying conditions.
-
•
Steroid use can result in disease progression of Kaposi sarcoma (KS), so the benefits of therapy must be weighed against the risks before steroid initiation in patients with co-occurring KS and COVID-19 pneumonia.
-
•
The COVID-19 pandemic has resulted in limited access to HIV preventive and treatment services, resulting in worsening control of the HIV epidemic.
1. Introduction
The impact of the COVID 19 pandemic has been devastating globally and there still remains a paucity of research regarding its epidemiology, pathogenesis, disease course and treatment. Management becomes more complicated when COVID pneumonia is diagnosed in special populations that's are chronically ill including immunodeficiency syndromes, end stage comorbidities, concurrent pulmonary disorders, cancers and pregnancy. Theoretically, patients with Human Immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) are more susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, risk of mortality among persons living with HIV (PLWH) who become infected with SARS-CoV 2 is largely unknown [[1], [2], [3]].
We report a challenging case of AIDS-related disseminated KS in a young patient with concomitant COVID-19 infection. Due to reduced access to health care and HIV treatment barriers caused by the COVID-19 pandemic, the patient developed concomitant KS and COVID-19, ultimately resulting in multi-organ failure leading to his demise.
2. Case presentation
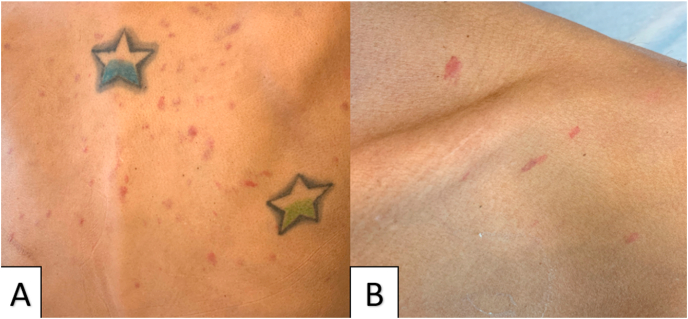
A 37-year-old African-American male with a past medical history of HIV, nonadherence with antiretroviral therapy (ART) and post-traumatic stress disorder presented to outside hospital with productive cough. The patient was nonadherent with antiretroviral therapy (ART) since the beginning of the pandemic and was having difficulty scheduling outpatient appointments. He reported being complaint with ART prior to the beginning of COVID-19 pandemic. He tested positive for SARS-CoV-2 via reverse transcriptase polymerase chain reaction testing and was initially treated conservatively at home. Subsequently the patient developed worsening cough, new onset shortness of breath, fatigue, dysphagia and odynophagia requiring hospitalization. Upon arrival, the patient was hypoxic on room air and required 5 L of supplemental oxygen via nasal canula. His vital signs were otherwise normal. On physical exam, the patient was noted to have multiple red violaceous macules and papules on his back, chest, and roof of his mouth (Fig. 1). He was also found to have an oral thrush on his tongue.(seeTable 1)
Fig. 1.
Multiple scattered red violaceous macules and papules seen on back (Panel A) and front of chest (Panel B). Pertinent laboratory workup at admission is summarized in Table 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Pertinent laboratory abnormalities noted at admission.
| Laboratory marker (units) | Results (normal ranges) |
|---|---|
| Sodium (mmol/L) | 129 (136–145) |
| WBC (k/mcL) | 6.94 (4.4–11.30) |
| Hemoglobin (g/dl) | 8.1 (14–17.4) |
| Platelets (k/mcL) | 50 (145–445) |
| CD4 count (per ul) | 5 (441–2156/μL) |
| HIV viral load (per ml) | 56 (0) |
| LDH (U/L) | 322 (110–216) |
| Ferritin (ng/ml) | 1978 (30–400 ng/ml) |
| CRP (mg/dl) | 5 (<0.5) |
| Procalcitonin (ng/ml) | 3.38 (<0.1) |
WBC: white blood cell count, HIV: Human immunodeficiency virus, LDH: lactate dehydrogenase, CRP: C-reactive protein.
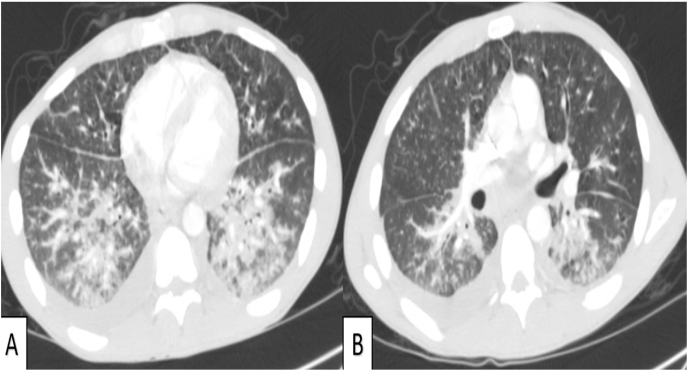
Computed tomography (CT) chest showed predominant bilateral lower lobe, peribronchovascular consolidations and ground glass opacities with associated bronchial wall thickening concerning for KS (Fig. 2).
Fig. 2.
Bilateral lower lobe consolidations and ground glass opacities (Panel A); peribronchovascular consolidations and bronchial thickening (Panel B).
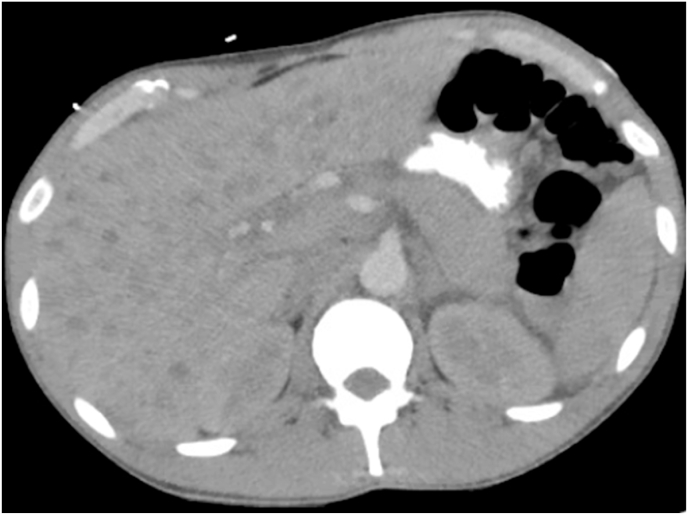
CT abdomen showed diffuse esophageal thickening consistent with esophagitis as well as numerous hypodense liver lesions (Fig. 3).
Fig. 3.
Multiple hypodense lesions are noted in liver parenchyma.
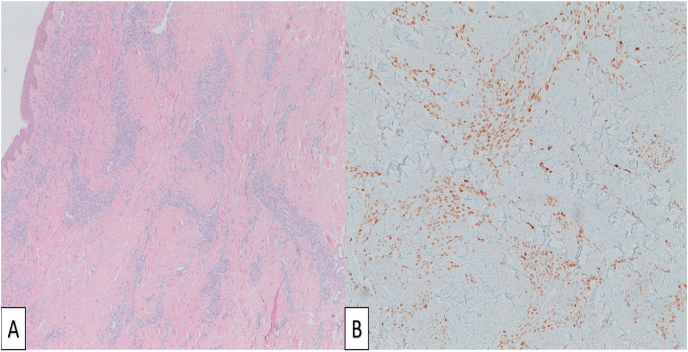
A skin tissue biopsy was performed which showed findings consistent with KS (Fig. 4).
Fig. 4.
Hematoxylin and eosin stain shows proliferating spindled nuclear morphology cells surrounding an irregular dermal proliferation of slit-like vascular spaces (Panel A). Immunohistochemical stain shows HHV-8 positive cells (Panel B).
The patient was started on a 10-day course of dexamethasone 6 mg daily and 5-day course of Remdesivir for COVID-19 pneumonia. Due to concerns of superimposed community acquired pneumonia, the patient received a 7-day course of ampicillin-sulbactam and azithromycin. Antiretroviral therapy (bictegravir-emtricitabine-tenofovir alafenamide) was resumed and the patient was started on trimethoprim-sulfamethoxazole for Pneumocystis Jiroveci pneumonia (PJP) prophylaxis. The patient received a course of fluconazole for presumed candida-related esophagitis which resulted in complete resolution of odynophagia. Due to concerns for worsening COVID-19 pneumonia, a shared decision was made by the interdisciplinary discussion to hold chemotherapy until completion of therapies for COVID-19.
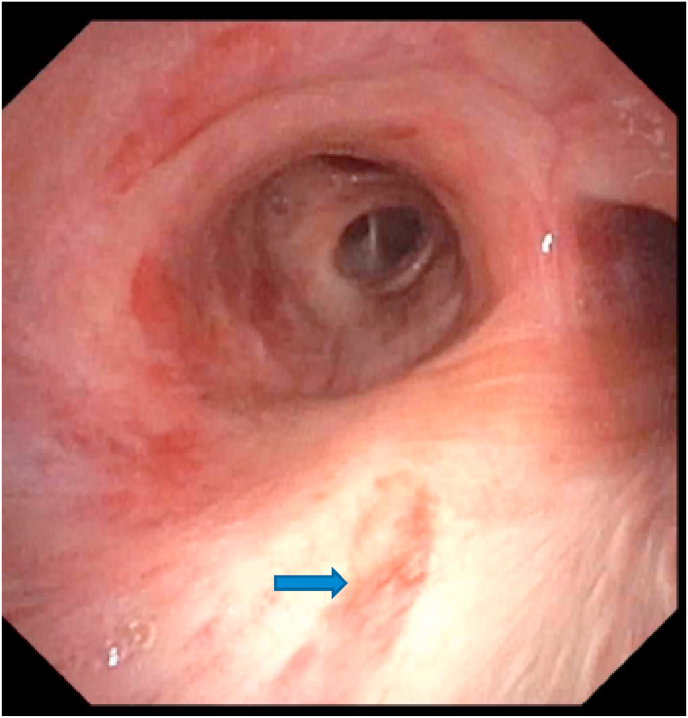
Given concern for pulmonary involvement of KS, a bronchoscopy was performed on day 10 of hospitalization which unveiled a flat elongated violaceous mucosal lesion in the right mainstem bronchus suspicious for KS (Fig. 5).
Fig. 5.
Flat elongated violaceous mucosal lesion in right mainstem bronchus (marked by blue arrow) suggestive of KS. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Bronchoalveolar lavage fluid was collected from the right lower lobe which tested positive for Human herpes virus-8 (HHV-8), while other microbiological studies including fungal, acid-fast bacilli, bacterial cultures and PJP PCR were negative. Bronchial biopsy was deferred due to high risk of bleeding. In light of this constellation of findings, the patient was diagnosed with disseminated KS. The patient was observed over subsequent days following bronchoscopy due to concern for development of immune reconstitution inflammatory syndrome (IRIS). Initially, the patient reported an improvement in symptoms and oxygen requirements decreased. On day 15 of hospitalization, the patient began having hemoptysis in the setting of thrombocytopenia and underlying pulmonary KS. Due to worsening shortness of breath and hypoxia the patient was transferred to the medical intensive care unit. He was eventually intubated due to persistent hypoxia and respiratory distress on day 18 of hospitalization. Subsequently, the patient developed multi-organ failure including cardiogenic shock and acute kidney injury requiring continuous renal replacement therapy. Superimposed bacterial and fungal infection were ruled out. The patient then underwent tracheostomy due to prolonged dependence on mechanical ventilator support. He was started on paclitaxel due to concerns for worsening KS. Due to worsening multi-organ failure and prolonged ICU stay with no meaningful functional recovery, goals of care discussions were initiated. A shared decision was made by the family and treatment team and the patient's goals of care were changed to comfort measures. The patient passed away shortly afterwards.
3. Discussion
COVID-19-HIV coinfection has primarily been reported in males over 50 years old, with the most frequently cited symptoms being fever (74.5%) and cough (58%) [4]. Clinical and radiological presentation of COVID-19 in patients who are HIV-positive is similar to presentation in those who are uninfected with HIV [5]. There has been limited literature regarding association of COVID 19 pneumonia with HIV and its effect on disease course. A recent metanalysis concluded that HIV remains a significant risk factor for acquiring SARS-CoV-2 infection and is associated with a higher risk of mortality, primarily due to underlying comorbidities [6]. However, another prospective study, provided evidence that HIV coinfection does not significantly impact presentation, hospital course, or outcomes of patients infected with SARS-CoV-2, when compared with matched non-HIV patients [2,7]. An important factor that contributes to poor outcomes in HIV patient's, is the reduction in access to health care that has aggravated during the pandemic [8]. Studies have shown that among patients with HIV infection, risk factors of older age, male sex and sub-Saharan descent are all associated with more severe symptoms when combined with COVID-19 infection.
[9]. Interestingly, a prior report highlighted the case of a patient with KS who developed a skin rash after acquiring COVID-19 infection, which suggests that the hyper-inflammatory state associated with COVID-19 may lead to proliferation of HHV8 and result in recurrence of KS [10]. Approach to treatment of COVID-19 in PLWH should be same as that of the general population with a few exceptions. Ideally steroids remains the mainstay of COVID pneumonia treatment, however in patients with HIV, standard COVID-19 therapy may induce reactivation of KS-associated herpesvirus which can predispose patients with untreated HIV to develop KS [3]. Our case described this perplexing dilemma as a difficult decision had to be made after weighting risks and benefits of steroid therapy.
KS is a rare angio-proliferative tumor associated with HHV-8, which is classically seen in males with low CD4 count and in those who are nonadherent with antiretroviral therapy (ART) such as our patient [11]. It can manifest locally in a single organ or become a multisystemic disease. It can manifest as painless violaceous lesions involving mucocutaneous tissues or affect multiple organs.
Typically, the skin, oral mucosa, lung, lymph nodes, bones and gastrointestinal tract are involved. CD8 cells >1000/μl and CD4:CD8 ratio ≤0.5 are also associated with increased incidence of KS [11]. Radiologically, KS can present with diffuse grand glass opacities and consolidations in the peribronchovascular regions. Definitive diagnosis of KS requires biopsy of the involved organ showing abnormal spindle cells of endothelial origin, aberrant proliferating blood vessels and extravasated blood cells surrounded by inflammatory cells [12]. The prognosis of AIDS-related KS depends on the stage of disease, though disseminated KS has a 5-years survival of 41%.
Immunohistochemical stains test positive for HHV-8 in virus-infected cells.
Management of COVID-19 pneumonia in a patient with AIDS-related disseminated KS poses a clinical challenge. Although steroids remain a mainstay therapy for COVID pneumonia [13], immunosuppression can predispose patients with uncontrolled HIV to develop KS, meaning that a clear distinction between disease etiologies must be made prior to initiation of therapy. Combined ART is first line therapy for limited KS but systemic chemotherapy is recommended in disseminated cases. Specifically, pegylated liposomal doxorubicin (45% response rate) and paclitaxel are considered first line and the decision to use either drug should be individualized based on side effect profile [14,15]. However, chemotherapy may cause progression of COVID-19 pneumonia so clinical judgment should be employed from case to case. In this scenario, no specific clinical guidelines exist, so the decisions regarding use of steroids and/or chemotherapy will be based on the stage and relative severity of a given patient's KS and COVD-related disease.
A secondary finding highlighted in our case was the delay and difficulty in access to care that has resulted due to the COVID-19 pandemic. Our patient reported difficulty continuing treatment of HIV and receiving support for post-traumatic stress disorder during this time. Eventually he became non adherent with his medications, which worsened his current disease state and predisposed him to opportunistic infections.
4. Conclusion
We presented a novel case of disseminated AIDS-related KS in the setting of COVID-19 infection. Due to challenges posed by the COVID-19 pandemic, our patient was unable to access preventive care for HIV, resulting in numerous complications. Despite aggressive treatment of AIDS-related KS and COVID-19 pneumonia, the patient had a protracted ICU stay and shortly afterwards passed away. HIV is a significant risk factor for acquiring SARS-CoV-2 infection and therefore Centers for Disease Control and Prevention (CDC) guidelines, recommends priority consideration of the SARS-CoV-2 vaccine for PLWH. Additionally, better access to HIV treatment services is critical in allowing patients to begin ART sooner, thus preventing such potentially fatal complications.
Category
Case report.
Prior presentations
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The other authors report no conflicts of interest.
References
- 1.Lesko C.R., Bengtson A.M. HIV and COVID-19: intersecting epidemics with many unknowns. Am. J. Epidemiol. 2021;190(1):10–16. doi: 10.1093/aje/kwaa158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karmen-Tuohy S., Carlucci P.M., Zervou F.N., Zacharioudakis I.M., Rebick G., Klein E., Reich J., Jones S., Rahimian J. Outcomes among HIV-positive patients hospitalized with COVID-19. J. Acquir. Immune Defic. Syndr. 2020;85(1):6–10. doi: 10.1097/QAI.0000000000002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J., Dai L., Barrett L., Post S.R., Qin Z. SARS-CoV-2 proteins and anti-COVID-19 drugs induce lytic reactivation of an oncogenic virus. bioRxiv. 2020:2020. doi: 10.1038/s42003-021-02220-z. 10.02.324228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirzaei H., McFarland W., Karamouzian M., Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2021;25(1):85–92. doi: 10.1007/s10461-020-02983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vizcarra P., Pérez-Elías M.J., Quereda C., Moreno A., Vivancos M.J., Dronda F., Casado J.L., Moreno S., Pérez-Elías M.J., Fortún J., Navas E., Quereda C., Dronda F., Del Campo S., López-Vélez R., Cobo Reinoso J., Casado J.L., Moreno A., Norman F., Martín-Dávila P., Hermida J.M., Pérez Molina J.A., Monge B., Pintado V., Serrano-Villar S., Sánchez-Conde M., Chamorro S., Escudero R., Gioia F., Comeche B., Crespillo C., Herrera S., Ron R., Martínez-Sanz J., Pons-Guillén M., Vivancos M.J., Vizcarra P. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. The Lancet HIV. 2020;7(8):e554–e564. doi: 10.1016/S2352-3018(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ssentongo P., Heilbrunn E.S., Ssentongo A.E., Advani S., Chinchilli V.M., Nunez J.J., Du P. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci. Rep. 2021:6283. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sarwari A.R. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34(13):F3–f8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown L.B., Spinelli M.A., Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr. Opin. HIV AIDS. 2021;16(1):63–73. doi: 10.1097/COH.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etienne N., Karmochkine M., Slama L., Pavie J., Batisse D., Usubillaga R., Letembet V.-A., Brazille P., Canouï E., Slama D., Joumaa H., Canoui-Poitrine F., Segaux L., Weiss L., Viard J.-P., Salmon D., Team C.-I. HIV infection and COVID-19: risk factors for severe disease. AIDS. 2020;34(12):1771–1774. doi: 10.1097/QAD.0000000000002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leoni E., Cerati M., Finzi G., Lombardo M., Sessa F. COVID-19 and HHV8 first spotted together: an affair under electron microscopy. J. Eur. Acad. Dermatol. Venereol. 2021;35(5):e311–e312. doi: 10.1111/jdv.17123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poizot-Martin I., Lions C., Cheret A., Rey D., Duvivier C., Jacomet C., Allavena C., Huleux T., Bani-Sadr F., Obry-Roguet V., Makinson A. Kaposi sarcoma in people living with HIV: incidence and associated factors in a French cohort between 2010 and 2015. AIDS. 2020;34(4):569–577. doi: 10.1097/QAD.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 12.Szajerka T., Jablecki J. Kaposi's sarcoma revisited. AIDS Rev. 2007;9(4):230–236. [PubMed] [Google Scholar]
- 13.Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarchoan R., Uldrick T.S. HIV-associated cancers and related diseases. N. Engl. J. Med. 2018;378(11):1029–1041. doi: 10.1056/NEJMra1615896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lebbe C., Garbe C., Stratigos A.J., Harwood C., Peris K., Marmol V.d., Malvehy J., Zalaudek I., Hoeller C., Dummer R., Forsea A.M., Kandolf-Sekulovic L., Olah J., Arenberger P., Bylaite-Bucinskiene M., Vieira R., Middleton M., Levy A., Eggermont A.M., Battistella M., Spano J.P., Grob J.J., Pages C. Diagnosis and treatment of Kaposi's sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC) Eur. J. Cancer. 2019;114:117–127. doi: 10.1016/j.ejca.2018.12.036. [DOI] [PubMed] [Google Scholar]