Abstract
Background
Myocardial infarction is a cardiac adverse event associated with 5-fluorouracil (5-FU). There are limited data on the incidence, risk, and prognosis of 5-FU-associated myocardial infarction.
Objectives
The aim of this study was to examine the risk for myocardial infarction in patients with gastrointestinal (GI) cancer treated with 5-FU compared with age- and sex-matched population control subjects without cancer (1:2 ratio).
Methods
Patients with GI cancer treated with 5-FU between 2004 and 2016 were identified within the Danish National Patient Registry. Prevalent ischemic heart disease in both groups was excluded. Cumulative incidences were calculated, and multivariable regression and competing risk analyses were performed.
Results
A total of 30,870 patients were included in the final analysis, of whom 10,290 had GI cancer and were treated with 5-FU and 20,580 were population control subjects without cancer. Differences in comorbid conditions and select antianginal medications were nonsignificant (P > 0.05 for all). The 6-month cumulative incidence of myocardial infarction was significantly higher for 5-FU patients at 0.7% (95% CI: 0.5%-0.9%) versus 0.3% (95% CI: 0.3%-0.4%) in population control subjects, with a competing risk for death of 12.1% versus 0.6%. The 1-year cumulative incidence of myocardial infarction for 5-FU patients was 0.9% (95% CI: 0.7%-1.0%) versus 0.6% (95% CI: 0.5%-0.7%) among population control subjects, with a competing risk for death of 26.5% versus 1.4%. When accounting for competing risks, the corresponding subdistribution hazard ratios suggested an increased risk for myocardial infarction in 5-FU patients, compared with control subjects, at both 6 months (hazard ratio: 2.10; 95% CI: 1.50-2.95; P < 0.001) and 12 months (hazard ratio: 1.39; 95% CI: 1.05-1.84; P = 0.022).
Conclusions
Despite a statistically significantly higher 6- and 12-month risk for myocardial infarction among 5-FU patients compared with population control subjects, the absolute risk for myocardial infarction was low, and the clinical significance of these differences appears to be limited in the context of the significant competing risk for death in this population.
Key Words: cardiotoxicity, 5-fluorouracil, gastrointestinal cancer, myocardial infarction
Abbreviations and Acronyms: 5-FU, 5-fluorouracil; GI, gastrointestinal; IQR, Interquartile range; MI, myocardial infarction
Central Illustration
5-Fluorouracil (5-FU) is a commonly used chemotherapeutic agent in the treatment of solid malignancies internationally,1,2 including colorectal and head and neck tumors. The most common adverse cardiac events associated with 5-FU are chest pain, presenting as atypical chest pain, angina, or acute coronary syndromes including myocardial infarction (MI).3,4 Other less frequent manifestations of cardiotoxicity include atrial fibrillation and other arrhythmias,5, 6, 7 myocarditis and pericarditis,8 heart failure,9 and MI-related death.10, 11, 12, 13 Dose- and time-dependent coronary vasospasm resulting in both myocyte and endothelial injury may precede these events.14
Managing adverse events by discontinuing cancer treatment is possible, but premature chemotherapy discontinuation may compromise the desired oncological outcomes. Data on adverse events are variable and often based on relatively small case series or small retrospective observational studies with reoccurrence of cardiac complications ranging from 20% to 100%.15
Using nationwide electronic health care record data from Denmark, we examined the 6- and 12-month risk for MI and death in patients with gastrointestinal (GI) cancer treated with 5-FU compared with age- and sex-matched population control subjects.
Methods
Study population
Patients with GI cancer at any stage treated with 5-FU between 2004 and 2016 were identified in the Danish National Patient Registry. Risk set matching was used to identify background population control subjects matched on the basis of age and sex, as well as year and month equivalent to 5-FU treatment initiation, in a 1:2 ratio. For patients with GI cancer, the time-to-event analysis was defined as the duration of 5-FU treatment, and the control subjects were matched to that time. Patients treated with 5-FU and control subjects with pre-existing ischemic heart disease were excluded.
Setting and data sources
This nationwide registry-based study from Denmark including 5-FU-treated patients with GI cancer and population control subjects was performed using the encrypted unique identifier (the Danish Civil Personal Registration number) given to each Danish resident upon birth or immigration. This unique identifier enables linkage on a personal level among nationwide registry data sources. The Danish Civil Registration System also includes data on date of birth and sex.16 In this study, we extracted data on selected in- and outpatient diagnosis codes from the Danish National Patient Registry to define comorbid conditions including hypertension, hypercholesterolemia, diabetes, chronic pulmonary obstructive disease, heart failure, atrial fibrillation, and chronic kidney disease, as well as MI as the outcome. In addition, we used Anatomical Therapeutic Chemical codes for selected prescription medication from the Danish National Prescription Registry to define certain comorbid conditions not solely on hospital contact diagnoses but also on whether certain prescription drugs were dispensed. Specifically, we defined diabetes and hypertension as either a relevant hospital-based diagnosis or dispensed prescription for antidiabetic or antihypertensive drugs, respectively. For the prescription drug–related definition of diabetes, we required that at least 1 antidiabetic drug was dispensed in a 180-day period before study inclusion. For the prescription drug–related definition of hypertension, we required that at least 2 prescribed antihypertensive drugs were dispensed in 2 consecutive 180-day periods prior to inclusion, consistent with prior study definitions.17 Moreover, we also extracted data on select antianginal medications, including nitrates, beta-blockers, and calcium blockers in a 180-day time period prior to study inclusion to understand the potential differences between 5-FU patients and control subjects with respect to these prescription drugs. Last, we used the Danish Cause of Death Registry to obtain information on date of death and presumed cause of death. Specific codes are provided in Supplemental Tables 3 to 5.
Outcomes
The primary outcome was MI. Secondary outcomes were the competing risk for all-cause mortality and presumed cardiovascular cause of death.
Statistics
Categorical variables are presented as counts and percentages and continuous data as median and interquartile range. Aalen-Johansen and Kaplan-Meier estimates were used to determine the cumulative incidence of MI and all-cause mortality, respectively. Given the lack of administrative censoring, we used logistic regression analyses to obtain absolute and relative risks for MI for 5-FU-treated patients relative to control subjects standardized to the age, sex, comorbidity, and pharmacotherapy distributions of all included subjects.18 Age, sex, hypertension, hypercholesterolemia, diabetes, chronic obstructive pulmonary disease, chronic kidney disease, heart failure, and atrial fibrillation as well as selected antianginal medications including nitrates, beta-blockers, and calcium-channel blockers were included as covariates.
Standardization ensured that the age, sex, comorbidity, and pharmacotherapy distributions were equally distributed between 5-FU-treated patients and control subjects. Relative hazards were further calculated from a Cox proportional hazards model including the aforementioned covariates. Furthermore, a Fine and Gray regression analysis with the same covariates was conducted to calculate the subdistribution hazard ratios in the presence of competing risks. Additional analyses comparing 5-FU patients with versus without pre-existing ischemic heart disease were also performed. We also investigated the 5-day and 1-month incident risks for MI after consecutive 5-FU administrations. A 2-sided P value <0.05 was considered to indicate statistical significance. Data management was performed using SAS version 9.4 (SAS Institute) and data analysis using R version 3.6.1 (R Foundation for Statistical Computing).
Ethics
The study was approved by the data-responsible institute in the North Denmark Region of Denmark (internal ID number 2016-3). In Denmark, ethical approval is not required for registry-based studies.
Results
Patients and characteristics
A total of 30,870 patients (46.4% women; mean age 65 years; range: 58-71 years) were included in the final analysis, of whom 10,290 had GI cancer and were treated with 5-FU and 20,580 were control subjects.
Comorbid conditions including diabetes, chronic obstructive pulmonary disease, chronic kidney disease, heart failure, and atrial fibrillation as well as selected antianginal medications including nitrates, beta-blockers, and calcium blockers are noted in Table 1; the baseline prevalence differed slightly between the 2 groups. In addition, the percentage of patients treated with other chemotherapeutic agents, including irinotecan, oxaliplatin, bevacizumab, cetuximab, and panitumumab are also noted in Table 1. Characteristics of patients with versus without pre-existing ischemic heart disease and in comparison with control subjects are shown in Supplemental Table 1.
Table 1.
Study Population
| Patients With GI Cancer (n = 10,290) | Control Subjects (n = 20,580) | |
|---|---|---|
| Age, y | 65 (58-71) | 65 (58-71) |
| Male | 5,511 (53.6) | 11,022 (53.6) |
| Hypertension | 3,499 (34) | 6,646 (32.3) |
| Hypercholesterolemia | 1,750 (17) | 4,692 (22.8) |
| Diabetes | 1,028 (10) | 1,819 (8.8) |
| Chronic pulmonary obstructive disease | 490 (4.8) | 928 (4.5) |
| Heart failure | 159 (1.5) | 527 (2.6) |
| Atrial fibrillation | 457 (4.4) | 910 (4.4) |
| Chronic kidney disease | 159 (1.5) | 282 (1.4) |
| Nitrates | 192 (1.9) | 736 (3.6) |
| Calcium channel blockers | 1,331 (12.9) | 2,696 (13.1) |
| Beta-blockers | 1,022 (9.9) | 2,631 (12.8) |
| Cholesterol medication | 1,633 (15.9) | 4,451 (21.6) |
| Aspirin | 915 (8.9) | 2,811 (13.7) |
| P2Y12 inhibitors | 136 (1.3) | 472 (2.3) |
| Vitamin K antagonist | 324 (3.1) | 621 (3.0) |
| Novel oral anticoagulants | 95 (0.9) | 203 (1.0) |
| Cotreated with irinotecan | 5,087 (49.4) | NA |
| Cotreated with oxaliplatin | 5,002 (48.6) | NA |
| Cotreated with bevacizumab | 2,088 (20.3) | NA |
| Cotreated with cetuximab | 1,256 (12.2) | NA |
| Cotreated with panitumumab | 409 (4.0) | NA |
Values are median (interquartile range) or n (%).
GI = gastrointestinal; NA = not available.
Cumulative incidence of MI and Kaplan-Meier estimates of all-cause mortality
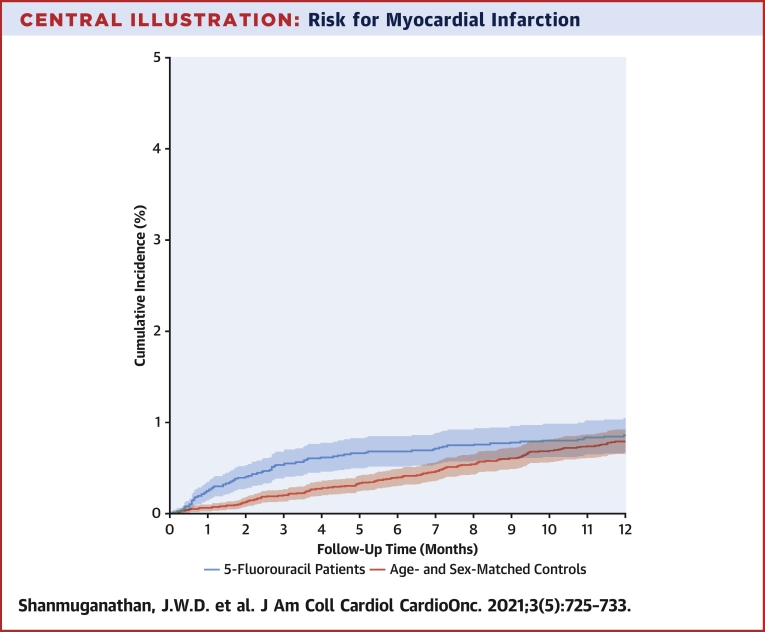
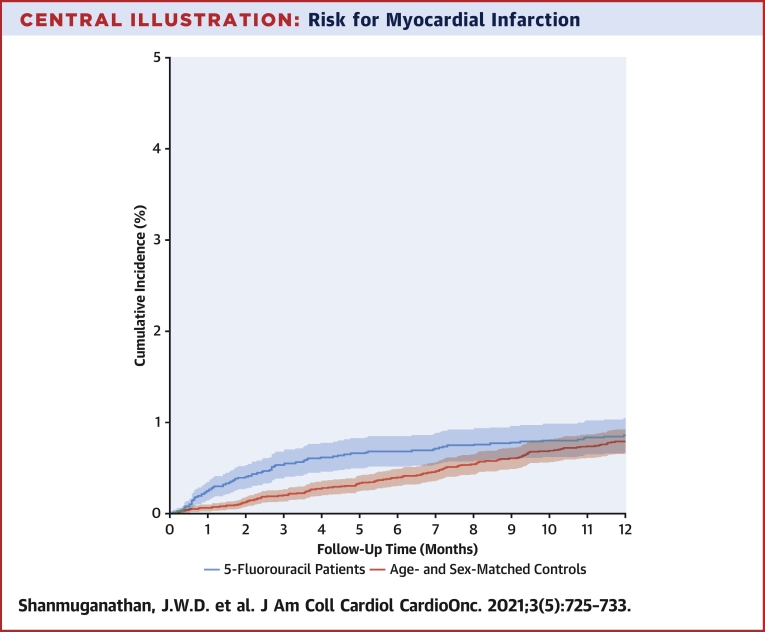
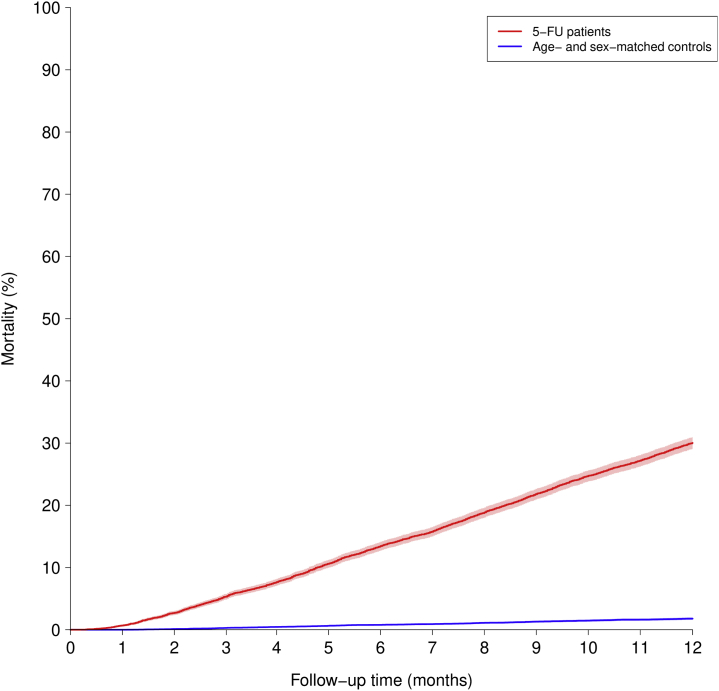
Overall, only 20 subjects (0.1%) were lost to follow-up for the 180-day outcome, and only 35 subjects (0.1%) were lost to follow-up for the 1-year outcome. The 6-month cumulative incidence of MI was significantly higher for 5-FU-treated patients with GI cancer, at 0.7% compared with 0.3% among population control subjects (Central Illustration), with a competing risk for death of 12.1% versus 0.6%. The corresponding 1-year cumulative incidences were 0.9% and 0.6% for 5-FU-treated patients and population control subjects (Central Illustration), with a competing risk for death of 26.5% versus 1.4% (Figure 1). Differences in the cumulative incidences of MI in 5-FU-treated patients in relation to cotreatment with chemotherapeutic agents including irinotecan, oxaliplatin, bevacizumab, cetuximab, and panitumumab were small and are shown in Supplemental Table 2.
Central Illustration.
Risk for Myocardial Infarction
Comparison of 5-fluorouracil (5-FU)–treated patients with gastrointestinal cancer (red) and control group (blue). The 6-month cumulative incidence of myocardial infarction was significantly higher for 5-FU-treated patients at 0.7% compared with 0.3% among population control subjects (P < 0.001). The corresponding 1-year cumulative incidences were 0.9% and 0.6% for 5-FU-treated patients and population control subjects (P = 0.051).
Figure 1.
Mortality Risk
Comparison of 5-fluorouracil (5-FU)–treated patients with gastrointestinal cancer (red) and control group (blue). Six-month competing risk for death was 12.1% versus 0.6%. The corresponding 1-year competing risk for death was 26.5% versus 1.4%.
Standardized and competing risks analysis
The standardized 180-day MI absolute risks were 0.7% (95% CI: 0.5%-0.9%) for 5-FU-treated patients versus 0.3% (95% CI: 0.3%-0.4%) for control subjects (P < 0.001). In comparison, the standardized 1-year MI risk difference was of borderline statistical significance, 0.9% (95% CI: 0.7%-1.0%) versus 0.6% (95% CI: 0.5%-0.7%) (P = 0.051). The average risk ratio standardized to the age, sex, selected comorbidity, and antianginal pharmacotherapy distributions of all subjects for the 180-day risk for MI for 5-FU patients versus control subjects was 2.09 (95% CI: 1.39-2.80). The corresponding 1-year risk for MI was of borderline significance at 1.38 (95% CI: 1.00-1.77) . To allow for censoring and competing risks, we also used Cox proportional hazards models to evaluate the risk for MI. At 6 months, the hazard ratio was significant at 2.19 (95% CI: 1.55-3.10; P < 0.001), and similarly, at 12 months, the hazard ratio was statistically significant, but attenuated, at 1.54 (95% CI: 1.16-2.05; P = 0.003). The corresponding subdistribution hazard ratios from a Fine and Gray model were 2.10 (95% CI: 1.50-2.95; P < 0.001) at 6 months and 1.39 (95% CI: 1.05-1.84; P = 0.022) at 12 months. Altogether, these findings demonstrate a low absolute risk for MI in 5-FU patients with GI malignancy but one that is statistically significantly greater than in control subjects.
Timing of MI
Additionally, we investigated the 5-day and 1-month incident risks for MI after consecutive 5-FU administrations and found that 36 of 65 patients diagnosed with MI (55.4%) had MI events within 5 days of 5-FU administration, and 49 of 65 (75.4%) had events within 1 month of 5-FU administration.
Furthermore, of the 65 patients diagnosed with MI, 23 continued with 5-FU treatment with no hospital admission for reinfarction, and all were alive 3 months post-MI.
MI risk in patients with pre-existing ischemic heart disease
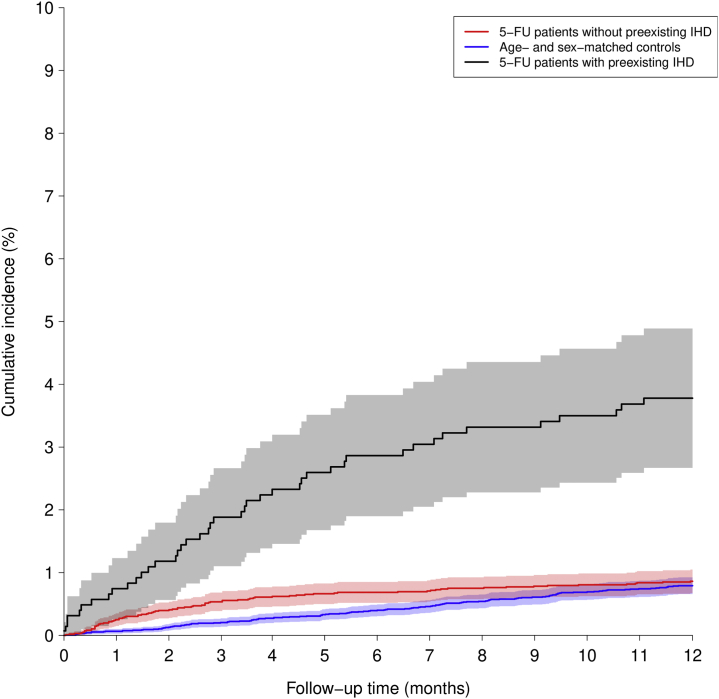
Figure 2 shows that the cumulative incidence of MI in patients with pre-existing ischemic heart disease was significantly higher than in patients without pre-existing ischemic disease, with the standardized 180-day MI risk being 1.7% (95% CI: 0.9%-2.4%; P = 0.012) and the standardized 1-year MI risk being 1.8% (95% CI: 1.1%-2.5%; P = 0.014). The corresponding standardized relative risk for MI among 5-FU patients with pre-existing ischemic heart disease versus 5-FU patients without pre-existing ischemic heart disease shows significant differences in MI risk at 6 months (risk ratio: 2.45; 95% CI: 1.15-3.76) and 1 year (risk ratio: 2.07; 95% CI: 1.09-3.06).
Figure 2.
Risk for Myocardial Infarction in Different Groups
Comparison of 5-fluorouracil (5-FU)–treated patients with gastrointestinal (GI) cancer without ischemic heart disease (IHD) (red), 5-FU-treated patients with GI cancer with pre-existing IHD (black), and control group (blue). The 6-month and 1-year cumulative incidence of myocardial infarction was significantly higher for 5-FU-treated patients with pre-existing IHD compared with the other 2 groups.
Cardiovascular death
The 180-day risk for presumed cardiovascular death for the control group was 0.26%, 1.55% in 5-FU patients without pre-existing ischemic heart disease, and 3.15% in 5-FU patients with pre-existing ischemic heart disease. For the 1-year risk for presumed cardiovascular death, the corresponding figures were 0.51%, 2.65%, and 5.72% for the control group, 5-FU patients without pre-existing ischemic heart disease, and 5-FU patients with pre-existing ischemic heart disease, respectively.
Discussion
In this study, we evaluated the risk for MI and death in patients with GI cancer treated with 5-FU versus age- and sex-matched population control subjects. We evaluated the clinically relevant time points of 6 months and 1 year, as 5-FU regimens are administered over a 6-month period with up to 6 cycles of treatment. The half-year cumulative incidence of MI was significantly higher for 5-FU-treated patients at 0.6%, compared with 0.3% among population control subjects. At 1 year, the risk was attenuated but remained significant in Cox proportional hazards models and Fine and Gray analysis. In evaluating 5-FU administration data, a total of 36 of 65 patients (55.4%) diagnosed with MI experienced MI events within 5 days of 5-FU administration, and this was 49 of 65 (75.4%) within 1 month of 5-FU administration. As such, the absolute risk for MI was overall low, and its effect on mortality is difficult to interpret because of a large competing risk for death.
Furthermore 23 of the 65 patients diagnosed with MI continued with 5-FU treatment, and all were alive 3 months post-MI. Patients with pre-existing ischemic heart disease have significantly higher risk for MI both at 180 days and at 1 year compared with patients without pre-existing ischemic heart disease. In line with our study, prior studies have demonstrated an incidence of MI during the first cycle of 5-FU of 0.3% to 2%. However, the exact timing of MI has not been fully delineated.14 Although our study shows higher 6- and 12-month risk for MI among 5-FU patients compared with population control subjects, overall, the absolute numbers are small.
In a study comparing cardiac toxicity between 5-FU and raltitrexed,19 a total of 155 patients received 5-FU, and 4.5% were diagnosed with MI. In that study, the median time from 5-FU therapy initiation to the onset of cardiac symptoms was 6 days. Other common cardiac adverse events included angina (86%). There was no difference in the type or timing between adverse events that occurred while receiving 5-FU or capecitabine (the oral formulation of 5-FU). Nine patients underwent angiography and 1 patient underwent a thallium scan shortly after experiencing angina episodes. One patient was found to have a left anterior descending coronary artery stenosis and a critical circumflex coronary artery stenosis on angiography that required stenting. The remaining 8 patients had no detectable coronary abnormalities. These results are consistent with previous reports that cardiac toxicities with thymidylate synthase inhibitors frequently occur despite normal coronary blood vessels.20
Our study reported a competing risk for death of 26.5% in 5-FU patients compared with 1.4% in control subjects in the first year. Although cancer remains the primary cause of death, there are cardiovascular concerns in this population. Malignancy is associated with a hypercoagulable state, as cancer cells can activate the coagulation cascade and secrete acute phase reactants, increasing thrombotic risk.21 The risk for thrombosis may be further exacerbated by antiplatelet therapy interruption in cases of surgery. Thrombocytopenia,22,23 malignant GI cancer,24 and metastatic hepatic disease can also significantly increase bleeding risk in patients with cancer. Comorbid conditions such as anemia due to impaired erythropoiesis, hemolysis, chemotherapy, nutritional deficiencies, and immune-mediated mechanisms25 serve to further increase both ischemic and hemorrhagic risk. Major bleeding and thrombotic events are known to be associated with mortality.26 All of these factors are potential confounding factors in this observational study and may to some extent explain the increased rates of MI in patients treated with 5-FU.
Other studies have evaluated cardiac toxicity with 5-FU. A study by Zafar et al27 focused on vasospasm induced by 5-FU, whereas our study focused on MI induced by 5-FU. They found that there was no significant difference in progression-free survival, overall mortality, or cancer-specific mortality between patients who developed vasospasm and those who did not. Altogether, we found that there was higher 6-month and 1-year risk for MI among 5-FU patients compared with population control subjects, although the magnitude of the effect decreased over time, and the overall absolute risk for MI is small, likely because of the competing risk for death in the cancer cohort.28
Study limitations
Cancer itself can increase the risk for MI because of inflammation and other shared mechanisms, and as such, the risk for MI among 5-FU-treated patients with GI cancer in comparison with population control subjects may be subject to confounding, and our results should therefore be interpreted with caution. However, as the relative risk for MI was only slightly elevated, and the absolute risk was low, we therefore suggest that administration of intravenous 5-FU carries a very small risk for MI. We did not compare with other GI cancer groups, but comparison with a GI cancer group not treated with 5-FU but instead treated with surgery, radiation, and/or other conventional or targeted cancer therapies answers a slightly different research question and also holds a risk for introducing confounding by indication. We could not obtain the exact doses of 5-FU to evaluate whether there is a dose-dependent relationship between exposure and outcome of MI. It is hard to clearly differentiate whether it is the cancer itself in the 5-FU-treated patients or the 5-FU-induced cardiotoxicity that may be the underlying mechanism for MI. Important clinical characteristics are lacking, including the stages of GI cancer and the dosing regimens of the treatment with 5-FU. Regarding the coding of 5-FU, all treatment regimens were coded according to a national coding system. This coding system reports all drugs administered, but there was no information regarding dosing. Thus, it is possible to discriminate among the different 5-FU regimens according to the prescribed schedule, but it is not be possible to obtain detailed dosing information (ie, whether the 5-FU bolus in a FOLFIRI regimen was omitted), making it difficult to draw any conclusions regarding MI risk according to regimen. Several other cardiotoxic events, including heart failure, may be attributed to 5-FU exposure, but these were outside the scope of the present study, and further studies addressing such additional cardiovascular events are warranted.
Conclusions
To our knowledge, this is the largest published report of 5-FU-associated MI in patients with GI cancer. There was higher 6- and 12-month risk for MI among 5-FU-treated patients with GI cancer compared with population control subjects, although the overall absolute risk for MI was small, potentially because of the competing risk for mortality in the cancer cohort. Among those diagnosed with MI, more than half of the events occurred within 5 days of 5-FU administration. However, the absolute risk for MI was low, and the clinical significance of these differences appears to be limited in the context of the significant competing risk for death in this population. This may allow patients with GI cancer who develop cardiotoxicity (angina, vasospasm, etc) to be successfully rechallenged with 5-FU therapy.29 Further studies identifying patients at risk for MI following 5-FU treatment are warranted.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Our study demonstrates that patients without pre-existing ischemic heart disease who developed 5-FU-associated MI had significantly higher 6-month and 1-year risk compared with matched population control subjects. Most patients who were diagnosed with MI experienced MI within 5 days of 5-FU administration.
TRANSLATIONAL OUTLOOK: We hypothesize that patients with 5-FU-treated GI-cancer without pre-existing ischemic heart disease have a lower prevalence of MI compared with patients with ischemic heart disease because of lower cardiovascular risk factors. Further studies identifying risk factors for MI following 5-FU treatment are warranted, as are additional studies related to other cardiac toxicities and strategies to mitigate and manage cardiac toxicity risk.
Funding Support and Author Disclosures
Dr El-Galaly was previously employed at Roche; and has received speaker fees from Abbvie. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Grem J.L. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Invest New Drugs. 2000;18(4):299–313. doi: 10.1023/a:1006416410198. [DOI] [PubMed] [Google Scholar]
- 2.Myers C.E. The pharmacology of the fluoropyrimidines. Pharmacol Rev. 1981;33(1):1–15. [PubMed] [Google Scholar]
- 3.de Forni M., Malet-Martiono M.C., Jaillais J., et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: a prospective clinical study. J Clin Oncol. 1992;10(11):1795–1801. doi: 10.1200/JCO.1992.10.11.1795. [DOI] [PubMed] [Google Scholar]
- 4.Lestuzzi C., Vaccher E., Talamini R., et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: an underestimated risk. Ann Oncol. 2014;25(5):1059–1064. doi: 10.1093/annonc/mdu055. [DOI] [PubMed] [Google Scholar]
- 5.Stewart T., Pavlakis N., Ward M. Cardiotoxicity with 5-fluorouracil and capecitabine: more than just vasospastic angina. Intern Med J. 2010;40(4):303–307. doi: 10.1111/j.1445-5994.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 6.Hrovatin E., Elda Viel, Lestuzzi C., et al. Severe ventricular dysrhythmias and silent ischemia during infusion of the antimetabolite 5-fluorouracil and cis-platin. J Cardiovasc Med (Hagerstown) 2006;7(8):637–640. doi: 10.2459/01.JCM.0000237914.12915.dd. [DOI] [PubMed] [Google Scholar]
- 7.Talapatra K., Rajesh I., Rajesh B. Transient asymptomatic bradycardia in patients on infusional 5-fluorouracil. J Cancer Res Ther. 2007;3(3):169–171. doi: 10.4103/0973-1482.37412. [DOI] [PubMed] [Google Scholar]
- 8.Calik A.N., Celiker E., Velibey Y. Initial dose effect of 5-fluorouracil: rapidly improving severe, acute toxic myopericarditis. Am J Emerg Med. 2012;30(1):257.e1–257.e3. doi: 10.1016/j.ajem.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Robben N.C., Pippas A.Q., Moore J.O. The syndrome of 5-fluorouracil cardiotoxicity. An elusive cardiopathy. Cancer. 1993;71(2):493–509. doi: 10.1002/1097-0142(19930115)71:2<493::aid-cncr2820710235>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Manojlovic N., Babic D., Stojanovic Capecitabine cardiotoxicity—case reports and literature review. Hepatogastroenterology. 2008;55(85):1249–1256. [PubMed] [Google Scholar]
- 11.Kosmas C., Kallistratos M.S., Kopterides P., et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol. 2008;134(1):75–82. doi: 10.1007/s00432-007-0250-9. [DOI] [PubMed] [Google Scholar]
- 12.Saif M.W., Shah M.M., Shah A.R. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf. 2009;8(2):191–202. doi: 10.1517/14740330902733961. [DOI] [PubMed] [Google Scholar]
- 13.Jensen S.A., Hasbak P., Mortensen J. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J Clin Oncol. 2010;28(36):5280–5286. doi: 10.1200/JCO.2009.27.3953. [DOI] [PubMed] [Google Scholar]
- 14.Sara J.D., Kaur J., Khodadadi R., et al. 5-Fluorouracil and cardiotoxicity: a review. Ther Adv Med Oncol. 2018;10 doi: 10.1177/1758835918780140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker K., Erckenbrecht J.F., Häussinger D., et al. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs. 1999;57(4):475–484. doi: 10.2165/00003495-199957040-00003. [DOI] [PubMed] [Google Scholar]
- 16.Saif M.W., Tomita M., Ledbetter L. Capecitabine-related cardiotoxicity: recognition and management. J Support Oncol. 2008;6(1):41–48. [PubMed] [Google Scholar]
- 17.Pedersen C.B. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 18.Grant R.L. Converting an odds ratio to a range of plausible relative risks for better communication of research findings [published correction appears in BMJ. 2014;348:g2124] BMJ. 2014;348:f7450. doi: 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- 19.Khan K., Rane Jayant K., Cunningham D., et al. Efficacy and cardiotoxic safety profile of raltitrexed in fluoropyrimidines-pretreated or high-risk cardiac patients with GI malignancies: large single-center experience. Clin Colorectal Cancer. 2019;18(1):64–71.e1. doi: 10.1016/j.clcc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Deboever G., Hiltrop N., Cool M. Alternative treatment options in colorectal cancer patients with 5-fluorouracil- or capecitabine-induced cardiotoxicity. Clin Colorectal Cancer. 2013;12(1):8–14. doi: 10.1016/j.clcc.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Caine G.J., Stonelake P.S., Lip G. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4(6):465–473. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarthy C.P., Steg G., Bhatt D.L. The management of antiplatelet therapy in acute coronary syndrome patients with thrombocytopenia: a clinical conundrum. Eur Heart J. 2017;38(47):3488–3492. doi: 10.1093/eurheartj/ehx531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenoy C., Harjai K.J. Thrombocytopenia following percutaneous coronary intervention. J Interv Cardiol. 2011;24(1):15–26. doi: 10.1111/j.1540-8183.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- 24.Patel N.J., Pau D., Nalluri N., et al. Temporal trends, predictors, and outcomes of in-hospital gastrointestinal bleeding associated with percutaneous coronary intervention. Am J Cardiol. 2016;118(8):1150–1157. doi: 10.1016/j.amjcard.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Birgegard G., Aapro Matti S., Bokemeyer C., et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(suppl 1):3–11. doi: 10.1159/000083128. [DOI] [PubMed] [Google Scholar]
- 26.Mehran R., Pocock S., Nikolsky E., et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events), ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy), and HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trials. J Am Coll Cardiol Intv. 2011;4(6):654–664. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Zafar A., Drobni Z.D., Mosarla R., et al. The incidence, risk factors, and outcomes with 5-fluorouracil-associated coronary vasospasm. J Am Coll Cardiol CardioOnc. 2021;3(1):101–109. doi: 10.1016/j.jaccao.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andersen P., Keiding N. Interpretability and importance of functionals in competing risks and multistate models. Statist Med. 2012;31:1074–1088. doi: 10.1002/sim.4385. [DOI] [PubMed] [Google Scholar]
- 29.Padgimas A., Carver J.R. How to diagnose and manage patients with fluoropyrimidine-induced chest pain: a single center approach. J Am Coll Cardiol CardioOnc. 2020;2(4):650–654. doi: 10.1016/j.jaccao.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.