Abstract
In two-thirds of patients with Zollinger-Ellison syndrome (ZES), fasting serum gastrin (FSG) levels overlap with values seen in other conditions. In these patients, gastrin provocative tests are needed to establish the diagnosis of ZES. Whereas numerous gastrin provocative tests have been proposed, only the secretin, calcium and meal tests are widely used today. Many studies have analyzed gastrin provocative test results in ZES, but they are limited by small patient numbers and methodological differences. To address this issue, we report the results of a prospective National Institutes of Health (NIH) study of gastrin provocative tests in 293 ZES patients and compare these data with those from 537 ZES and 462 non-ZES patients from the literature. In 97–99% of gastrinoma patients an increase in serum gastrin post secretin or post calcium occurred. In NIH ZES patients with <10-fold increase in FSG, the sensitivity/specificity of the widely used criteria were: Δ secretin ≥200 pg/ml (83%/100%), Δ secretin >50% (86%/93%), Δ calcium ≥395 pg/ml (54%/100%) and Δ calcium >50% (78%/83%). A systematic analysis of the sensitivity and specificity of other possible criteria for a positive secretin or calcium test allowed us to identify a new criterion for secretin testing (Δ≥120 pg/ml) with the highest sensitivity/specificity (94%/100%) and to confirm the commonly used criterion for calcium tests (Δ≥395 pg/ml) (62%/100%). This analysis further showed that the secretin test was more sensitive than the calcium test (94% vs. 62%). Our results suggest that secretin stimulation should be used as first-line provocative test because of its greater sensitivity, simplicity and lack of side effects. In ZES patients with a negative secretin test, 38–50% have a positive calcium test. Therefore the calcium test should be considered in patients with a strong clinical suspicion of ZES but a negative secretin test. Furthermore, we found that some clinical (diarrhea, duration of medical treatment), laboratory (BAO) and tumoral (size, extent) characteristics correlate with the serum gastrin increase post secretin and post calcium. However, using the proposed criteria, the result of these provocative tests (i.e. positive or negative) is minimally influenced by these factors. Therefore, secretin and calcium provocative tests are reliable in patients with different clinical, laboratory and tumor characteristics. A systematic analysis of meal testing showed that 54–77% of ZES patients have a <50% postprandial serum gastrin increase. However, 9–20% of ZES patients had a >100% increase post meal, causing significant overlap with antral syndromes. Furthermore, we could not confirm the usefulness of meal tests for localization of duodenal gastrinomas. We conclude that the secretin test is a crucial element in the diagnosis of most ZES patients, the calcium test may be useful in selected patients, but our data suggest that the meal test is not helpful in the management of ZES. For secretin testing, the criterion with the highest sensitivity and specificity is an increase of ≥120 pg/ml, which should replace other criteria commonly used today.
Introduction
In the accompanying paper 30, we analyzed the role of fasting serum gastrin (FSG) and confirmed its importance in providing relevant information for diagnosis and management of ZES. However, like others 39,80,136,140,168,184,196,199,201,205,247,266,306,341,343,380, we found in the majority of patients, the determination of FSG, alone or in combination with acid secretion studies, cannot establish the diagnosis of ZES 30. In these patients, gastrin provocative tests are needed. Of the several tests developed, only secretin, calcium and meal stimulation are widely used in the today 78,110,111,165,165,167,196,219,238,250,397. However, the exact role of these provocative tests, the optimal testing procedures and criteria for positive responses remain controversial, so that some studies even question their overall usefulness 304,343,344. This has occurred because most existing studies have one or multiple limitations including small patient numbers, retrospective nature, non-standardized radioimmunoassay (RIA), different criteria for positivity, different testing procedures and lack of stratification for FSG < or > 10x normal110,120,152,165,167,196,248,249,295,344,360,381,397.
To address these issues, we analyzed the results of secretin, calcium and meal provocative tests in 293 ZES patients from a 31-year prospective NIH study. FSG was determined using well-established gastrin RIAs 30. Provocative tests were performed using well-established time points and standardized stimulant conditions 110,111. Because of the prospective nature of our study with regular follow-ups, including detailed imaging studies and surgical exploration 121,279,283,319,320,349, correlations of test results with clinical, laboratory and tumoral parameters were possible. We compared our data to results from 537 ZES patients from the literature. Furthermore, we determined the specificity of provocative tests by analyzing data from published non-ZES patients. This approach allowed us to determine the optimal criteria for positive secretin and calcium tests and to propose a new criterion for the secretin test with high sensitivity/specificity. In conjunction with the accompanying report 30, this extensive analysis allowed us to identify important guidelines for gastrin provocative tests in the clinical management of ZES patients.
Case History
The detailed history and clinical findings of this 38-year-old man with a 12-year history of recurrent upper gastrointestinal symptoms resistant to standard medical treatment are reported in the accompanying paper 30. The patient was hospitalized for an acute abdomen requiring surgical exploration for a perforated duodenal ulcer with an oversew as well as a vagotomy/pyloroplasty. His FSG was 221 pg/ml (normal <100) while taking omeprazole and he had an elevated serum calcium (2.41 mM, normal 2.05–2.50 mM). The possibility of ZES was raised and because no secretin was available, a calcium infusion study was performed. The calcium test results supported the diagnosis with his FSG increasing from 215 pg/ml to 1010 pg/ml after 180 minutes (Figure 1, middle panel). Subsequently, the patient was referred to the NIH where FSG levels were 200–250 pg/ml (normal <100) and basal acid output (BAO) was 9 mEq/hr 2 weeks after stopping omeprazole. A secretin test with 2-unit/kg bolus of GIH secretin showed an increase from 217 to 1150 pg/ml (Figure 1, left panel) and a meal test showed an increase of only 30% over basal (Figure 1, right panel), supporting the diagnosis of ZES. Imaging revealed three pancreatic lesions while further biochemical studies showed hyperparathyroidism and MEN1 (see accompanying paper, Figure 130). A parathyroidectomy was performed, followed by an abdominal exploration with resection of pancreatic/duodenal tumors and metastatic tumor in lymph nodes. Postoperatively, the secretin test was negative (i.e. <200 pg/ml increase) 110,238,397, but after three years became positive. The patient has remained asymptomatic with no evidence of recurrent tumor on imaging studies, however, the secretin test has remained positive.
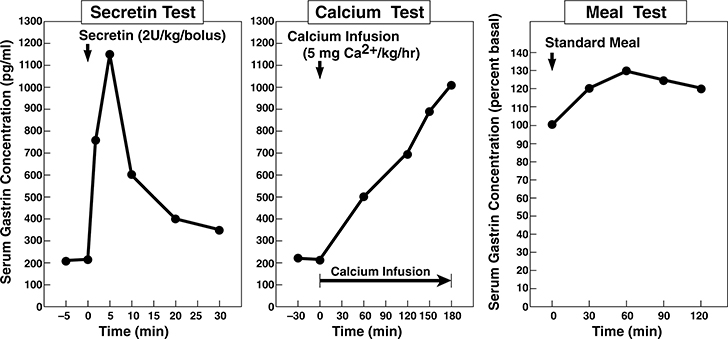
Figure 1.
Secretin, calcium and meal gastrin provocative test results from the ZES patient in the case presentation. Left Panel. Secretin (2 units/kg IV bolus) was given at zero time and serum gastrin was measured at −5,0,2,5,10,15,20 and 30 min post secretin injection. This ZES patient shows the rapid increase in serum gastrin post secretin characteristic of patients with ZES 78,110,153,196,238,250. Middle panel. A calcium infusion of 5 mg/kg/hr was started at 0 time and give for 3 hours as indicated by the solid bar. Serum gastrin levels were measured at −30,0,60,120,150 and 180 min after the infusion was begun. This ZES patient shows the characteristic slow rise in serum gastrin characteristic of patients with ZES 78,110,196,255,295. Right panel. A standard meal was given at 0 time and serum gastrin measured at −15, 0 30,60,90 and 120 min post meal. This ZES patient shows less than a 50% increase in serum gastrin post meal which is reported to be characteristic of patients with ZES and in contrast to patients with antral G cell hyperplasia/hyperfunction which have an exaggerated response (i.e.>100% increase) 7,9,167,196,204
Comment:
This case illustrates the difficulty in making the diagnosis of ZES with the increasing use of potent acid suppressants such as proton pump inhibitors (PPI’s). Therefore, the correct assessment of FSG and gastrin provocative tests have become essential in the diagnosis of ZES 67,144,160,231,244,257. In this patient, the diagnosis was complicated because of an initial FSG elevation of only 2-fold (characteristically increased >10-fold in 40–60% of ZES patients) 110,111,162,165,250. Furthermore, this moderate hypergastrinemia was detected while the patient was taking omeprazole, which can cause FSG elevations in patients without ZES 67,80,156,187 and the BAO was only moderately increased to 9 mEq/hr (mean 16–26 mEq/hr post gastric surgery in ZES patients)160,165,250,315,319. This case illustrates the importance of the secretin test in the diagnosis of ZES and shows that PPI’s should be discontinued prior to FSG determination. As false positive secretin tests have been reported in achlorhydric patients 98,141,219, gastrin provocative testing may not be reliable in patients treated with PPI’s, providing another reason PPI’s should be carefully stopped to determine the diagnosis of ZES. Lastly, this case illustrates the importance of assessing both the secretin test and the FSG level to determine cure 102,283,284. This patient underwent a gastrinoma resection and a parathyroidectomy, both of which can affect the FSG 29,72,123,143,157,159,197,234,263,276,281, and subsequently his FSG levels became normal. However, with time, although the FSG level remained normal, the secretin test became positive showing persistent ZES. This latter finding illustrates the particular importance of secretin testing in MEN1 patients post resection or post parathyroidectomy. Numerous patients with MEN1/ZES in the literature have been reported cured long-term. However, recent studies in MEN/1 ZES patients who underwent repetitive secretin testing show that the long-term cure rate is very low in this patient group (i.e. <1–2%) 210,274,283,286,333.
MATERIALS AND METHODS
All patients admitted to the National Institutes of Health (NIH) with a diagnosis of ZES over a 31-year period (1974–2005) who agreed to participate in the initial and follow-up evaluations were eligible for this study. Details on study organization, diagnostic criteria for ZES or MEN1 and the FSG determination have been described in the accompanying study 30 and the literature 15,16,117,123,220,224,283,320.
FSG levels were determined as described previously 21,30,56,102,110,319. All gastrin provocative tests were performed prior to any gastrinoma treatment in patients with diffuse metastases who subsequently underwent chemotherapy 379, treatment with α-interferon 298 or somatostatin analogues 334. FSG and provocative tests were performed prior to gastrinoma resection in all but 13 patients and were usually performed on the initial NIH admission. Furthermore, because correction of hypercalcemia by parathyroidectomy can affect both the FSG level and gastrin provocative test results 72,123,143,157,159,197,234,263,276, all patients with ZES and MEN1 had provocative testing performed prior to any treatment for hyperparathyroidism.
FSG determinations after stimulation with secretin were carried out in all patients, whereas calcium and standard meal tests were carried out routinely until 1995. Results of serum gastrin responses after stimulation were expressed as the relative rise over the pre-test value (), or the absolute increase expressed in pg/ml (Δ=gastrin max-FSG). For some criteria, the maximal FSG level after provocative stimulation was considered.
Secretin testing was performed using an intravenous bolus injection (2 U/kg body weight) of Secretin-Kabi (Ferring AB, Malmo, Sweden) 110. FSG concentrations were measured at −15, −5 and 0 minutes before as well as 2, 5, 10, 15, 20 and 30 minutes after secretin injection. Secretin test results were analyzed using the criterion of McGuigan and Wolfe 110,238 (Δ≥200 pg/ml); the criterion of Deveney and colleagues 78 (Δ≥110 pg/ml); another criterion of Deveney 77 (Δ>100 pg/ml); the criterion of Lamers and van Tongeren 196 (Δ>50%); the criterion of Modlin and colleagues 260 (Δ≥100%); the criterion of Malageleda 219, (gastrinmax≥335 pg/ml); and one of the criteria proposed by Poynard and Bonfils 301 (gastrinmax≥186 pg/ml).
The calcium infusion provocative test was performed as described previously 78,110 with calcium gluconate (10%) (5 mg calcium/kg per hr)(54 mg/kg per hr of calcium gluconate) given by intravenous infusion for 3 hours. FSG and calcium levels were measured simultaneously at 30 or 15 min before, immediate prior to as well as at 30, 60, 90, 120, 150 and 180 minutes after the calcium infusion. A rise in serum calcium ≥1.5 mEq/l was required for the test to be considered valid. Patients with hypercalcemia, renal disease, cardiac disease or unstable clinical condition did not undergo calcium testing. Calcium test results were analyzed using the criterion of Deveney 78, (Δ≥395 pg/ml); a second criterion of Deveney 77 (Δ>450 pg/ml); the criterion of Lamers and van Tongeren 196 (Δ>50%); the criterion proposed by Modlin and colleagues 260 (Δ≥100%); and the criterion of Malageleda 219, (gastrinmax≥326 pg/ml).
The standard meal test was performed as described previously 111 and consisted of 30 g of protein, 20 g of fat and 25 g of carbohydrate 111,196. FSG levels were measured at −15, 0, 30, 60, 90 and 120 min 111. Test results were analyzed using the criterion of Lamers and van Tongeren 196 (Δ>50%) and the criterion of Malageleda 219 (gastrinmax≥500 pg/ml). Meal tests were not performed if the patient had a prior total gastrectomy or if they could not take oral feedings.
Basal acid output (BAO), maximal acid output (MAO) and assessment of acid secretory control by anti-secretory medication were measured 30,223,225,228,245,310,319,355. Patient histories 30,123,320,409, laboratory investigations 30,117,319,320, endoscopy 30,36,122,320 and imaging studies using both conventional 109,206,227,292,297,332,353,354,382 and functional56,85,185,256,288,357 localization methods were performed. Based on the imaging studies and surgical exploration, patients were stratified into those with primary tumor only, with lymph node metastases and those with liver metastases. Lymph node and liver metastases were established by biopsy in all patients 350,384,409.
Surgical exploration was performed in all patients with ZES without MEN1, without diffuse liver metastases or an illness limiting life-expectancy and in patients with MEN1 with imageable lesions >2.5 cm as described previously 3,30,103,104,112,273,275,277,279,280,282,286,287,349,387. Patients with limited hepatic metastases considered resectable also underwent surgical exploration and resection 52,274,278,289,290,387. For the purpose of analysis of the effect of primary tumor location, patients were divided into those who had only a duodenal primary; a pancreatic primary; a lymph node primary; a primary in another non-duodenal, non-pancreatic, non-lymph node location; or a primary tumor in an unknown location. A primary tumor of the lymph node was defined as occurring in a patient who was disease-free [normal fasting gastrin, negative provocative test and negative imaging 102] post-resection of only a lymph node gastrinoma as described previously 14,273,283. Other non-duodenal-pancreatic-lymph node primary locations were defined as occurring in patients who were disease-free post resection of a gastrinoma from these sites 283,402,403.
Computed tomography or an MRI of the sella turcica and determinations of serum prolactin, calcium (total and ionized) and parathyroid hormone concentrations (using both an assay for the intact PTH molecule and with an antibody directed against the mid-portion of PTH) were also performed to determine if associated MEN1 was present as described previously 16,29,123,283.
Literature review of fasting serum gastrin and provocative tests in patients with ZES and in non-ZES control patients.
To compare our results to previously reported data, we attempted to identify all published cases of ZES with gastrin provocative test data. MEDLINE search and analysis was performed as described in the accompanying paper 30. For the provocative tests, the percent rise of FSG above baseline, Δ (%), and the absolute increase in FSG above baseline, Δ (pg/ml), were calculated. Maximal FSG values after provocative tests were normalized for the highest normal value used in each publication. Furthermore, we collected published data on secretin tests in non-ZES patients with and without achlorhydria. Only the secretin test results from literature patients treated with synthetic or GIH secretin were analyzed, not Boot’s secretin, because it can cause false positive responses and is no longer available 40,43,128,238,265,302,398. Furthermore, only secretin results from literature ZES patients in whom the secretin test was performed using an intravenous bolus administration of a dose equivalent to 2 units per kg body weight of secretin were used for comparison to the NIH results. Results of literature patients tested with 1 unit per kg, 75 total units or ≥3 units per kg were analyzed separately to determine the effect of secretin dose. Results from calcium testing in ZES and non-ZES patients were only included if the calcium test was performed with a calcium infusion over 3 hours using 5 mg calcium per kg per hour.
Statistical analysis
Statistical analysis was performed using the Student t test for unpaired values, the Mann-Whitney-U test, the Fisher’s exact test and the Chi-squared test using the computer programs Statview (SAS Institute, Casy, NC) and Statistica MAC (Statsoft, Tulsa, OK). P values <0.05 were considered significant. All continuous variables with a normal distribution are reported as mean ± SEM, otherwise the median is indicated. Regression lines were calculated using a least-squares analysis.
Results
General characteristics of NIH and literature ZES patients (Table 1)
Table 1.
Comparison of clinical characteristics, laboratory data and tumor features in 293 ZES patients seen at NIH and 537 ZES patients from the literature who underwent gastrin provocative testing.
| No. (%)* | |||
|---|---|---|---|
| Characteristic | NIH | Literature | p |
|
| |||
| Number of patients | 293 | 537 | |
| Male gender | 160/293 (55) | 264/436 (61) | 0.11 |
| Age at evaluation (yr) | |||
| Mean ± SEM [Range] | 47.9 ± 0.6 [15–71] | 46.3 ± 0.65 [15–80] | 0.15 |
| MEN1 present | 85/293 (29) | 105/489 (21) | 0.017† |
| Previous acid-reducing surgery‡ | 44 (15) | 93 (17) | 0.39 |
| Acid secretion (no previous acid-reducing surgery)§ | |||
| BAO (mEq/hr) | |||
| Mean ± SEM [Range] | 43.4 ± 1.6 [1.8–159] | 28.8 ± 1.7 [1.9–143] | 0.0077† |
| MAO (mEq/hr) | |||
| Mean ± SEM [Range] | 63.5 ± 2.1 [3.8–159] | 52.1 ± 2.3 [4–149] | <0.0001† |
| Acid secretion (previous acid-reducing surgery)** | |||
| BAO (mEq/hr) | |||
| Mean ± SEM [Range] | 27.1 ± 3.1 [2–94] | 16.5 ± 1.7 [1–66] | 0.0009† |
| MAO (mEq/hr) | |||
| Mean ± SEM [Range] | 38.3 ± 4.8 [2–140] | 29.4 ± 3.2 [3–90] | 0.14 |
| Gastrin†† | |||
| Fold fasting serum gastrin‡‡ | |||
| Median [Range] | 6.37 [0.96–5500] | 4.83 [0.13–50000] | <0.0001† |
| Δ secretin (pg/ml) | |||
| Median [Range] | 744 [0–3000000] | 600 [−536–4000000] | 0.048† |
| Δ calcium (pg/ml) | |||
| Median [Range] | 850 [–10–208000] | 994 [0–168000] | 0.29 |
| Δ meal (pg/ml) | |||
| Median [Range] | 212 [−40000–60000] | 132 [−1750–35102] | 0.082 |
| Primary tumor localization | |||
| Pancreatic | 81/293 (28) | 107/300 (36) | 0.036† |
| Duodenum | 110/293 (38) | 68/300 (23) | <0.0001† |
| Other§§ | 36/293 (12) | 35/300 (12) | 0.81 |
| Unknown*** | 78/293 (27) | 96/300 (32) | 0.15 |
| Tumor extent | |||
| Localized disease)††† | 247/293 (84) | 160/227 (70) | 0.0004† |
| Distant metastases ‡‡‡ | 46/293 (16) | 67/227 (30) | 0.0004† |
Abbreviations: ZES: Zollinger-Ellison syndrome, NIH: National Institutes of Health, yr: years, SEM: standard error of the mean, MEN1: multiple endocrine neoplasia type 1, BAO: basal acid output, MAO: maximal acid output.
Numbers in denominator are numbers of patients with data available.
indicates a significant difference (p<0.05).
Including patients with partial gastrectomy (literature 66, NIH 23), vagotomy/drainage (literature 45, NIH 17) and total gastrectomy (literature 15, NIH 6).
BAO data from 230 literature and 240 NIH patients, MAO data from 152 literature and 202 NIH patients without previous acid-reducing surgery.
BAO data from 73 literature and 39 NIH patients, MAO data from 45 literature and 36 NIH patients with previous acid-reducing surgery..
Data on fasting serum gastrin available for all patients, secretin tests for 355 literature and 279 NIH patients, calcium stimulation for 212 literature and 207 NIH patients, the meal test data for 112 literature and 229 NIH patients. The Δ calculated for the stimulation tests corresponds to the difference between the gastrin value showing the greatest change after stimulation and the basal gastrin value.
Expressed in fold of the highest normal value.
‘Other’ includes 16 primary tumors of the lymph node, 4 gastric, 6 ovary, 5 liver, 2 jejunal for the literature data; 20 lymph node, 5 liver, 3 stomach 2 bile duct, 2 heart, 1 jejunum, 1 omentum and 1 lung tumor for the NIH patients.
‘Unknown’ includes patients without tumor evidence on imaging studies who did not undergo surgical exploration or for whom surgical data was not available.
Only patients with no evidence of liver or other distant metastases
Including 60 patients with liver metastases, 1 lung, 3 bone, 1 peritoneal and 1 duodenal for the literature patients and 45 liver metastases for the NIH patients.
Gastrin provocative test results from 293 NIH and 537 literature ZES patients were analyzed and their clinical/laboratory/tumor characteristics compared (Table 1). In agreement with most large series 49,60,92,96,171,253,312,320,339,343,360,383, 29% of NIH patients had ZES with MEN1. This was significantly higher (p=0.017) than 21% of literature ZES patients. Similar to most large series of ZES patients 320, there was a slight male predominance both in the NIH and literature patients. Acid hypersecretion, which is a constant feature of ZES 11,164,166,199,217,240,248–251,315,319,323,343,360,377,383,391, was higher in NIH than in literature patients (Table 1). Increased maximal acid output, which is another characteristic feature of ZES 165,320, was elevated in both groups. Hypergastrinemia, a recognized constant feature of ZES 161,164,166,250, was significantly more pronounced in NIH than in literature patients (median 6.4-fold vs. 4.8-fold increase). The median FSG increase after secretin or calcium (150%) and after a standard meal (33%) was similar in NIH and literature patients (Table 1). In accordance with most recent series 142,145,166,275,279,283,285,286,356, duodenal gastrinomas were more frequent than pancreatic gastrinomas among NIH patients (Table 1). This was not true for literature patients, possibly reflecting the fact that in most early series, the duodenum was not systematically explored at surgery 275,279,280,285,363. In both the NIH and literature groups, primary gastrinomas occurred in 12% of patients in non-duodenal/non-pancreatic locations (Table 1), including lymph nodes and some rare extra-abdominal locations 1,118,160,166,226,270,339. Similar to most series 160,166,339, in one third of patients, the site of the primary was not established. In accordance with recent studies, < 50% of patients had distant metastases and one-third had local lymph node metastases 14,160,166,273,283,286. In contrast to studies of 1960–1980 60,92,319,320,347,360,383, prior gastric acid-reducing surgery occurred in <20% of patients, while in most patients, gastric hypersecretion was controlled either with histamine H2-receptor antagonists or PPI’s 49,60,63,92,119,160,161,163,166,383,397.
In accordance with previous studies 110,143,159 sporadic and MEN1/ZES patients had comparable FSG levels (642 vs. 748 pg/ml) and FSG levels after secretin (1513 vs. 2192 pg/ml) or calcium (1550 vs. 2895 pg/ml). However, sporadic ZES patients had significantly lower postprandial gastrin values (810 vs. 1550 pg/ml, p<0.05).
Results of gastrin provocative tests in NIH and literature patients (Tables 2 and 3)
Table 2.
Results of provocative tests with secretin, calcium or a standard meal in ZES patients seen at NIH and Zollinger-Ellison patients from the literature.
| No. (%) | |||
|---|---|---|---|
| NIH | Literature | p | |
|
| |||
| Δ post secretin (pg/ml) * | (n=280) | (n=355) | |
| Decrease | 1 (1) | 3 (1) | 0.44 |
| No change | 3 (1) | 2 (1) | 0.46 |
| Increase | |||
| 1–100 | 13 (4) | 9 (2) | 0.15 |
| 101–199 | 19 (7) | 28 (8) | 0.60 |
| 200–500 | 72 (26) | 111 (32) | 0.12 |
| 501–999 | 48 (17) | 61 (17) | 0.99 |
| 1000–4999 | 73 (26) | 102 (28) | 0.46 |
| ≥ 5000 | 51 (18) | 39 (11) | 0.0095† |
|
| |||
| Δ post calcium (pg/ml) ‡ | (n=208) | (n=212) | |
| Decrease | 3 (1) | 0 (0) | 0.081 |
| No change | 3 (1) | 3 (2) | 0.98 |
| Increase | |||
| 1–100 | 13 (7) | 7 (3) | 0.18 |
| 101–199 | 17 (8) | 11 (5) | 0.22 |
| 200–500 | 46 (22) | 48 (23) | 0.90 |
| 501–999 | 32 (16) | 37 (17) | 0.57 |
| 1000–4999 | 64 (31) | 69 (33) | 0.69 |
| ≥ 5000 | 30 (14) | 37 (17) | 0.40 |
|
| |||
| Δ post meal (% change from basal) § | (n=238) | (n=112) | |
| Decrease | |||
| ≤ 49% | 30 (13) | 10 (9) | 0.31 |
| 50–99 | 2 (1) | 1 (1) | 0.96 |
| ≥ 100 | 0 (0) | 0 (0) | 0.99 |
| No change | 7 (3) | 9 (8) | 0.033† |
| Increase | |||
| 0–49 | 96 (40) | 58 (52) | 0.044† |
| 50–99 | 55 (23) | 17 (15) | 0.087 |
| ≥ 100 | 48 (20) | 17 (15) | 0.26 |
i.v. bolus of 2 UI/kg GIH secretin. Shown is the maximal absolute increase in serum gastrin (in pg/ml) after secretin injection. Literature data prior to 1980 are from 51,68,69,78,79,115,124,132,196,200,232,233,254,255,291,293,296,371,374,393; literature data from 1980–1989 are from 4,18,20,38,54,73,74,89,91,95,97,99,100,113,125,127,130,134,138,157,169,170,175,178,179,188,218,222,239,240,242,252,258,259,261,272,305,309,315,316,321,322,325,326,341,345,362,364,365,375,395,396,401,407,408,411; literature data from 1990–2005 are from 5,7,8,17,27,35,37,57,61,65,66,70,71,86,94,105,116,131,150,154,172–174,180,202,211,213,241,243,271,314,317,327,328,346,363,367,370,376,392,406,410,412.
indicates a significant difference (p<0.05).
I.v. infusion of 5 mg/kg/hr calcium or equivalent. Shown is the maximal absolute increase in serum gastrin (in pg/ml) after beginning of calcium infusion. Literature data are from 10,12,13,23,25,46,47,53,54,62,64,69,75,76,78,94,100,107,115,124,139,146,153,175,181,190,196,207–209,214,232,233,235,237,241,255,262,265,267,296,305,318,324,330,335,338,341,347,358,360,366,368,372,376,385,389.
Table 3.
Distribution of fasting serum gastrin values and evaluation of different proposed criteria for positive results with provocative tests using secretin, calcium and a standardized meal. Data from 537 ZES patients from the literature and 293 ZES patients seen at NIH.
| Variable | No. of patients (%) | |||||
|---|---|---|---|---|---|---|
| NIH |
Literature* |
|||||
| All patients | Patients with FSG < 10-fold increase | All patients | Patients with FSG < 10-fold increase | |||
|
| ||||||
| Fasting serum gastrin | ||||||
| Number of patients | 293 | 190 | 537 | 400 | ||
| Fasting serum gastrin (fold normal) | ||||||
| ≤ 1.0 | 1 (0.3) | 1 (0.5) | 20 (3.7) | 20 (5) | ||
| 1.1 – 4.9 | 111 (38) | 111 (58) | 257 (48) | 257 (64) | ||
| 5.0 – 9.9 | 78 (27) | 78 (41) | 123 (23) | 123 (31) | ||
| ≥ 10 | 103 (35) | 0 (0) | 137 (25) | 0 (0) | ||
| Secretin test | ||||||
| Number of patients | 280 | 181 | 355 | 273 | ||
| Change of serum gastrin after secretin | ||||||
| Δ > 100 pg/ml† | 264 (94) | 168 (93) | 341 (96) | 262 (96) | ||
| Δ‡ ≥ 110 pg/ml§ | 262 (93) | 166 (92) | 341 (96) | 262 (96) | ||
| Δ ≥ 200 pg/ml** | 245 (87) | 151 (83) | 313 (88) | 237 (87) | ||
| Δ > 50%†† increase‡‡ | 237 (84) | 155 (86) | 286 (81) | 229 (84) | ||
| Δ >100% increase§§ | 178 (63) | 112 (62) | 201 (57) | 165 (60) | ||
| Maximal serum gastrin value after secretin | ||||||
| gastrin max. ≥ 186 pg/ml*** | 280 (100) | 180 (100) | 353 (99) | 271 (99) | ||
| gastrin max. ≥ 335 pg/ml††† | 261 (93) | 162 (89) | 345 (97) | 263 (96) | ||
| Calcium test | ||||||
| Number of patients | 208 | 142 | 212 | 157 | ||
| Change of serum gastrin after calcium | ||||||
| Δ ≥ 450 pg/ml† | 134 (64) | 73 (51) | 148 (70) | 100 (64) | ||
| Δ > 395 pg/ml§ | 139 (67) | 77 (54) | 159 (75) | 110 (70) | ||
| Δ > 50% increase‡‡ | 164 (79) | 111 (78) | 190 (90) | 143 (91) | ||
| Δ >100% increase§§ | 118 (57) | 83 (58) | 158 (75) | 120 (76) | ||
| Maximal serum gastrin value after calcium | ||||||
| gastrin max. ≥ 326 pg/ml††† | 201 (97) | 135 (95) | 195 (92) | 141 (90) | ||
| Meal test | ||||||
| Number of patients | 238 | 162 | 112 | 79 | ||
| Change of serum gastrin after meal | ||||||
| < 50% increase‡‡ | 136 (57) | 87 (54) | 78 (67) | 61 (77) | ||
| <100% increase | 190 (80) | 131 (81) | 95 (85) | 72 (91) | ||
| Maximal serum gastrin value after meal | ||||||
| gastrin max. > 500 pg/ml††† | 175 (74) | 100 (62) | 76 (68) | 45 (57) | ||
Literature data are from publications listed in Table 2.
Criterion proposed by Deveney 77
The Δ calculated corresponds to the difference between the gastrin value showing the greatest change after stimulation and the basal gastrin value.
Criterion proposed in publication 78
Criterion proposed in publication238
% change (increase or decrease) is calculated as the difference between the gastrin value showing the greatest change after stimulation and the basal gastrin value divided by the basal value and multiplied by 100.
Criterion proposed in publication 196
Criterion proposed in publication 260
Criterion proposed in publication 301
Criterion proposed in publication 219
To differentiate ZES from other conditions causing hypergastrinemia, various gastrin provocative tests have been developed. Today, the most commonly used are the secretin, calcium and meal tests 40,78,110,111,153,164–167,189,196,238,243,247,295,397. In previous studies, small number of ZES cases, different methodologies and failure to consider separately patients with FSG<10-fold and >10-fold increased have led to controversy about the role of gastrin provocative tests in ZES 110,304,343,344. It is especially important to consider separately patients with FSG< and >10-fold increased because the combination of FSG >10-fold increased and hyperchlorhydria (preferably pH<2) is generally considered to be pathognomonic of ZES 40,119,142,248,250,319,360,381,397. Therefore, in the present study, both the NIH and literature patients were stratified into those with FSG<10 fold or ≥10-fold increased.
The provocative test results (secretin, calcium, meal) were similar in NIH and literature ZES patients (Table 2). In particular, no change or a decrease in FSG levels after secretin or calcium were exceptional, occurring in < 2% of NIH and literature patients (Table 2). The median FSG increase after secretin was similar in NIH and literature patients (i.e. 744 and 600 pg/ml, Table 1), as was the median change after calcium (850 and 994 pg/ml, Table 1). In both groups, few patients (2–7%) had an absolute increase in FSG ≤100 pg/ml or ≤200 (5–8%). The distribution of FSG levels was also generally similar in the NIH and literature ZES patients (Table 3, top). Because only 25–35% of NIH or literature ZES patients have a FSG ≥ 10 fold increased (Table 3), our results confirm that approximately two-thirds of all FSG levels in ZES fall into a non-diagnostic range (i.e. <10 fold increased with acidic gastric pH). Therefore, these FSG values will overlap with those seen in other conditions with hypergastrinemia/hyperchlorhydria (i.e. H. pylori infection, antral G cell hyperplasia/hyperfunction, renal failure, post small bowel resection, gastric antral obstruction 7,9,158,165,167,204,246,250,397) and gastrin provocative tests are needed.
Numerous criteria have been proposed for a positive gastrin provocative test. The most widely used secretin test criterion, (Δ ≥200 pg/ml), is derived from a review of 14 original studies by McGuigan and Wolfe 238. In these studies, different secretin preparations (GIH, Boots), doses and modes of administration (bolus, infusion) were used. The Δ ≥200 pg/ml criterion was selected because it allowed the diagnosis of a maximal number of ZES patients without false positives. In that review, this criterion was reported to have a sensitivity of 100% when 2 units/kg bolus injection of secretin was performed. However, we found it to be less sensitive, with 87% of all NIH and 88% of all literature patients having a positive secretin test (Table 3). The sensitivity decreased even further to 83% for the NIH and 87% for the literature ZES patients when the clinically relevant group of patients with FSG <10-fold increased were considered (Table 3).
Deveney proposed Δ secretin≥110 pg/ml and Δ calcium>395 pg/ml had the greatest discriminatory value 78. When applied to our populations, 93% of all NIH patients and 96% of all literature patients had a positive secretin test, whereas 67% of all NIH and 75% of all literature patients had a positive calcium test. The secretin test’s sensitivity remained unchanged in patients with FSG<10-fold increased, whereas the calcium test sensitivity decreased to 54 and 70%. In another publication 77, the authors proposed slightly different criteria (Δ secretin>100 pg/ml, Δ calcium≥450 pg/ml), which lead to comparable results in our study (Table 3).
Lamers and van Tongeren 196 proposed FSG increases >50% for the secretin and calcium test and <50% for the meal were characteristic of ZES. In our study, 84% of all NIH and 81% of all literature patients had a positive secretin test, 79 and 90% had a positive calcium test and 57 and 67%, respectively, had a positive meal test (Table 3). These percentages decreased slightly when only patients with a <10-fold FSG increase were considered (Table 3). However, 20% of all NIH and 15% of all literature ZES patients had a ≥100% increase post meal, a value considered characteristic of antral G-cell hyperplasia 7,9,108,158,167,204,336,347. In patients with FSG<10-fold increased, it was 19%/9%, respectively (Tables 2, 3).
Malagelada et al. 219 proposed criteria on the basis of the highest FSG value after stimulation. In their study, the authors used maximal FSG levels post provocation of 335 pg/ml, 326 pg/ml and 500 pg/ml for secretin, calcium and meal tests, respectively. In our study, 93% of all NIH and 97% of all literature patients had a positive secretin test, 97% and 92% had a positive calcium test and 74% and 68%, respectively, had a positive meal test (Table 3). These percentages decreased slightly in patients with a <10-fold FSG increase (Table 3).
Modlin et al. 260 proposed a FSG increase >100% post secretin or calcium to diagnose ZES. In our study, 63% of all NIH and 57% of literature patients had a positive secretin test, whereas 57 and 75%, respectively, had a positive calcium test (Table 3). These percentages did not change significantly in patients with FSG<10-fold increased were considered (Table 3).
Poynard and Bonfils 301 proposed that a combination of 4 criteria gave the best sensitivity and specificity with the best single criterion being a maximal FSG after secretin ≥186 pg/ml. With these criteria, 100% of NIH and 99% of literature patients had a positive secretin test and these percentages were identical in patients with a <10-fold FSG increase (Table 3).
In both the NIH and the literature ZES patients, the criterion of an increase of either 110 or 100 pg/ml post secretin had a greater sensitivity than the criterion of a 200 pg/ml increase (p<0.003).
Correlation of FSG and serum gastrin increase after gastrin provocative tests (Figure 2)
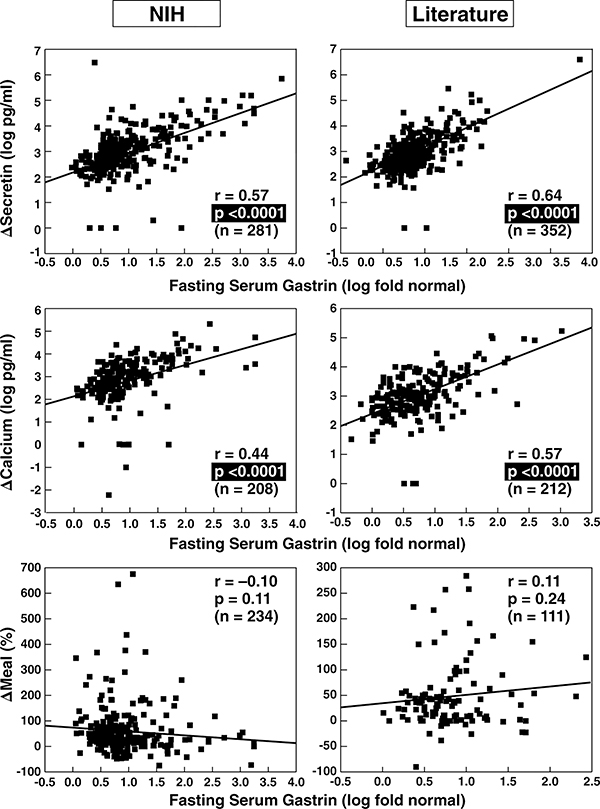
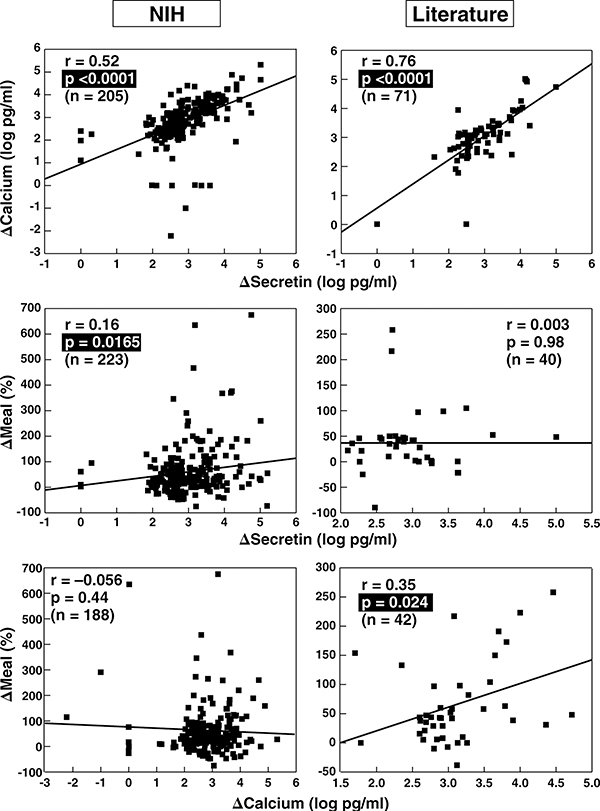
Figure 2.
Correlation between fasting serum gastrin and secretin, calcium and meal provocative test results in ZES patients. Data from 280 NIH patients and 355 from the literature are shown. The correlation between the fasting serum gastrin level expressed as a fold increase and the absolute change in serum gastrin concentration after secretin injection (upper panels) or calcium infusion (middle panels) as well as the relative change in serum gastrin concentration after a standard meal (lower panels). Each point represents data from one patient. Indicated are the regression line and the correlation coefficient (r) using a least-squares analysis. Literature data are from publications listed in Table 2.
To investigate the influence of FSG levels on provocative testing, we correlated the FSG and the absolute increase in FSG after secretin (Δ secretin), calcium (Δ calcium) and a meal (Δ meal) in NIH and literature patients. The magnitude of the FSG correlated well with Δ secretin (Figure 2, top panel) and Δ calcium (Figure 2, middle panel) in NIH and literature patients, whereas there was no correlation with Δ meal (Figure 2, bottom panel).
To analyze if these correlations still exist after correction for higher FSG values, we correlated the relative increase in serum gastrin after secretin or calcium with FSG levels and found no significant correlation (r=[−0.068] [−0.18], data not shown), suggesting that the magnitude of the FSG contributes to the higher Δ response but is not associated with a proportional gastrin release post stimulation.
Influence of clinical and laboratory variables on gastrin provocative tests in NIH and literature ZES patients (Tables 4–7, Figure 3)
Table 4.
Effect of different clinical characteristics and tumor features on gastrin levels after secretin, calcium and meal stimulation in 293 Zollinger-Ellison patients seen at NIH*
| Δ Secretin (pg/ml)† | Δ Calcium (pg/ml)† | Δ Meal (%)* | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | Variable present | Variable absent | Variable present | Variable absent | Variable present | Variable absent |
|
| ||||||
| Age at onset ≤ 40 years | ||||||
| Median [Range] | 831 [0–3000000] | 732 [−150–156000] | 697 [−166–208000] | 935 [−10–75250] | 45§ [−48–2376] | 30§ [−75–8265] |
| Age at diagnosis ≤ 47 years | ||||||
| Median [Range] | 972 [0–3000000] | 686 [−150–47618] | 830 [−166–208000] | 865 [−10–75250] | 43 [−73–675] | 30 [−75–8265] |
| Caucasian race | ||||||
| Median [Range] | 673 [−150–3000000] | 1129 [41–158000] | 754 [−166–208000] | 1332 [24–46000] | 38 [−48–2376] | 33 [−75–8265] |
| Duration from onset to diagnosis ≤ 3 years | ||||||
| Median [Range] | 899 [−150–700000] | 710 [0–3000000] | 919 [−166–208000] | 691 [0–75250] | 33§ [−73–346] | 48§ [−75–8265] |
| Duration from onset to 1st NIH evaluation ≤ 4.9 years | ||||||
| Median [Range] | 686 [−150–3000000] | 813 [0–700000] | 856 [−166–208000] | 709 [0–75250] | 35 [−48–675] | 40 [−75–8265] |
| Prior PPI therapy | ||||||
| Median [Range] | 1390 [0–103000] | 686 [−150–3000000] | 1660 [1–208000] | 724 [−166–75250] | 53 [−21–2376] | 36 [−75–8265] |
| Duration of prior medical treatment ≤ 1.4 years | ||||||
| Median [Range] | 646‡ [0–103000] | 930‡ [−150–3000000] | 814 [−10–208000] | 959 [−166–53600] | 35 [−75–635] | 42 [−73–8265] |
| Prior gastrinoma resection | ||||||
| Median [Range] | 571 [67–158000] | 744 [−150–3000000] | 501 [460–10144] | 850 [−166–208000] | 55 [−39–138] | 37 [−75–8265] |
| History of following symptoms at 1st NIH evaluation: | ||||||
| Pain | ||||||
| Median [Range] | 720 [0–158000] | 767 [−150–3000000] | 842 [−10–208000] | 818 [−166–75250] | 34 [−75–8265] | 49 [−38–635] |
| Diarrhea | ||||||
| Median [Range] | 1024‡ [0–700000] | 463‡ [−150–3000000] | 935 [−166–208000] | 691 [−10–25105] | 37 [−75–2376] | 36 [−45–8265] |
| Esoph. stricture/dysphagia | ||||||
| Median [Range] | 1377 [0–101650] | 680 [−150–3000000] | 3260 [212–46000] | 814 [−166–208000] | 64§ [−11–675] | 35§ [−75–8265] |
| Hx of confirmed ulcer | ||||||
| Median [Range] | 744 [0–700000] | 680 [−150–3000000] | 850 [−166–208000] | 814 [0–75250] | 34 [−48–8265] | 53 [−75–635] |
| Primary lymph node tumor | ||||||
| Median [Range] | 1130 [355–8944] | 798 [0–3000000] | 2116 [15–8892] | 850 [0–208000] | 27 [−47–368] | 36 [−73–2376] |
| Tumor extent: | ||||||
| Primary only | ||||||
| Median [Range] | 715 [102–300000] | 1305 [0–700000] | 711 [174–9380] | 1410 [0–208000] | 35 [−45–675] | 37 [−73–2376] |
| Liver metastases | ||||||
| Median [Range] | 904 [0–156000] | 744 [−150–3000000] | 575 [0–53600] | 856 [−166–208000] | 22§ [−73–182] | 41§ [−75–8265] |
| Lymph node metastases | ||||||
| Median [Range] | 1485§ [2–700000] | 644§ [0–3000000] | 1420 [0–208000] | 850 [0–75250] | 37 [−73–2376] | 35 [−45–675] |
| Size of largest resected tumor: | ||||||
| ≤ 1 cm | ||||||
| Median [Range] | 471‡ [102–26446] | 981‡ [−150–3000000] | 532§ [15–6803] | 1014§ [−166–208000] | 51 [−32–259] | 34 [−75–8265] |
| ≥ 3 cm | ||||||
| Median [Range] | 1686‡ [0–3000000] | 657‡ [−150–700000] | 838 [0–75250] | 842 [−166–208000] | 36 [−73–2376] | 38 [−75–8265] |
Abbreviations: see table 1; esoph., esophageal; Hx, history.
For continuous variables, the median value is used to divide the patients into 2 groups.
Δ secretin, Δ Calcium and Δ meal were calculated as indicated in Methods.
P < 0.01
P < 0.05
Table 7.
Effect of various clinical characteristics, laboratory data and tumor features on serum gastrin levels after a standard test meal in 208 patients seen at NIH and 112 ZES patients from the literature.
| Δ Meal (%)* | ||||||
|---|---|---|---|---|---|---|
| NIH | Literature† | |||||
|
|
|
|||||
| Variable‡ | Variable Present | Variable Absent | p | Variable Present | Variable Absent | p |
|
| ||||||
| Age at evaluation > 48 years | ||||||
| median [range] | 32 [−75–8265] | 42 [−48–675] | 0.17 | 38 [−10–155] | 31 [−90–900] | 0.45 |
| Male gender | ||||||
| median [range] | 33 [−75–8265] | 44 [−73–675] | 0.18 | 24 [−25–173] | 40 [−90–900] | 0.40 |
| MEN1 | ||||||
| median [range] | 59 [−75–186] | 34 [−48–8265] | 0.019§ | 38 [−90–191] | 37 [−38–900] | 0.99 |
| Prior acid-reducing surgery | ||||||
| median [range] | 24 [−42–8265] | 41 [−75–2376] | 0.17 | 50 [−25–284] | 36 [−90–900] | 0.14 |
| BAO > 15 (prior acid-reducing surgery) | ||||||
| Median [Range] | 24 [−40–8265] | 28 [−42–83] | 0.67 | 75 [−25–284] | 50 [27–191] | >0.99 |
| MAO > 51 (no prior acid-reducing surgery) | ||||||
| Median [Range] | 46 [−75–675] | 26 [−45–635] | 0.067 | 10 [−10–900] | 37 [−7–153] | 0.43 |
| Primary tumor size > 1.5 cm (all pts.) | ||||||
| Median [Range] | 28 [−75–675] | 38 [−73–2376] | 0.26 | No Data** | No Data | |
| Primary tumor size ≥ 3 cm (all pts.) | ||||||
| Median [Range] | 22 [−75–675] | 36 [−75–2376] | 0.30 | No Data | No Data | |
| Primary localization pancreas | ||||||
| Median [Range] | 26 [−75–190] | 39 [−73–2376] | 0.054 | 58 [−25–284] | 46 [−7–900] | 0.89 |
| Primary localization duodenum | ||||||
| Median [Range] | 48 [−73–2376] | 26 [−75–675] | 0.0081§ | 82 [−7–223] | 55 [−25–900] | 0.95 |
| Localized disease†† | ||||||
| Median [Range] | 39 [−75–8265] | 26 [−73–182] | 0.088 | 49 [−38–900] | 34 [−25–284] | 0.92 |
Abbreviations: See Table 1. Pts., patients; mts., metastases.
‘Δ meal (%)was calculated as described in Methods.
Literature data are from publications listed in Table 2.
For continuous variables, the median of the total population (NIH+literature) is used to divide the patients into 2 groups.
* indicates a significant difference (p<0.05).
‘No Data’ indicates that information was available on less than 10 patients.
‘Localized disease’ includes all patients without evidence for liver or other distant metastases.
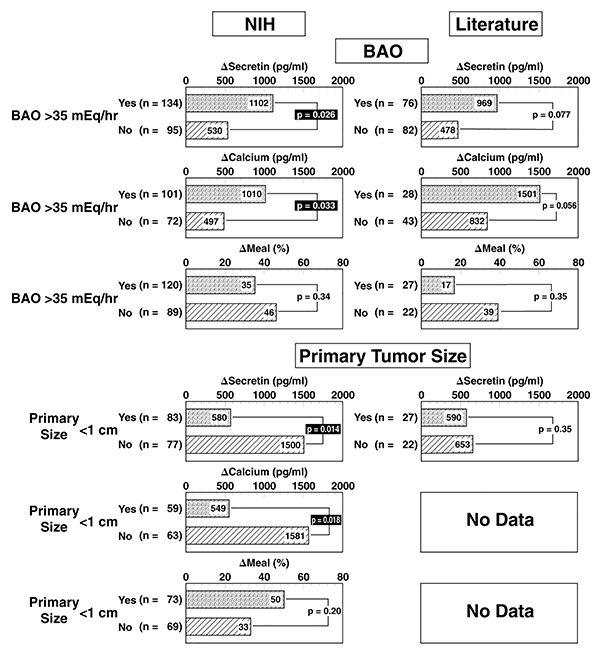
Figure 3.
Effect of the magnitude of basal hyperchlorhydria or primary tumor size on provocative test results after stimulation with secretin, calcium or a standard meal. Indicated is the median value of the absolute (secretin, calcium) serum gastrin increase after stimulation in ZES patients from NIH and from the literature. The meal results are expressed as the median percentage gastrin increase over the pretreatment value. Literature data are from publications listed in Table 2. Insufficient data on primary tumor size in patients in the literature was found to allow comparison of this variable in literature patients. Only patients without previous gastric acid-reducing surgery were included in the analysis of the effect of BAO and only patients without liver metastases were included in the analysis of the effect of primary tumor size. Numbers in parenthesis refer to number of patients with the indicated variable.
To determine whether clinical or laboratory variables might influence gastrin provocative testing, these variables were correlated with the increase in FSG levels after stimulation. In NIH patients, extensive data was available allowing a detailed analysis (Table 4), while the effect of only selective variables could be be compared in literature patients (Tables 5–7).
Table 5.
Effect of various clinical characteristics, laboratory data and tumor features on serum gastrin levels after secretin stimulation in 355 ZES patients from the literature and 280 patients seen at NIH.
| Δ Secretin (pg/ml)* | ||||||
|---|---|---|---|---|---|---|
| NIH | Literature† | |||||
|
|
|
|||||
| Variable‡ | Variable Present | Variable Absent | p | Variable Present | Variable Absent | p |
|
| ||||||
| Age at evaluation > 48 years | ||||||
| median [range] | 732 [−150–156000] | 772 [0–3000000] | 0.47 | 585 [−1–291350] | 770 [−25–171000] | 0.28 |
| Male gender | ||||||
| median [range] | 644 [0–95500] | 1455 [−150–3000000] | 0.0075§ | 600 [−536–4000000] | 624 [−1–291350] | 0.85 |
| MEN1 | ||||||
| median [range] | 1400 [0–3000000] | 678 [−150–103000] | 0.062 | 515 [50–291350] | 624 [−536–171000] | 0.20 |
| Prior acid-reducing surgery | ||||||
| median [range] | 964 [66–700000] | 686 [−150–300000] | 0.30 | 680 [61–35500] | 594 [−536–4000000] | 0.36 |
| BAO > 15 (prior acid-red. surgery) | ||||||
| Median [Range] | 463 [66–31200] | 1712 [618–158000] | 0.0015§ | 271 [70–30500] | 860 [61–17000] | 0.060 |
| MAO > 51 (no prior acid-red. surgery) | ||||||
| Median [Range] | 700 [2–3000000] | 580 [0–101650] | 0.26 | 728 [100–46500] | 750 [−25–291350] | 0.84 |
| Primary tumor size > 1.5 cm (all pts.) | ||||||
| Median [Range] | 793 [0–103000] | 790 [41–3000000] | 0.92 | 653 [181–38000] | 590 [141–5100] | 0.59 |
| Primary tumor size ≥ 3 cm (all pts.) | ||||||
| Median [Range] | 667 [0–56527] | 830 [41–3000000] | 0.82 | 653 [181–38000] | 590 [141–9000] | 0.76 |
| Primary localization pancreas | ||||||
| Median [Range] | 720 [0–158000] | 830 [88–3000000] | 0.24 | 600 [40–291350] | 700 [−25–85000] | 0.94 |
| Primary localization duodenum | ||||||
| Median [Range] | 798 [88–3000000] | 831 [0–56527] | 0.96 | 683 [−25–291350] | 600 [45–8198] | 0.68 |
| Localized disease** | ||||||
| Median [Range] | 732 [−150–3000000] | 1129 [0–156000] | 0.97 | 536 [−25–85000] | 1132 [−536–291350] | 0.12 |
Abbreviations: See Table 1. Pts., patients; mets., metastases.
‘Δ secretin’ corresponds to the maximal absolute change in serum gastrin levels (expressed in pg/ml) after injection of 2 units of secretin.
Literature data are from publications listed in Table 2.
For continuous variables, the median of the total population (NIH+literature) is used to divide the patients into 2 groups.
indicates a significance difference (p<0.05)
‘Localized disease’ includes all patients without evidence for liver or other distant metastases.
In the NIH patients, age, race, MEN1 status, disease duration, prior treatment with PPI’s, the presence/absence of most common ZES symptoms (pain, heartburn) or the presence/absence of manifestations of severe disease (confirmed ulcer, esophageal disease, pyloric obstruction) did not effect secretin or calcium provocative testing (Tables 4, 5, 6). In contrast, higher Δ secretin and Δ calcium levels were seen with female gender and high BAO’s, whereas higher Δ secretin levels only were seen in the presence of diarrhea or with a long duration of antisecretory drug treatment. Correlation of clinical or laboratory parameters with the literature data did not demonstrate a relationship between higher Δ secretin or Δ calcium values with higher BAO’s or gender (Tables 5, 6, Figure 3). Similar to NIH patients, no relationship with age, MEN1 status or prior gastric-acid reducing surgery was seen (Tables 5, 6). In contrast to the Δ secretin and Δ calcium results in NIH ZES patients, higher Δ meal levels were present in younger patients, patients with a longer disease history, patients with heartburn as an initial symptom (data not shown) or with severe esophageal disease (stricture/dysphagia) and the presence of MEN1, but not with BAO or MAO levels (Tables 4, 7, Figure 3). In the literature ZES patients, no correlations were seen with the Δ meal value and acid secretory levels or clinical parameters (Table 7, Figure 3).
Table 6.
Effect of various clinical characteristics, laboratory data and tumor features on serum gastrin levels calcium stimulation in 208 patients seen at NIH and 212 ZES patients from the literature.
| Δ Calcium (pg/ml)* | ||||||
|---|---|---|---|---|---|---|
| NIH | Literature† | |||||
|
|
|
|||||
| Variable‡ | Variable Present | Variable Absent | p | Variable Present | Variable Absent | p |
|
| ||||||
| Age at evaluation > 48 years | ||||||
| median [range] | 865 [−10–75250] | 835 [−166–208000] | 0.78 | 1000 [0–116047]] | 1000 [0–95000] | 0.41 |
| Male gender | ||||||
| median [range] | 1453 [−10–25105] | 3825 [−166–208000] | 0.016§ | 912 [0–95000] | 1121 [0–116047] | 0.15 |
| MEN1 | ||||||
| median [range] | 1222 [−166–17590] | 774 [−10–208000] | 0.25 | 980 [48–116047] | 980 [0–95000] | 0.66 |
| Prior acid-reducing surgery | ||||||
| median [range] | 1230 [0–53600] | 826 [−166–208000] | 0.24 | 1115 [68–92140] | 980 [0–168000] | 0.33 |
| BAO > 15 (prior acid-reducing surgery) | ||||||
| Median [Range] | 535 [0–53600] | 4420 [705–25105] | 0.0013§ | 975 [68–22724] | 912 [145–92140] | 0.74 |
| MAO > 51 (no prior acid-reducing surgery) | ||||||
| Median [Range] | 850 [−166–208000] | 701 [1–46000] | 0.92 | 1595 [0–168000] | 980 [0–7993] | 0.15 |
| Primary tumor size > 1.5 cm (all pts.) | ||||||
| Median [Range] | 1182 [0–208000] | 697 [15–17590] | 0.17 | No Data** | No Data | |
| Primary tumor size ≥ 3 cm (all pts.) | ||||||
| Median [Range] | 838 [0–75250] | 888 [1–208000] | 0.58 | No Data | No Data | |
| Primary localization pancreas | ||||||
| Median [Range] | 958 [0–75250] | 814 [1–208000] | 0.79 | 691 [48–29000] | 1170 [0–95000] | 0.80 |
| Primary localization duodenum | ||||||
| Median [Range] | 698 [1–208000] | 1035 [0–75250] | 0.60 | 1170 [0–9890] | 691 [48–95000] | 0.85 |
| Localized disease†† | ||||||
| Median [Range] | 850 [−166–208000] | 602 [0–53600] | 0.28 | 1122 [0–28650] | 1235 [0–95000] | 0.87 |
Abbreviations: See Table 1. Pts., patients; mts., metastases.
‘Δ Calcium’ corresponds to the maximal absolute change in serum gastrin levels (expressed in pg/ml) after infusion of 5 mg/kg/hr. calcium for 3 hours. Indicated are the median and the range for each group of patients.
Literature data are from publications listed in Table 2.
For continuous variables, the median of the total population (NIH+literature) is used to divide the patients into 2 groups.
indicates a significant difference (p<0.05).
‘No Data’ indicates that information was available on less than 10 patients.
‘Localized disease’ includes all patients without evidence for liver or other distant metastases.
Influence of tumoral variables on provocative test results in NIH and literature ZES patients (Tables 4–7, Figures 3, 4)
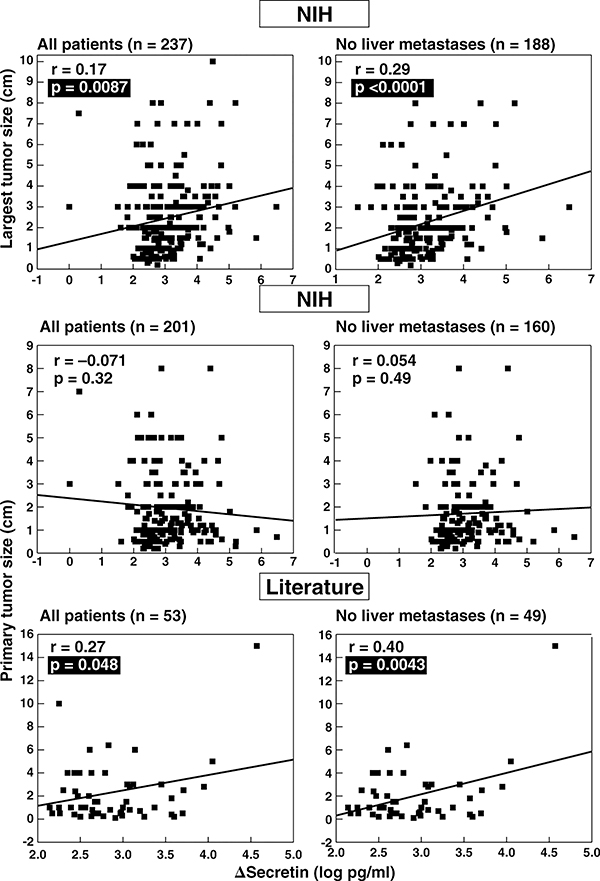
Figure 4.
Correlation between tumor size and secretin test results in ZES patients. The top panels show the correlation between the size of the largest tumor found at surgery or by imaging and the absolute change in serum gastrin after secretin injection (Δ secretin) in ZES patients from the NIH with and without liver metastases. The Δ secretin is expressed as the log of the change is serum gastrin with secretin. The middle and bottom panels show correlations of primary tumor size in ZES patients from NIH and from the literature with the Δ secretin. Each point represents data from one patient. Indicated are the regression line and the correlation coefficient (r) using a least-squares analysis. Literature data are from publications listed in Table 2. Tumor size is expressed as cm in diameter.
In general, primary tumor location had no effect on the Δ secretin or Δ calcium levels in NIH or literature ZES patients (Tables 4–6). Tumor size was associated with an increased Δ secretin and Δ calcium response (Tables 4–6 and Figures 3, 4) in NIH patients more than in the literature ZES patients.
In contrast to secretin and calcium tests, the meal test was not influenced by tumor size in the NIH ZES patients. However, in NIH patients only, a duodenal tumor was associated with a higher Δ meal response, whereas the presence of liver metastases was associated with a lower meal response (Tables 4, 7).
To further investigate the relationship between tumor size/disease extent and secretin provocative testing, we correlated the size of the largest tumor and the primary tumor size with Δ secretin in NIH ZES patients (Figure 4). In the literature patients, only primary tumor size was available for this analysis (Figure 4, lower panel). In NIH ZES patients, there was a significant correlation between the largest tumor size and Δ secretin in all patients and this correlation was even stronger in patients without liver metastases (Figure 4, top panel). No correlation existed with primary tumor size in the NIH patients with or without liver metastases (Figure 4, middle panel). However, in literature ZES patients, a weakly positive correlation between primary tumor size and Δ secretin existed in all patients, which increased in significance in patients without liver metastases (Figure 4, lower panel).
Correlation between various provocative test results in NIH and literature ZES patients (Figure 5)
Figure 5.
Correlation between secretin, calcium and meal provocative test results in ZES patients from the NIH (n=223) and the literature (n=71). Represented is the correlation between the absolute changes in serum gastrin concentration after secretin injection (Δ secretin) or calcium infusion (Δ calcium) (upper panels); the correlation between Δ secretin and the relative change in serum gastrin concentration after a standard test meal (Δ meal)(middle panels) and the correlation between Δ calcium and Δ meal (bottom panels). Each point represents data from one patient. Indicated are the regression line and the correlation coefficient (r) using a least-squares analysis. Literature data are from publications listed in Table 2.
As secretin and calcium provocative tests were influenced by comparable factors, we further studied the correlation between these different tests. We found a close correlation between Δ secretin and Δ calcium both in NIH and literature patients (Figure 5, top panel). This correlation remained unchanged if relative increases (% increase) post secretin and post calcium were correlated (data not shown). However, there was no correlation or a weak correlation between Δ secretin and Δ meal or Δ calcium and Δ meal in any patient group (Figure 5, middle and bottom panel).
Influence of different variables on the positivity of the various provocative tests in NIH and literature ZES patients (Table 8)
Table 8.
Influence of clinical variables, acid secretion data and tumor features on the results of the secretin stimulation test in 280 ZES-patients seen at NIH and 355 patients from the literature.
| Variable present* | ||||||
|---|---|---|---|---|---|---|
| NIH | Literature† | |||||
|
|
|
|||||
| Variable‡ | Δ Secretin ≥ 200 | Δ secretin < 200 | p | Δ Secretin ≥ 200 | Δ secretin < 200 | p |
|
|
|
|||||
| Age ≥ 48 years | 131 (53) | 15 (43) | 0.24 | 131 (50) | 23 (62) | 0.15 |
| Male gender | 137 (56) | 22 (55) | 0.97 | 156 (60) | 8 (19) | <0.0001§ |
| MEN1 present | 71 (29) | 12 (30) | 0.31 | 56 (18) | 6 (9.5) | 0.50 |
| Previous acid-reducing surgery | 38 (16) | 4 (10) | 0.62 | 57 (18) | 19 (51) | <0.0001§ |
| BAO > 35 (no acid-reducing surgery) | 120 (60) | 17 (52) | 0.23 | 67 (49) | 9 (47) | 0.92 |
| MAO > 51 (no acid-reducing surgery) | 110 (63) | 18 (58) | 0.47 | 38 (45) | 4 (40) | >0.99 |
| Primary size > 1.5 cm (all patients) | 71 (39) | 13 (65) | 0.027§ | 21 (44) | 1 (20) | 0.39 |
| Primary size > 1.3 cm (no liver metastases) | 63 (42) | 4 (44) | >0.99 | 22 (49) | 0 (0) | 0.11 |
| Primary size ≤ 1 cm (all patients) | 90 (50) | 7 (35) | 0.2 | 24 (50) | 8 (44) | 0.69 |
| Primary size ≤ 1 cm (no liver metastases) | 78 (52) | 5 (55) | >0.99 | 28 (56) | 4 (22) | 0.10 |
| Primary size ≥ 3 cm (all patients) | 40 (22) | 9 (35) | 0.023§ | 14 (29) | 7 (39) | 0.42 |
| Primary size ≥ 3 cm (no liver metastases) | 24 (26) | 3 (25) | 0.18 | 11 (24) | 13 (65) | 0.0018§ |
| Pancreatic primary tumor | 65 (28) | 15 (37) | 0.17 | 61 (47) | 8 (44) | 0.84 |
| Duodenal primary tumor | 99 (40) | 8 (20) | 0.0017§ | 50 (38) | 7 (39) | 0.97 |
| Other primary tumor | 32 (13) | 3 (7.5) | 0.59 | 22 (17) | 4 (22) | 0.52 |
| Localized disease | 212 (86) | 26 (72) | 0.026§ | 111 (74) | 13 (65) | 0.37 |
Abbreviations: see table 1.
Indicated is the number and percentage of patients with the indicated variable present, subdivided according to the secretin stimulation test. Data for literature patients is derived from the publications cited in Figure 1. The total number of patients in each group can be calculated using the number and percentage values.
Literature data are from publications listed in Table 2.
For continuous variables, the median of the total population (NIH + Literature) is used to divide patients into 2 groups.
indicates a significant difference (p<0.05).
To analyze whether clinical, laboratory or tumor variables could also influence provocative test results, i.e. positive or negative, we investigated the effect of the presence or absence of these variables on the positivity of secretin (Table 8), calcium (data not shown) or meal test (data not shown). In general, there was little concordance between those variables and the positivity of the secretin test in NIH or literature ZES patients (Table 8). This might partially be due to the small numbers of patients in some of the variable groups. The only variable associated with a positive secretin test in both the NIH and literature patients was the presence of a large primary tumor (Table 8). In contrast, only in the NIH ZES patients, a gastrinoma located in the duodenum and the presence of localized disease were associated with a positive secretin test result (Table 8). In the literature ZES patients only, male gender and lack of previous acid-reducing surgery associated with increased likelihood of a positive result (Table 8).
With the calcium test using the Δ calcium≥395 pg/ml criterion 78, the most commonly used criterion 166,397, no clinical, laboratory or tumor variable affected the positivity in ZES patients. No clinical, laboratory or tumoral variable, except MEN1 status or previous gastric acid-reducing surgery, influenced the occurrence of <50% increase in serum gastrin post meal, a response characteristic of ZES patients 31,195,196,347 (data not shown).
Comparison of four doses of secretin for provocative testing (Table 9)
Table 9.
Comparison of results with four different doses of secretin (synthetic or GIH) used for provocative testing using multiple proposed criteria for positive tests for ZES patients from the literature.
| No. of patients (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Secretin dose |
1 unit/kg* |
75 units† |
2 units/kg‡ |
3 units/kg§ |
||||
| All patients | FSG < 10-fold increase | All patients | FSG < 10-fold increase | All patients | FSG < 10-fold increase | All patients | FSG < 10-fold increase | |
|
|
|
|||||||
| Number of patients | 116 | 91 | 28 | 19 | 355 | 273 | 42 | 14 |
| Change of serum gastrin after secretin** | ||||||||
| Δ > 100 pg/ml | 92 (79) | 68 (75) | 27 (96) | 18 (95) | 343 (97) | 264 (97) | 30 (71) | 8 (57) |
| Δ ≥ 110 pg/ml | 92 (79) | 68 (75) | 25 (89) | 16 (84) | 341 (96) | 262 (96) | 30 (71) | 8 (57) |
| Δ ≥ 200 pg/ml | 81 (70) | 58 (64) | 20 (71) | 11 (58) | 313 (88) | 237 (87) | 28 (66) | 5 (36) |
| Δ > 50% increase | 89 (76) | 69 (76) | 26 (93) | 17 (89) | 286 (81) | 229 (84) | 19 (63) | 10 (71) |
| Δ >100% increase | 59 (51) | 46 (51) | 25 (89) | 16 (84) | 201 (57) | 165 (60) | 15 (50) | 8 (57) |
| Max. serum gastrin value after secretin | ||||||||
| Gastrin max. ≥ 186 pg/ml | 97 (84) | 72 (79) | 28 (100) | 19 (100) | 353 (99) | 271 (99) | 41 (98) | 13 (93) |
| Gastrin max. ≥ 335 pg/ml | 93 (80) | 68 (75) | 25 (89) | 16 (84) | 345 (97) | 263 (96) | 34 (81) | 6 (43) |
Data are from publications listed in table 2
Δ secretin corresponds to the absolute (pg/ml) or relative (%) change in serum gastrin concentration after secretin injection.
In the past, different doses of secretin have been used for provocative testing (Table 9). While 2 units of secretin per kg body weight is the most commonly used dose today, in some studies, doses of 1 unit/kg, 75 units, 3 units/kg or even higher doses have been used (Table 9). We compared the percentage of positive tests using different doses of secretin in literature patients (Table 9). Similar to some studies 189,219,380, but not others 153,229,329,405 there was no apparent secretin dose-response effect using any criterion either for all patients or for patients with FSG<10-fold increased. Specifically, results with 1 unit/kg or 3 units/kg secretin were similar and no increased detection occurred with the higher dose (Table 9). Moreover, secretin tests with 75 units and 2 units/kg also gave similar results (Table 9).
FSG and secretin provocative test results in non-ZES patients (Table 10)
Table 10.
Fasting serum gastrin distribution and evaluation of different proposed criteria for positive results for provocative tests using secretin and calcium in 462 patients from the literature without ZES.
| No. of patients (%)* | ||
|---|---|---|
| Literature non-ZES patients without achlorhydria† | Literature non-ZES patients with achlorhydria‡ | |
|
| ||
| Fasting serum gastrin | ||
| Number of patients | 147 | 25 |
| Fasting serum gastrin (fold normal) | ||
| ≤ 1.0 | 76 (52) | 0 (0) |
| 1.1 – 4.9 | 66 (45) | 5 (20) |
| 5.0 – 9.9 | 4 (3) | 10 (40) |
| ≥ 10 | 1 (1) | 10 (10) |
| Secretin test | ||
| Number of patients (Δ pg/ml) | 462 | 27 |
| Number of patients (Δ %) | 223 | 27 |
| Number of patients (absolute increase) | 134 | 27 |
| Change of serum gastrin after secretin | ||
| Δ > 100 pg/ml§ | 1 (0.22) | 9 (33) |
| Δ** ≥ 110 pg/ml†† | 0 (0) | 9 (33) |
| Δ ≥ 200 pg/ml‡‡ | 0 (0) | 5 (18) |
| Δ > 50%§§ increase*** | 16 (7) | 2 (7) |
| Δ >100% increase††† | 3 (1) | 0 |
| Maximal serum gastrin value after secretin | ||
| gastrin max. ≥ 186 pg/ml‡‡‡ | 17 (13) | 27 (100) |
| gastrin max. ≥ 335 pg/ml§§§ | 5 (4) | 26 (96) |
| Calcium test | ||
| Number of patients (Δ pg/ml) | 100 | |
| Number of patients (Δ %) | 105 | |
| Number of patients (absolute increase) | 59 | |
| Change of serum gastrin after calcium | ||
| Δ > 450 pg/ml§ | 0 (0) | |
| Δ ≥ 395 pg/ml | 0 (0) | |
| > 50% increase | 17 (17) | |
| >100% increase | 10 (9.5) | |
| Maximal serum gastrin value after calcium | ||
| gastrin max. ≥ 326 pg/ml | 1 (1.7) | |
Abbreviations: see table 1.
Patients with antral G-cell hyperplasia, moderate hypochlorhydria, peptic ulcer disease and patients with normal gastric function were included. Data are from 7,8,28,42,69,78,81,99,157,189,192,193,196,219,229,255,295,307,318,331,344,380
Criterion proposed by Deveney 77
The Δ calculated corresponds to the difference between the gastrin value showing the greatest change after stimulation and the basal gastrin value. Data available for 437 patients.
Criterion proposed in publication 78
Criterion proposed in publication238
% change (increase or decrease) is calculated as the difference between the gastrin value showing the greatest change after stimulation and the basal gastrin value divided by the basal value and multiplied by 100.
Criterion proposed in publication 196
Criterion proposed in publication 260
Criterion proposed in publication 301
Criterion proposed in publication 219
To assess the specificity of secretin and calcium provocative tests, we analyzed results from the literature in non-ZES patients (Table 10). Because high FSG levels and false positive gastrin provocative tests can occur in patients with achlorhydria 42,98,141,192,219, we analyzed results separately for this patient group. Of the 462 subjects without ZES and without achlorhydria who underwent secretin testing (normal controls, patients with antral G-cell hyperplasia, moderate hypochlorhydria or peptic ulcer disease), FSG levels were reported in 147 patients and the majority had either a normal or only moderately elevated FSG value (Table 10). None of the 462 non-ZES patients had a positive secretin test using the Δ≥200 or Δ≥110pg/ml criterion and 1 out of 462 (0.22%) had Δ>100 pg/ml (Table 10). Sixteen patients (7%) had a positive secretin test using the >50% criterion, 3 (1%) using the criterion Δ>100%, 17 patients (13%) using the criterion of Δ ≥186 pg/ml and 5 patients (4%) using the criterion of ≥ 335 pg/ml increase (Table 10). Hence, overall, the Δ criteria had better specificity than the % increase or maximal gastrin value criteria (Table 10). In patients with achlorhydria, false positive secretin tests were more common (Table 10), emphasizing the importance of excluding these patients. One hundred non-ZES subjects without achlorhydria who underwent calcium provocative tests could be found in the literature (Table 10). Overall, false positive calcium tests occurred more frequently with the percentage criteria.
Sensitivity and specificity of different criteria for positive provocative tests using secretin (Table 11)
Table 11.
Comparison of the sensitivity and specificity of different criteria for positive results with provocative tests using secretin in 1097 NIH and literature patients.
| No. of patients (%) | |||||
|---|---|---|---|---|---|
| NIH+Literature ZES-patients* |
Literature non-ZES patients† |
Sensitivity‡ (%) | Specificity‡ (%) | ||
| Change of serum gastrin after secretin | All patients | Patients with FSG < 10-fold increase§ | Patients without achlorhydria only | ||
|
|
|
|
|||
| Number of patients | 635 | 453 | 462 | ||
| Δ > 100 pg/ml | 605 (95) | 430 (95)*** | 1 (0.22) | 95 | 99.8 |
| Δ** ≥ 110 pg/ml | 603 (95) | 428 (94)*** | 0 (0) | 94 | 100 |
| Δ ≥ 200 pg/ml | 558 (88) | 388 (87) | 0 (0) | 87 | 100 |
| Δ ≥ 190 pg/ml | 562 (89) | 391 (86) | 0 (0) | 86 | 100 |
| Δ ≥ 180 pg/ml | 572 (90) | 400 (88) | 0 (0) | 88 | 100 |
| Δ ≥ 170 pg/ml | 576 (91) | 403 (89) | 0 (0) | 89 | 100 |
| Δ ≥ 160 pg/ml | 579 (91) | 406 (90) | 0 (0) | 90 | 100 |
| Δ ≥ 150 pg/ml | 582 (92) | 408 (90) | 0 (0) | 90 | 100 |
| Δ ≥ 140 pg/ml | 588 (93) | 414 (91)* | 0 (0) | 91 | 100 |
| Δ ≥ 130 pg/ml | 591 (93) | 417 (92)** | 0 (0) | 92 | 100 |
| Δ ≥ 120 pg/ml | 600 (94) | 425 (94)*** | 0 (0) | 94 | 100 |
Data for literature patients are from publications listed in table 2.
Data are from publications listed in Table 12.
Sensitivity and specificity calculated for the clinically relevant group of patients with a fasting serum gastrin < 10-fold increase.
*p<0.05, **p<0.01 and *** p<0.003 compared to sensitivity of Δ ≥ 200 pg/ml criterion.
The Δ calculated corresponds to the absolute change in serum gastrin above basal after stimulation with secretin.
Data from ZES and non-ZES patients were used to estimate sensitivity and specificity of secretin tests in the clinically relevant group of patients with <10-fold FSG increase (Table 11). As the Δ criteria offered better specificity (Table 10), only these criteria were considered for analysis. Both the proposed Δ criteria of >100 77, Δ≥110 78 and ≥ 200 pg/ml 238 increase were analyzed as well as new possible criteria of an increase of ≥120, ≥130, ≥140, ≥150, ≥160, ≥170, ≥180 or ≥190 pg/ml (Table 11). As the highest increase in serum gastrin occurring in a normal subject was 101 pg/ml, delta criteria of ≥110 to ≥200 pg/ml had 100% specificity. The sensitivity of the proposed criterion of Δ≥ 200 pg/ml was 87%, which is significantly less (p<0.05) than the 91% sensitivity of a Δ≥140 criterion, highly significantly different (p<0.01) from Δ ≥ 130 pg/ml and even more significantly different (p<0.003) from a criterion of Δ ≥ 120 pg/ml. From this analysis, the criterion with 100% specificity and highest sensitivity is Δ secretin ≥ 120 pg/ml.
Discussion
In this study, we prospectively analyzed the increase in serum gastrin after provocative tests in 293 ZES patients and compared our results to data from 537 patients from the literature. In the accompanying paper 30, we found that the determination of FSG levels alone cannot establish the diagnosis in two-thirds of ZES patients, because FSG levels of ZES patients significantly overlap with those seen in idiopathic peptic disease or other non-ZES conditions 78,151,152,217. Therefore, several gastrin provocative tests have been developed. These tests use different stimuli including calcium infusion or bolus injection 196,295,318,380, infusion or bolus injection of secretin 31,153,192,196,247,252, administration of a meal 31,196,348 and injection of glucagon 26,69,153,183,183,265,343,360 or bombesin 22–24,158. Only the secretin (bolus), calcium (infusion) and meal tests are generally used today 78,110,111,167,196,219,238,250,397. Due to methodological inconsistencies in previous studies, the criteria for each of these tests remain an area of controversy and some studies even question their overall usefulness.
Since the initial description of the ability of secretin to stimulate an exaggerated serum gastrin increase in ZES patients 137,153, the secretin test has been increasingly used in the diagnosis of ZES 110,238,394,397. Although some studies have proposed that this increased response represents an exaggerated normal response 40,44,45, there is strong evidence that secretin can stimulate gastrin release by direct interaction with gastrinoma cells. Secretin receptors occur on gastrinoma cells 84, secretin stimulates gastrin release from isolated gastrinoma cells 58,93,129,129,147 and this effect can be inhibited by somatostatin 58.
In the literature, some ZES patients have no increase in FSG after a bolus injection of secretin 73,94,219,229,230,233,247,318,341,343,344,351,360,405,406. The percentage of these patients varies from 0% in most studies 78,150,157,189,196,240,255,315 to 7–75% 229,247,318,343,344,360. In larger series however, most patients had a significant increase in FSG post secretin (median 262–1103 pg/ml in 10 large studies 66,78,100,150,196,218,232,315,341,412). In our study, only 1.4% of literature or NIH ZES patients had no increase in serum gastrin after secretin, demonstrating that >98% respond to secretin. Our results demonstrate that in ZES patients, no response to secretin injection is very uncommon.
Numerous studies have proposed criteria for a positive secretin test 77,78,110,196,219,260,301. However, these criteria vary widely, which is primarily due to multiple methodological differences. Some studies are limited by small patient numbers (<25 patients) and patients with FSG< or >10-fold increased are usually considered together, although provocative tests are of clinical relevance only in the former group 78,110,152,196,342,344,360. Moreover, different protocols and different secretin preparations have been used (Boots, GIH, synthetic), which is relevant because false positive secretin tests have been reported with Boots secretin 41,238,398. Different modes for secretin administration have been employed (infusion, bolus injection) 238,247,249,250,252,301 and different doses used. The secretin dose could be relevant because some studies 153,229,238,405, but not others 153,229,329,405, report that different doses may give different results. Our systematic analysis of secretin test results in patients receiving different doses of secretin (1 U/kg, 75 U total, 2 U/kg, ≥3 U/kg) showed no dose-response effect (Table 9). These results are similar to some studies 189,219,380, but not others 153,229,329,405, and led us to conclude that there is no need to increase the usual 2 units/kg dose or to repeat a secretin study with a higher dose if the initial study was negative, as suggested by others 229,405. Furthermore, secretin test protocols frequently varied in the time points for collection of blood samples. This is important because some studies did not measure gastrin 2 minutes after secretin injection 196,255. In one study 110 however, 6% of ZES patients had their only positive response 2 minutes post injection. Moreover, these studies were further limited by small patient numbers (<25 patients) and the fact that patients with FSG< or >10-fold increased were considered together, although gastrin provocative tests are of clinical relevance only in the former group 78,110,152,196,342,344,360.
Three different types of criteria for a positive secretin test have been proposed: 3 criteria based on the absolute increase in FSG post secretin (>100, ≥110, ≥200 pg/ml) 77,78,110, 2 criteria based on the relative increase (i.e. >50%, >100%) 196,260 and 2 criteria based on the maximal FSG level post secretin (i.e. ≥186 or ≥335 pg/ml) 219,301. Based on a review of 122 cases from the literature 238, the criterion of ≥200 pg/ml increase post secretin has been most widely used since 1980 in the United States 110,119,166,397. Because we had a large number of patients, we could systematically assess the sensitivity of the various proposed criteria, both in all patients and the clinically important subset of ZES patients with FSG<10-fold increased 78,110,152,196,342,344,360. In NIH and literature ZES patients, 3 criteria had sensitivities of 90–100% (Δ>100 pg/ml, Δ≥110pg/ml, gastrin max. ≥186 pg/ml) and 3 had sensitivities of 85–89% (Δ≥200 pg/ml, Δ>50%, gastrin max.≥335 pg/ml). One proposed criterion, Δ>100%, had a low sensitivity of only 60–62%, suggesting it is not generally useful. Because ZES is an uncommon condition (1–3 cases/year/million population) and hypergastrinemia due to H. pylori infection or other conditions is much more frequent 78,90,151,152,203,217,236,246,337, it is very important to limit false positive responses, so that the specificity may be the most important factor in determining an optimal secretin test criterion. To assess the specificity of the six different secretin test criteria with high sensitivity (85–100%), we analyzed the results from 489 non-ZES patients’ responses from the literature (Table 10), including 462 without and 27 subjects with achlorhydria. Because there was an unacceptably high false positive rate varying from 7% to 100% in achlorhydric patients, our analysis confirms the proposal that secretin test results are not reliable in these subjects 42,98,141,192,219. After exclusion of achlorhydric patients, the 3 absolute increase criteria (i.e.Δ >100, ≥110, ≥ 200 pg/ml) gave very low false positive rates (0–0.5%) resulting in a specificity of 99.8–100%, whereas the remaining highly sensitive criteria (Δ>50%, gastrin max. ≥186 pg/ml, gastrin max. ≥ 335 pg/ml) gave higher false positive rates of 4–13%. Our analysis demonstrated that the proposed criteria with the highest sensitivity and specificity are Δ≥100 pg/ml [95%, 99.8%], Δ≥110 pg/ml [94%, 100%] and Δ≥200 pg/ml [87%, 100%]. Because the sensitivity of the Δ>100 and Δ≥110 criteria was significantly higher than that of Δ≥200 pg/ml, we tried to identify a new criterion with maximal sensitivity and 100% specificity. This criterion is a Δ≥120 pg/ml, which has 94% sensitivity and a specificity of 100%. Both the Δ≥120 and Δ≥130 pg/ml criteria had a 100% specificity and each had a significantly higher sensitivity than the commonly used Δ≥200 pg/ml criterion (Table 11). The Δ≥120 pg/ml criterion had the same sensitivity as the previously proposed Δ≥110 criterion 78 (i.e. 94%) and its sensitivity was not significantly different from that of the Δ>100 criterion 77. However, in the non-ZES patients without achlorhydria, one patient had an increase in serum gastrin after secretin of 101 pg/ml and 4 patients had an increase of 100 pg/ml. Therefore, we conclude that Δ≥120 pg/ml was the criterion with maximal sensitivity and 100% specificity. Criteria of ≥110 and ≥120 pg/ml both had equal sensitivity and 100% specificity, but because of the importance of limiting a false positive result to the greatest extent, we recommend a criterion of Δ≥120 pg/ml rather than 110 pg/ml as recommended by others 78.
Based on these results, we suggest that a secretin test should be performed with 2 units GIH or synthetic secretin per kg body weight, it should include an assessment of serum gastrin before secretin injection, and 2, 5, 10, 15 and 20 minutes 110 after secretin. An absolute increase in serum gastrin ≥ 120 pg/ml should be the criterion for positivity because it has the highest sensitivity with 100% specificity (Table 11). When this new criterion is applied to the 453 NIH and literature ZES patients in our study with FSG<10-fold increased, it allows the correct diagnosis of 37 ZES patients that would have had a negative test with the commonly used the 200 pg/ml criterion.
Numerous studies show serum calcium levels can greatly influence FSG in ZES patients with or without MEN1. Intravenous administration of calcium augments basal hypergastrinemia 69,78,157,181,197,212,237,295,311,361,368,368,386, whereas a reduction of calcium levels by EDTA administration 157,388 or correction of hyperparathyroidism by parathyroidectomy 59,87,88,127,153,159,276,361,368,369,393 diminishes hypergastrinemia in ZES patients. This calcium-induced augmentation of FSG in ZES patients is the basis of the calcium provocative test 69,197,262,295,360,368. Subsequent studies have shown that this effect is likely due to a direct action of calcium on gastrinoma cells 2,33,91,93,399,400. In fact, calcium can increase gastrin release from isolated gastrinoma cells 2,33,91,93,399,400. Furthermore, these cells possess both calcium sensing receptors and various calcium channels 126,155,378 whose activation could stimulate gastrin secretion.
In previous reports 69,78,192,196,197,219,255,295,343,360, very few ZES patients did not respond to an intravenous calcium infusion 195,233,351 and the median FSG increase was 438–4325 pg/ml in 8 larger series 23,78,110,196,232,262,318,341. In our study, 6 NIH and 3 literature ZES patients did not respond to calcium, demonstrating that calcium evokes an increase in serum gastrin in >97% of ZES patients. The median increase was 850 pg/ml in NIH and 994 pg/ml in literature patients.
Numerous authors have proposed different criteria for a positive calcium test 77,78,196,219,260. With the exception of two studies advocating a bolus injection of calcium 318,380, these studies used very similar protocols (dose of calcium, time of infusion, time points for blood collection). Nevertheless, their results are limited by small patient numbers and the fact that patients with a FSG<10 and >10-fold increased are considered together. Three different types of criteria have been proposed for a positive calcium test: 2 criteria based on the absolute increase in FSG post calcium (≥450, ≥395 pg/ml) 77,78, 2 criteria based on the relative increase (>50%, >100%) 196,260 and 1 criterion based on the maximum FSG level post calcium (≥326 pg/ml) 219. In the United States, the criteria of ≥395 pg/ml and >50% are widely used 78,110,196. We found that 1 criterion had >90% sensitivity (gastrin max. ≥326 pg/ml), 1 criterion had 79–90% sensitivity (Δ>50% increase) and 3 criteria had 57–76% sensitivity (Δ≥450, ≥395 pg/ml, >100%). In patients with <10-fold increase in FSG the sensitivities of the two criteria based on absolute increases dropped 5–13%, whereas there was no relevant decrease in sensitivity for the other criteria. For the two criteria generally used in the United States (Δ≥395 pg/ml or >50% increase), the sensitivities in the NIH/literature patients were 54/70% and 78/91%, respectively. As discussed above, avoiding false positive tests is especially important. An analysis of the available data from 105 non-ZES patients who underwent calcium testing allowed us to assess the specificity of the different criteria. As the calcium test may be falsely positive in up to 50 % of non-ZES patients with achlorhydria 196,219, only non-ZES patients without achlorhydria were included in this analysis. The Δ>50% and Δ>100% criteria gave an unacceptably high number of 10–17% false positives, whereas only one non-ZES patient exceeded the gastrin max. ≥326 pg/ml criterion. However, this last criterion is not clinically useful because most patients with a FSG<10-fold increased (64% in our study) have a fasting serum gastrin ≥326 pg/ml prior to any stimulation. No non-ZES patient without achlorhydria in the literature had an increase in serum gastrin post calcium ≥ 395 pg/ml, so that the Δ≥450 and Δ≥395 pg/ml criteria have 100% specificity. The ≥395 pg/ml criterion had a greater sensitivity than the ≥450 pg/ml criterion, although this difference did not quite reach statistical significance (p>0.1). Nevertheless, it could be clinically relevant because the calcium test is generally used as second-line test in patients with strong clinical suspicion of ZES but a negative secretin test. Therefore, maximal sensitivity, along with 100% specificity, is crucial for choosing the optimal criterion. Taking this into account, we conclude that Δ≥395 pg/ml should be used. However, even with this criterion, the sensitivity of the calcium test is significantly lower than that of the secretin test (63 vs. 94%, p<0.01). Furthermore, unlike with secretin, numerous side-effects are reported with the infusion of calcium, including abdominal pain, nausea, vomiting, headache, phlebitis, shortening of Q-T interval on ECG, paresthesias, diuresis, severe fatigue, palpitations, arrhythmias and a rise in blood pressure 110,177,196,255,318,380. These complications lead to early termination of the infusion in some patients 318. Moreover, the calcium infusion test takes longer than the secretin test (3 hr. vs. 30 min) and requires a physician’s attendance. In addition, the secretin test may be superior to calcium testing in distinguishing antral from tumoral hypergastrinemia 182,190,196,347. For these reasons, we conclude the calcium test should not be used routinely as the first-line test in patients suspected of having ZES. However, in one previous study 110, 33% of ZES patients with a negative secretin test had a positive calcium test. Two smaller series report secretin-negative ZES patients with a positive response to calcium infusion 318 or injection 380, whereas another study reports negative calcium tests in all secretin-negative ZES patients 196. In our study, 38% of NIH and 50% of literature ZES patients with a negative secretin test (applying the Δ≥120 pg/ml criterion) had a positive calcium test (by the Δ≥395 pg/ml criterion). Therefore, we conclude that the calcium test is useful in patients with a clinical suspicion of ZES but a negative secretin test.
Ingestion of food is a physiological stimulus for the release of gastrin from the antral and duodenal mucosa 31. In patients with hypergastrinemia of antral origin, i.e. atrophic gastritis or antral G-cell hyperfunction, a condition reported to occur in H. pylori infection 9, feeding induces an increase >100% in FSG31,83,114,158,176,204,300. In ZES patients, gastrin produced by the tumor is the main source of serum gastrin and early studies reported no major increase in FSG after ingestion of a test meal, suggesting that feeding does not stimulate gastrin release from gastrinomas 31,152,158,190,195,264,347. Therefore, it was proposed that a standard meal test could differentiate hypergastrinemia of antral and tumoral origin 31,347. This is an important distinction because antral G-cell hyperplasia/hyperfunction can mimic ZES clinically and cause hyperchlorhydria/hypergastrinemia 6,7,167,186,194,204,299,397. However, other studies have reported ZES patients with large increases in FSG after feeding and questioned the value of the meal test 69,111,196,343. The mechanism of postprandial FSG increase in ZES patients is controversial. Some studies suggest the gastrin could be of antral origin in patients having both ZES and antral syndromes 106, while other authors propose the gastrin release is mediated by the tumor 111, possibly involving release of a gastrin-stimulating agent, e.g. secretin, from the duodenum 359,360. Some reports suggest that previous gastric surgery could influence the increase in gastrin post meal in ZES patients 69,111,195,360. The small number of ZES patients investigated, the different proportion of patients with prior gastric surgery and differences in tumor characteristics could partly explain the large discrepancies between some of these studies (Table 12). In our study, 39% of NIH and 20% of literature patients had no increase in serum gastrin post meal. In the remaining patients, the median increase was 132 pg/ml among NIH and 212 pg/ml among literature ZES patients. In five larger series, the median increase in FSG post meal was 55–2269 pg/ml 69,158,196,198,360. In our present study, meal test results did not differ in ZES patients with or without prior acid-reducing surgery (median increase 175 vs. 223 pg/ml, p=0.2).
Table 12.
Comparison of provocative test results using secretin, calcium or a standard meal in ZES patients from large studies, the remaining literature and NIH using different criteria for positive testing. Indicated are the percentage of all patients and the percentage of patients with FSG < 10-fold increase (in brackets) with positive tests at a given criterion. The patients analyzed in the individual studies indicated were excluded of the analysis of literature data.
| Secretin test | Deveney* 1977 | Mc Callum 1979 | Stabile 1980 | Feurle 1982 | Malegelada 1983 | Richardson 1985 | Corletto 1993 | Imamura 1994 | Zimmer 1996 | Literature * | NIH |
| (n=10) | (n=15) | (n=21) | (n=16) | (n=10) | (n=22) | (n=13) | (n=11) | (n=10) | (n=227) | (n=309) | |
| Reference | 78 | 232 | 341 | 100 | 218 | 315 | 66 | 150 | 412 | ||
| Δ≥110 pg/ml † | 100% (100%) | 100% (100%) | 95% (100%) | 94% (93%) | 90% (90%) | 91% (89%) | 100% (100%) | 100% (100%) | 100% (100%) | 96% (96%) | 93% (92%) |
| Δ≥200 pg/ml ‡ | 80% (80%) | 80% (70%) | 95% (100%) | 87% (86%) | 90% (90%) | 86% (83%) | 100% (100%) | 91% (89%) | 100% (100%) | 87% (85%) | 87% (83%) |
| > 50% increase § | 80% (80%) | 87% (100%) | 81% (94%) | 87% (86%) | 70% (70%) | 77% (78%) | 100% (100%) | 45% (44%) | 70% (70%) | 81% (85%) | 84% (86%) |
|
| |||||||||||
| Calcium test | Morrow** 1975 | Deveney 1977 | Lamers 1977 | McCallum 1979 | Stabile 1980 | Basso 1981 | Romanus 1983 | Literature ** | NIH | ||
| (n=14) | (n=12) | (n=15) | (n=13) | (n=19) | (n=11) | (n=13) | (n=113) | (n=208) | |||
|
Reference
|
262 | 78 | 196 | 232 | 341 | 23 | 318 | ||||
| Δ≥395 pg/ml ‡‡ | 64% (58%) | 75% (75%) | 87% (89%) | 62% (44%) | 74% (67%) | 55% (50%) | 92% (88%) | 76% (72%) | 67% (54%) | ||
| > 50% increase § | 86% (83%) | 92% (92%) | 87% (89%) | 100% (100%) | 100% (100%) | 100% (100%) | 100% (100%) | 85% (87%) | 79% (78%) | ||
|
| |||||||||||
| Meal test | Creutzfeld†† 1975 | Strauss 1975 | Thompson 1975 | Lamers 1977 | Malagelada 1978 | Jansen 1982 | Literature ** | NIH | |||
| (n=9) | (n=7) | (n=11) | (n=9) | (n=6) | (n=7) | (n=63) | (n=208) | ||||
| Reference | 69 | 347 | 360 | 196 | 216 | 157 | |||||
| <50% increase ‡‡ | 22% (40%) | 86% (86%) | 18% (20%) | 89% (87%) | 33% (67%) | 100% (100%) | 81% (82%) | 57% (54%) | |||
| < 100% increase § | 56% (40%) | 86% (86%) | 54% (80%) | 100% (100%) | 50% (100%) | 100% (100%) | 94% (96%) | 80% (81%) | |||
Abbreviations: see Table 1
Studies including ≥ 10 patients are listed individually, smaller studies are grouped under “Literature”. Literature data are from publications listed in Table 2.
Criterion proposed in publication 77
Criterion proposed in publication 238
Criterion proposed in publication 196
Abbreviations: see Table 1
Studies including ≥ 10 patients are listed individually, smaller studies are grouped under “Literature”. Literature data are from publications listed in Table 2.
The 6 largest studies are listed individually, smaller studies are grouped under “Literature”. Literature data are from publications listed in Table 2.
Criterion proposed in publication 78
Lamers and van Tongeren proposed a postprandial increase <50% to be characteristic of ZES 196. In another study, a maximal serum gastrin value post meal >500 pg/ml was suggested as criterion. However, this latter criterion is of little clinical value because it does not differentiate antral hypergastrinemia from ZES and is not applicable to ZES patients with a FSG >500 pg/ml. In our study, 57% of NIH and 67% of literature ZES patients had an increase in serum gastrin post meal <50%. These numbers change to 54 and 77%, among patients with FSG<10-fold increased. However, 20% of NIH and 15% of literature ZES patients had an increase in FSG post meal ≥100%, a value reported to be characteristic of antral G-cell hyperplasia 7,9,108,111,158,167,204,336,347. Because of this overlap of meal test results in patients with hypergastrinemia of antral or tumoral origin, we conclude, in accordance with others 69,111,219, that the meal test is not useful in the diagnosis of ZES.
Only very limited information is available on correlations of gastrin provocative test results with clinical, laboratory or tumoral features in ZES. However, these correlations might be useful because they could define a subgroup of clinically relevant patients. Moreover, such correlations could identify patients in whom provocative test results are not conclusive because they are biased by different factors. In accordance with previous studies 196,318, we found a positive correlation (r=0.52–0.56) between the absolute increase in FSG after secretin and calcium (Figure 5). This correlation remained unchanged if the relative (%) increases were correlated, suggesting that the correlation is not due to the influence of FSG levels on both variables, but rather reflects common factors may be involved in determining the response to both stimulants. Our study did not identify these factors, but they could include the response of the gastrinoma to external stimuli, the amount of gastrin stored or shared pathways of stimulation. Similar to other studies 42,315, we found that secretin and calcium test results were both correlated with FSG values when the absolute increases post stimulation (Δ) were considered (Figure 2). However, this correlation was not significant after correction for higher FSG values by correlating the relative increases. We found a correlation of secretin and calcium test results with BAO levels and with some clinical findings (duration of medical treatment, diarrhea) associated with gastric hyperacidity 319 (Tables 4–6 and Figure 3). Most interestingly, MEN1 patients, who frequently have hypercalcemia due to hyperparathyroidism 29,123,221, did not show a significantly greater serum gastrin response to secretin (Table 5). This finding is surprising because hypercalcemia facilitates secretin-induced gastrin release in ZES patients 157,196,318. In our study, we found that larger tumors are associated with a higher response to secretin or calcium (Tables 4–6, Figures 3, 4). The size of the largest tumor correlates better with FSG increases post secretin than primary tumor size. This correlation is even stronger in patients without liver metastases, suggesting that the absolute tumor mass rather than the characteristics of the primary tumor influences the response to secretin. While these clinical, laboratory or tumor variables had some influence on the magnitude of the serum gastrin response to secretin, they had little effect on the positivity of the provocative test results. This finding has clinical importance because it shows that clinical, laboratory or tumor features do not bias provocative test results with secretin or calcium and that these tests can therefore be performed in subgroups of ZES patients with different characteristics.
As discussed above, our data suggest that the meal test is generally not helpful in the diagnosis of ZES. However, correlations of the increase in serum gastrin post meal in ZES patients with clinical, laboratory and tumor features might allow some insight into ongoing controversies concerning meal-stimulated gastrin release. Some authors propose that the postprandial gastrin release in ZES patients could be mediated by the release of a gastrin-stimulating agent, such as secretin, from the duodenum 359,360. If secretin were involved in postprandial gastrin release, patients with increases in serum gastrin post meal might have a proportional response to secretin. Therefore a significant correlation might exist between secretin and meal test results. However, we did not find such a correlation (Figure 5). Because Billroth II resection results in markedly diminished meal-stimulated secretin release 268,269, our finding of similar meal test results in ZES patients with or without previous Billroth II resection further invalidates the hypothesis of a secretin-mediated mechanism for postprandial gastrin release in ZES patients. It has been suggested that the increase in serum gastrin post meal observed in some ZES patients could be due to coexisting antral G-cell hyperplasia/hyperfunction 106. Because the meal response is markedly affected by gastrinoma resection in ZES patients111, this proposal is unlikely. MEN1/ZES patients are reported to have an increased incidence of antral syndromes in some studies 106, but not in others 111. Our data do not support this proposal, because we found that prior antrectomy does not influence meal test results in ZES patients. However, our data did show that NIH MEN1/ZES patients had a significantly higher median FSG increase post meal than sporadic ZES patients (Table 7) and a significantly higher percentage of these patients had a ≥50% postprandial FSG increase. This difference could be related to other factors than an increased occurrence of antral syndromes, such as concomitant hyperparathyroidism with hypercalcemia, which could affect meal-stimulated hormone release. It has been suggested that duodenal gastrinomas are associated with higher postprandial FSG increases than pancreatic tumors 196, whereas another large study did not find such a correlation 111. In our study, we found that patients with duodenal gastrinomas have larger postprandial serum gastrin increases (Table 7). However, this did not affect meal test results, i.e. the percentage of patients with ≥ 50% increase in serum gastrin post meal. Therefore our result is similar to another study 111 and suggests that the meal test is not useful in localizing duodenal gastrinomas.
Our results for secretin testing in general show similarities to most other series including ≥10 patients, however, they also show some important differences (Table 12). Specifically, we found the most widely used criterion of ≥200 pg/ml does not have a sensitivity of 100% as reported in a number of studies 66,238,341,412 or a low sensitivity of 70–80% as reported in other studies78,232. Our results agree with a number of other series reporting a sensitivity of 83–86% 100,315 (Table 12). Similar to most studies, we found the criterion of Δ≥110 pg/ml to have a higher sensitivity (92–100%) and the >50% criterion to be less sensitive than either the Δ≥110 or Δ≥200 pg/ml criteria (Table 12). For the calcium test, a number of studies reported a higher sensitivity than we found, especially for the 50% criterion 23,78,232,318,341, while others had results comparable to those of our study 262 (Table 12). This difference in sensitivity cannot be due to a difference in the amount of calcium infused because in most studies, 5 mg/kg/hr of calcium was infused for 3 hours and all patients had at least a 1.5 mEq/l increase in serum calcium. The meal test results in our study are comparable to results reported in some series, 69,347,360, but markedly differ from results of other studies which reported no ZES patients with a ≥100% increase in serum gastrin post meal 157,196,216 (Table 12).
The systematic analysis of gastrin provocative test results in 293 NIH and 537 literature ZES patients presented in this paper allowed us to draw several important conclusions which are summarized in Table 13. We could show a new criterion, Δ≥120%, which had the highest sensitivity and specificity. When this new criterion is applied, the secretin test is highly sensitive (94%) and specific (100%) and therefore play a crucial role in the diagnosis of ZES in the two-thirds of patients with non-diagnostic FSG values. This new criterion had greater sensitivity (p<0.003) than the usually used criterion of Δ≥200 pg/ml with equal specificity (i.e. 100%) and allowed the detection of 37 more ZES patients in the NIH and literature groups than the Δ≥200 pg/ml criterion. We applied the same analysis to calcium test results and could confirm the usefulness of the Δ≥395 pg/ml criterion proposed by others 78. However, even with this criterion, the calcium test has only a 62% sensitivity. Furthermore, multiple side-effects have been reported with intravenous calcium infusion. Therefore, the calcium test should not be used as first-line provocative test. However, the calcium test may be useful in patients with a negative secretin test, but strong clinical suspicion of ZES because it will be positive in 38–50% of these ZES patients. We could not confirm the utility of the meal test, because we found significant overlap of meal test results in patients with ZES and antral syndromes. We found the magnitude of the gastrin response to secretin and calcium to correlate with BAO values and some clinical findings reflecting hyperchlorhydria as well as with tumor size and extent. However, the results (i.e. positive or negative) of these tests were only minimally influenced by these factors. The magnitude of the meal-induced gastrin response correlated with some clinical findings, including MEN1 status, and with tumor location. However, meal test results were only minimally influenced by these factors, so that the meal test is not useful for tumor localization.
Table 13.
Key findings of an analysis of gastrin provocative tests in NIH and literature ZES patients.
| A. Secretin provocative tests show: |
| 1. An increase in serum gastrin post secretin (Δ secretin) in 99% of all patients and 99% of patients with a fasting serum gastrin (FSG) <10-fold increased. |
| 2. In 453 ZES patients with FSG <10-fold increased, the sensitivity of proposed criteria: Δ≥110 (94%), Δ≥120 (94%), Δ≥200 pg/ml (87%), Δ>50% (85%), Δ>100% (61%). |
| 3. In 462 non-ZES patients without achlorhydria, the specificity of these proposed criteria was: Δ≥110 (100%), Δ≥120 (100%), Δ≥200 (100%), Δ>50% (93%), Δ>100% (99%). |
| 4. A new criterion of Δ≥120 pg/ml was found to have the highest sensitivity (94%) and specificity (100%) and should be used in the future. |
| 5. Δ secretin significantly correlates with BAO but not MAO as well as with some clinical findings reflecting hyperchlorhydria (diarrhea, duration of medical treatment). |
| 6. Δ secretin correlates with tumor size and disease extent. |
|
|
| B. Calcium provocative tests show: |
| 1. An increase in serum gastrin post calcium (Δ calcium) in 98% of all patients and 97% of patients with FSG <10-fold increased. |
| 2. In 299 ZES patients with FSG<10-fold increased, the sensitivity of proposed criteria was: Δ≥450 (58%), Δ≥395 (62%), Δ>50% (85%), Δ>100% (68%). |
| 3. In 105 non-ZES patients without achlorhydria, the specificity of these proposed criteria was: Δ≥450 (100%), Δ≥395 (100%), Δ>50% (83%), Δ>100% (90%). |
| 4. A Δ ≥395 pg/ml gives maximal sensitivity (62%) with 100% specificity and should be used as criterion for a positive test. |
| 5. The calcium test has a significantly lower sensitivity than the secretin test (62% vs. 94%) and has greater side effects. Therefore it should not be used as first-line provocative test. |
| 6. Is positive (Δ≥395 pg/ml) in 38–50% of patients with a negative secretin test. |
| 7. Δ calcium significantly correlates with BAO but not MAO values. There is no significant correlation with clinical symptoms or tumor characteristics. |
|
|
| Meal provocative tests show: |
| 1. An increase in serum gastrin post meal (Δ meal) in 84% of all patients and 85% of patients with a FSG <10-fold increased. |
| 2. In 241 ZES patients with a FSG <10-fold increased: a) 61% have a <50% Δ meal. b) 16% have Δ meal ≥100% increase overlapping with the response in antral syndromes. |
| 3. Because of this overlap, the standard meal test is not clinically useful in the diagnosis of ZES. |
| 4. Δ meal test correlates with some clinical findings (time to diagnosis, age of onset, presence of MEN1, heartburn, esophageal disease) and with tumor location. |
Acknowledgements
The authors thank the patients and their relatives who participated in the study. We thank the NIH Clinical Center 9D nursing staff, endoscopy nurses, the past and present fellows of the NIH-Georgetown University-Washington VA Gastroenterology Training Program. We thank members of the Nuclear Medicine and Diagnostic Radiology Branch, especially Drs. James C. Reynolds and John L. Doppman for their support. We thank the numerous referring physicians as well as the physicians who assisted us in the follow-up of some of these patients, particularly Dr. David C. Metz (University of Pennsylvania) and Dr. Jeffrey A. Norton (Stanford University). We would like to thank the members of the Metabolic Diseases Branch (MDB), NIDDK, who participated in the care and investigation of a number of the patients.
This research is partially supported by the Intramural Research Program of the NIDDK, NIH.
Abbreviations used in this article:
- Δ calcium
Increase in serum gastrin post calcium
- Δ meal
Increase in serum gastrin post meal
- Δ secretin
Increase in serum gastrin post secretin
- BAO
Basic acid output
- FSG
Fasting serum gastrin
- GIH secretin
Gastro-intestinal hormones secretin, Karolinska Institute, Stockholm, Sweden
- MAO
Maximal acid output
- MEN1
Multiple Endocrine Neoplasia type 1
- MRI
Magnetic resonance imaging
- NIH
National Institutes of Health
- PPI
Proton pump inhibitor
- PTH
Parathormone
- RIA
Radioimmunoassay
- ZES
Zollinger-Ellison syndrome
Reference List
- 1.Abou-Saif A, Lei J, McDonald TJ, Chakrabarti S, Waxman I, Shojamanesh H, Schrump DS, Kleiner DE, Gibril F, Jensen RT. A new cause of Zollinger-Ellison syndrome: non-small cell lung cancer. Gastroenterology. 2001;120:1271–1278. [DOI] [PubMed] [Google Scholar]
- 2.Ahlman H, Ahlund L, Dahlstrom A, Theodorsson E. Expression of gastrointestinal endocrine tumours in culture systems. Arch Histol Cytol. 1989;52 Suppl:233–240. [DOI] [PubMed] [Google Scholar]
- 3.Alexander HR, Fraker DL, Norton JA, Barlett DL, Tio L, Benjamin SB, Doppman JL, Goebel SU, Serrano J, Gibril F, Jensen RT. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998;228:228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison MC, Renfrew CC, Webb WJ, Chappell ME, Pounder RE. Neuroendocrine islet cell tumour producing gastrin and ACTH in a patient with calcifying chronic pancreatitis. Gut. 1985;26:426–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alspaugh JA. Severe, recurrent peptic ulcer disease: a case of the Zollinger-Ellison syndrome. J Tenn Med Assoc. 1995;88:353–354. [PubMed] [Google Scholar]
- 6.Annibale B, Aprile MR, Ferraro G, Marignani M, Angeletti S, D’Ambra G, Caruana P, Bordi C, Delle Fave G. Relationship between fundic endocrine cells and gastric acid secretion in hypersecretory duodenal ulcer diseases. Aliment Pharmacol Ther. 1998;12:779–788. [DOI] [PubMed] [Google Scholar]
- 7.Annibale B, De Magistris L, Corleto V, D’Ambra G, Marignani M, Iannoni C, Delle Fave G. Zollinger-Ellison syndrome and antral G-cell hyperfunction in patients with resistant duodenal ulcer disease. Aliment Pharmacol Ther. 1994;8:87–93. [DOI] [PubMed] [Google Scholar]
- 8.Annibale B, Delle Fave G, Azzoni C, Corleto V, Camboni G, D’Ambra G, Pilato FP, Bordi C. Three months of octreotide treatment decreases gastric acid secretion and argyrophil cell density in patients with Zollinger-Ellison syndrome and antral G-cell hyperfunction. Aliment Pharmacol Ther. 1994;8:95–104. [DOI] [PubMed] [Google Scholar]
- 9.Annibale B, Rindi G, D’Ambra G, Marignani M, Solcia E, Bordi C, Delle Fave G. Antral gastrin cell hyperfunction and helicobacter pylori infection. Aliment Pharmacol Ther. 1996;10:607–615. [DOI] [PubMed] [Google Scholar]
- 10.Antonioli DA, Dayal Y, Dvorak AM, Banks PA. Zollinger-Ellison syndrome. Cure by surgical resection of a jejunal gastrinoma containing growth hormone releasing factor. Gastroenterology. 1987;92:814–823. [PubMed] [Google Scholar]
- 11.Aoyagi T, Summerskill WH. Gastric secretion with ulcerogenic islet cell tumor. Importance of basal acid output. Arch Intern Med. 1966;117:667–672. [PubMed] [Google Scholar]
- 12.Arenas Mirave JI, Chantar C, Escartin P, Boixeda D, Pina R, Hernandez RF, Gil P. [Zollinger-Ellison syndrome type II. Preoperative diagnosis by means of gastrin stimulation tests]. Rev Clin Esp. 1978;148:411–414. [PubMed] [Google Scholar]
- 13.Arnold R, Fuchs K, Siewert R, Peiper HJ, Creutzfeldt W. [Morphology, clinical features, diagnosis, and treatment of Zollinger-Ellison syndrome (author’s transl)]. Dtsch Med Wochenschr. 1974;99:607–616. [DOI] [PubMed] [Google Scholar]
- 14.Arnold WS, Fraker DL, Alexander HR, Weber HC, Jensen RT. Apparent lymph node primary gastrinoma. Surgery. 1994;116:1123–1130. [PubMed] [Google Scholar]
- 15.Asgharian B, Chen YJ, Patronas NJ, Venzon DJ, Peghini P, Reynolds JC, Vortmeyer A, Zhang ZP, Gibril F, Jensen RT. Meningiomas may be a component tumor of MEN1. Clin Cancer Res. 2004;10:869–880. [DOI] [PubMed] [Google Scholar]
- 16.Asgharian B, Turner ML, Gibril F, Entsuah LK, Serrano J, Jensen RT. Cutaneous tumors in patients with MEN1 and gastrinomas: Prospective study of frequency and development of criteria with high sensitivity and specificity. J Clin Endocrinol Metab. 2004;89:5328–5336. [DOI] [PubMed] [Google Scholar]
- 17.Baffy G, Boyle JM. Association of Zollinger-Ellison syndrome with pancreatitis: report of five cases. Dig Dis Sci. 2000;45:1531–1534. [DOI] [PubMed] [Google Scholar]
- 18.Bahn RS, Scheithauer BW, vanHeerden JA, Laws ER, Horvath E, Gharib H. Nonidentical expressions of multiple endocrine neoplasia, type I, in identical twins. Mayo Clin Proc. 1986;61:689–696. [DOI] [PubMed] [Google Scholar]
- 19.Bali JP, Balmes JL, Fournajoux J, Cayrol B, Khazrai H. Effects of secretin on immuno-reactive serum gastrin in two cases of Zollinger-Ellison syndrome. Digestion. 1972;7:277–283. [DOI] [PubMed] [Google Scholar]
- 20.Baltas B, Nemeth J, Remak G, Csanadi J, Karacsonyi S. [Diagnostic and therapeutic problems in Zollinger-Ellison syndrome]. Zentralbl Chir. 1986;111:224–232. [PubMed] [Google Scholar]
- 21.Bashir S, Gibril F, Ojeaburu J, Asgharian B, Entsuah L, Ferraro G, Crafa P, Bordi C, Jensen RT. Prospective study of the ability of histamine, serotonin or chromogranin A levels to identify gastric carcinoids in patients with gastrinomas. Aliment Pharmacol Ther. 2002;16:1367–1382. [DOI] [PubMed] [Google Scholar]
- 22.Basso N, LeZoche E, Giri S, Percoco M, Speranza V. Acid and gastrin levels after bombesin and calcium infusion in patients with incomplete antrectomy. Am J Dig Dis. 1977;22:125–128. [DOI] [PubMed] [Google Scholar]
- 23.Basso N, LeZoche E, Materia A, Passaro J Jr., Speranza V. Studies with bombesin in Zollinger-Ellison syndrome. Br J Surg. 1981;68:97–100. [DOI] [PubMed] [Google Scholar]
- 24.Basso N, LeZoche E, Speranza V. Studies with bombesin in man. World J Surg. 1979;3:579–585. [DOI] [PubMed] [Google Scholar]
- 25.Bastagli A, Nosenzo MA, Galimberti A, Vitri P, Rovati V. [Gastrinoma and MEN I]. Ann Ital Chir. 1992;63:573–576. [PubMed] [Google Scholar]
- 26.Becker HD, Reeder DD, Thompson JC. Effect of glucagon on circulating gastrin. Gastroenterology. 1973;65:28–35. [PubMed] [Google Scholar]
- 27.Behr T, Becker W, Koch W, Grebmeier J, Wolf F. [Somatostatin receptor scintigraphy in neuroendocrine tumors exemplified by a patient with hepatic metastases of gastrinoma]. Z Gastroenterol. 1994;32:100–104. [PubMed] [Google Scholar]
- 28.Benson L, Lundqvist G, Wide L, Akerstrom G, Oberg K, Ljunghall S. Effects of secretin on parathyroid hormone and calcium in normal subjects, patients with hyperparathyroidism and patients with gastrinoma. Acta Med Scand. 1985;217:205–211. [DOI] [PubMed] [Google Scholar]
- 29.Benya RV, Metz DC, Venzon DJ, Fishbeyn VA, Strader DB, Orbuch M, Jensen RT. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am J Med. 1994;97:436–444. [DOI] [PubMed] [Google Scholar]
- 30.Berna MJ, Hoffmann KM, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients and comparison with 2229 cases from the literature. Medicine (Baltimore). 2006;10:12–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berson SA, Yalow RS. Radioimmunoassay in gastroenterology. Gastroenterology. 1972;62:1061–1084. [PubMed] [Google Scholar]
- 32.Blair EL, Grund ER, Miller IT, Redd JD, Sanders DJ, Thompson MH, Venables CW. Metiamide in the Zollinger-Ellison syndrome. Am J Dig Dis. 1975;20:1123–1130. [DOI] [PubMed] [Google Scholar]
- 33.Bold RJ, Lowry PS, Ishizuka J, Battey JF, Townsend CM Jr., Thompson JC. Bombesin stimulates the in vitro growth of a human gastric cancer cell line. J Cell Physiol. 1994;161:519–525. [DOI] [PubMed] [Google Scholar]
- 34.Bollen ECM, Lamers CB, Jansen JB, Larsson LI, Joosten HJ. Zollinger-Ellison syndrome due to a gastrin-producing ovarian cystadenocarcinoma. Br J Surg. 1981;68:776–777. [DOI] [PubMed] [Google Scholar]
- 35.Bordi C, Azzoni C, D’Adda T, Bertele A, Volpi R, Franze A. Endocrine cell replacement of oxyntic glands in Zollinger-Ellison syndrome: a role for female sex hormones? Endocr Pathol. 1995;6:345–354. [DOI] [PubMed] [Google Scholar]
- 36.Bordi C, Azzoni C, Ferraro G, Corleto VD, Gibril F, Delle Fave G, Lubensky IA, Venzon DJ, Jensen RT. Sampling strategies for analysis of enterochromaffin-like cell changes in Zollinger-Ellison syndrome. Am J Clin Pathol. 2000;114:419–425. [DOI] [PubMed] [Google Scholar]
- 37.Bordi C, D’Adda T, Ceda GP, Bertele A, Pilato FP, Ceresini G, Baggi MT, Missale. G Glycoprotein hormone alpha subunit in endocrine cells of human oxyntic mucosa. Studies on its relation to neuroendocrine tumors. Hepato-Gastroenterol. 1990;37:108–114. [PubMed] [Google Scholar]
- 38.Bordi C, DeVita O, Pilato FP, Carfagna G, D’Adda T, Missale G, Peracchia A. Multiple islet cell tumors with predominance of glucagon-producing cells and ulcer disease. Am J Clin Pathol. 1987;88:153–161. [DOI] [PubMed] [Google Scholar]
- 39.Bradley EL3, Galambos JT. Diagnosis of gastrinoma by the secretin suppression test. Surg Gynecol Obstet. 1976;143:784–788. [PubMed] [Google Scholar]
- 40.Brady CE III. Secretin provocation test in the diagnosis of Zollinger-Ellison syndrome. Am J Gastroenterol. 1991;86:129–134. [PubMed] [Google Scholar]
- 41.Brady CE. Release of gastrin by secretin. Gastroenterology. 1989;97:1354. [DOI] [PubMed] [Google Scholar]
- 42.Brady CE III, Hyatt JR, Utts SJ. Is the gastrin response to secretin provocation a function of antral G-cell mass? Results in the hypergastrinemia of acid hyposecretion. J Clin Gastroenterol. 1989;11:27–32. [DOI] [PubMed] [Google Scholar]
- 43.Brady CE, Johnson RC, Williams JR, Boran KLU. False-positive serum gastrin stimulation due to impure secretin. Gastroenterology. 1979;76:1106. [DOI] [PubMed] [Google Scholar]
- 44.Brady CE, Utts SJ, Dev J. Secretin provocation in normal and duodenal ulcer subjects. Is the gastrin rise in Zollinger-Ellison syndrome paradoxic or exaggeration? Dig Dis Sci. 1987;32:232–238. [DOI] [PubMed] [Google Scholar]
- 45.Brady CE, Utts SJ, Hyatt JR, Dev J. Secretin provocation: gastrin results in various clinical situations. Am J Gastroenterol. 1988;83:130–135. [PubMed] [Google Scholar]
- 46.Burcharth F, Stage JG, Stadil F, Jensen LI, Fischermann K. Localization of gastrinoma by transhepatic portal catheterization and gastrin assay. Gastroenterology. 1979;77:444–450. [PubMed] [Google Scholar]
- 47.Buysschaert M, Fiasse R, Rahier J, Leonard JP, Lammens P, Kestens PJ, Dive C. [Recent advances in the diagnosis of the Zollinger-Ellison syndrome (author’s transl)]. Acta Gastroenterol Belg. 1977;40:7–26. [PubMed] [Google Scholar]
- 48.Cadiot G, Helie C, Vallot T, Marmuse JP, Cosnes J, Riche A, Mignon M. [Stenosing esophagitis in Zollinger-Ellison syndrome]. Gastroenterol Clin Biol. 1994;18:1018–1020. [PubMed] [Google Scholar]
- 49.Cameron AJ, Hoffman HN. Zollinger-Ellison syndrome; Clinical features and long-term follow-up. Mayo Clin Proc. 1974;49:44–51. [PubMed] [Google Scholar]
- 50.Capitaine Y, Rime F. [Diagnosis of Zollinger-Ellison syndrome (ZES). Responsibility of the clinician]. Rev Med Suisse Romande. 1982;102:845–852. [PubMed] [Google Scholar]
- 51.Carlson HE, Levine GA, Goldberg NJ, Hershman JM. Hyperprolactinemia in multiple endocrine adenomatosis, type 1. Arch Intern Med. 1978;138:1807–1808. [PubMed] [Google Scholar]
- 52.Carty SE, Jensen RT, Norton JA. Prospective study of aggressive resection of metastatic pancreatic endocrine tumors. Surgery. 1992;112:1024–1031. [PubMed] [Google Scholar]
- 53.Cavina E [Current therapeutic problems in the Zollinger-Ellison syndrome. Personal cases]. Minerva Med. 1982;73:3015–3021. [PubMed] [Google Scholar]
- 54.Cavina E, Pagliai EF, Goletti O, Chiarugi M, Chirico A. Pylorus preserving pancreatoduodenectomy for gastrinoma. A case report. Ital J Surg Sci. 1986;16:291–295. [PubMed] [Google Scholar]
- 55.Chagnon JP, Barge J, Henin D, Blanc D. [Recklinghausen’s disease with digestive localizations associated with gastric acid hypersecretion suggesting Zollinger-Ellison syndrome]. Gastroenterol Clin Biol. 1985;9(1):65–69. [PubMed] [Google Scholar]
- 56.Cherner JA, Doppman JL, Norton JA, Miller DL, Krudy AG, Raufman JP, Collen MJ, Maton PN, Gardner JD, Jensen RT. Selective venous sampling for gastrin to localize gastrinomas. A prospective study. Ann Intern Med. 1986;105:841–847. [DOI] [PubMed] [Google Scholar]
- 57.Cherner JA, Sawyers JL. Benefit of resection of metastatic gastrinoma in multiple endocrine neoplasia type I. Gastroenterology. 1992;102:1049–1053. [DOI] [PubMed] [Google Scholar]
- 58.Chiba T Release of gastrin by secretin. Gastroenterology. 1989;97:1354. [DOI] [PubMed] [Google Scholar]
- 59.Christiansen J, Aagaard P. Parathyroid adenoma and gastric acid secretion. Scand J Gastroenterol. 1972;7:445–449. [DOI] [PubMed] [Google Scholar]
- 60.Christlieb AR, Schuster MM. Zollinger-Ellison syndrome. Arch Intern Med. 1964;114:381–388. [DOI] [PubMed] [Google Scholar]
- 61.Clark CJ, Ryan JA. Zollinger-Ellison syndrome caused by solitary gastrinoma blocking the pancreatic duct: Enucleation and pancreatic duct repair. Surgery. 2005;138:111–112. [DOI] [PubMed] [Google Scholar]
- 62.Cocco AE, Conway SJ. Zollinger-Ellison syndrome associated with ovarian mucinous cystadenocarcinoma. N Engl J Med. 1975;293:485–486. [DOI] [PubMed] [Google Scholar]
- 63.Collen MJ, Howard JM, McArthur KE, Raufman JP, Cornelius MJ, Ciarleglio CA, Gardner JD, Jensen RT. Comparison of ranitidine and cimetidine in the treatment of gastric hypersecretion. Ann Intern Med. 1984;100:52–58. [DOI] [PubMed] [Google Scholar]
- 64.Conte N, LoGiudice C, Valmachino VG, Manente P, Fioretti D, Gasparoni P, Pedrazzoli S. Zollinger-Ellison syndrome associated with primary hyperparathyroidism and pituitary prolactin-secreting adenoma (MEN1). Ital J Gastroenterol. 1981;13:196–198. [Google Scholar]
- 65.Corbetta S, Pizzocaro A, Peracchi M, Beck-Peccoz P, Faglia G, Spada A. Multiple endocrine neoplasia type 1 in patients with recognized pituitary tumours of different types. Clin Endocrinol. 1997;47:507–512. [DOI] [PubMed] [Google Scholar]
- 66.Corleto V, Annibale B, D’Ambra G, Saggioro A, Ferrua B, Cassetta MR, Della Fave G. Efficacy of long-term therapy with low doses of omeprazole in the control of gastric acid secretion in Zollinger-Ellison syndrome patients. Aliment Pharmacol Ther. 1993;7:167–173. [DOI] [PubMed] [Google Scholar]
- 67.Corleto VD, Annibale B, Gibril F, Angeletti S, Serrano J, Venzon DJ, Delle Fave G, Jensen RT. Does the widespread use of proton pump inhibitors mask, complicate and/or delay the diagnosis of Zollinger-Ellison syndrome? Aliment Pharmacol Ther. 2001;15:1555–1561. [DOI] [PubMed] [Google Scholar]
- 68.Crane SA, Summers RW, Heeringa WG. Long-term cimetidine and anticholinergic therapy in patients with gastrinoma. Am J Surg. 1979;138:446–450. [DOI] [PubMed] [Google Scholar]
- 69.Creutzfeldt W, Arnold R, Creutzfeldt C, Track NS. Pathomorphologic, biochemical and diagnostic aspects of gastrinomas (Zollinger-Ellison syndrome). Hum Pathol. 1975;6:47–76. [DOI] [PubMed] [Google Scholar]
- 70.Croffie JM, Fitzgerald JF, Grosfeld JL, Chong SK, Heifetz SA. Marked gastric mucosal hyperplasia without definite peptic ulceration related to a multifocal hepatic gastrinoma. J Pediatr Gastroenterol Nutr. 1994;19:315–321. [DOI] [PubMed] [Google Scholar]
- 71.D’Adda T, Corleto V, Pilato FP, Baggi MT, Robutti F, Delle Fave G, Bordi C. Quantitative ultrastructure of endocrine cells of oxyntic mucosa in Zollinger-Ellison syndrome. Correspondence with light microscopic findings. Gastroenterology. 1990;99:17–26. [DOI] [PubMed] [Google Scholar]
- 72.Danielides IC, Mellow MH. Effect of acute hypercalcemia on human esophageal motility. Gastroenterology. 1978;75:1115–1119. [PubMed] [Google Scholar]
- 73.de Broucker F, Caudron C, Sournia V, Adamsbaum C, Levesque M. [Zollinger-Ellison syndrome of ovarian origin. Apropos of a case with a review of the literature]. J Radiol. 1989;70:111–114. [PubMed] [Google Scholar]
- 74.de la Pierre M, Arico S, Capussotti L, Dellepiane M, Marucci M, Gandini G, Imarisio P. [Description of a case of Zollinger-Ellison syndrome]. Minerva Med. 1984;75:1153–1158. [PubMed] [Google Scholar]
- 75.Debas HT, Soon-Shlong P, McKenzie AD, Bogoch A, Greig JH, Dunn WL, Magill AB. Use of secretin in the roentgenologic and biochemical diagnosis of duodenal gastrinoma. Am J Surg. 1983;145:408–411. [DOI] [PubMed] [Google Scholar]
- 76.DeLellis RA, Gagel RF, Kaplan MM, Curtis LE. Gastrinoma of duodenal G-cell origin. Cancer. 1976;38:201–208. [DOI] [PubMed] [Google Scholar]
- 77.Deveney CW, Deveney KE, Jones S, Jaffe BM. Calcium and secretin tests in diagnosis of gastrinoma. Gastroenterology. 1976;70:968. [Google Scholar]
- 78.Deveney CW, Deveney KS, Jaffe BM, Jones RS, Way LW. Use of calcium and secretin in the diagnosis of gastrinoma (Zollinger-Ellison syndrome). Ann Intern Med. 1977;87:680–686. [DOI] [PubMed] [Google Scholar]
- 79.Deveney CW, Deveney KS, Way LW. The Zollinger-Ellison syndrome--23 years later. Ann Surg. 1978;188:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dhillo WS, Jayasena CN, Lewis CJ, Martin NM, Tang KC, Meeran K, Todd JF. Plasma gastrin measurement cannot be used to diagnose a gastrinoma in patients on either proton pump inhibitors or histamine type-2 receptor antagonists. Ann Clin Biochem. 2006;43:153–155. [DOI] [PubMed] [Google Scholar]
- 81.Di Mario F, Plebani M, Gottardello L, Battaglia G, Vianello F, Farinati F, Del Favero G. Role of serum fasting gastrin in screening for hypergastrinemic syndromes in duodenal ulcer disease. Clin Biochem. 1992;25:121–124. [DOI] [PubMed] [Google Scholar]
- 82.Diepstraten A, Driessen WM, Jansen JB, Lamers CB. Fallibility of gastrin level as an indicator of complete excision of a gastrinoma. Br J Surg. 1990;77:1403–1405. [DOI] [PubMed] [Google Scholar]
- 83.DiMagno EP, Go VL. Malabsorption secondary to antral gastrin-cell hyperplasia. Mayo Clin Proc. 1974;49:727–730. [PubMed] [Google Scholar]
- 84.Ding WQ, Kuntz S, Bohmig M, Wiedenmann B, Miller LJ. Dominant negative action of an abnormal secretin receptor arising from mRNA missplicing in a gastrinoma. Gastroenterology. 2002;122:500–511. [DOI] [PubMed] [Google Scholar]
- 85.Doppman JL, Miller DL, Chang R, Maton PN, London JF, Gardner JD, Jensen RT, Norton JA. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology. 1990;174:25–29. [DOI] [PubMed] [Google Scholar]
- 86.Duarte P, Luz Z, Lourenco I, Reis B, Alvelos A. [Gastrinoma localized in the pylorus in a patient with recurrent peptic ulcer]. Acta Med Port. 1992;5:393–396. [PubMed] [Google Scholar]
- 87.Duncan LA, Marynick SP. Glucagonoma and dacarbazine [letter]. Ann Intern Med. 1982;97:930. [DOI] [PubMed] [Google Scholar]
- 88.Dutta P, Wallace MR, Wrong OM, Taylor S, Welbourn RB. Familial multiple endocrine adenopathy (primary hyperparathyroidism and Zollinger-Ellison syndrome) in two siblings. Proc R Soc Med. 1968;61:658–660. [PMC free article] [PubMed] [Google Scholar]
- 89.Eberle F, Martini GA, Scheuer A, Rohr G, Sohl R, Bischof W, Wahl R. Thymus carcinoid tumor and multiple endocrine neoplasia type I (MEN1). Internist (Berl). 1982;23:718–724. [PubMed] [Google Scholar]
- 90.el-Omar EM, Penman ID, Ardill JE, Chittajallu RS, Howie C, McColl KE. Helicobacter pylori infection and abnormalities of acid secretion in patients with duodenal ulcer disease. Gastroenterology. 1995;109:681–691. [DOI] [PubMed] [Google Scholar]
- 91.Ellison EC, Gower WR, Elkhammas E, Woltering EA, Sparks J, O’Doriosio TM, Fabri PJ. Characterization of the in vivo and in vitro inhibition of gastrin secretion from gastrinoma by a somatostatin analogue (SMS 201–995). Am J Med. 1986;81:56–64. [DOI] [PubMed] [Google Scholar]
- 92.Ellison EH, Wilson SD. The Zollinger-Ellison syndrome: Re-appraisal and evaluation of 260 registered cases. Ann Surg. 1964;160:512–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Elouaer-Blanc L, Sobhani I, Ruszniewski P, Duet M, Lehy T, Mignon M, Bonfils S, Lewin MJ. Gastrin secretion by gastrinoma cells in long-term culture. Am J Physiol. 1988;255:G596–G602. [DOI] [PubMed] [Google Scholar]
- 94.Erdozain JC, Martin de AC, Moreira VF, Moreno A. [Zollinger-Ellison syndrome with basal gastrin and non-diagnostic stimulation tests (secretin and calcium)]. Med Clin (Barc ). 1993;100:37–38. [PubMed] [Google Scholar]
- 95.Fallucca P, Delle Fave G, Giangrande L, Del Balzo P, De Magistris L, Carratu R. Effect of somatostatin on gastrin, insulin and glucagon secretion in two patients with Zollinger-Ellison syndrome. J Endocrinol Invest. 1981;4:451–453. [DOI] [PubMed] [Google Scholar]
- 96.Farley DR, Van Heerden JA, Grant CS, Miller LJ, Ilstrup DM. The Zollinger-Ellison syndrome. A collective surgical experience. Ann Surg. 1992;215:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fegan C, Sunter JP, Miller IA. Menetrier’s disease complicated by development of the Zollinger-Ellison syndrome. Br J Surg. 1985;72:929–930. [DOI] [PubMed] [Google Scholar]
- 98.Feldman M, Schiller LR, Walsh JH, Fordtran JS, Richardson CT. Positive intravenous secretin test in patients with achlorhydria-related hypergastrinemia. Gastroenterology. 1987;93:59–62. [DOI] [PubMed] [Google Scholar]
- 99.Feldman M, Walsh JH, Richardson CT. Serum gastrin response to secretin after vagotomy. Dig Dis Sci. 1980;25:921–923. [DOI] [PubMed] [Google Scholar]
- 100.Feurle GE, Helmstaeder V, Hoevels J, Wenzel-Herzer G, Klempa J. [Changes in the diagnosis and treatment of Zollinger-Ellison syndrome]. Dtsch Med Wohenschr. 1982;107:697–704. [DOI] [PubMed] [Google Scholar]
- 101.Fiasse R, DeGraef J, Pauwels S, Woussen-Colle MC, Rahier J, Kestens PJ, Dive C. [Hypergastrinemia. Physiopathological and clinical aspects with a study of a series of Zollinger-Ellison cases]. Acta Gastroenterol Belg. 1982;45:293–309. [PubMed] [Google Scholar]
- 102.Fishbeyn VA, Norton JA, Benya RV, Pisegna JR, Venzon DJ, Metz DC, Jensen RT. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993;119:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fraker DL, Jensen RT. Pancreatic endocrine tumors. In: DeVita VT, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. Philadelphia, PA: Lippincott-Raven Publishers; 1997;1678–1704. [Google Scholar]
- 104.Fraker DL, Norton JA, Alexander HR, Venzon DJ, Jensen RT. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fries W, Moschino P, Chierichetti F, Carniato S, Martines D, Martin A, Cecchetto A, Ricciardi G, Stracca-Pansa V, Naccarato R, Corsini A. Changing approach to gastroenteropancreatic tumours. Case report of a gastrinoma metastasis localized by somatostatin-receptor scintigraphy. Ital J Gastroenterol. 1996;28:220–224. [PubMed] [Google Scholar]
- 106.Friesen SR. The development of endocrinopathies in the prospective screening of two families with multiple endocrine adenopathy, type I. World J Surg. 1979;3:753–764. [DOI] [PubMed] [Google Scholar]
- 107.Friesen SR, McGuigan JE. Ectopic apudocarcinomas and associated endocrine hyperplasias of the foregut. Ann Surg. 1975;182:371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Friesen SR, Schimke RN, Pearse AG. Genetic aspects of the Z-E syndrome: prospective studies in two kindreds: antral gastrin cell hyperplasia. Ann Surg. 1972;176:370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frucht H, Doppman JL, Norton JA, Miller DL, Dwyer AJ, Frank JA, Vinayek R, Maton PN, Jensen RT. Gastrinomas: Comparison of MR Imaging with CT, angiography and US. Radiology. 1989;171:713–717. [DOI] [PubMed] [Google Scholar]
- 110.Frucht H, Howard JM, Slaff JI, Wank SA, McCarthy DM, Maton PN, Vinayek R, Gardner JD, Jensen RT. Secretin and calcium provocative tests in the Zollinger-Ellison syndrome: A prospective study. Ann Intern Med. 1989;111:713–722. [DOI] [PubMed] [Google Scholar]
- 111.Frucht H, Howard JM, Stark HA, McCarthy DM, Maton PN, Gardner JD, Jensen RT. Prospective study of the standard meal provocative test in Zollinger-Ellison syndrome. Am J Med. 1989;87:528–536. [DOI] [PubMed] [Google Scholar]
- 112.Frucht H, Norton JA, London JF, Vinayek R, Doppman JL, Gardner JD, Jensen RT, Maton PN. Detection of duodenal gastrinomas by operative endoscopic transillumination: a prospective study. Gastroenterology. 1990;99:1622–1627. [DOI] [PubMed] [Google Scholar]
- 113.Furuta GT, Bloss RS, Lifschitz CH, Khalil K, Shulman RJ. Colonic interposition after esophagogastrectomy in a child with Zollinger-Ellison syndrome. J Pediatr Surg. 1989;24:1146–1148. [DOI] [PubMed] [Google Scholar]
- 114.Ganguli PC, Elder JB, Polak JM, Pearse AG. Antral-gastrin cell hyperplasia in peptic-ulcer disease (Letter). Lancet. 1974;1:1288. [DOI] [PubMed] [Google Scholar]
- 115.Garcia JC, Carney JA, Stickler GB, Telander RL, Malagelada JR. Zollinger-Ellison syndrome and neurofibromatosis in a 13-year-old boy. J Pediatr. 1978;93:982–984. [DOI] [PubMed] [Google Scholar]
- 116.Gaztambide S, Vazquez JA. Short- and long-term effect of a long-acting somatostatin analogue, lanreotide (SR-L) on metastatic gastrinoma. J Endocrinol Invest. 1999;22:144–146. [DOI] [PubMed] [Google Scholar]
- 117.Gibril F, Chen Y-J, Schrump DS, Vortmeyer A, Zhuang ZP, Lubensky IA, Reynolds JG, Louie JV, Entsuah L, Huang K, Asgharian B, Jensen RT. Prospective study of thymic carcinoids in patients with Multiple Endocrine Neoplasia Type 1. J Clin Endocrinol Metab. 2003;88:1066–1081. [DOI] [PubMed] [Google Scholar]
- 118.Gibril F, Curtis LT, Termanini B, Fritsch MK, Lubensky IA, Doppman JL, Jensen RT. Primary cardiac gastrinoma causing Zollinger-Ellison syndrome. Gastroenterology. 1997;112:567–574. [DOI] [PubMed] [Google Scholar]
- 119.Gibril F, Jensen RT. Zollinger-Ellison syndrome revisited: diagnosis, biologic markers, associated inherited disorders, and acid hypersecretion. Curr Gastroenterol Rep. 2004;6:454–463. [DOI] [PubMed] [Google Scholar]
- 120.Gibril F, Lindeman RJ, Abou-Saif A, Shojamanesh H, Roy PK, Peghini PL, Reynolds JC, Lubensky IA, Jensen RT. Retained gastric antrum syndrome. A forgotten, treatable cause of refractory peptic ulcer disease. Dig Dis Sci. 2001;46:610–617. [DOI] [PubMed] [Google Scholar]
- 121.Gibril F, Reynolds JC, Doppman JL, Chen CC, Venzon DJ, Termanini B, Weber HC, Stewart CA, Jensen RT. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas: a prospective study. Ann Intern Med. 1996;125:26–34. [DOI] [PubMed] [Google Scholar]
- 122.Gibril F, Reynolds JC, Roy PK, Peghini PL, Doppman JL, Jensen RT. Ability of somatostatin receptor scintigraphy to identify patients with localized gastric carcinoids: A prospective study. J Nucl Med. 2000;41:1646–1656. [PubMed] [Google Scholar]
- 123.Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine. 2004;83:43–83. [DOI] [PubMed] [Google Scholar]
- 124.Gibson RG, Mihas AA, Hirschowitz BI, Courington DP, Ho KJ. Benign duodenal gastrinoma associated with the Zollinger-Ellison syndrome. Alabama J Med Sci. 1977;14:387–393. [PubMed] [Google Scholar]
- 125.Glowniak JV, Shapiro B, Vinik AI, Glaser B, Thompson NW, Cho KJ. Percutaneous transhepatic venous sampling of gastrin: value in sporadic and familial islet-cell tumors and G-cell hyperfunction. N Engl J Med. 1982;307:293–297. [DOI] [PubMed] [Google Scholar]
- 126.Goebel SU, Peghini PL, Goldsmith PK, Spiegel AM, Gibril F, Raffeld M, Jensen RT, Serrano J. Expression of the calcium-sensing receptor in gastrinomas. J Clin Endocrinol Metab. 2000;85:4131–4137. [DOI] [PubMed] [Google Scholar]
- 127.Gogel HK, Buckman MT, Cadieux T, McCarthy DM. Gastric secretion and hormonal interactions in multiple endocrine neoplasia type 1. Arch Intern Med. 1985;145:855–859. [PubMed] [Google Scholar]
- 128.Gogel HK, Cadieux D, Strickland RG. The use of Boot’s secretin in the secretin stimulation test. Gastroenterology. 1980;79:773–774. [PubMed] [Google Scholar]
- 129.Gower WR Jr., Ellison EC, Knierim TH, Elkhammas EA, O’Dorisio TM, Fabri PJ. Gastrinoma in vitro: morphological and physiological studies of primary cell cultures. Gastroenterology. 1990;98:936–954. [DOI] [PubMed] [Google Scholar]
- 130.Greenstein RJ, Clain DJ, Straus E, Yalow RS. Distribution, molecular forms, and bioactivity of immunoreactive gastrin in a patient with metastatic gastrinoma. Am J Gastroenterol. 1987;82:886–889. [PubMed] [Google Scholar]
- 131.Griffith JL, Cummings OW, Hirschowitz BI. Development of sustained achlorhydria in a patient with the Zollinger-Ellison syndrome treated with omeprazole [published erratum appears in Gastroenterology 1992 Mar;102(3):1096]. Gastroenterology. 1991;101(1):242–246. [DOI] [PubMed] [Google Scholar]
- 132.Groarke JF, Haggstrom GD, Halpern NB, Hirschowitz BI. Zollinger-Ellison syndrome unresponsive to cimetidine. Am J Gastroenterol. 1979;72:168–170. [PubMed] [Google Scholar]
- 133.Groza P, Vasilescu E, Petrescu L. [Clinical information on gastric secretion and gastrinemia after gastric resection, in Zollinger-Ellison syndrome and in pernicious anemia]. Physiologie. 1980;17:175–181. [PubMed] [Google Scholar]
- 134.Guzzetti A, Premoli P. [Zollinger-Ellison syndrome]. Chir Ital. 1982;34:735–744. [PubMed] [Google Scholar]
- 135.Hacki WH. [Diagnosis and curative therapy in Zollinger-Ellison syndrome]. Schweiz Med Wochenschr. 1985;115:575–581. [PubMed] [Google Scholar]
- 136.Hage E, Hendel L, Gustafsen J, Hendel J. Histopathology of the gastric oxyntic mucosa in two different patient groups during long-term treatment with omeprazole. Eur J Gastroenterol Hepatol. 2003;15:781–789. [DOI] [PubMed] [Google Scholar]
- 137.Hansky J, Soveny C, Korman MG. Effect of secretin on serum gastrin as measured by radioimmunoassay. Gastroenterology. 1971;61:62–68. [PubMed] [Google Scholar]
- 138.Hanssen LE, Schrumpf E, Jacobsen MB, Kolbenstvedt AN, Kolmannskog F, Bergan A, Dolva LO. Extended experience with recombinant alpha-2b interferon with or without hepatic artery embolization in the treatment of midgut carcinoid tumors. Acta Oncol. 1991;30:523–527. [DOI] [PubMed] [Google Scholar]
- 139.Hausamen TU, Fritsch WP, Scholten T, Jungblut R, Strohmeyer G. [Treatment of peptic ulcer in the Zollinger-Ellison syndrome with histamin H2-receptor antagonists (author’s transl)]. Dtsch Med Wochenschr. 1977;102:1709–1712. [DOI] [PubMed] [Google Scholar]
- 140.Hirschowitz BI, Mohnen J, Shaw S. Long-term treatment with lansoprazole for patients with Zollinger-Ellison syndrome. Aliment Pharmacol Ther. 1996;10:507–522. [DOI] [PubMed] [Google Scholar]
- 141.Hixson LJ, Morgan TR, Sampliner RE. False-positive secretin-KABI provocation test associated with achlorhydria. J Clin Gastroenterol. 1988;10:201–204. [DOI] [PubMed] [Google Scholar]
- 142.Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19:675–697. [DOI] [PubMed] [Google Scholar]
- 143.Hoffmann KM, Gibril F, Entsuah LK, Serrano J, Jensen RT. Patients with Multiple Endocrine Neoplasia type 1 with Gastrinomas Have an Increased Risk of Severe Esophageal Disease Including Stricture, and the Premalignant Condition, Barrett’s Esophagus. J Clin Endocrinol Metab. 2006;91:204–212. [DOI] [PubMed] [Google Scholar]
- 144.Howard JM, Chremos AN, Collen MJ, McArthur KE, Cherner JA, Maton PN, Ciarleglio CA, Cornelius MJ, Gardner JD, Jensen RT. Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology. 1985;88:1026–1033. [DOI] [PubMed] [Google Scholar]
- 145.Howard TJ, Zinner MJ, Stabile BE, Passaro E Jr. Gastrinoma excision for cure. A prospective analysis. Ann Surg. 1990;211:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hutcheon DF, Bayless TM, Cameron JL, Baylin SB. Hormone-mediated watery diarrhea in a family with multiple endocrine neoplasms. Ann Intern Med. 1979;90:932–934. [DOI] [PubMed] [Google Scholar]
- 147.Imamura M, Adachi H, Takahashi K, Noguchi M, Mizutani N, Nakagawa M, Tobe T. Gastrin release from gastrinoma cells stimulated with secretin. Dig Dis Sci. 1982;27:1130–1136. [DOI] [PubMed] [Google Scholar]
- 148.Imamura M, Takahashi K. Use of selective arterial secretin injection test to guide surgery in patients with Zollinger-Ellison syndrome. World J Surg. 1993;17:433–438. [DOI] [PubMed] [Google Scholar]
- 149.Imamura M, Takahashi K, Adachi H, Minematsu S, Shimada Y, Naito M, Suzuki T, Tobe T, Azuma T. Usefulness of selective arterial secretin injection test for localization of gastrinoma in the Zollinger-Ellison syndrome. Ann Surg. 1987;205:230–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Imamura M, Takahashi K, Isobe Y, Hattori Y, Satomura K, Tobe T. Curative resection of multiple gastrinomas aided by selective arterial secretin injection and intraoperative secretin test. Ann Surg. 1989;210:710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ippoliti AF. Zollinger-Ellison syndrome: provocative diagnostic tests. Ann Intern Med. 1977;87:787–788. [DOI] [PubMed] [Google Scholar]
- 152.Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology. 1973;65:140–165. [PubMed] [Google Scholar]
- 153.Isenberg JI, Walsh JH, Passaro E Jr., Moore EW, Grossman ME. Unusual effect of secretin on serum gastrin, serum calcium and gastric acid secretion in a patient with suspected Zollinger- Ellison syndrome. Gastroenterology. 1972;62:626–631. [PubMed] [Google Scholar]
- 154.Ishido H, Yamashita N, Kitaoka M, Tanaka Y, Ogata E. A case of ectopic ACTH syndrome associated with Zollinger-Ellison syndrome: long term survival with chemical adrenalectomy. Endocrine J. 1994;41:171–176. [DOI] [PubMed] [Google Scholar]
- 155.Itami A, Kato M, Komoto I, Doi R, Hosotani R, Shimada Y, Imamura M. Human gastrinoma cells express calcium-sensing receptor. Life Sci. 2001;70:119–129. [DOI] [PubMed] [Google Scholar]
- 156.Jansen JB, Klinkenberg-Knol EC, Meuwissen SG, De Bruijne JW, Festen HP, Snel P, Luckers AE, Biemond I, Lamers CB. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology. 1990;99:621–628. [DOI] [PubMed] [Google Scholar]
- 157.Jansen JB, Lamers CB. Effects of changes in serum calcium on secretin-stimulated serum gastrin in patients with Zollinger-Ellison syndrome. Gastroenterology. 1982;83:173–178. [PubMed] [Google Scholar]
- 158.Jansen JB, Lamers CB. Serum gastrin responses to bombesin and food in patients with hypergastrinemia. Dig Dis Sci. 1982;27:303–307. [DOI] [PubMed] [Google Scholar]
- 159.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. [DOI] [PubMed] [Google Scholar]
- 160.Jensen RT. Zollinger-Ellison syndrome. In: Doherty GM, Skogseid B, eds. Surgical Endocrinology: Clinical Syndromes. Philadelphia: Lippincott Williams & Wilkins; 2001;291–344. [Google Scholar]
- 161.Jensen RT. Consequences of long-term proton pump blockade: Highlighting insights from studies of patients with gastrinomas. Basic Clin Pharmacol Toxicol. 2006;98:4–19. [DOI] [PubMed] [Google Scholar]
- 162.Jensen RT. Gastrinoma as a model for prolonged hypergastrinemia in man. In: Walsh JH, ed. Gastrin. New York, NY: Raven Press Publishing Co.; 1993;373–393. [Google Scholar]
- 163.Jensen RT. Use of omeprazole and other proton pump inhibitors in the Zollinger-Ellison syndrome. In: Olbe L, ed. Milestones in Drug Therapy. Basel, Switzerland: Birkhauser Verlag AG Publish. Co.; 1999;205–221. [Google Scholar]
- 164.Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol Toxicol. 2002;91:333–350. [DOI] [PubMed] [Google Scholar]
- 165.Jensen RT, Doppman JL, Gardner JD. Gastrinoma. In: Go VLW, Brooks FA, DiMagno EP, Gardner JD, Lebenthal E, Scheele GA, eds. The Exocrine Pancreas: Biology, Pathobiology and Disease. New York: Raven Press; 1986;727–744. [Google Scholar]
- 166.Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, eds. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press Publishing Co.; 1993;931–978. [Google Scholar]
- 167.Jensen RT, Gardner JD, Raufman JP, Pandol SJ, Doppman JL, Collen MJ. Zollinger-Ellison syndrome: current concepts and management. Ann Intern Med. 1983;98:59–75. [DOI] [PubMed] [Google Scholar]
- 168.Johnson JA, Fabri PJ, Lott JA. Serum gastrins in Zollinger-Ellison syndrome: identification of localized disease. Clin Chem. 1980;26:867–870. [PubMed] [Google Scholar]
- 169.Joshi VV, Nord KS, Hanna M. Case 3. Renal gastrinoma with Zollinger-Ellison syndrome. Pediatr Pathol. 1986;6:475–476. [DOI] [PubMed] [Google Scholar]
- 170.Kandler C, Holle A. [Malignant gastrinoma]. Zentralbl Chir. 1985;110:305–310. [PubMed] [Google Scholar]
- 171.Kaplan EL, Horvath K, Udekwu A, Straus F, Schark C, Ferguson DJ, Skinner DB. Gastrinomas: a 42-year experience. World J Surg. 1990;14:365–375. [DOI] [PubMed] [Google Scholar]
- 172.Kato H, Shimozawa E, Kojima T, Tanabe T. A case report of Zollinger Ellison syndrome and review of the literature. Jpn J Surg. 1991;21:105–109. [DOI] [PubMed] [Google Scholar]
- 173.Kato M, Imamura M, Hosotani R, Shimada Y, Doi R, Itami A, Komoto I, Kosaka MTT, Konishi J. Curative resection of microgastrinomas based on the intraoperative secretin test. World J Surg. 2000;24:1425–1430. [DOI] [PubMed] [Google Scholar]
- 174.Katoh H, Yabana T, Yachi A, Tanabe T. Malignant Zollinger-Ellison syndrome. Am Surg. 1990;56:360–363. [PubMed] [Google Scholar]
- 175.Kaur U, Dilawari JB, Kataria RN, Anand BS, Bhushnurmath SR, Dash RJ. Zollinger Ellison syndrome: a case report. J Assoc Physicians India. 1983;31:546–549. [PubMed] [Google Scholar]
- 176.Keuppens F, Willems G, DeGraef J, Woussen-Colle MC. Antral gastrin cell hyperplasia in patients with peptic ulcer. Ann Surg. 1980;191:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kiewiet RM, Ponssen HH, Janssens EN, Fels PW. Ventricular fibrillation in hypercalcaemic crisis due to primary hyperparathyroidism. Neth J Med. 2004;62:94–96. [PubMed] [Google Scholar]
- 178.King CE, Toskes PP. Nutrient malabsorption in the Zollinger-Ellison Syndrome. Arch Intern Med. 1983;143:349–351. [PubMed] [Google Scholar]
- 179.Kitagawa M, Hayakawa T, Kondo T, Shibata T, Sakai Y, Ohno H, Nimura Y, Hayakawa N, Kamiya J, Kondo S. Gastrinoma in a mesenteric lymph node. Am J Gastroenterol. 1989;84:660–662. [PubMed] [Google Scholar]
- 180.Ko TC, Flisak M, Prinz RA. Selective intra-arterial methylene blue injection: a novel method of localizing gastrinoma. Gastroenterology. 1992;102:1062–1064. [DOI] [PubMed] [Google Scholar]
- 181.Kolts BE, Herbst CA, McGuigan JE. Calcium and secretin-stimulated gastrin release in the Zollinger- Ellison syndrome. Ann Intern Med. 1974;81:758–762. [DOI] [PubMed] [Google Scholar]
- 182.Korman MG, Soveny C, Hansky J. Paradoxical effect of secretin on serum immunoreactive gastrin in the Zollinger-Ellison syndrome. Digestion. 1973;8:407–416. [DOI] [PubMed] [Google Scholar]
- 183.Korman MG, Soveny C, Hansky J. Effect of glucagon on serum gastrin. II. Studies in pernicious anaemia and the Zollinger-Ellison syndrome. Gut. 1973;14:459–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kothary PC, Fabri PJ, Gower W, O’Dorisio TM, Ellis J, Vinik AI. Evaluation of NH2-terminus gastrins in gastrinoma syndrome. J Clin Endocrinol Metab. 1986;62:970–974. [DOI] [PubMed] [Google Scholar]
- 185.Krudy AG, Doppman JL, Jensen RT, Norton JA, Collen MJ, Shawker TH, Gardner JD, McArthur KK, Gorden P. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. Am J Roentgenol. 1984;143:585–589. [DOI] [PubMed] [Google Scholar]
- 186.Kwan CP, Tytgat GN. Antral G-cell hyperplasia: a vanishing disease? Eur J Gastroenterol Hepatol. 1995;7:1099–1103. [DOI] [PubMed] [Google Scholar]
- 187.Lamberts R Morphological changes of the human gastric mucosa under long-term proton pump inhibitor therapy and their clinical relevance. Microsc Res Tech. 2000;48:357–366. [DOI] [PubMed] [Google Scholar]
- 188.Lamberts SW, Tilanus HW, Klooswijk AI, Bruining HA, van der Lely AJ, de Jong FH. Successful treatment with SMS 201–995 of Cushing’s syndrome caused by actopic adrenocorticotropin secretion from a metastatic gastrin-secreting pancreatic islet cell carcinoma. J Clin Endocrinol Metab. 1988;67(5):1080–1083. [DOI] [PubMed] [Google Scholar]
- 189.Lamers CB. Clinical usefulness of the secretin provocation test. J Clin Gastroenterol. 1981;3:255–259. [DOI] [PubMed] [Google Scholar]
- 190.Lamers CB, Buis JT, Van Tongeren JH. Secretin-stimulated serum gastrin levels in hyperparathyroid patients from families with multiple endocrine adenomatosis type 1. Ann Intern Med. 1977;86:719–724. [DOI] [PubMed] [Google Scholar]
- 191.Lamers CB, Festen HP, Van Tongeren JH. Long-term treatment with histamine H2-receptor antagonists in Zollinger-Ellison syndrome. Am J Gastroenterol. 1978;70:286–291. [PubMed] [Google Scholar]
- 192.Lamers CB, Jansen JB, Roeffen W. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology. 1981;80:1615–1616. [PubMed] [Google Scholar]
- 193.Lamers CB, Rotter JI, Jansen JB. Gastrin cell function in familial multiple endocrine neoplasia type I. Gut. 1988;29:1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lamers CB, Ruland CM, Joosten HJ, Verkooyen HC, Van Tongeren JH, Rehfeld JF. Hypergastrinemia of antral origin in duodenal ulcer. Am J Dig Dis. 1978;23:998–1002. [DOI] [PubMed] [Google Scholar]
- 195.Lamers CB, Van Tongeren JH. Postprandial serum gastrin levels in patients with combined hypergastrinaemia and hyperchlorhydria. Br J Surg. 1979;66:547–549. [DOI] [PubMed] [Google Scholar]
- 196.Lamers CB, Van Tongeren JHM. Comparative study of the value of calcium, secretin, and meal stimulated increase in serum gastrin in the diagnosis of the Zollinger-Ellison syndrome. Gut. 1977;18:128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lamers CB, Van Tongeren JHM. Serum gastrin response to acute and chronic hypercalcemia in man: studies on the value of calcium stimulated serum gastrin levels in the diagnosis of Zollinger-Ellison syndrome. Eur J Clin Invest. 1977;7:315–317. [DOI] [PubMed] [Google Scholar]
- 198.Lamers CBHW, Lind T, Moberg S, Jansen JBMJ, Olbe L. Omeprazole in Zollinger-Ellison syndrome: effects of a single dose and of long term treatment in patients resistant to histamine H2-receptor antagonists. N Engl J Med. 1984;310:758–761. [DOI] [PubMed] [Google Scholar]
- 199.Larkin CJ, Joy ES, Ardill JE, Johnston CF, Collins JS, Buchanan KD. Gastrinomas and the change in their presentation and management in Northern Ireland, UK, from 1970 to 1996. Eur J Gastroenterol Hepatol. 1998;10:947–952. [DOI] [PubMed] [Google Scholar]
- 200.Larkworthy W Cimetidine in the mangement of a case of the Zollinger-Ellison syndrome. In: Burland WL, Simkins MA, eds. Cimetidine. Amsterdam: Excerpta Medica; 1977;326. [Google Scholar]
- 201.Lehy T, Mignon M, Cadiot G, Elouaer-Blanc L, Ruszniewski P, Lewin MJ, Bonfils S. Gastric endocrine cell behavior in Zollinger-Ellison patients upon long-term potent antisecretory treatment. Gastroenterology. 1989;96:1029–1040. [DOI] [PubMed] [Google Scholar]
- 202.Leimer M, Kurtaran A, Smith-Jones P, Raderer M, Havlik E, Angelberger P, Vorbeck F, Niederle B, Herold C, Virgolini I. Response to treatment with yttrium 90-DOTA-lanreotide of a patient with metastatic gastrinoma. J Nucl Med. 1998;39:2090–2094. [PubMed] [Google Scholar]
- 203.Levi S, Beardshall K, Haddad G, Playford R, Ghosh P, Calam J. Campylobacter pylori and duodenal ulcers: the gastrin link [see comments]. Lancet. 1989;1:1167–1168. [DOI] [PubMed] [Google Scholar]
- 204.Lewin KJ, Yang K, Ulick T, Elashoff JD, Walsh J. Primary gastrin-cell hyperplasia. Am J Surg Pathol. 1984;8:821–832. [PubMed] [Google Scholar]
- 205.Lloyd-Davies KA, Rutgersson K, Solvell L. Omeprazole in the treatment of Zollinger-Ellison syndrome: a 4-year international study. Aliment Pharmacol Ther. 1988;2:13–32. [DOI] [PubMed] [Google Scholar]
- 206.London JF, Shawker TH, Doppman JL, Frucht HH, Vinayek R, Stark HA, Miller LS, Miller DL, Norton JA, Gardner JD, Jensen RT. Zollinger-Ellison syndrome: prospective assessment of abdominal US in the localization of gastrinomas. Radiology. 1991;178:763–767. [DOI] [PubMed] [Google Scholar]
- 207.Long TT3, Barton TK, Draffin R, Reeves WJ, McCarty KS Jr. Conservative management of the Zollinger-Ellison syndrome. Ectopic gastrin production by an ovarian cystadenoma. J A M A. 1980;243:1837–1839. [PubMed] [Google Scholar]
- 208.Lye WC, Lee KO, Tay HH. Multiple endocrine neoplasia type 1 -- presenting with impotence. Singapore Med J. 1990;31:277–279. [PubMed] [Google Scholar]
- 209.Lyons DF, Eisen BR, Clark MR, Pysher TJ, Kem KC. Concurrent Cushing’s and Zollinger-Ellison syndromes in a patient with islet cell carcinoma: case report and review of the literature. Am J Med. 1984;76:729–733. [DOI] [PubMed] [Google Scholar]
- 210.MacFarlane MP, Fraker DL, Alexander HR, Norton JA, Jensen RT. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-Type 1. Surgery. 1995;118:973–980. [DOI] [PubMed] [Google Scholar]
- 211.MacGillivray DC, Rushin JM, Zeiger MA, Shakir KM. The significance of gastrinomas found in peripancreatic lymph nodes. Surgery. 1991;109:558–562. [PubMed] [Google Scholar]
- 212.Macleod AF, Ayers B, Young AE, Medd WE, Sonksen PH. Resolution of hypergastrinaemia after parathyroidectomy in multiple endocrine neoplasia syndrome type I (MEN type I). Clin Endocrinol (Oxf). 1987;26:693–698. [DOI] [PubMed] [Google Scholar]
- 213.Mai HD, Sanowski RA. Regression of duodenal gastrinomas in a patient with multiple endocrine neoplasia type I after parathyroidectomy. Gastrointest Endosc. 1992;38:706–708. [DOI] [PubMed] [Google Scholar]
- 214.Mainardi M, Maxwell V, Sturdevant RA, Isenberg JI. Inhibition of gastric acid secretion by metiamide in Zollinger- Ellison syndrome. Am J Dig Dis. 1975;20:280–281. [DOI] [PubMed] [Google Scholar]
- 215.Maiter D, Lambert M, Alhajje A, Dugernier T, Crabbe J, Fiasse R. Multiple endocrine neoplasia type I (MEN I). A wide range of clinical presentation. Report of three cases. Acta Clin Belg. 1986;41:89–95. [DOI] [PubMed] [Google Scholar]
- 216.Malagelada JR. Pathophysiological responses to meals in the Zollinger-Ellison syndrome: 1. Paradoxical postprandial inhibition of gastric secretion. Gut. 1978;19:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Malagelada JR, Davis CS, O’Fallon WM, Go VL. Laboratory diagnosis of gastrinoma. I. A prospective evaluation of gastric analysis and fasting serum gastrin levels. Mayo Clin Proc. 1982;57:211–218. [PubMed] [Google Scholar]
- 218.Malagelada JR, Edis AJ, Adson MA, Van Heerden JA, Go VLW. Medical and surgical options in the management of patients with gastrinoma. Gastroenterology. 1983;84:1524–1532. [PubMed] [Google Scholar]
- 219.Malagelada JR, Glanzman SL, Go VL. Laboratory diagnosis of gastrinoma. II: A prospective study of gastrin challenge tests. Mayo Clin Proc. 1982;57:219–226. [PubMed] [Google Scholar]
- 220.Marx S, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–494. [DOI] [PubMed] [Google Scholar]
- 221.Marx SJ, Agarwal SK, Kester MB, Heppner C, Kim YS, Skarulis MC, Goldsmith PK, Saggar SK, Park SY, Spiegel AM, Burns AL, Debelenko LV, Zhuang Z, Lubensky IA, Liotta LA, Emmert-Buck MR, Guru SC, Manickam P, Crabtree J, Erdos MR, Collins FS, Chandrasekharappa SC. Multiple endocrine neoplasia type 1: clinical and genetic features of the hereditary endocrine neoplasias. Recent Prog Horm Res. 1999;54:397–439. [PubMed] [Google Scholar]
- 222.Mathus-Vliegen EM, Tytgat GN. [An extreme form of hypochloremia]. Ned Tijdschr Geneeskd. 1985;129:625–628. [PubMed] [Google Scholar]
- 223.Maton PN, Frucht H, Vinayek R, Wank SA, Gardner JD, Jensen RT. Medical management of patients with Zollinger-Ellison syndrome who have had previous gastric surgery: A prospective study. Gastroenterology. 1988;94:294–299. [DOI] [PubMed] [Google Scholar]
- 224.Maton PN, Gardner JD, Jensen RT. Cushing’s syndrome in patients with Zollinger-Ellison syndrome. N Engl J Med. 1986;315:1–5. [DOI] [PubMed] [Google Scholar]
- 225.Maton PN, Lack EE, Collen MJ, Cornelius MJ, David E, Gardner JD, Jensen RT. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology. 1990;99:943–950. [DOI] [PubMed] [Google Scholar]
- 226.Maton PN, Mackem SM, Norton JA, Gardner JD, O’Dorisio TM, Jensen RT. Ovarian carcinoma as a cause of Zollinger-Ellison syndrome. Natural history, secretory products and response to provocative tests. Gastroenterology. 1989;97:468–471. [DOI] [PubMed] [Google Scholar]
- 227.Maton PN, Miller DL, Doppman JL, Collen MJ, Norton JA, Vinayek R, Slaff JI, Wank SA, Gardner JD, Jensen RT. Role of selective angiography in the management of Zollinger- Ellison syndrome. Gastroenterology. 1987;92:913–918. [DOI] [PubMed] [Google Scholar]
- 228.Maton PN, Vinayek R, Frucht H, McArthur KA, Miller LS, Saeed ZA, Gardner JD, Jensen RT. Long-term efficacy and safety of omeprazole in patients with Zollinger-Ellison syndrome: a prospective study. Gastroenterology. 1989;97:827–836. [DOI] [PubMed] [Google Scholar]
- 229.Matsui T, Iida M, Nanbu T, Kohrogi N. [A study of secretin dosage of secretin provocation test in the Zollinger-Ellison syndrome]. Nippon Shokakibyo Gakkai Zasshi. 1985;82:288–295. [PubMed] [Google Scholar]
- 230.Maye P, Bisetti A, Burger A, Docter R, Gaillard R, Griessen M, Pelte MF. Hyperprealbuminemia, euthyroid hyperthyroxinemia, Zollinger-Ellison-like syndrome and hypercorticism in a pancreatic endocrine tumour. Acta Endocrinol (Copenh). 1989;120:87–91. [DOI] [PubMed] [Google Scholar]
- 231.McArthur KE, Collen MJ, Maton PN, Cherner JA, Howard JM, Ciarleglio CA, Cornelius MJ, Jensen RT, Gardner JD. Omeprazole: effective, convenient therapy for Zollinger-Ellison syndrome. Gastroenterology. 1985;88:939–944. [DOI] [PubMed] [Google Scholar]
- 232.McCallum RW, Walsh JH. Relationship between lower esophageal sphincter and serum gastrin concentration in Zollinger-Ellison syndrome and other clinical settings. Gastroenterology. 1979;76:76–81. [PubMed] [Google Scholar]
- 233.McCarthy DM, Olinger EJ, May RJ, Long BW, Gardner JD. H2-histamine receptor blocking agents in the Zollinger-Ellison syndrome. Experience in seven cases and implications for long-term therapy. Ann Intern Med. 1977;87:668–675. [DOI] [PubMed] [Google Scholar]
- 234.McCarthy DM, Peikin SR, Lopatin RN, Long BW, Spiegel A, Marx S, Brennan A. Hyperparathyroidism a reversible cause of cimetidine-resistant gastric hypersecretion. Br Med J. 1979;1:1765–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.McColl KE, Buchanan NM, Laferla G, Hearns J, Buchanan K, Crean GP. Effects of nifedipine on gastric acid secretion and gastrin release in man. Gut. 1987;28:455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.McColl KE, El-Omar E. Helicobacter pylori and disturbance of gastric function associated with duodenal ulcer disease and gastric cancer. Scand J Gastroenterol Suppl. 1996;215:32–37. [DOI] [PubMed] [Google Scholar]
- 237.McGuigan JE, Colwell JA, Franklin J. Effect of parathyroidectomy on hypercalcemic hypersecretory peptic ulcer disease. Gastroenterology. 1974;66:269–272. [PubMed] [Google Scholar]
- 238.McGuigan JE, Wolfe MM. Secretin injection test in the diagnosis of gastrinoma. Gastroenterology. 1980;79:1324–1331. [PubMed] [Google Scholar]
- 239.Mee AS, Bornman PC, Marks IN. Conservative surgery in the Zollinger-Ellison syndrome. Br J Surg. 1984;71:423–424. [DOI] [PubMed] [Google Scholar]
- 240.Mee AS, Ismail S, Bornman PC, Marks IN. Changing concepts in the presentation, diagnosis and management of the Zollinger-Ellison syndrome. Q J Med. 1983;52:256–267. [PubMed] [Google Scholar]
- 241.Melendez N, Almazan J, Hernandez C, De la Cerda R, Arista J, Kershenobich D. Diarrea como expresion clinica de gastrinoma. Informe de un caso. Rev Gastroenterol Mex. 1993;58:229–232. [PubMed] [Google Scholar]
- 242.Meryn S, Straus E. A new rapid gastrin radioimmunoassay. Dig Dis Sci. 1986;31:567–570. [DOI] [PubMed] [Google Scholar]
- 243.Metz DC, Buchanan M, Purich E, Fein S. A randomized controlled crossover study comparing synthetic porcine and human secretins with biologically derived porcine secretin to diagnose Zollinger-Ellison Syndrome. Aliment Pharmacol Ther. 2001;15:669–676. [DOI] [PubMed] [Google Scholar]
- 244.Metz DC, Jensen RT. Advances in gastric antisecretory therapy in Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995;240–257. [Google Scholar]
- 245.Metz DC, Strader DB, Orbuch M, Koviack PD, Feigenbaum KM, Jensen RT. Use of omeprazole in Zollinger-Ellison: A prospective nine-year study of efficacy and safety. Aliment Pharmacol Ther. 1993;7:597–610. [DOI] [PubMed] [Google Scholar]
- 246.Metz DC, Weber HC, Orbuch M, Strader DB, Lubensky IA, Jensen RT. Helicobacter pylori infection: a reversible cause of hypergastrinemia and hyperchlorhydria which can mimic Zollinger-Ellison syndrome. Dig Dis Sci. 1995;40:153–159. [DOI] [PubMed] [Google Scholar]
- 247.Mignon M, Accary JP, Bonfils S. Signification diagnostique du test a la secretine dans le syndrome de Zollinger-Ellison. Gastroenterol Clin Biol. 1977;1:425–434. [PubMed] [Google Scholar]
- 248.Mignon M, Bonfils S. Diagnosis and treatment of Zollinger-Ellison syndrome. Bailliere’s Clin Gastroenterol. 1988;2:677–698. [DOI] [PubMed] [Google Scholar]
- 249.Mignon M, Cadiot G. Diagnostic and therapeutic criteria in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. J Intern Med. 1998;243:489–494. [DOI] [PubMed] [Google Scholar]
- 250.Mignon M, Jais P, Cadiot G, Yedder D, Vatier J. Clinical features and advances in biological diagnostic criteria for Zollinger-Ellison syndrome. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent Advances in Research and Management. Series: Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995;223–239. [Google Scholar]
- 251.Mignon M, N’Go Y, Gobet B, Piper A, Duet M, Vatier J. Sensitivity and specificity of cut-off points values for acid output and serum gastrin in basal state and upon secretin for the diagnosis of Zollinger-Ellison syndrome: A study of 57 postoperative ulcer patients. Gastroenterology. 1990;98:A227. [Google Scholar]
- 252.Mignon M, Rigaud D, Cambray S, Chayvialle JA, Accary JP, Rene E, Vatier J, Bonfils S. A comparative evaluation of secretin bolus and secretin infusion as secretin provocation tests in the Zollinger-Ellison syndrome. Scand J Gastroenterol. 1985;20:791–797. [DOI] [PubMed] [Google Scholar]
- 253.Mignon M, Ruszniewski P, Haffar S, Rignaud D, Rene E, Bonfils S. Current approach to the management of tumoral process in patients with gastrinoma. World J Surg. 1986;10:703–710. [DOI] [PubMed] [Google Scholar]
- 254.Mihas AA, Ceballos R, Mihas A, Gibson RG. Zollinger-Ellison syndrome associated with ductal adenocarcinoma of the pancreas. N Engl J Med. 1978;298:144–147. [DOI] [PubMed] [Google Scholar]
- 255.Mihas AA, Hirschowitz BI, Gibson RG. Calcium and secretin as provocative stimuli in the Zollinger- Ellison syndrome. Digestion. 1978;17:1–10. [DOI] [PubMed] [Google Scholar]
- 256.Miller DL, Doppman JL, Metz DC, Maton PN, Norton JA, Jensen RT. Zollinger-Ellison syndrome: technique, results and complications of portal venous sampling. Radiology. 1992;182:235–241. [DOI] [PubMed] [Google Scholar]
- 257.Miller LS, Vinayek R, Frucht H, Gardner JD, Jensen RT, Maton PN. Reflux esophagitis in patients with Zollinger-Ellison syndrome. Gastroenterology. 1990;98:341–346. [DOI] [PubMed] [Google Scholar]
- 258.Miyata M, Nakao K, Sakamoto T, Tsumori T, Hamaji M, Himeno S, Kawashima Y. Removal of mesenteric gastrinoma: a case report. Surgery. 1986;99:245–248. [PubMed] [Google Scholar]
- 259.Moattari AR, Deftos LJ, Vinik AI. Effects of sandostatin on plasma chromogranin-A levels in neuroendocrine tumors. J Clin Endocrinol Metab. 1989;69:902–905. [DOI] [PubMed] [Google Scholar]
- 260.Modlin IM, Jaffe BM, Sank A, Albert D. The early diagnosis of gastrinoma. Ann Surg. 1982;196:512–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mony M, Lavoie S, Lemire S. [Zollinger-Ellison syndrome: long-term treatment with ranitidine]. Union Med Can. 1986;115:149–150. [PubMed] [Google Scholar]
- 262.Morrow DJ, Passaro E Jr. Calcium infusion test before and after total gastrectomy in the Zollinger-Ellison syndrome. Am J Surg. 1975;129:62–66. [DOI] [PubMed] [Google Scholar]
- 263.Mowschenson PM, Rosenberg S, Pallota J, Silen W. Effect of hyperparathyroidism and hypercalcemia on lower esophageal sphincter pressure. Am J Surg. 1982;143:36–39. [DOI] [PubMed] [Google Scholar]
- 264.Mukai K, Grotting JC, Greider MH, Rosai J. Retrospective study of 77 pancreatic endocrine tumors using the immunoperoxidase method. Am J Surg Pathol. 1982;6:387–399. [DOI] [PubMed] [Google Scholar]
- 265.Nakanome C, Ishimori A, Goto Y, Yamazaki T, Kameyama J, Sasaki I, Inui M, Furukawa Y, Komatsu K. Clinical significance of glucagon provocation test in the diagnosis of hypergastrinemia. Gastroenterol Jpn. 1981;16:213–222. [DOI] [PubMed] [Google Scholar]
- 266.Nikou GC, Toubanakis C, Nikolaou P, Giannatou E, Marinou K, Safioleas M, Karamanolis D. Gastrinomas associated with MEN-1 syndrome: new insights for the diagnosis and management in a series of 11 patients. Hepatogastroenterology. 2005;52:1668–1676. [PubMed] [Google Scholar]
- 267.Nishiwaki H, Kawazoe Y, Yamashita T, Satake K, Sowa M. A case of jejunal gastrinoma diagnosed by percutaneous transhepatic portal venous sampling. Gastroenterology. 1992;27:405–410. [DOI] [PubMed] [Google Scholar]
- 268.Nishiwaki H, Nishimori T, Teramura M, Satake K, Soma M, Umeyama K. [Results of long follow-up study for peptic ulcer patients treated with gastrectomy]. Nippon Geka Gakkai Zasshi. 1988;89:1789–1795. [PubMed] [Google Scholar]
- 269.Nishiwaki H, Satake K, Kitamura T, So S, Umeyama K. Postprandial plasma secretin response in patients following gastrectomy. Surg Gynecol Obstet. 1983;156:69–72. [PubMed] [Google Scholar]
- 270.Noda S, Norton JA, Jensen RT, Gay WA Jr. Surgical resection of intracardiac gastrinoma. Ann Thorac Surg. 1999;67:532–533. [DOI] [PubMed] [Google Scholar]
- 271.Nomura T, Fukudo S, Muranaka M, Iwahashi S, Sasaki M, Satake M, Sasaki I, Shibata C, Matsuno S. Impact of stress on serum gastrin in Zollinger-Ellison syndrome. Am J Gastroenterol. 1993;88:1432–1435. [PubMed] [Google Scholar]
- 272.Nord KS, Joshi V, Hanna M, Khademi M, Saad S, Marquis J, Pelzman H, Verner E. Zollinger-Ellison syndrome associated with a renal gastrinoma in a child. J Pediatr Gastroenterol Nutr. 1986;5:980–986. [DOI] [PubMed] [Google Scholar]
- 273.Norton JA, Alexander HA, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: A prospective study. Ann Surg. 2003;237:650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann Surg. 2004;239:617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Norton JA, Cornelius MJ, Doppman JL, Maton PN, Gardner JD, Jensen RT. Effect of parathyroidectomy in patients with hyperparathyroidism, Zollinger-Ellison syndrome and multiple endocrine neoplasia Type I: A prospective study. Surgery. 1987;102:958–966. [PubMed] [Google Scholar]
- 277.Norton JA, Cromack DT, Shawker TH, Doppman JL, Comi R, Gorden P, Maton PN, Gardner JD, Jensen RT. Intraoperative ultrasonographic localization of islet cell tumors. A prospective comparison to palpation. Ann Surg. 1988;207:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Norton JA, Doherty GM, Fraker DL, Alexander HR, Doppman JL, Venzon DJ, Gibril F, Jensen RT. Surgical treatment of localized gastrinoma within the liver: A prospective study. Surgery. 1998;124:1145–1152. [DOI] [PubMed] [Google Scholar]
- 279.Norton JA, Doppman JL, Collen MJ, Harmon JW, Maton PN, Gardner JD, Jensen RT. Prospective study of gastrinoma localization and resection in patients with Zollinger-Ellison syndrome. Ann Surg. 1986;204:468–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: Results of a 10-year prospective study. Ann Surg. 1992;215:8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Norton JA, Fang TD, Jensen RT. Surgery for Gastrinoma and Insulinoma in Multiple Endocrine Neoplasia Type 1. J Natl Compr Canc Netw. 2006;4:148–153. [DOI] [PubMed] [Google Scholar]
- 282.Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, Jensen RT. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Norton JA, Fraker DL, Alexander HR, Venzon DJ, Doppman JL, Serrano J, Goebel SU, Peghini P, Roy PK, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. [DOI] [PubMed] [Google Scholar]
- 284.Norton JA, Gibril F, Jensen RT. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:2096–2097. [DOI] [PubMed] [Google Scholar]
- 285.Norton JA, Jensen RT. Unresolved surgical issues in the management of patients with the Zollinger-Ellison syndrome. World J Surg. 1991;15:151–159. [DOI] [PubMed] [Google Scholar]
- 286.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Norton JA, Jensen RT. Current surgical management of Zollinger-Ellison syndrome (ZES) in patients without multiple endocrine neoplasia-type 1 (MEN1). Surg Oncol. 2003;12:145–151. [DOI] [PubMed] [Google Scholar]
- 288.Norton JA, Shawker TH, Doppman JL, Miller DL, Fraker DL, Cromack DT, Gorden P, Jensen RT. Localization and surgical treatment of occult insulinomas. Ann Surg. 1990;212:615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Norton JA, Sugarbaker PH, Doppman JL, Wesley RA, Maton PN, Gardner JD, Jensen RT. Aggressive resection of metastatic disease in selected patients with malignant gastrinoma. Ann Surg. 1986;203:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Norton JA, Warren RS, Kelly MG, Zurek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134:1057–1065. [DOI] [PubMed] [Google Scholar]
- 291.Oberg K, Bostrom H, Fahrenkrug J, Dymling JF, Shaffalitsky de Muckadell OB, Lundqvist G. Streptozotocin treatment of a pancreatic tumour producing VIP and gastrin associated with Verner-Morrison syndrome. Acta Med Scand. 1979;206:223–227. [DOI] [PubMed] [Google Scholar]
- 292.Orbuch M, Doppman JL, Strader DB, Fishbeyn VA, Benya RV, Metz DC, Jensen RT. Imaging for pancreatic endocrine tumor localization: recent advances. In: Mignon M, Jensen RT, eds. Endocrine Tumors of the Pancreas: Recent advances in research and management. Frontiers of Gastrointestinal Research. Basel, Switzerland: S. Karger; 1995;268–281. [Google Scholar]
- 293.Orchard JL, Peternel WW. Cimetidine therapy in Zollinger-Ellison syndrome. JAMA. 1977;237:2221. [PubMed] [Google Scholar]
- 294.Pannall P, van den Berg WP. The Zollinger-Ellison syndrome. Case report. S Afr Med J. 1974;48:2489–2491. [PubMed] [Google Scholar]
- 295.Passaro E Jr., Basso N, Walsh JH. Calcium challenge in the Zollinger-Ellison syndrome. Surgery. 1972;72:60–67. [PubMed] [Google Scholar]
- 296.Peurifoy JT, Gomez LG, Thompson JC. Separate pancreatic gastrin cell and beta-cell adenomas: report of a patient with multiple endocrine adenomatosis type 1. Arch Surg. 1979;114:956–958. [DOI] [PubMed] [Google Scholar]
- 297.Pisegna JR, Doppman JL, Norton JA, Metz DC, Jensen RT. Prospective comparative study of ability of MR imaging and other imaging modalities to localize tumors in patients with Zollinger-Ellison syndrome. Dig Dis Sci. 1993;38:1318–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Pisegna JR, Slimak GG, Doppman JL, Strader DB, Metz DC, Fishbeyn VA, Benya RV, Orbuch M, Fraker DL, Norton JA, Maton PN, Jensen RT. An evaluation of human recombinant alpha interferon in patients with metastatic gastrinoma. Gastroenterology. 1993;105:1179–1183. [DOI] [PubMed] [Google Scholar]
- 299.Polak JM, Stagg B, Pearse AG. Two types of Zollinger-Ellison syndrome: immunofluorescent, cytochemical and ultrastructural studies of the antral and pancreatic gastrin cells in different clinical states. Gut. 1972;13:501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Poulsen J, Delikaris P, Larsson LI, Amdrup E. Antral hypergastrinemia--a report of three cases. Digestion. 1987;37:211–216. [DOI] [PubMed] [Google Scholar]
- 301.Poynard T, Mignon M, Accary JP, Bonfils S. Sensitivity, specificity and predictive values of the secretin infusion test in the diagnosis of gastrinoma. Gastroenterol Clin Biol. 1986;10:492–496. [PubMed] [Google Scholar]
- 302.Primrose JN, Beastall GA. Boots secretin and the Zollinger-Ellison syndrome. J Clin Gastroenterol. 1984;6:187. [DOI] [PubMed] [Google Scholar]
- 303.Primrose JN, Maloney M, Wells M, Bulgim O, Johnson D. Gastrin-producing ovarian mucinous cystadenomas: a cause of the Zollinger-Ellison syndrome. Surgery. 1988;104:830–833. [PubMed] [Google Scholar]
- 304.Primrose JN, Ratcliffe JG, Joffe SN. Assessment of the secretin provocation test in the diagnosis of gastrinoma. Br J Surg. 1980;67:744–746. [DOI] [PubMed] [Google Scholar]
- 305.Procacciante F, Citone G, Guaitoli P, Montesani C, Ribotta G. [Surgery of Zollinger-Ellison syndrome]. Minerva Chir. 1981;36:1291–1302. [PubMed] [Google Scholar]
- 306.Proye C, Pattou F, Carnaille B, Paris JC, d’Herbomez M, Marchandise X. Intraoperative gastrin measurements during surgical management of patients with gastrinomas: experience with 20 cases. World J Surg. 1998;22:643–650. [DOI] [PubMed] [Google Scholar]
- 307.Quatrini M, Basilisco G, Conte D, Bardella MT, Bozzani A, Bianchi PA. Secretin-induced gastrin response in the Zollinger-Ellison syndrome and chronic duodenal ulcer patients before and after cimetidine treatment. Am J Gastroenterol. 1984;79:345–347. [PubMed] [Google Scholar]
- 308.Raddatz D, Horstmann O, Basenau D, Becker H, Ramadori G. Cushing’s syndrome due to ectopic adrenocorticotropic hormone production by a non-metastatic gastrinoma after lonterm conservative treatment of Zollinger-Ellison syndrome. Ital J Gastroenterol Hepatol. 1998;30:636–640. [PubMed] [Google Scholar]
- 309.Ramakrishnan T Zollinger-Ellison syndrome presenting as reflux esophagitis and stricture. J La State Med Soc. 1981;133:189–190. [PubMed] [Google Scholar]
- 310.Raufman JP, Collins SM, Pandol SJ, Korman LY, Collen MJ, Cornelius MJ, Feld MK, McCarthy DM, Gardner JD, Jensen RT. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 1983;84:108–113. [PubMed] [Google Scholar]
- 311.Reeder DD, Becker HD, Thompson JC. Effect of intravenously administered calcium on serum gastrin and gastric secretion in man. Surg Gynecol Obstet. 1974;138:847–851. [PubMed] [Google Scholar]
- 312.Regan PT, Malagelada JR. A reappraisal of clinical, roentgenographic, and endoscopic features of the Zollinger-Ellison syndrome. Mayo Clin Proc. 1978;53:19–23. [PubMed] [Google Scholar]
- 313.Reusch J, Keck AM, Link KH, Mohr HH. [The coexistence of 2 different neuroendocrine tumors of the upper gastrointestinal tract and pancreas]. Dtsch Med Wochenschr. 1998;123:1472–1477. [DOI] [PubMed] [Google Scholar]
- 314.Richards ML, Gauger P, Thompson NW, Giordano TJ. Regression of type II gastric carcinoids in multiple endocrine neoplasia type 1 patients with Zollinger-Ellison syndrome after surgical excision of all gastrinomas. World J Surg. 2004;28:652–658. [DOI] [PubMed] [Google Scholar]
- 315.Richardson CT, Peters MN, Feldman M, McClelland RN, Walsh JH, Cooper KA, Willeford G, Dickerman RM, Fordtran JS. Treatment of Zollinger-Ellison syndrome with exploratory laparotomy, proximal gastric vagotomy, and H2-receptor antagonists. A prospective study. Gastroenterology. 1985;89:357–367. [DOI] [PubMed] [Google Scholar]
- 316.Rodl W, Nebel G, Harms D. [Computer tomography in juvenile malignant gastrinoma]. Rofo. 1983;139:206–208. [DOI] [PubMed] [Google Scholar]
- 317.Rolny P, Ekman R, Sjolund K, Wickbom G, Ohrn PG. Endoscopic diagnosis and treatment of duodenal gastrinoma. Gastrointest Endosc. 1990;36:511–513. [DOI] [PubMed] [Google Scholar]
- 318.Romanus ME, Neal JA, Dilley WG, Leight GS, Linehan WM, Santen RJ, Farndon JR, Jones RS, Wells SA, Jr. Comparison of four provocative tests for the diagnosis of gastrinoma. Ann Surg. 1983;197:608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Roy P, Venzon DJ, Feigenbaum KM, Koviack PD, Bashir S, Ojeaburu JV, Gibril F, Jensen RT. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis - A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore). 2001;80:189–222. [DOI] [PubMed] [Google Scholar]
- 320.Roy P, Venzon DJ, Shojamanesh H, Abou-Saif A, Peghini P, Doppman JL, Gibril F, Jensen RT. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000;79:379–411. [DOI] [PubMed] [Google Scholar]
- 321.Ruszniewski P, Girard F, Benamouzig R, Mignon M, Bonfils S. Long acting somatostatin treatment of paraneoplastic Cushing’s syndrome in a case of Zollinger-Ellison syndrome. Gut. 1988;29:838–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Samaha JK. Zollinger-Ellison syndrome. J Tenn Med Assoc. 1980;73:496–500. [PubMed] [Google Scholar]
- 323.Sanchez RE, Longmire WP Jr., Passaro E Jr. Acid secretion and serum gastrin levels in the Zollinger-Ellison syndrome. Calif Med. 1972;116:1–7. [PMC free article] [PubMed] [Google Scholar]
- 324.Sanzenbacher LJ, King DR, Zollinger RM. Prognostic implications of calcium-mediated gastrin levels in the ulcerogenic syndrome. Am J Surg. 1973;125:116–121. [DOI] [PubMed] [Google Scholar]
- 325.Sategna-Guidetti C, Imarisio P, de la PM. [Secretin stimulation and the gastrinemic response. Usefulness for diagnosis of Zollinger-Ellison syndrome]. Recenti Prog Med. 1982;72:744–752. [PubMed] [Google Scholar]
- 326.Schaub N, Schlup M, Kummer H, Wegmann W. [Long-term suppression of acid secretion with omeprazole use in metastatic gastrinoma]. Schweiz Med Wochenschr. 1988;118:1660–1666. [PubMed] [Google Scholar]
- 327.Schmidt J, Boedeker H, Zirngibl H. [Posterior resection of of the head of the pancreas in malignant duodenal gastrinoma]. Chirurg. 1997;68:425–428. [DOI] [PubMed] [Google Scholar]
- 328.Schroder W, Holscher AH, Beckurts T, Richter TH, Hofler H, Siewert JR. Duodenal microgastrinomas associated with Zollinger-Ellison syndrome. Hepato-Gastroenterol. 1996;43:1465–1469. [PubMed] [Google Scholar]
- 329.Schrumpf E, Petersen H, Berstad A, Myren J, Rosenlund B. The effect of secretin on plasma gastrin in the Zollinger- Ellison syndrome. Scand J Gastroenterol. 1973;8:145–150. [PubMed] [Google Scholar]
- 330.Schwartz DL, White JJ, Saulsbury F, Haller JA. Gastrin response to calcium infusion: an aid to the improved diagnosis of Zollinger-Ellison syndrome in children. Pediatrics. 1974;54:599–602. [PubMed] [Google Scholar]
- 331.Scofield H, Kaufman CE. Case report: gastrocolic fistula mimicking Zollinger-Ellison syndrome. J Med Sci. 1992;303:405–406. [DOI] [PubMed] [Google Scholar]
- 332.Seetharamaiah GS, Kurosky A, Desai RK, Dallas JS, Prabhakar BS. A recombinant extracellular domain of the thyrotropin (TSH) receptor binds TSH in the absence of membranes. Endocrinology. 1994;134:549–554. [DOI] [PubMed] [Google Scholar]
- 333.Sheppard BC, Norton JA, Doppman JL, Maton PN, Gardner JD, Jensen RT. Management of islet cell tumors in patients with Multiple Endocrine Neoplasia: A prospective study. Surgery. 1989;106:1108–1117. [PubMed] [Google Scholar]
- 334.Shojamanesh H, Gibril F, Louie A, Ojeaburu JV, Bashir S, Abou-Saif A, Jensen RT. Prospective study of the anti-tumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinomas. Cancer. 2002;94:331–343. [DOI] [PubMed] [Google Scholar]
- 335.Siewert R, Jennewein HM, Arnold R, Creutzfeldt W. The lower oesophageal sphincter in the Zollinger-Ellison syndrome. Ger Med. 1973;3:101–102. [PubMed] [Google Scholar]
- 336.Skogseid B, Oberg K, Benson L, Lindgren PG, Lorelius LE, Lundquist G, Wide L, Wilander E. A standardized meal stimulation test of the endocrine pancreas for early detection of pancreatic endocrine tumors in multiple endocrine neoplasia type 1 syndrome: five years experience. J Clin Endocrinol Metab. 1987;64:1233–1240. [DOI] [PubMed] [Google Scholar]
- 337.Smith JT, Pounder RE, Nwokolo CU, Lanzon-Miller S, Evans DG, Graham DY, Evans DJ, Jr. Inappropriate hypergastrinaemia in asymptomatic healthy subjects infected with Helicobacter pylori. Gut. 1990;31:522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Snyder N, Hughes W. Basal and calcium-stimulated gastroesophageal sphincter pressure in patients with Zollinger-Ellison syndrome. Gastroenterology. 1977;72:1240–1243. [PubMed] [Google Scholar]
- 339.Soga J, Yakuwa Y. The gastrinoma/Zollinger-Ellison syndrome: statistical evaluation of a Japanese series of 359 cases. J Hep Bil Pancr Surg. 1998;5:77–85. [DOI] [PubMed] [Google Scholar]
- 340.Somers G, Vanden HK, Segers O, Bossuyt A. Iodine-123 MIBG imaging in a generalized pancreatic polypeptide-gastrin-serotonin secreting tumor. Clin Nucl Med. 1988;13:352–355. [DOI] [PubMed] [Google Scholar]
- 341.Stabile BE, Braunstein GD, Passaro E Jr. Serum gastrin and human chorionic gonadotropin in the Zollinger- Ellison syndrome. Arch Surg. 1980;115:1090–1095. [DOI] [PubMed] [Google Scholar]
- 342.Stadil F, Stage G, Rehfeld JF, Efsen F, Fischerman K. Treatment of Zollinger-Ellison syndrome with streptozotocin. N Engl J Med. 1976;294:1440–1442. [DOI] [PubMed] [Google Scholar]
- 343.Stage JG, Stadil F. The clinical diagnosis of the Zollinger-Ellison syndrome. Scand J Gastroenterol Suppl. 1979;53:79–91. [PubMed] [Google Scholar]
- 344.Stage JG, Stadil F, Rehfeld JF, Fahrenkrug J, Muckadell OB. Secretin and the Zollinger-Ellison syndrome: reliability of secretin tests and pathogenetic role of secretin. Scand J Gastroenterol. 1978;13:501–511. [DOI] [PubMed] [Google Scholar]
- 345.Stevens MH, Thirlby RC, Richardson CT. Lack of effect of parathyroidectomy or calcium channel blockade on serum gastrin concentration and gastric acid secretion in a patient with hyperparathyroidism and Zollinger-Ellison syndrome. Surgery. 1987;101:108–113. [PubMed] [Google Scholar]
- 346.Straus E, Raufman JP. The Brooklyn gastrinomas. Mt Sinai J Med. 1992;59:125–128. [PubMed] [Google Scholar]
- 347.Straus E, Yalow RS. Differential diagnosis of hypergastrinemia. In: Thompson JC, ed. Gastrointestinal hormones. Austin,Texas: Austin: University of Texas Press; 1975;99–113. [Google Scholar]
- 348.Straus E, Yalow RS, Solomon A. Differential diagnosis in hyperchlorhydric hypergastrinemia. Gastroenterology. 1974;66:867. [Google Scholar]
- 349.Sugg SL, Norton JA, Fraker DL, Metz DC, Pisegna JR, Fishbeyn V, Benya RV, Shawker TH, Doppman JL, Jensen RT. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sutliff VE, Doppman JL, Gibril F, Yu F, Serrano J, Venzon DJ, Jensen RT. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J Clin Oncol. 1997;15:2420–2431. [DOI] [PubMed] [Google Scholar]
- 351.Takasu A, Shimosegawa T, Fukudo S, Asakura T, Uchi M, Kimura K, Kashimura J, Satoh K, Koizumi M, Sasaki I, Hongo M, Suzuki T, Toyota T. Duodenal gastrinoma--clinical features and usefulness of selective arterial secretin injection test. J Gastroenterol. 1998;33:728–733. [DOI] [PubMed] [Google Scholar]
- 352.Tebar Masso FJ, Ascaso Gimilio J, Ramirez Munoz L, Lozano Teruel MG. [MEN1 with involvement of 4 endocrine organs]. Med Clin (Barc). 1985;84:80–81. [PubMed] [Google Scholar]
- 353.Termanini B, Gibril F, Doppman JL, Reynolds JC, Stewart CA, Sutliff VE, Venzon DJ, Jensen RT. Distinguishing small hepatic hemangiomas from vascular liver metastases in patients with gastrinoma: Use of a somatostatin-receptor scintigraphic agent. Radiology. 1997;202:151–158. [DOI] [PubMed] [Google Scholar]
- 354.Termanini B, Gibril F, Reynolds JC, Doppman JL, Chen CC, Stewart CA, Sutliff V, Jensen RT. Value of somatostatin receptor scintigraphy: A prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112:335–347. [DOI] [PubMed] [Google Scholar]
- 355.Termanini B, Gibril F, Sutliff VE III, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–430. [DOI] [PubMed] [Google Scholar]
- 356.Thom AK, Norton JA, Axiotis CA, Jensen RT. Location, incidence and malignant potential of duodenal gastrinomas. Surgery. 1991;110:1086–1093. [PubMed] [Google Scholar]
- 357.Thom AK, Norton JA, Doppman JL, Chang R, Miller DL, Jensen RT. Prospective study of the use of intraarterial secretin injection and portal venous sampling to localize duodenal gastrinomas. Surgery. 1992;112(#6):1002–1008. [PubMed] [Google Scholar]
- 358.Thompson JC, Reeder DD, Bunchman HH. Clinical role of serum gastrin measurements in the Zollinger-Ellison syndrome. Am J Surg. 1972;124:250–261. [DOI] [PubMed] [Google Scholar]
- 359.Thompson JC, Reeder DD, Bunchman HH, Becker HD, Brandt EN, Jr. Effect of secretin on circulating gastrin. Ann Surg. 1972;176:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Thompson JC, Reeder DD, Villar HV, Fender HR. Natural history and experience with diagnosis and treatment of the Zollinger-Ellison syndrome. Surg Gynecol Obstet. 1975;140:721–739. [PubMed] [Google Scholar]
- 361.Thompson MH, Sanders DJ, Grund ER. The relationship of the serum gastrin and calcium concentrations in patients with multiple endocrine neoplasia type 1. Br J Surg. 1976;63:779–783. [DOI] [PubMed] [Google Scholar]
- 362.Thompson NW, Bondeson AG, Bondeson L, Vinik A. The surgical treatment of gastrinoma in MEN I syndrome patients. Surgery. 1989;106:1081–1085. [PubMed] [Google Scholar]
- 363.Thompson NW, Pasieka J, Fukuuchi A. Duodenal gastrinomas, duodenotomy, and duodenal exploration in the surgical management of Zollinger-Ellison syndrome. World J Surg. 1993;17:455–462. [DOI] [PubMed] [Google Scholar]
- 364.Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum. A cause of failed operations for the Zollinger-Ellison syndrome. Ann Surg. 1989;209:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Thompson NW, Vinik AI, Eckhauser FE, Strodel WE. Extrapancreatic gastrinomas. Surgery. 1985;98:1113–1120. [PubMed] [Google Scholar]
- 366.Tiengo A, Fedele D, Marchiori E, Nosadini R, Muggeo M. Suppression and stimulation mechanisms controlling glucagon secretion in a case of islet-cell tumor producing glucagon, insulin, and gastrin. Diabetes. 1976;25:408–412. [DOI] [PubMed] [Google Scholar]
- 367.Tiomny E, Brill S, Baratz M, Messer G, Greif F, Moshkowitz M, Gilat T. Primary Liver Gastrinoma. J Clin Gastroenterol. 1997;24:188–191. [DOI] [PubMed] [Google Scholar]
- 368.Trudeau WL, McGuigan JE. Effects of calcium on serum gastrin levels in the Zollinger- Ellison syndrome. N Engl J Med. 1969;281:862–866. [DOI] [PubMed] [Google Scholar]
- 369.Turbey WJ, Passaro E Jr. Hyperparathyroidism in the Zollinger-Ellison syndrome. Influence of hypercalcemia on clinical course. Arch Surg. 1972;105:62–66. [DOI] [PubMed] [Google Scholar]
- 370.van den Born BJ, Koopmans RP, Fliers E, Hart W. [Clinical thinking and decision making in practice. Unexplained rectal blood loss in a patient with multiple endocrine neoplasia type 1 syndrome]. Ned Tijdschr Geneeskd. 2002;146:712–717. [PubMed] [Google Scholar]
- 371.Vandeweghe M, Schutyser J, Braxel K, Vermeulen A. A case of multiple endocrine adenomatosis with primary amenorrhoea. Postgrad Med. 1978;54:618–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Varas Lorenzo MJ, Curto Cardus JA, Sans SM, Carrera PM. [Zollinger-Ellison syndrome type II due to diffuse hyperplasia of the pancreatic islet cells (author’s transl)]. Med Clin (Barc ). 1979;73:68–72. [PubMed] [Google Scholar]
- 373.Varas Lorenzo MJ, Sitges SA. [The secretin test in the diagnosis of hypergastrinemia]. Rev Esp Enferm Apar Dig. 1978;54:571–582. [PubMed] [Google Scholar]
- 374.Veldhuis JD, Green JE III, Kovacs E, Worgul TJ, Murray FT, Hammond JM. Prolactin-sescreting pituitary adenomas. Am J Med. 1979;67:830–837. [DOI] [PubMed] [Google Scholar]
- 375.Vellar D, Henderson M, Vellar ID, Desmond P. The Zollinger-Ellison syndrome: a review of the St. Vincent’s hospital, Melbourne experience. Aust N Z J Surg. 1985;55:455–462. [DOI] [PubMed] [Google Scholar]
- 376.Vezzadini C, Poggioli R, Casoni I, Vezzadini P. Use of calcium provocative test in the diagnosis of gastroenteropancreatic endocrine tumors. Panminerva Med. 1996;38:255–258. [PubMed] [Google Scholar]
- 377.Vezzadini P, Sternini C, Bonora G, Labo G. Gastric acid secretion in Zollinger-Ellison syndrome. Pan Med. 1984;26:93–96. [PubMed] [Google Scholar]
- 378.von Blankenfeld G, Turner J, Ahnert-Hilger G, John M, Enkvist MO, Stephenson F, Kettenmann H, Wiedenmann B. Expression of functional GABAA receptors in neuroendocrine gastropancreatic cells. Pflugers Arch. 1995;430:381–388. [DOI] [PubMed] [Google Scholar]
- 379.von Schrenck T, Howard JM, Doppman JL, Norton JA, Maton PN, Smith FP, Vinayek R, Frucht H, Wank SA, Gardner JD, Jensen RT. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology. 1988;94:1326–1334. [DOI] [PubMed] [Google Scholar]
- 380.Wada M, Komoto I, Doi R, Imamura M. Intravenous calcium injection test is a novel complementary procedure in differential diagnosis for gastrinoma. World J Surg. 2002;26:1291–1296. [DOI] [PubMed] [Google Scholar]
- 381.Walsh JH, Grossman MI. Gastrin (second of two parts). N Engl J Med. 1975;292:1377–1384. [DOI] [PubMed] [Google Scholar]
- 382.Wank SA, Doppman JL, Miller DL, Collen MJ, Maton PN, Vinayek R, Slaff JI, Norton JA, Gardner JD, Jensen RT. Prospective study of the ability of computerized axial tomography to localize gastrinomas in patients with Zollinger-Ellison syndrome. Gastroenterology. 1987;92:905–912. [DOI] [PubMed] [Google Scholar]
- 383.Way L, Goldman L, Dunphy JE. Zollinger-Ellison syndrome. An analysis of twenty-five cases. Am J Surg. 1968;116(2):293–304. [DOI] [PubMed] [Google Scholar]
- 384.Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA, Jensen RT. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. [DOI] [PubMed] [Google Scholar]
- 385.Weinhausel A, Kaserer K, Vierhapper H, Niederle B, Haas OA, Study Group of Multiple Endocrine Neoplasia Austria. Multiple endocrine neoplasia type 1 (MEN1) in Austria. Wien Klin Wochenschr. 2002;114:252–257. [PubMed] [Google Scholar]
- 386.Weltman MD, Wing JR, Diamond TH, Botha JR, Myburgh JA. Time-dependent changes in serum gastrin measurements after parathyroidectomy. S Afr Med J. 1989;27:8–9. [PubMed] [Google Scholar]
- 387.Wiedenmann B, Jensen RT, Mignon M, Modlin CI, Skogseid B, Doherty G, Oberg K. Preoperative diagnosis and surgical manangement of neuroendocrine gastroenteropancreatic tumors: general recommendations by a consensus workshop. World J Surg. 1998;22:309–318. [DOI] [PubMed] [Google Scholar]
- 388.Wiersinga WM, Schellekens AP, Thornton WE, Touber JL, Tijtgat GN. Modulation of gastrin release by acute changes in plasma calcium. Digestion. 1978;18:290–293. [DOI] [PubMed] [Google Scholar]
- 389.Wiersinga WM, Tytgat GN. Clinical recovery owing to target parietal cell failure in a patient with Zollinger-Ellison syndrome. Gastroenterology. 1977;73:1413–1417. [PubMed] [Google Scholar]
- 390.Wilson SD, Singh RB, Kalkhoff RK, Go VLW. Does hyperparathyroidism cause hypergastrinemia? Surgery. 1976;80:231–237. [PubMed] [Google Scholar]
- 391.Winship DH. Problems in the diagnosis of Zollinger-Ellison-syndrome by analysis of gastric secretion. In: Demling I, Ottenjann R, eds. Non-insulin-producing tumors of the pancreas. Modern aspects on Zollinger-Ellison-syndrome and gastrin. Erlangen, Stuttgart: Georg Thieme Verlagh; 1968;129–140. [Google Scholar]
- 392.Winter TC3, Freeny PC, Nghiem HV. Extrapancreatic gastrinoma localization: value of arterial-phase helical CT with water as an oral contrast agent. AJR Am J Roentgenol. 1996;166:51–52. [DOI] [PubMed] [Google Scholar]
- 393.Wolfe JHN, Pinkerton JR, Mackie DB, Gent AE. Pneumopericardium from recurrent peptic ulceration in a patient with multiple endocrine adenomatosis. Brit J Clin Pract. 1979;33:113–115. [PubMed] [Google Scholar]
- 394.Wolfe MM. Diagnosis of gastrinoma: much ado about nothing [comment]. Ann Intern Med. 1989;111:697–699. [DOI] [PubMed] [Google Scholar]
- 395.Wolfe MM, Alexander RW, McGuigan JE. Extrapancreatic, extraintestinal gastrinoma: effective treatment by surgery. N Engl J Med. 1982;306(25):1533–1536. [DOI] [PubMed] [Google Scholar]
- 396.Wolfe MM, Jain DK, Edgerton JR. Zollinger-Ellison syndrome associated with persistently normal fasting serum gastrin concentrations. Ann Intern Med. 1985;103:215–217. [DOI] [PubMed] [Google Scholar]
- 397.Wolfe MM, Jensen RT. Zollinger-Ellison syndrome: Current concepts in diagnosis and management. N Engl J Med. 1987;317:1200–1209. [DOI] [PubMed] [Google Scholar]
- 398.Wollmuth RL, Wagonfeld JB. False-positive secretin test [letter]. Ann Intern Med. 1978;88:718–719. [DOI] [PubMed] [Google Scholar]
- 399.Woltering EA, Ellison EC, O’Dorisio TM, Hitchcock C, Roberts K, Stephens R, Sparks J, Carey LC. Gastrin release from dispersed gastrinoma cells: effects of calcium and calcium ionophore (A23187). J Surg Res. 1985;39:331–337. [DOI] [PubMed] [Google Scholar]
- 400.Woltering EA, Ellison EC, O’Dorisio TM, Sparks J, Howe B, Dyben T. Somatostatin-like peptides alter calcium but not secretin sensitivity of gastrinoma cells. J Surg Res. 1986;40:605–610. [DOI] [PubMed] [Google Scholar]
- 401.Woodtli W, Gemsenjager E, Heitz PU, Gloor F, Rosch W, Bosseckert H. [Endocrine tumours (APUDomas) of the duodenum--a cooperative study]. Schweiz Rundsch Med Prax. 1982;71:1045–1053. [PubMed] [Google Scholar]
- 402.Wu PC, Alexander HR, Bartlett DL, Doppman JL, Fraker DL, Norton JA, Gibril F, Jensen RT. A prospective analysis of the frequency, location, and curability of ectopic (non-pancreaticoduodenal, non-nodal) gastrinoma. Surgery. 1997;122:1176–1182. [DOI] [PubMed] [Google Scholar]
- 403.Wulbrand U, Wied M, Zofel P, Goke B, Arnold R, Fehmann H. Growth factor receptor expression in human gastroenteropancreatic neuroendocrine tumours. Eur J Clin Invest. 1998;28:1038–1049. [DOI] [PubMed] [Google Scholar]
- 404.Wyke RJ, Hill GL, Axon ATR. A review of the Zollinger-Ellison syndrome--with particular reference to a patient treated with cimetidine. Postgrad Med J. 1979;55:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Yamamoto C, Aoyagi K, Iwata K, Morita I, Hotokezaka M, Funakoshi S, Sakamoto K, Iida M, Sakisaka S. Double doses of secretin contribute to diagnosis of Zollinger-Ellison syndrome in secretin and selective arterial secretion injection tests--a case report. Dig Dis Sci. 2005;50:2034–2036. [DOI] [PubMed] [Google Scholar]
- 406.Yamamoto M, Mine H, Maehara Y, Sugimachi K. The Zollinger-Ellison syndrome with acute bleeding pancreatitis. Hepatogastroenterology. 2003;50:430–431. [PubMed] [Google Scholar]
- 407.Yanda RJ, Ostroff JW, Ashbaugh CD, Guis MS, Goldberg HI. Zollinger-Ellison syndrome in a patient with normal screening gastrin level. Dig Dis Sci. 1989;34:1929–1932. [DOI] [PubMed] [Google Scholar]
- 408.Yap ILE, Chua KL, Ti TK, Tan LKA, Yuen R. Zollinger-Ellison syndrome--Report of four cases and review of literature. Ann Acad Med, Singapore. 1983;12:577–583. [PubMed] [Google Scholar]
- 409.Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. [DOI] [PubMed] [Google Scholar]
- 410.Zahner J, Borchard F, Schmitz U, Schneider W. [Thymus carcinoid in multiple endocrine neoplasms]. Dtsch Med Wochenschr. 1994;119:135–140. [DOI] [PubMed] [Google Scholar]
- 411.Ziemniak JA, Madura M, Adamonis AJ, Olinger EJ, Dreyer M, Schentag JJ. Failure of cimetidine in Zollinger-Ellison syndrome. Dig Dis Sci. 1983;28:976–980. [DOI] [PubMed] [Google Scholar]
- 412.Zimmer T, Stolzel U, Bader M, Koppenhagen K, Hamm B, Buhr H, Riecken EO, Wiedenmann B. Endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localisation of insulinomas and gastrinomas. Gut. 1996;39:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]