Abstract
The 21st Century Cures Act mandates immediate availability of test results upon request. The Cures Act does not require that patients be informed of results, but many organizations send notifications when results become available. Our medical center implemented 2 sequential policies: immediate notifications for all results, and notifications only to patients who opt in. We used over 2 years of data from Vanderbilt University Medical Center to measure the effect of these policies on rates of patient-before-clinician result review and patient-initiated messaging using interrupted time series analysis. When releasing test results with immediate notification, the proportion of patient-before-clinician review increased 4-fold and the proportion of patients who sent messages rose 3%. After transition to opt-in notifications, patient-before-clinician review decreased 2.4% and patient-initiated messaging decreased 0.4%. Replacing automated notifications with an opt-in policy provides patients flexibility to indicate their preferences but may not substantially alleviate clinicians’ messaging workload.
Keywords: patient portal, policy, 21st Century Cures Act, test results
INTRODUCTION
The Information Blocking Rule of the 21st Century Cures Act (Cures Act) mandates the immediate electronic availability of electronic health information, including test results, upon patient request.1 Improved access to health data can help patients take greater control of their health care and supports coordination and information sharing.2 Before the Cures Act, health systems commonly released a subset of health information through the patient portal.3 Information considered sensitive or easily misinterpreted was often delayed so that clinicians could review and follow-up on results. Many health systems, including ours, used a tiered system in which results considered routine (such as lipid profiles) were released immediately, but potentially concerning results (such as anatomic pathology reports) were delayed. With limitations to how commercially available electronic health record software can be configured, many health systems now comply with the Cures Act by delivering all electronic health information immediately to the patient portal, whether patients ask for it or not. Many patients now see test results before their healthcare team can review them.4 For test results that are difficult to interpret or emotionally charged, receiving results before follow-up with a clinician could be confusing or distressing.5 Patients who see their results before the clinician does may also be more likely to reach out with questions that otherwise would have been delivered by the clinician as part of results release. In turn, this potentially avoidable messaging volume increases clinical work and may contribute to professional burnout.6
The Cures Act does not require health systems to inform patients when results become available. Health systems can, for example, notify patients by email whenever a result is delivered, deliver results without notifying patients, or allow patients to select their notification preferences. Patients have varied preferences about how and when to receive test results.7 Allowing patients to choose a notification policy might reduce the negative emotional effects of receiving results by ensuring notifications are delivered in accordance with their preferences. It could also make it easier for patients to defer looking at results until they have a follow-up visit, which might reduce the rate at which patients send messages asking for interpretation.
The objective of this study was to measure the effect of different notification practices on rates of patient-before-clinician results review and patient-initiated messaging to their clinicians. We leveraged Vanderbilt University Medical Center’s (VUMC) January 2021 transition to Cures Act compliance when all results became immediately available via patient portal and an April 2021 notification policy change to conduct an interrupted time series analysis.
MATERIALS AND METHODS
This study was approved by the Vanderbilt University Institutional Review Board with a waiver of informed consent. We extracted data from the electronic health record on outpatient test results released to adult patients and subsequent messages sent via patient portal between June 1, 2020, and December 31, 2022. VUMC historically categorized test results for immediate release or delayed release after 1 day (eg, thyroid function tests), 3 days (eg, radiology reports), 7 days (eg, testing for sexually transmitted infections), or 14 days (eg, anatomic pathology).3 VUMC began Cures Act compliance on January 20, 2021, by removing delays for adult patients. On April 15, 2021, VUMC discontinued automatic notifications and invited patients to opt in to notifications. The new policy was accompanied by an explanatory email to all portal account holders inviting them to select their notification preference.
We measured individual test results as the unit of analysis. We conducted an interrupted time series analysis to estimate the effect of changes in notification policies on (1) the likelihood that a patient viewed results before their clinician and (2) the likelihood that a patient sent an electronic message via patient portal to their clinician after viewing their results. We estimated segmented logistic regression models to assess both changes following Cures Act compliance and following implementation of passive result delivery. Models included 2 key binary variables of interest. The first indicator took on a value of 1 if the test result was released following Cures Act Compliance (January 20, 2021, to April 14, 2021), and the second took on a value of 1 if the test result was released following implementation of passive delivery (April 15, 2021, to December 31, 2022). Odds ratios were converted to average marginal effects. In sensitivity analyses, we also interacted with linear time with the binary indicators to allow for potential changes in slope. These interaction terms were nonsignificant and slope changes are not visually apparent in descriptive results, so they are not reported. Models controlled for patient sex, race, ethnicity, primary payer, and age. Age was categorized into 10-year bins to allow for potential nonlinear effects on study outcomes. We estimated both overall models and stratified our analysis by the historic release categories to evaluate heterogeneity by test sensitivity.
RESULTS
Our sample included 368 831 unique patients who received 4 973 207 test results through the patient portal (Supplementary Table S1). The mean age was 47.2 years (standard deviation 17.9), and most patients were White (267 979 [72.7%]), non-Hispanic (286 397 [77.6%]), and women (232 476 [63.0%]). Approximately 90.3% of patients reviewed at least 1 test result, and patients collectively reviewed 80.8% of released test results. Before Cures Act compliance, 3 565 384 (71.5%) were released to patients immediately, and 1 416 823 (28.5%) were released after a delay.
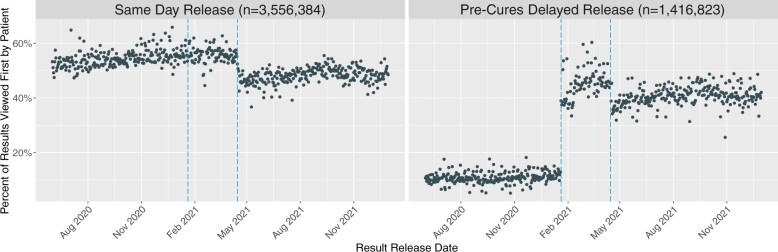
After Cures Act compliance, we observed a sustained increase in the proportion of test results that were reviewed by patients before a clinician (Figure 1). Table 1 illustrates patient-before-clinician review rates across historic release categories. There was an increase, from 11.5% (95% confidence interval [CI], 11.4–11.6) to 45.6% (95% CI, 45.2–46.0) in the proportion of patient-before-clinician review among results previously released after a delay. The largest change in patient-before-clinician review, from 4.7% to 56.6%, was among the most sensitive results—those previously classified for release after 14 days.
Figure 1.
Proportion of results viewed first by patients relative to notification policy. Each point indicates the daily percentage of test results reviewed by a patient before their clinician.
Table 1.
Adjusted and unadjusted interrupted time series of the daily percentage of test results reviewed by patients before their Clinician
| Pre-Cures Act release rules | Pre-Cures Act | Post-Cures Acta increment |
Postnotification policyb increment |
||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Immediate release | 53.7 | 2.2*** | 2.4*** | −6.9*** | −6.9*** |
| One-day delay | 26.9 | 25.3*** | 25.6*** | −7.3*** | −7.1*** |
| Three-day delay | 8.4 | 28.9*** | 29.7*** | −0.4*** | −0.4*** |
| Seven-day delay | 6.3 | 46.2*** | 46.5*** | −4.0*** | −4.2*** |
| Fourteen-day delay | 4.7 | 51.9*** | 51.9*** | −7.8*** | −7.6*** |
| All delay categories | 11.5 | 34.0*** | 34.1*** | –2.5*** | −2.4*** |
Significance compared to Pre-Cures period (June 1, 2020–January 19, 2021).
Significance compared to Post-Cures period (January 20, 2021–April 15, 2021).
P < .001.
The proportion of patients who sent a message after reviewing a sensitive result increased after Cures Act compliance from 6.2% (95% CI, 6.2–6.3) to 6.3% (95% CI, 6.2–6.6) within 6 hours of message review and from 12.4% (95% CI, 12.3–12.4) to 15.4% (95% CI, 15.2–15.5) within 72 hours (Table 2). This change corresponds to a median of 25 additional patients sending messages within 6 hours and 111 additional patients sending messages within 72 hours. The proportion of patients who messaged their clinician after reviewing the most sensitive results increased from 5.8% (95% CI, 5.6–6.0) to 8.5% (95% CI, 8.0–8.9) within 6 hours and from 10.7% (95% CI, 10.5–10.9) to 15.4% (95% CI, 14.8–16.1) after 72 hours.
Table 2.
Adjusted and unadjusted interrupted time series of the daily percentage of patients who sent messages within 6 and 72 hours after reviewing a test result before their clinician
| Pre-Cures Act release rules | Pre-Cures Act | Post-Cures Acta increment |
Postnotification policyb increment |
||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Messaging within 6 h of a reviewed result | |||||
| Immediate release | 6.4 | 0.4*** | 0.4*** | −0.7*** | −0.7*** |
| One-day delay | 7.1 | −0.4 | −0.3 | −0.6*** | −0.7*** |
| Three-day delay | 6.3 | 0.7*** | 0.7*** | −0.6*** | −0.6*** |
| Seven-day delay | 5.8 | −0.2 | −0.9*** | −0.2 | 0.2 |
| Fourteen-day delay | 5.8 | 2.6*** | 2.7*** | −1.6*** | −1.6*** |
| All delay categories | 6.2 | 0.3*** | 0.1* | −0.5*** | −0.4*** |
| Messaging within 72 h of a reviewed result | |||||
| Immediate release | 17.7 | 1.2*** | 1.3*** | −1.3*** | −1.3*** |
| One-day delay | 15.9 | 3.4*** | 3.5*** | −2.1*** | −2.0*** |
| Three-day delay | 12.0 | 4.0*** | 4.1*** | −1.4*** | −1.4*** |
| Seven-day delay | 11.1 | 2.2*** | 1.6*** | 0.8*** | 1.4*** |
| Fourteen-day delay | 10.7 | 4.6*** | 4.7*** | –2.0*** | −1.9*** |
| All delay categories | 12.4 | 3.0*** | 3.0*** | −0.5*** | −0.4*** |
Significance compared to Pre-Cures period (June 1, 2020–January 19, 2021).
Significance compared to Post-Cures period (January 20, 2021–April 15, 2021).
P < .05.
P < .001.
Within the first month after automatic notifications were discontinued, 42% of patients who received results turned notifications back on. After the notification policy change, patient-before-clinician review decreased by 6.9% (95% CI, −7.1 to −6.7) for results that were always categorized as immediate release and decreased by 2.4% (95% CI, −2.6 to −2.2) for previously delayed results. Messaging after reviewing a previously delayed result similarly decreased by 0.4% (95% CI, −0.5 to −0.3) within 6 hours and by 0.4% (95% CI, −0.6 to −0.2) within 72 hours. This change corresponds to a median of 14 fewer patients sending messages within 6 hours and 17 fewer patients sending messages within 72 hours. The change in notification policy was associated with a significant decrease in messaging across most categories. Among the most sensitive results, the proportions of patients sending messages after 6 and 72 hours decreased by 1.6% (95% CI, −2.0 to −1.2) and 1.9% (95% CI, −2.5 to −1.3).
DISCUSSION
After our medical center transitioned to Cures Act compliance with an immediate results notification policy, the proportion of previously delayed test results reviewed first by patients before a clinician increased 4-fold, and the percentage of patients who sent a message within 72 hours of reviewing a result increased by 3%. After the center dropped default notifications and invited patients to choose whether to receive notifications, patient-before-clinician review of previously delayed results dropped 2.4% and messaging decreased 0.4%. Understanding how notification policies influence result review trends provides critical insight into balancing patient preferences while limiting undue emotional distress.
Limitations to commercially available electronic health record software have led many health systems to implement the Cures Act regulation by immediately releasing all electronic health information through the patient portal.4 Patient portals do not have the ability to allow patients to specify granular preferences such as delaying notification of test results that may be emotionally charged (eg, a new cancer diagnosis). However, it is possible to allow patients to choose whether to receive immediate results notifications for all results.
Improved data availability benefits the patient-clinician relationship and represents a marked transformation in patients’ opportunity to take ownership of their health care. However, immediate release of test results may also have unintended consequences to patient wellbeing. Most patients want unrestricted access to their health records8 and we have recently shown that a large majority of patients also want immediate results release.7 However, the practice of immediately releasing test results without interpretation remains controversial.9 Results released via patient portal are often not accompanied with adequate guidance or context to interpret sensitive or abnormal findings, which contributes to emotional distress.5
We show an important impact of changing the notification policy, in which releasing results with immediate notification was followed by sharp increases in patient-before-clinician results review and patient-initiated messaging. Replacing automated notifications with an opt-in policy modestly attenuated both effects. There remains legitimate concern regarding the impact of releasing results on clinical workflow and messaging work. The increase in messaging leads to additional administrative work and contributes to interruptions, which are major job dissatisfiers and components of professional burnout.10 Allowing patients to opt in to immediate notifications can allow patients greater flexibility to indicate their preferences and maintain known benefits of improved information availability while modestly reducing adverse effects on clinical workflow.
This study has limitations. First, our data were generated at a single academic medical center and results may not be generalizable to other organizations. Second, this was a retrospective observational study. There is potential for additional confounding variables that were not addressed in this study and there may be inherent limitations to establishing causal relationships. Third, we analyzed trends in patient-initiated messaging following result review without consideration for message content and tone. Fourth, it is possible that additional unknown changes to clinical workflow or clinician behavior influenced result review and messaging behavior. For example, with the new result release policy, clinicians might have compensated by increasing precounseling and improving communication around the release of test results as an approach to reduce worry.8 Finally, given the ongoing evolution of digital patient engagement and changing patient expectations regarding information and patient-clinician communication, the relationship between result notification and patient behavior may continue to evolve. Additional research should further investigate different notification policies and patient preferences across diverse patient populations, study settings and clinical scenarios.
CONCLUSION
Improved availability of electronic health data supports patients to take ownership of their health care. However, if not thoughtfully implemented, immediate release of test results can negatively impact patient wellbeing and provider workflow. We found a small but significant decrease in patient-before-provider review of test results and patient-initiated messages after transitioning to an opt-in notification policy for new test results. Understanding how notification policies influence result review and messaging trends provides important insight into balancing patient preference while limiting emotional distress and modestly reducing negative consequences to clinical workflow.
Supplementary Material
Contributor Information
Bryan D Steitz, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Nana Addo Padi-Adjirackor, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Kevin N Griffith, Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Thomas J Reese, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
S Trent Rosenbloom, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Jessica S Ancker, Department of Biomedical Informatics, Vanderbilt University Medical Center, Nashville, Tennessee, USA; Department of Health Policy, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
AUTHOR CONTRIBUTIONS
BDS, TJR, and JSA conceived of the study. BDS acquired the data, designed the analysis plan, and drafted the article. NAP-A and KNG designed the analysis plan and conducted the analysis. TJR, STR, and JSA developed the analysis plan and oversaw all research procedures. All authors critically reviewed each manuscript draft and approved the final submission.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
CONFLICT OF INTEREST STATEMENT
The authors do not have any competing interests to declare.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly due to IRB requirement in regard to protected health information and patient identifiers. The data will be shared upon reasonable request to the corresponding author.
REFERENCES
- 1. 21st Century Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification Program; 2020. https://www.federalregister.gov/documents/2020/05/01/2020-07419/21st-century-cures-act-interoperability-information-blocking-and-the-onc-health-it-certification.
- 2. Ross SE, Lin C-T.. The effects of promoting patient access to medical records: a review. J Am Med Inform Assoc 2003; 10 (2): 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steitz BD, Wong JIS, Cobb JG, et al. Policies and procedures governing patient portal use at an Academic Medical Center. JAMIA Open 2019; 2 (4): 479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Steitz BD, Sulieman L, Wright A, et al. Association of immediate release of test results to patients with implications for clinical workflow. JAMA Netw Open 2021; 4 (10): e2129553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giardina TD, Menon S, Parrish DE, et al. Patient access to medical records and healthcare outcomes: a systematic review. J Am Med Inform Assoc 2014; 21 (4): 737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murphy DR, Meyer AND, Russo E, et al. The burden of inbox notifications in commercial electronic health records. JAMA Intern Med 2016; 176 (4): 559–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steitz BD, Turer RW, Lin C-T, et al. Perspectives of patients about immediate access to test results through an online patient portal. JAMA Netw Open 2023; 6 (3): e233572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. D'Costa SN, Kuhn IL, Fritz Z.. A systematic review of patient access to medical records in the acute setting: practicalities, perspectives and ethical consequences. BMC Med Ethics 2020; 21 (1): 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giardina TD, Baldwin J, Nystrom DT, Sittig DF, Singh H.. Patient perceptions of receiving test results via online portals: a mixed-methods study. J Am Med Inform Assoc 2018; 25 (4): 440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gregory ME, Russo E, Singh H.. Electronic health record alert-related workload as a predictor of burnout in primary care providers. Appl Clin Inform 2017; 8 (3): 686–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly due to IRB requirement in regard to protected health information and patient identifiers. The data will be shared upon reasonable request to the corresponding author.