SUMMARY
Background:
The prevalence of liver fibrosis detected by non-invasive imaging in alpha-1-antitrypsin (AAT) deficiency has not been systematically assessed.
Aims:
We conducted a systematic review and meta-analysis to determine the prevalence of significant fibrosis and advanced fibrosis in AAT deficiency based on non-invasive imaging.
Methods:
Medline and Embase electronic databases were searched for studies from inception to November 13th, 2022, that provided data for the prevalence of fibrosis in adults with AAT deficiency. A generalized linear mixed model with Clopper-Pearson intervals was used to pool single-arm outcomes.
Results:
Of the 214 records identified, 8 studies were included. Five studies assessed fibrosis using vibration-controlled transient elastography. The prevalence of significant fibrosis (defined as ≥ 7.1 kPA) in Z homozygosity, Z heterozygosity, and non-carrier status was 22.10% (5 studies, 95%CI: 17.07 – 28.12) and 9.24% (3 studies, 95%CI: 4.68 – 17.45) and 5.38% (1 study, 95%CI: 3.27 – 8.73), respectively, P<.0001, and the prevalence of advanced fibrosis (defined as ≥ 9.5 kPa) was 8.13% (5 studies, 95%CI: 4.60 – 13.96), 2.96% (3 studies, 95%CI: 1.49 – 5.81), and 1.08% (1 study, 95%CI: 0.35 – 3.28), respectively, P=.003. There were limited data regarding the use of magnetic resonance elastography or acoustic radiation force impulse to assess for fibrosis.
Conclusion:
More than one in five adult individuals with AAT deficiency and Z homozygosity harbor significant fibrosis, and nearly one in ten harbors advanced fibrosis. The risk of fibrosis increases incrementally with the frequency of Pi*Z mutations.
Keywords: Cirrhosis, AATD, transient elastography
Graphical Abstract

INTRODUCTION
Alpha1-antitrypsin (AAT) deficiency is one of the commonest genetic diseases and is an important but under-diagnosed cause of cirrhosis and hepatocellular carcinoma (HCC)1–3. AAT is a protease inhibitor that is formed in hepatocytes and secreted into the circulation, preventing damage to the lung parenchyma by inhibiting neutrophil elastase. Severe AAT deficiency is most commonly caused by the homozygous Pi*Z (Glu342Lys) mutation (Pi*ZZ genotype) in the SERPINA1 gene, which leads to a misfolded protein (Z-alpha-1 antitrypsin), leading to insufficient hepatic clearance, accumulation in hepatocytes and liver injury4. Likewise, heterozygous Pi*Z carriage is associated with hepatic injury and fibrosis, although to a lesser extent than homozygous Pi*Z mutations5.
Fibrosis stage is an important determinant of liver-related outcomes and mortality among individuals with AAT deficiency2. Although liver biopsy remains the gold standard for fibrosis assessment, it is limited by its invasive nature, potential complications, and sampling variability. Noninvasive imaging tests of fibrosis, such as vibration-controlled transient elastography (VCTE), magnetic resonance elastography (MRE), and acoustic radiation force impulse (ARFI) are not prone to these limitations and may be useful tools for risk stratifying patients with AAT deficiency. As novel therapies for adults with AAT deficiency emerge, it is important to quantify the burden of adults with AAT deficiency and significant liver disease6. Recent data suggest that a substantial proportion of adults with AAT deficiency harbor significant liver fibrosis7–10. However, the prevalence of significant or advanced fibrosis among individuals with AAT deficiency due to Z homozygosity or Z heterozygosity has not been systematically assessed. Through a systematic review and meta-analytic approach, we provide estimates for the prevalence of significant (≥F2), and advanced (F3–F4) liver fibrosis based on non-invasive imaging tests, among adults with AAT deficiency.
METHODS
Search Strategy and Inclusion Criteria
This study was conducted per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). A systematic search was conducted on Medline and Embase electronic databases for studies published from database inception to November 13, 2022, and an additional manual search of article references was conducted to ensure comprehensiveness (supplementary material 1). When data in the original studies were not reported, the corresponding authors of the original studies were contacted to provide additional data. The search terms included keywords for alpha 1-antitrypsin deficiency and elastography, including “((AAT or AATD or antitrypsin or antitrypsins).tw. or alpha 1-Antitrypsin Deficiency/) and ((MRE or elastography or fibroscan or VCTE or ARFI or Acoustic Radiation Force Impulse or stiffness or LSM.tw. or exp Elasticity Imaging Techniques/))”. Citations and duplicate removals were handled with Rayyan11 and the references of included articles were subsequently screened to ensure a comprehensive search. Two authors (CHN, KEC) independently screened abstracts and conducted full-text reviews to ensure the eligibility of studies for inclusion; disputes were resolved by the consensus of an independent author (DQH). Only original articles, including cross-sectional and cohort studies of adult patients (≥18 years) with AAT deficiency, were included. Reviews, commentaries, conference abstracts, and editorials were excluded. Studies involving pediatric populations (individuals aged <18 years) or a combined cohort of pediatric and adult populations where it was not possible to extract data specific to adults were excluded. When multiple studies were generated from the same database, only data from the most updated study was included.
Study objectives
The primary objective was to estimate the pooled prevalence of significant fibrosis (≥F2) in AAT deficiency based on non-invasive imaging tests of fibrosis, including VCTE, MRE, and ARFI. The co-primary objective was to estimate the pooled prevalence of advanced fibrosis (F3–F4) in AAT deficiency based on non-invasive imaging tests of fibrosis. The prevalence of significant fibrosis and advanced fibrosis were stratified by the presence of Z homozygosity (Pi*ZZ genotype), Z heterozygotes (heterozygous Pi*Z carriage), and non-carriers (MM). We defined significant fibrosis and advanced fibrosis as a liver stiffness measurement (LSM) by VCTE of ≥7.1 kPa and ≥9.5 kPa respectively, as these thresholds were utilized in the majority of the included articles. Due to the limited number of studies assessing liver stiffness by MRE and ARFI, these data were in the form of a systematic review.
Statistical Analysis and Quality Assessment
All statistical analyses were conducted in rStudio (4.2.0). In the pooling of single-arm outcomes, a generalized linear mixed model with Clopper-Pearson intervals in a random effects model12,13 was used14,15. Statistical heterogeneity was assessed via I2 and the Q test.16,17. A p-value<0.1 was used to determine the statistical significance for heterogeneity. Comparisons between Z homozygosity (Pi*ZZ genotype), Z heterozygotes (heterozygous Pi*Z carriage), and non-carriers (MM) were computed in subgroup analyses. Based on the pooled proportions of single-arm outcomes, the respective relative risks (RR) were calculated as the ratio of pooled proportions of clinically significant and advanced fibrosis in each subgroup18. 95% confidence intervals (CI) were estimated using the Katz-logarithmic method and the p-value was calculated after a natural log transformation of the relative risk z-score19. A p-value ≤0.05 was considered as the threshold for statistical significance. Quality assessment was conducted using the Joanna Briggs Institute (JBI) Critical Appraisal Tools that measures the appropriateness of studies reporting prevalence data on the basis of sample size adequacy, methodology and statistical analyses20.
RESULTS
Summary of included articles
The initial search from Medline and Embase yielded a total of 214 articles. One article was identified by an independent manual search. After the removal of duplicates and irrelevant articles, 25 papers remained for full-text review (figure 1). A total of eight articles were included in the study. Liver fibrosis was assessed non-invasively by VCTE, MRE, and ARFI respectively in the included primary articles. The summary of study characteristics and quality assessment based on the JBI Critical Appraisal Checklist assessment tool are shown in Table 1.
Figure 1.
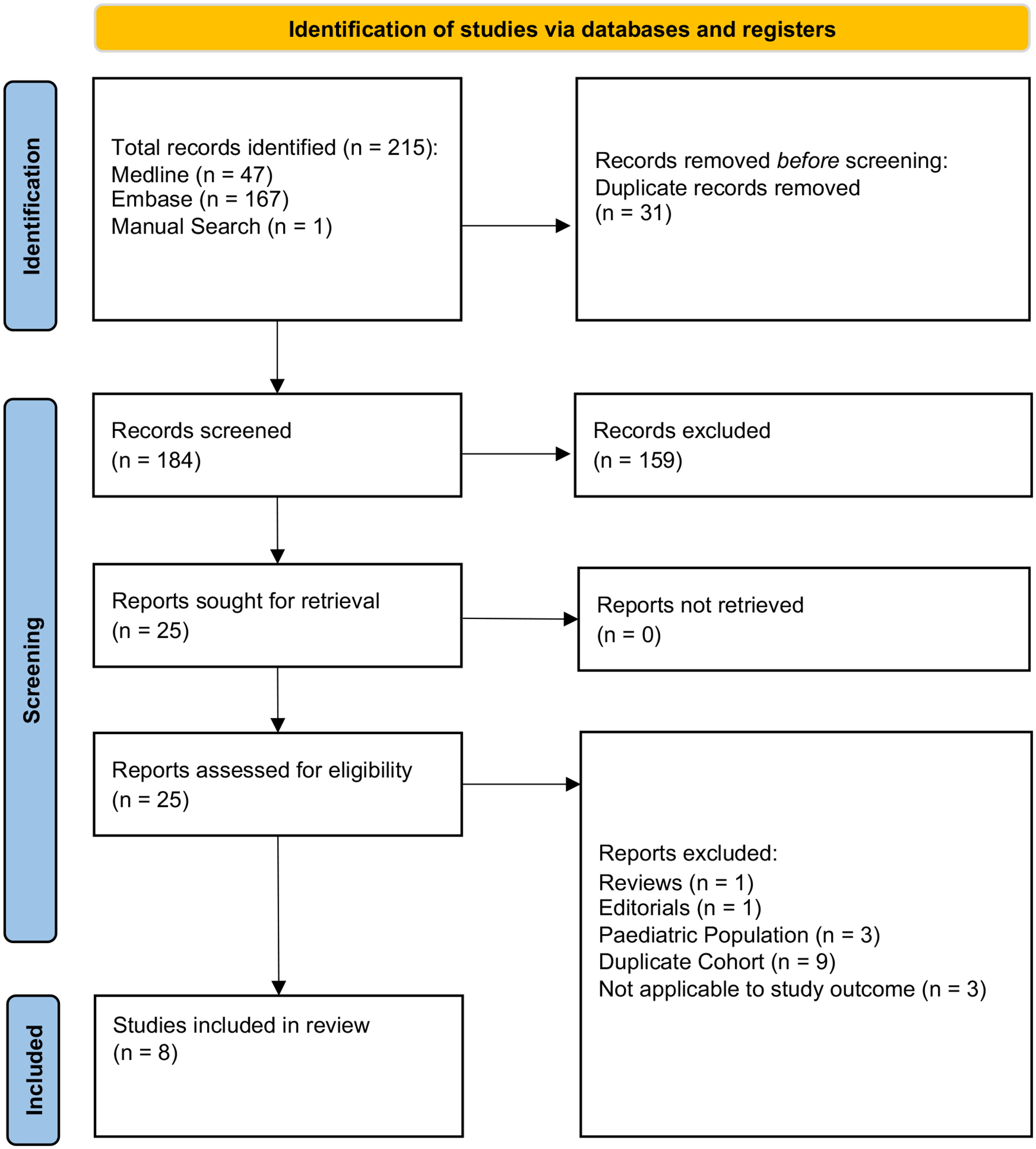
PRISMA flowchart
Table 1.
Summary of included articles
| Author, year | Location | Recruitment of participants | Non-invasive imaging test | Total sample size | Baseline characteristics | Quality assessment | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean, years) | Male (%) | Z homozygosity (Pi*ZZ genotype) | Z heterozygosity (Heterozygous Pi*Z carriage) | Non-carriers | ||||||
| Kim et al10, 2016 | USA | Participants of a prospective study at a tertiary center | MRE | 33 | 62.33 | 57.58 | 11 | - | 11 | 9 |
| Mostafavi et al23, 2017 | Sweden | Swedish National Neonatal AAT screening program | ARFI | 211 | 38.47 | 46.45 | 87 | 32 | 92 | 8 |
| Clark et al7, 2018 | USA/Canada | AATD patient registries, patient outreach events, and clinics at University of Florida | VCTE | 94 | 51.00 | 35.11 | 94a | - | - | 9 |
| Mandorfer et al27, 2018 | Austria | Patients with AATD at a tertiary center | VCTE | 41 | 54.20 | 59.52 | 28 | 13 | - | 7 |
| Reiter et al22, 2018 | Germany | Patients with AATD and healthy volunteers recruited prospectively | MRE/ ARFI/ 2D-SWE | 31 | 53.94 | 51.61 | 11 | 4 | 16 | 8 |
| Guillaud et al26, 2019 | France | Patients with AATD at a hospital | VCTE | 29 | 46.37 | 67.86 | 29b | - | - | 7 |
| Pons et al25, 2021 | Spain | Multicentre study of patients recruited from clinics and hospitalsc | VCTE | 148 | 54.96 | 50.68 | 81 | 67 | - | 7 |
| Fromme et al9, 2022 | Multinationald | Alpha-1 Liver initiative registry | VCTE | 1104e | 52.92 | 50.82 | 586 | 239f | 279 | 9 |
Abbreviations: MRE, Magnetic Resonance Elastography; ARFI, Acoustic Radiation Force Impulse; VCTE, Vibration-controlled Transient Elastography; 2D SWE, 2-Dimentional Shear Wave Elastography; AATD, alpha1-antitrypsin deficiency
Only 87 individuals with valid VCTE
Only 28 individuals with valid VCTE
Outpatient Pneumology Clinics of three AATD reference centres in Spain (Vall d’Hebron University Hospital, Barcelona, University Hospital Complex of Vigo, and Hospital Clínico San Carlos, Madrid)
United Kingdom/ Portugal/ Spain/ Italy/ Austria/ Belgium/ Ireland/ Denmark/ Poland/ USA
Cohort 2
Only 190 individuals with valid VCTE
Prevalence of significant fibrosis
A total of 5 studies (1,359 individuals) provided data for the prevalence of significant fibrosis (≥F2) based on LSM by VCTE. The pooled prevalence of significant fibrosis (LSM by VCTE ≥7.1 kPa) was highest in Z homozygotes (5 studies, 22.10%; 95% CI: 17.07 – 28.12; I2: 45.00%), followed by Z heterozygotes (3 studies, 9.24%; 95% CI: 4.68 – 17.45; I2: 40.00), and non-carriers (1 study, 5.38%; 95% CI: 3.27 – 8.73), P<.0001 (figure 2a). In a test for subgroup difference, the prevalence of significant fibrosis was significantly higher in Z homozygotes compared to Z heterozygotes (P=.01) and non-carriers (P<.0001) respectively (supplementary material 2).
Figure 2.
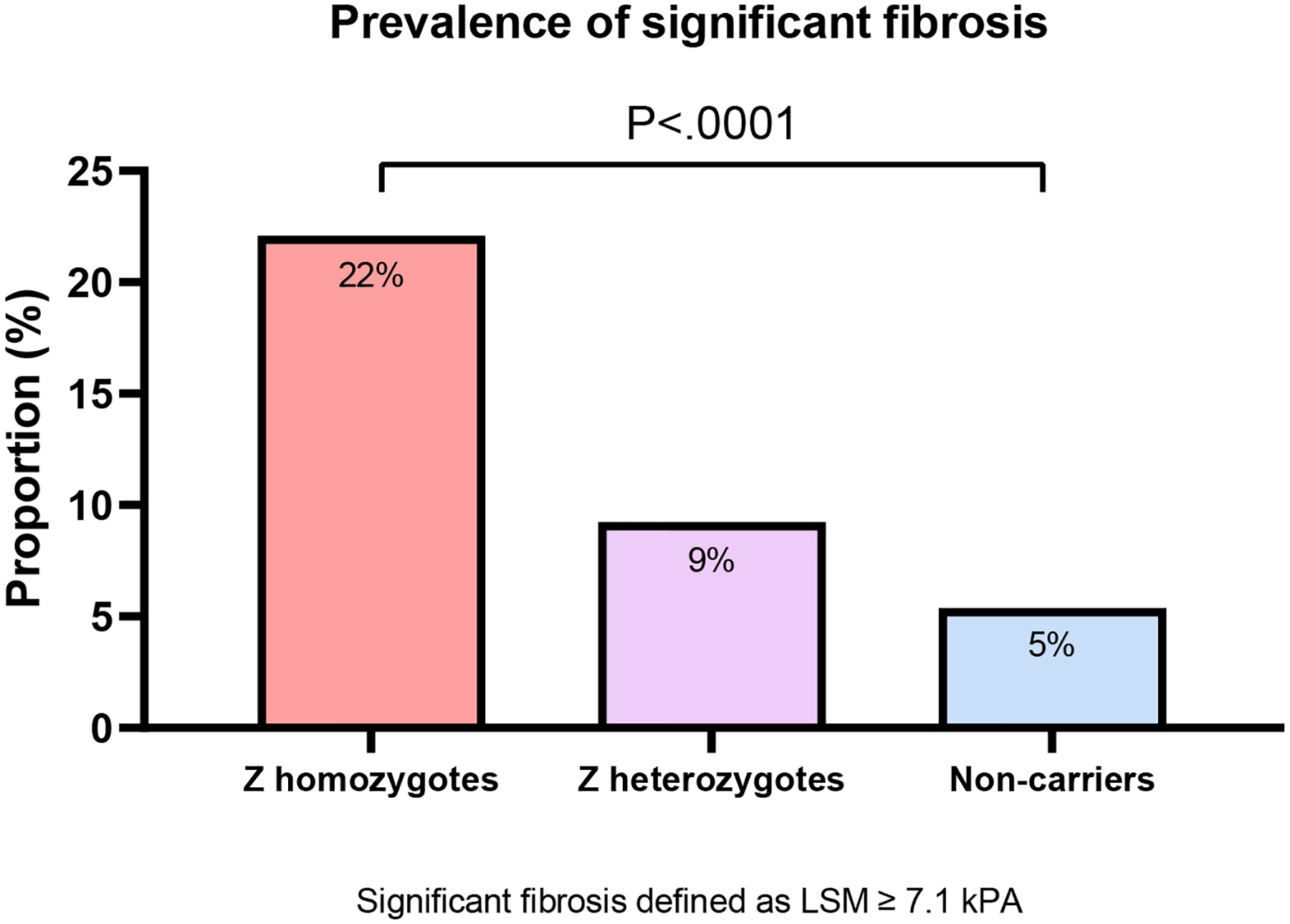
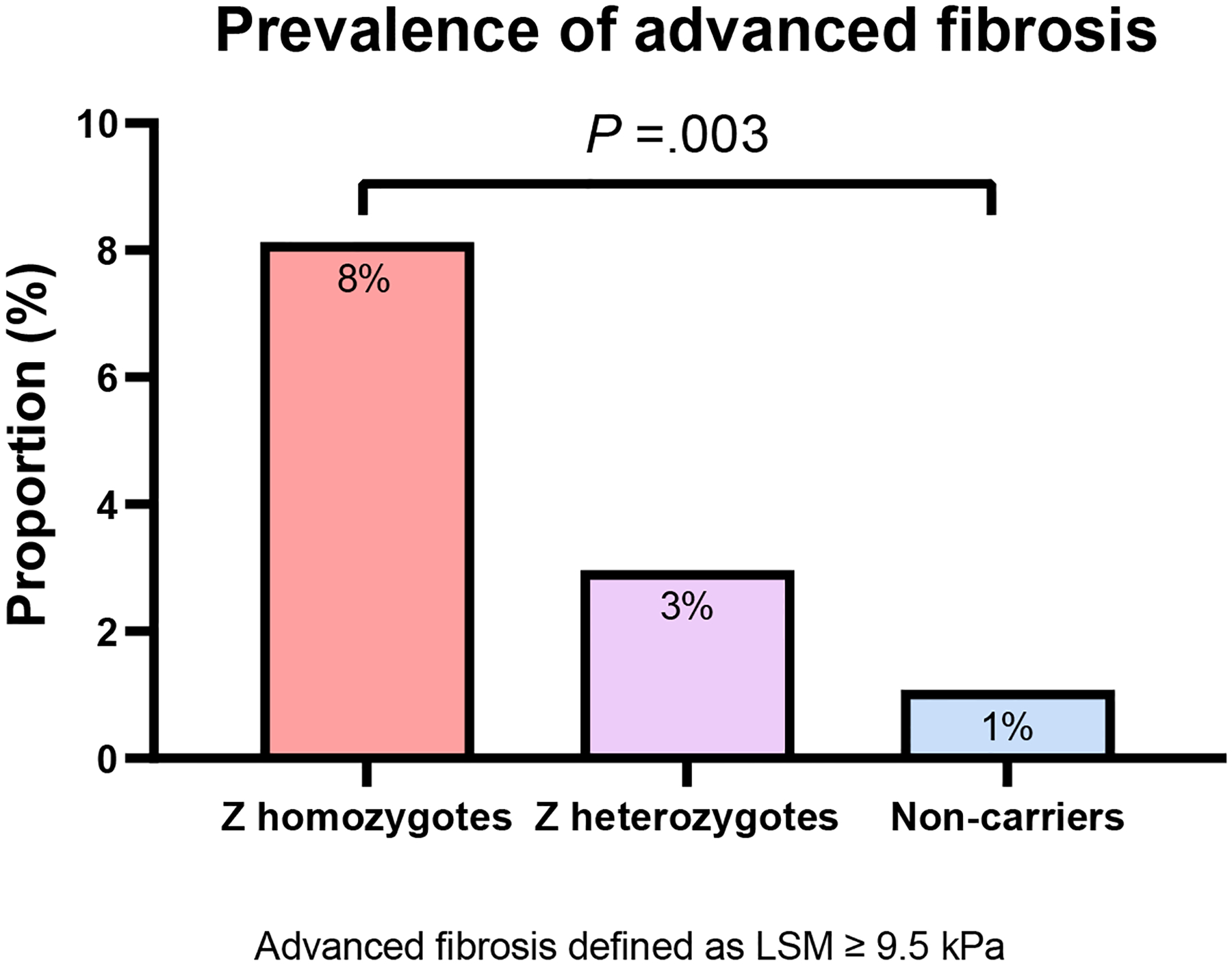
Prevalence of (A) significant fibrosis and (B) advanced fibrosis in adults with alpha-1-antitrypsin deficiency
Abbreviations: LSM, liver stiffness measurement
Prevalence of advanced fibrosis
A total of 5 studies (1,359 individuals) provided data for the prevalence of advanced fibrosis (F3–F4) based on LSM by VCTE. The pooled prevalence of advanced fibrosis (≥9.5 kPa) was 8.13% (5 studies, 95% CI: 4.60 – 13.96; I2: 50.00%) in Z homozygotes, 2.96% (3 studies, 95% CI: 1.49 – 5.81; I2: 0.00%) in Z heterozygotes, and 1.08% (1 study, 95% CI: 0.35 – 3.28) in non-carriers (figure 2b). The prevalence of advanced fibrosis was significantly higher in Z homozygotes than in Z heterozygotes (P=.02) and non-carriers (P=.001) (supplementary material 3).
Assessment of fibrosis by MRE and ARFI
The limited number of studies utilizing MRE or ARFI precluded a pooled analysis and the findings are reported in the form of a systematic review (Table 2). Kim et al. conducted a prospective study comparing 11 individuals with AAT deficiency who were Z homozygotes against 11 age- and sex-matched healthy controls and 11 NAFLD patients. A total of 44.4% of the individuals who were Z homozygotes were assessed to have any fibrosis (defined by the authors as LSM by MRE ≥3.0 kPa)10.
Table 2.
Systematic review of studies that determined the prevalence of fibrosis by MRE or ARFI in alpha-1-antitrypsin deficiency
| Author, year | Location | Non-invasive imaging test | Study cohort | Findings |
|---|---|---|---|---|
| Kim et al10, 2016 | USA | MRE | 11 individuals with Z homozygosity, compared with 11 non-carriers, and 11 with NAFLD | 44.4% of individuals who were Z homozygotes were assessed to have any fibrosis (defined by the authors as LSM by MRE ≥3.0 kPa) |
| Mostafavi et al23, 2017 | Sweden | ARFI | 87 individuals with Z homozygosity, 32 with Z heterozygosity, and 92 non-carriers | The prevalence of significant liver fibrosis (defined by the authors as > 1.30 m/s) was estimated to be 25%, 13%, and 22% among individuals with Z homozygosity, Z heterozygosity, and non-carriers respectively. The prevalence of cirrhosis (defined as >1.80 m/s) was 25%, 20%, and 18%, in Z homozygosity, Z heterozygosity, and non-carriers, respectively |
| Reiter et al22, 2018 | Germany | MRE/ ARFI | 11 individuals with Z homozygosity, 4 with Z heterozygosity, and 16 non-carriers | 54.5% with Z homozygosity had significant fibrosis (defined by the authors as > 1.30 m/s), and 27.2% with Z homozygosity had advanced fibrosis (defined by the authors as > 1.80 m/s) based on ARFI. Prevalence of significant fibrosis and advanced fibrosis determined by MRE not reported. |
Abbreviations: MRE, Magnetic Resonance Elastography; ARFI, Acoustic Radiation Force Impulse; VCTE, Vibration-controlled Transient Elastography; LSM, liver stiffness measurement; NAFLD, nonalcoholic fatty liver disease; AAT, alpha-one antitrypsin
Two studies assessed liver fibrosis in patients with AAT deficiency using ARFI. Reiter et al. determined that the prevalence of significant (≥F2) fibrosis (thresholds based on a meta-analysis) among 11 individuals who were Z homozygotes was 54.5% and the prevalence of advanced fibrosis was 27.2%, while all 3 with Z heterozygosity did not have significant fibrosis21,22. Mostafavi et al. assessed liver stiffness using ARFI in a prospective study of individuals with AAT deficiency (32 individuals who were Z homozygotes, 15 who were Z heterozygotes), compared to 51 non-carriers (MM). The prevalence of significant liver fibrosis (defined by the authors as > 1.30 m/s) was estimated to be 25%, 13%, and 22% among individuals who were Z homozygotes, heterozygotes, and non-carriers, respectively. The prevalence of cirrhosis (defined as >1.80 m/s) was 25%, 20%, and 18%, among individuals who were Z homozygotes, heterozygotes, and non-carriers, respectively23
DISCUSSION
Main findings
We estimated the pooled prevalence of significant and advanced fibrosis in adults with AAT deficiency. The pooled prevalence of significant fibrosis (≥F2) based on LSM by VCTE in Z homozygosity, Z heterozygosity, and non-carrier status was 22%, 9%, and 5%, respectively while the pooled prevalence of advanced fibrosis (F3–F4) was 8%, 3%, and 1%, respectively. The prevalence of fibrosis appeared to increase incrementally with the number of Pi*Z mutations. There were limited data from studies that utilized magnetic resonance elastography or acoustic radiation force impulse to determine the presence of fibrosis in AAT deficiency.
In context with current literature
A prospective study of 94 patients with AAT deficiency that underwent liver biopsy determined that LSM by VCTE is a clinically applicable and reasonably accurate non-invasive imaging test for detecting significant and advanced fibrosis7. Several single-center and multicenter studies have reported the prevalence of significant and advanced fibrosis8,9,24–27. However, a pooled analysis of the prevalence of significant or advanced fibrosis among patients with AAT deficiency has not been reported.
Strengths and limitations
This is the first meta-analysis to systematically evaluate the prevalence of fibrosis in patients with AAT deficiency. However, it is not without limitations. Data for MRE and ARFI were limited and precluded a pooled analysis. In addition, there were limited data for the Pi*SS genotype, as well as in subgroups such as by sex, precluding meaningful subgroup analyses or meta-regression for the association of demographic data with the risk of fibrosis. The data were dominated by a large study that contributed a majority of the included patients, which may have introduced bias into the results. The thresholds used to define significant and advanced fibrosis have not been specifically validated in the context of AAT deficiency. An individual patient data meta-analysis comparing fibrosis stage on histology and LSM would have been ideal and allowed for assessment of the interaction between LSM and risk factors for fibrosis.
Implications for clinical practice and future research
More than one in five adults with Z homozygosity have significant fibrosis, and nearly one in ten have advanced fibrosis (F3–F4). These data affirm the clinical utility of VCTE for assessing fibrosis in AAT deficiency. As novel therapies for AAT deficiency emerge, these data help quantify the burden of liver disease in AAT deficiency and have important implications for sample size estimation in clinical trials. Further studies are required to define and validate the optimal liver stiffness thresholds for significant and advanced fibrosis in AAT deficiency.
In summary, we determined that a substantial proportion of patients with AAT deficiency harbor significant and advanced fibrosis, and the prevalence of fibrosis appears to increase incrementally with the number of Pi*Z mutations.
Supplementary Material
Funding
R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18-2-0026). D.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01).
Competing interests
R.L. serves as a consultant or advisory board member for Anylam/Regeneron, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse Bio, Inipharm, Intercept, Ionis, Janssen, Merck, Metacrine, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Promethera, Sagimet, 89bio, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, Genfit, Gilead, Intercept, Inventiva, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Pfizer, pH Pharma, and Siemens. He is also co-founder of Liponexus. D.H serves as an advisory board member for Eisai. P.M serves as a consultant of advisory member for Ipsen, Eisai, Abbvie, Sanofi, Gilead Sciences, Evive Biotech, Novo Nordisk, Bayer Healthcare, Intercept, Surrozen, and Pfizer. H.C.P lectures and receives advisory board fees from Intercept, Genfit, Promethera Bioscience, Orphalan, Novo Nordisk and Roche Portugal.
Data availability statement:
Data are publicly available and ethics approval was not required.
REFERENCES
- 1.Strnad P, McElvaney NG, Lomas DA. Alpha(1)-Antitrypsin Deficiency. N Engl J Med. 2020;382(15):1443–1455. [DOI] [PubMed] [Google Scholar]
- 2.Townsend SA, Edgar RG, Ellis PR, Kantas D, Newsome PN, Turner AM. Systematic review: the natural history of alpha-1 antitrypsin deficiency, and associated liver disease. Aliment Pharmacol Ther. 2018;47(7):877–885. [DOI] [PubMed] [Google Scholar]
- 3.Bowlus CL, Willner I, Zern MA, et al. Factors associated with advanced liver disease in adults with alpha1-antitrypsin deficiency. Clin Gastroenterol Hepatol. 2005;3(4):390–396. [DOI] [PubMed] [Google Scholar]
- 4.Narayanan P, Mistry PK. Update on Alpha-1 Antitrypsin Deficiency in Liver Disease. Clinical liver disease. 2020;15(6):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strnad P, Buch S, Hamesch K, et al. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut. 2019;68(6):1099–1107. [DOI] [PubMed] [Google Scholar]
- 6.Strnad P, Mandorfer M, Choudhury G, et al. Fazirsiran for Liver Disease Associated with Alpha(1)-Antitrypsin Deficiency. N Engl J Med. 2022;387(6):514–524. [DOI] [PubMed] [Google Scholar]
- 7.Clark VC, Marek G, Liu C, et al. Clinical and histologic features of adults with alpha-1 antitrypsin deficiency in a non-cirrhotic cohort. J Hepatol. 2018;69(6):1357–1364. [DOI] [PubMed] [Google Scholar]
- 8.Hamesch K, Mandorfer M, Pereira VM, et al. Liver Fibrosis and Metabolic Alterations in Adults With alpha-1-antitrypsin Deficiency Caused by the Pi*ZZ Mutation. Gastroenterology. 2019;157(3):705–719.e718. [DOI] [PubMed] [Google Scholar]
- 9.Fromme M, Schneider CV, Pereira V, et al. Hepatobiliary phenotypes of adults with alpha-1 antitrypsin deficiency. Gut. 2021. [DOI] [PubMed] [Google Scholar]
- 10.Kim RG, Nguyen P, Bettencourt R, et al. Magnetic resonance elastography identifies fibrosis in adults with alpha-1 antitrypsin deficiency liver disease: a prospective study. Aliment Pharmacol Ther. 2016;44(3):287–299. [DOI] [PubMed] [Google Scholar]
- 11.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell A, Fairbrother M, Jones K. Fixed and random effects models: making an informed choice. Quality & Quantity. 2019;53(2):1051–1074. [Google Scholar]
- 13.Tufanaru C, Munn Z, Stephenson M, Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. International journal of evidence-based healthcare. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 14.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934:404–413. [Google Scholar]
- 15.Schwarzer G, Chemaitelly H, Abu‐Raddad LJ, Rücker G. Seriously misleading results using inverse of Freeman‐Tukey double arcsine transformation in meta‐analysis of single proportions. Research synthesis methods. 2019;10(3):476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher J What is heterogeneity and is it important? Bmj. 2007;334(7584):94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tay PWL, Xiao J, Tan DJH, et al. An Epidemiological Meta-Analysis on the Worldwide Prevalence, Resistance, and Outcomes of Spontaneous Bacterial Peritonitis in Cirrhosis. Front Med (Lausanne). 2021;8:693652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz D, Baptista J, Azen S, Pike M. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics. 1978:469–474. [Google Scholar]
- 20.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. International journal of health policy and management. 2014;3(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. European Radiology. 2013;23(11):3040–3053. [DOI] [PubMed] [Google Scholar]
- 22.Reiter R, Wetzel M, Hamesch K, et al. Comparison of non-invasive assessment of liver fibrosis in patients with alpha1-antitrypsin deficiency using magnetic resonance elastography (MRE), acoustic radiation force impulse (ARFI) Quantification, and 2D-shear wave elastography (2D-SWE). PLoS ONE. 2018;13(4):e0196486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mostafavi B, Diaz S, Tanash HA, Piitulainen E. Liver function in alpha-1-antitrypsin deficient individuals at 37 to 40 years of age. Medicine. 2017;96(12):e6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mostafavi B, Piitulainen E, Tanash HA. Survival in the Swedish cohort with alpha-1-antitrypsin deficiency, up to the age of 43–45 years. Int J Chron Obstruct Pulmon Dis. 2019;14:525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pons M, Núñez A, Esquinas C, et al. Utility of Transient Elastography for the Screening of Liver Disease in Patients with Alpha1-Antitrypsin Deficiency. Journal of clinical medicine. 2021;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillaud O, Dumortier J, Traclet J, et al. Assessment of liver fibrosis by transient elastography (Fibroscan(®)) in patients with A1AT deficiency. Clin Res Hepatol Gastroenterol. 2019;43(1):77–81. [DOI] [PubMed] [Google Scholar]
- 27.Mandorfer M, Bucsics T, Hutya V, et al. Liver disease in adults with α1-antitrypsin deficiency. United European Gastroenterol J. 2018;6(5):710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are publicly available and ethics approval was not required.


