| dbo:abstract
|
- La périfosine est un alkyl-lysophospholipide apparenté structurellement à la miltéfosine et agissant comme inhibiteur enzymatique de la protéine kinase B (Akt) et des phosphoinositide 3-kinases (PI3K). Il est en essai clinique comme médicament phospholipidique potentiel et était en étude pivot (phase III) en 2011 comme traitement contre la maladie de Kahler et le cancer du côlon ; le 2 avril 2012 était cependant annoncé que la périfosine n'avait pas passé la phase III comme traitement contre le cancer du côlon. (fr)
- Perifosine (also KRX-0401) is a former drug candidate that was under development for a variety of cancer indications. It is an alkyl-phospholipid structurally related to miltefosine. Perifosine interrupts the PI3K/AKT/mTOR pathway by acting as an allosteric AKT inhibitor targeting the pleckstrin homology domain of AKT. It was being developed by Keryx Biopharmaceuticals who had licensed it from Inc. In 2010, perifosine received orphan drug status in the U.S. for the treatment of multiple myeloma and neuroblastoma, and for multiple myeloma in the EU. However, both were later withdrawn. In 2011 it was in a phase III trial for colorectal cancer, and another for multiple myeloma. On April 2, 2012, it was announced that perifosine failed its phase III clinical trial for treatment of colon cancer. Detailed results were released in June 2012. On March 11, 2013 Aeterna Zentaris announced the discontinuing of Phase 3 clinical trial of perifosine for the treatment of relapsed and refractory multiple myeloma. (en)
- Perifosina (DCI; ou KRX-0401) é um fármaco antineoplásico desenvolvido pela Keryx Biopharmaceuticals e Aeterna Zentaris. Recebeu estatuto de medicamento órfão pelo FDA em julho de 2010, para tratamento de neuroblastoma. É um fármaco oncológico, utilizado no tratamento de mieloma múltiplo, câncer colorretal metatástico, câncer de rim e outros tipos de câncer. Aeterna Zentaris possui direitos de comercialização do fármaco em todos países exceto EUA (área da Keryx) e Coreia do Sul. (pt)
|
| dbo:alternativeName
| |
| dbo:iupacName
|
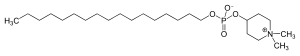
- 1,1-Dimethylpiperidinium-4-yl octadecyl phosphate (en)
|
| dbo:thumbnail
| |
| dbo:wikiPageID
| |
| dbo:wikiPageLength
|
- 5635 (xsd:nonNegativeInteger)
|
| dbo:wikiPageRevisionID
| |
| dbo:wikiPageWikiLink
| |
| dbp:imagealt
|
- Skeletal formula of perifosine (en)
- Space-filling model of the perifosine zwitterion (en)
|
| dbp:imagefile
|
- Perifosine-zwitterion-3D-spacefill.png (en)
- Perifosine.svg (en)
|
| dbp:imagesize
|
- 300 (xsd:integer)
- 320 (xsd:integer)
|
| dbp:iupacname
| |
| dbp:othernames
| |
| dbp:wikiPageUsesTemplate
| |
| dcterms:subject
| |
| gold:hypernym
| |
| rdf:type
| |
| rdfs:comment
|
- La périfosine est un alkyl-lysophospholipide apparenté structurellement à la miltéfosine et agissant comme inhibiteur enzymatique de la protéine kinase B (Akt) et des phosphoinositide 3-kinases (PI3K). Il est en essai clinique comme médicament phospholipidique potentiel et était en étude pivot (phase III) en 2011 comme traitement contre la maladie de Kahler et le cancer du côlon ; le 2 avril 2012 était cependant annoncé que la périfosine n'avait pas passé la phase III comme traitement contre le cancer du côlon. (fr)
- Perifosina (DCI; ou KRX-0401) é um fármaco antineoplásico desenvolvido pela Keryx Biopharmaceuticals e Aeterna Zentaris. Recebeu estatuto de medicamento órfão pelo FDA em julho de 2010, para tratamento de neuroblastoma. É um fármaco oncológico, utilizado no tratamento de mieloma múltiplo, câncer colorretal metatástico, câncer de rim e outros tipos de câncer. Aeterna Zentaris possui direitos de comercialização do fármaco em todos países exceto EUA (área da Keryx) e Coreia do Sul. (pt)
- Perifosine (also KRX-0401) is a former drug candidate that was under development for a variety of cancer indications. It is an alkyl-phospholipid structurally related to miltefosine. Perifosine interrupts the PI3K/AKT/mTOR pathway by acting as an allosteric AKT inhibitor targeting the pleckstrin homology domain of AKT. It was being developed by Keryx Biopharmaceuticals who had licensed it from Inc. In 2010, perifosine received orphan drug status in the U.S. for the treatment of multiple myeloma and neuroblastoma, and for multiple myeloma in the EU. However, both were later withdrawn. (en)
|
| rdfs:label
|
- Périfosine (fr)
- Perifosine (en)
- Perifosina (pt)
|
| owl:sameAs
| |
| prov:wasDerivedFrom
| |
| foaf:depiction
| |
| foaf:isPrimaryTopicOf
| |
| is dbo:wikiPageRedirects
of | |
| is dbo:wikiPageWikiLink
of | |
| is foaf:primaryTopic
of | |