Abstract
Free full text

In Vitro Formation of the Endoplasmic Reticulum Occurs Independently of Microtubules by a Controlled Fusion Reaction
Abstract
We have established an in vitro system for the formation of the endoplasmic reticulum (ER). Starting from small membrane vesicles prepared from Xenopus laevis eggs, an elaborate network of membrane tubules is formed in the presence of cytosol. In the absence of cytosol, the vesicles only fuse to form large spheres. Network formation requires a ubiquitous cytosolic protein and nucleoside triphosphates, is sensitive to N-ethylmaleimide and high cytosolic Ca2+ concentrations, and proceeds via an intermediate stage in which vesicles appear to be clustered. Microtubules are not required for membrane tubule and network formation. Formation of the ER network shares significant similarities with formation of the nuclear envelope. Our results suggest that the ER network forms in a process in which cytosolic factors modify and regulate a basic reaction of membrane vesicle fusion.
Introduction
Cellular organelles have characteristic shapes. Some organelles, such as lysosomes, vacuoles, endosomes, peroxisomes, synaptic vesicles, and various transport vesicles resemble spheres, likely the thermodynamically most stable form of a lipid vesicle. Other organelles have more complex shapes in which two membranes are closely apposed to one another. These include the nuclear envelope, the ER, the Golgi cisternae, and the mitochondrial cristae. How these structures are generated and maintained is largely unknown.
The ER forms an elaborate tubular and cisternal network that is continuous with the outer nuclear membrane. This network is dynamic; new membrane tubules are continuously formed, fuse with other tubules to form three-way junctions, and move relative to one another (Lee and Chen 1988). Microtubules are thought to be required for the formation and movement of the tubular ER network (Terasaki et al. 1986; Lee and Chen 1988). When cells are treated with nocodazole to depolymerize microtubules, the ER retracts from the cell periphery, and after removal of nocodazole, new ER membrane tubules often align with newly formed microtubules (Terasaki et al. 1986; Lee et al. 1989). Microscopy of live cells indicates that movement of membrane tubules can occur by three different mechanisms: (a) motor proteins pull on the membrane as they migrate along a microtubule; (b) the membrane is attached to the plus end of a microtubule and is dragged along as the microtubule grows or shrinks; and (c) the membrane attaches to microtubules that move as an intact entity through the cell (Lee and Chen 1988; Waterman-Storer and Salmon 1998). However, these experiments do not exclude that the primary role of microtubule-based movement is to position the ER at the periphery of the cell, rather than to form ER membrane tubules de novo. It is at least clear that the membrane network is not solely maintained by the microtubule network: upon treatment of tissue culture cells with nocodazole, the microtubules rapidly depolymerize, whereas the ER network retracts only slowly towards the cell center (Terasaki et al. 1986). In plant cells and S. cerevisiae, microtubules probably play no role in the formation of the ER network. In this case, the actin cytoskeleton is required for movement of ER tubules (Terasaki 1990; Liebe and Menzel 1995), although it is dispensable for maintenance of the ER structure in yeast (Prinz, W.A., L. Grzyb, J.A. Kahana, P.A. Silver, and T.A. Rapoport, manuscript submitted for publication).
In vitro experiments suggest a direct role of microtubules in the formation of an ER network. In these experiments, membranes and cytosol from chicken embryo fibroblasts or Xenopus laevis eggs were placed in a flow chamber, and video-enhanced differential interference contrast (DIC) microscopy was used to follow the movement of membrane tubules on a glass surface (Dabora and Sheetz 1988; Allan and Vale 1991; Waterman-Storer et al. 1995). It was found that microtubules are required to form a network of membrane tubules and that the movement of membrane tubules frequently occurs along microtubules by the action of motor proteins or by the attachment to the tip of growing microtubules (Allan and Vale 1994; Waterman-Storer et al. 1995; Steffen et al. 1997). However, the formation of a membrane network on a surface may not necessarily reflect the physiological situation in a cell.
The formation of membrane tubules or tubular networks has also been observed with Golgi membranes in vivo and in vitro. As with the ER, Golgi membrane tubules often coalign with microtubules, and formation in vitro depends on prepolymerized microtubules as well as motor proteins (Cooper et al. 1990; Lippincott-Schwartz et al. 1990; Allan and Vale 1991, Allan and Vale 1994; Fullerton et al. 1998). Other data indicate that Golgi tubule formation can occur independently of microtubules (Cluett et al. 1993; de Figueiredo et al. 1998).
An excellent system to study the de novo formation of an ER network is egg extracts from the frog Xenopus laevis (Allan and Vale 1991). In Xenopus eggs, ER membranes are abundant and stockpiled for ~2,000 cells that are formed during the rapid early cell divisions. The ER in fertilized eggs is present in small membrane tubules and vesicles that at some point during early development must fuse to reform the ER network. The machinery for the formation of the network must also be stored in the egg, since there is no synthesis of new material during the first cell divisions. In mammalian cells, the ER may remain largely intact, even during cell division (Warren 1993; Ellenberg et al. 1997), and its formation de novo may therefore be less extensive and thus more difficult to study.
Using Xenopus egg extracts, we have established an in vitro system for the formation of an ER network. Surprisingly, we find that microtubules or an actin scaffold are not required for the process. Rather, other cytosolic factors serve to incorporate a basic fusion reaction, which itself generates only large round vesicles, into a controlled reaction that results in a tubular network.
Materials and Methods
Preparation of Xenopus Egg Extract, Membranes, and Cytosol
Xenopus egg extract was prepared as described with minor modifications (Murray 1991; Newmeyer and Wilson 1991). Eggs from 10 frogs were dejellied, washed with 0.2 M NaCl, and then with buffer A (50 mM Hepes/KOH, pH 7.7, 50 mM potassium acetate, 2.5 mM MgCl2, 250 mM sucrose, 7 mM β-mercaptoethanol). All subsequent steps were carried out on ice. Eggs were transferred into SW28 tubes (Beckman) that contained 5 ml buffer A plus 10 μg/ml cytochalasin B, 50 μg/ml cycloheximide, and protease inhibitors (PI: 10 μg/ml leupeptin, 5 μg/ml chymostatin, 2.5 μg/ml elastatinal, 1 μg/ml pepstatin A, 10 μg/ml aprotinin). Excess buffer was removed and the eggs were crushed by centrifugation for 10 min at 10,000 rpm and 4°C in an HB4 rotor (Sorvall; DuPont Company). The egg extract was collected using a syringe via side puncture. 7 mM β-mercaptoethanol, 10 μg/ml cytochalasin B, 50 μg/ml cycloheximide, and PI were added, the egg extract was frozen in liquid nitrogen, and stored at −80°C.
To prepare membrane and cytosol fractions, the egg extract was centrifuged in an SW40 rotor (Beckman) for 1 h at 40,000 rpm and 2°C, resulting in sedimentation of a heavy membrane fraction. The supernatant was centrifuged in a 100.4 rotor (Beckman) for 1.5 h at 100,000 rpm and 2°C. The resulting supernatant contained the cytosol and was almost completely free of membranes. The pellet consisted of a clear layer with light membranes on top. The latter fraction was resuspended with very little buffer A, frozen in liquid nitrogen, and stored at −80°C. For some experiments, the light membranes were washed twice with 15 vol of buffer A to remove residual cytosol, and resuspended in buffer A. The absorption of the light membrane suspension in 1% SDS was between 20 and 40 OD (280 nm).
Mitotic egg extract was prepared from interphase extract by the addition of an energy regenerating system and 0.1 mg/ml glutathione S-transferase (GST)–cyclin B1 Δ90, that has GST fused to cyclin B1 with a deletion of the destruction box, followed by an incubation for 30 min at room temperature (Murray et al. 1989; Stukenberg et al. 1997). GST–cyclin B1 Δ90 was kindly provided by P. Stein (Harvard Medical School, Boston, MA). The histone H1 kinase activity of the extract was determined as described (Murray 1991).
Preparation of Various Membranes and Cytosols
Dog pancreas rough ER microsomes, yeast membranes and cytosol, and wheat germ cytosol were prepared as described by Walter and Blobel 1983, Panzner et al. 1995, and Prehn et al. 1990, respectively. Cytosol and membranes from rat or cow liver and cow pancreas were prepared essentially as described (Walter and Blobel 1983), except that the sucrose cushion was omitted. Rabbit reticulocyte lysate was from Promega.
Formation of ER Networks In Vitro
To form membrane networks, 10 μl of cytosol, 0.5–1 μl of the light membranes, and 0.5 μl of an energy regenerating system (1 mM ATP, 0.5 mM GTP, 20 mM creatine phosphate, 0.1 mg/ml creatine kinase) were mixed and incubated at room temperature for the indicated times (10–90 min). Afterwards, membranes were stained by pipetting 1 μl of the reaction mixture into a 2-μl drop of 0.1% (vol/vol) octadecyl rhodamine (Molecular Probes) in buffer A, and observed by fluorescence microscopy using an Axioplan II microscope (Zeiss) equipped with an Orca 12-bit cooled CCD camera (Hamamatsu Photonics). In the basic fusion reaction, cytosol was replaced with buffer A.
In experiments in which the membrane network was allowed to settle onto a glass surface, the reaction mixture was transferred to one well of a 10-well slide (ICN Pharmaceuticals) that was precoated for 5–10 min with 20 μl 5% BSA in buffer A. After incubation for 1 h at room temperature in a humidified chamber, bound membranes were carefully washed with buffer A, stained with 0.1% (vol/vol) octadecyl rhodamine in buffer A, and washed again with buffer A. A Delta Vision microscope system (Applied Precision Instruments) with a Zeiss Axiovert microscope and a PXL CCD camera (Photometrics Ltd.) was used to take images.
Simple flow chambers with a volume of ~10 μl were built with a slide, an 18-mm2 No. 1.5 coverslip, and two strips of double stick Scotch tape as a spacer (Waterman-Storer et al. 1995). Microtubules with an average length of 16 μm were prepared by incubating 3 mg/ml bovine brain tubulin (Cytoskeleton Inc.), 10% glycerol, and 1 mM GTP in BRB80 (80 mM Pipes, pH 6.8, 1 mM MgCl2, 1 mM EGTA) for 30 min at 35°C, and stabilized by the addition of 10 μM taxol. The flow chambers were precoated for 2 min with egg extract that was diluted 1:20 in buffer A (Allan and Vale 1991). Where indicated, 10 μl of 300 μg/ml microtubules was flowed into the chamber and allowed to attach to the glass surface for 5 min. Then, 10 μl egg extract, diluted 1:4 with buffer A, or a mixture of light membranes and cytosol was perfused into the flow chamber. After a 1-h incubation at room temperature in a humidified chamber, 5 μl of 0.1% (vol/vol) octadecyl rhodamine in buffer A was flowed into the chamber and membrane networks were observed by fluorescence microscopy.
Quantitation of network formation was done by counting and averaging the number of three-way junctions in 10 randomly selected fields with a size of 65 × 65 μm2 or 31 × 31 μm2 for networks on 10-well slides or in flow chambers, respectively (Allan 1998).
To confirm that the various inhibitors of microtubule polymerization prevent the formation of microtubules in our system, we visualized microtubules with Oregon green–labeled taxol (10 μM; a gift of Tim Mitchison, Harvard Medical School) in reactions that were preincubated with or without the inhibitors for 15 min on ice.
Colchicine, nocodazole, ionomycin, A23187, thapsigargin, cytochalasin D, and latrunculin A were dissolved in DMSO and added to the reaction mixture so that the final DMSO concentration was 1% (vol/vol) or less. DMSO at this concentration does not affect the in vitro reactions. Vinblastine was dissolved in water. 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) and derivatives were purchased from Molecular Probes.
To deplete ATP, hexokinase (0.2 U/μl) and glucose (50 mM) were added to the reaction mixture and incubated on ice for 5 min. GTPγS (Boehringer Mannheim) was added to the reaction mixture at a concentration of 1 mM. Treatment of cytosol or membranes with N-ethylmaleimide (NEM; 10 mM) was done for 15 min on ice, and unreacted NEM was quenched by the addition of 20 mM DTT. NEM-treated cytosol was used directly and NEM-treated membranes were pelleted and resuspended in buffer A. ATPγS (1 mM) was added to a basic fusion reaction with washed light membranes and buffer A, or to an ER formation reaction with washed light membranes and dialyzed cytosol. In both cases, an energy regenerating system with only 0.1 mM ATP was added, and the reaction mixture was incubated for 30 min on ice before transfer to room temperature. This procedure enhanced the inhibitory effect of ATPγS on ER formation. Proteinase K (2 mg/ml) and CaCl2 (2 mM) were included in the staining solution and thereby added to preformed networks. Proteinase K was gel-filtered into buffer A before use.
Depletion of Tubulin
Tubulin was removed from the cytosol in two different ways. Microtubules with a length of 2 μm were prepared by incubating 5 mg/ml bovine brain tubulin, 1 mM GTP, 30% glycerol in BRB80 for 10 min at 35°C, and then stabilized by the addition of 20 μM taxol and stored at room temperature. 1 μl of the microtubules was added to 100 μl cytosol together with 1 mM GTP/Mg2+, 5 μM taxol, and an energy regenerating system and incubated for 5 min at 25°C. The concentration of taxol was increased to 20 μM, followed by further incubation for 25 min at 25°C. The microtubules were then sedimented for 20 min at 75,000 rpm and 22°C in a 100.3 rotor (Beckman).
The second method to deplete tubulin used vinblastine, which forms aggregates with tubulin that can be separated from the cytosol by centrifugation. 50 μl cytosol was incubated with 100 μM vinblastine for 30 min on ice and then centrifuged as above. Depletion of tubulin from the supernatant was analyzed by quantitative immunoblotting using the DM1A antibody against α-tubulin (Sigma Chemical Co.).
Immunofluorescence Microscopy
For immunofluorescence microscopy, membrane networks were allowed to settle on the glass surface of a coverslip (see above), washed, and fixed with 1% glutaraldehyde in buffer A without β-mercaptoethanol for 15 min. An antibody against translocon-associated protein α (TRAPα) (Gorlich et al. 1990) was used as a marker for the ER and the mAb414 (BAbCO) as a marker for nuclear pores (Davis and Blobel 1986). Rhodamine-coupled concanavalin A (ConA) and Oregon green–coupled WGA (both from Molecular Probes) were used at a concentration of 0.1 mg/ml. Images were taken with the Delta Vision microscope system. To compare the intensity of ConA and WGA staining, the same settings on the microscope system were used.
Fluorescence Microscopy
BHK cells that stably express a fusion of the ER protein Sec61β to the green fluorescent protein were used to analyze the ER in cells. This cell line was generated as described and kindly provided by M. Rolls (Harvard Medical School) and P. Stein (Rolls et al. 1999). For analysis, the cells were fixed with 3% paraformaldehyde, 0.25% glutaraldehyde in PBS for 10 min, and unreacted glutaraldehyde was reduced with 10 mg/ml freshly prepared sodium borohydride for 10 min. Images were taken with the Zeiss microscope system.
Electron Microscopy (EM)
ER formation reactions were carried out in 200-μl reactions, the membrane structures were fixed for 15 min in 2 ml 1% glutaraldehyde in buffer A without sucrose and β-mercaptoethanol, and sedimented for 5 min at 10,000 rpm. The pellet was washed four times with 100 mM Hepes/KOH, pH 7.7, postfixed with 1% OsO4, and prepared for thin sectioning. Alternatively, ER formation reactions were carried out in a humidified chamber on EM grids. The grids were then briefly dipped into buffer A without sucrose and β-mercaptoethanol, fixed for 15 min in 1% glutaraldehyde in buffer A without sucrose and β-mercaptoethanol, washed with 200 mM Hepes/KOH, pH 7.7, and negatively stained with uranyl acetate.
Formation of Nuclei
Sperm chromatin was prepared as described by Murray 1991. Formation of nuclei was done by adding chromatin to an ER formation reaction with unwashed light membranes. Several images from different focal planes of nuclei were captured and deconvolved using the Delta Vision system. When indicated, a three-dimensional image was calculated.
Results
In Vitro Formation of a Membrane Network
To establish an in vitro ER formation system, we fractionated an interphase Xenopus egg extract into cytosol and membrane fractions. The eggs were crushed in a centrifuge at low speed, and the extract was further centrifuged to sediment a heavy membrane fraction. The supernatant was centrifuged again to pellet a light membrane fraction and to obtain cytosol that was almost completely free of residual membranes. When the light membranes were placed on a coverslip and stained with the hydrophobic fluorescent dye octadecyl rhodamine, a relatively homogeneous bright area was seen in the fluorescence microscope (Fig. 1 A). When they were viewed in the electron microscope after thin sectioning, they appeared as vesicles with a diameter of 100–200 nm (Fig. 2 B).
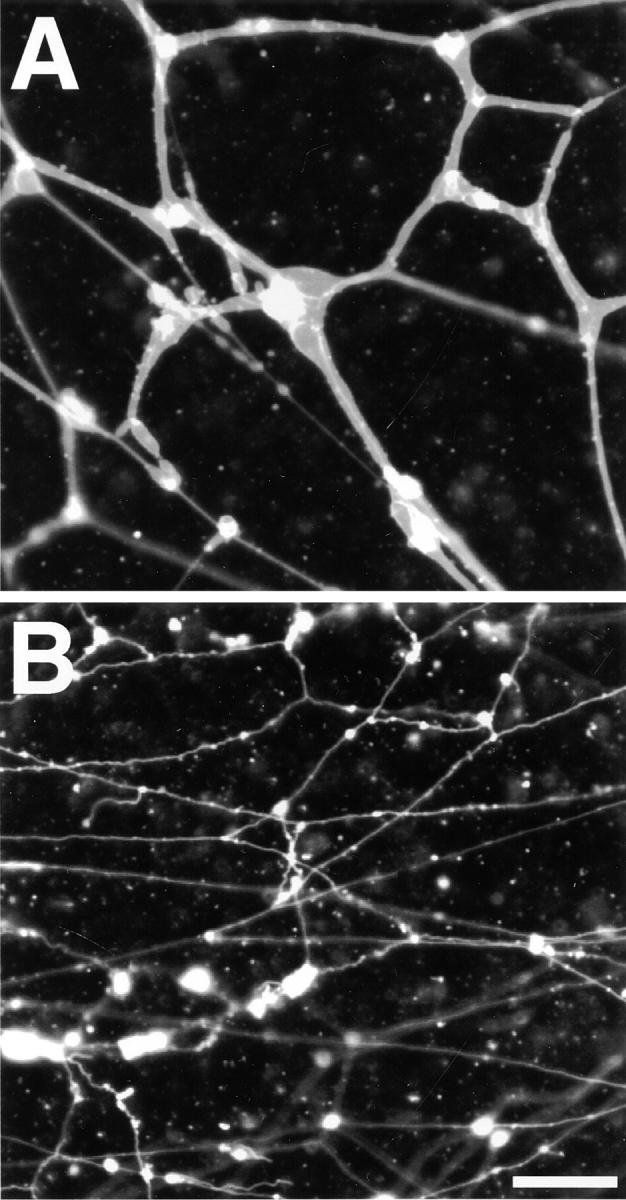
Formation of membrane networks in vitro requires cytosol. (A) The light membrane fraction was analyzed in a fluorescence microscope after staining with a hydrophobic fluorescent dye. (B) Washed light membranes were incubated for 60 min with cytosol and an energy regenerating system and visualized as in A. (C) As in B but without cytosol. (D) Washed light membranes were allowed to attach to the glass surface of a microscope slide and visualized as in A. (E) Washed light membranes were incubated with cytosol for 60 min, and analyzed as in D. (F) As in E but without cytosol. Bars: (A–C) 40 μm; (D–F) 10 μm.
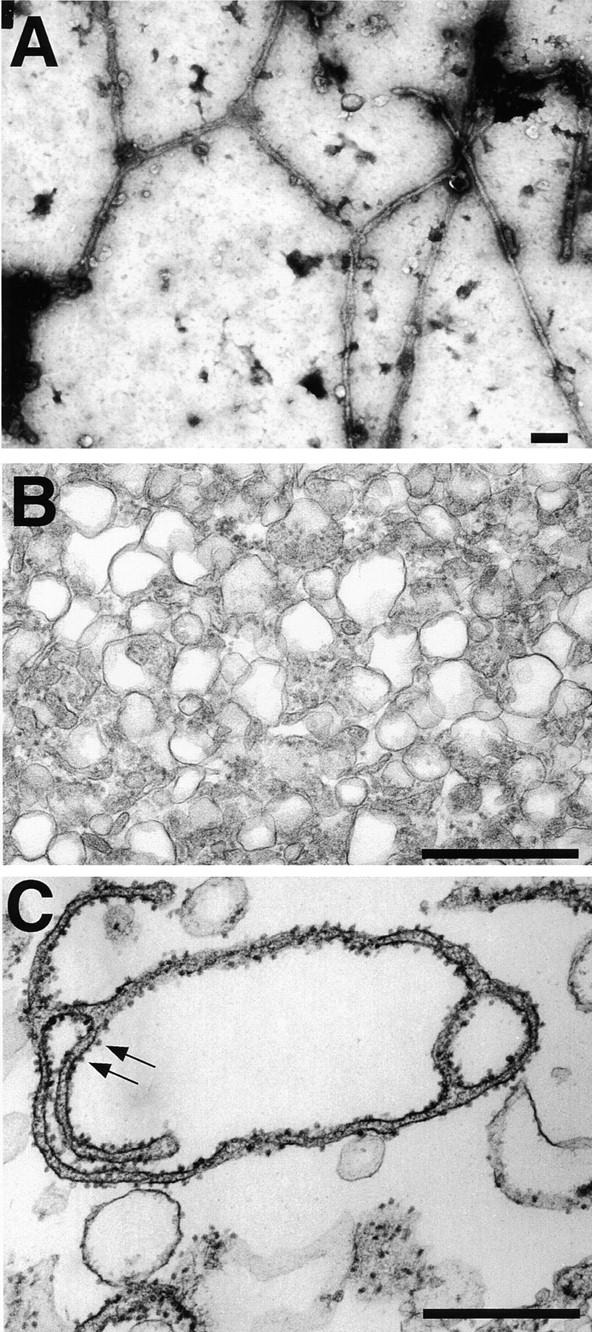
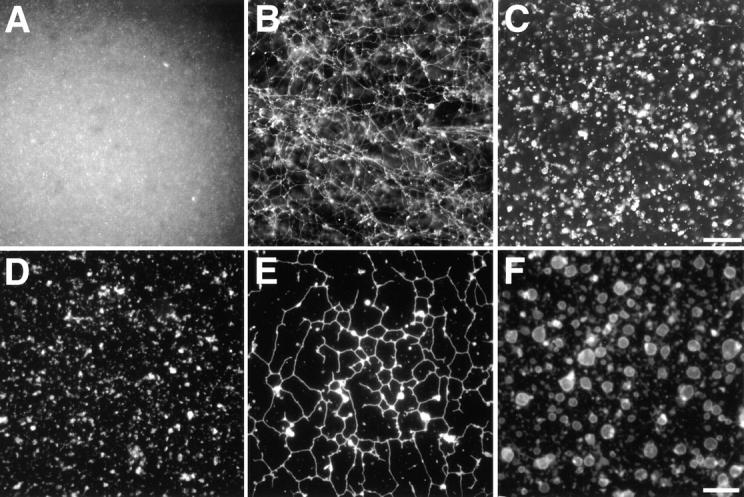
EM analysis of the membrane networks. (A) The membrane network was analyzed by EM after attachment to an EM grid and negative staining with uranyl acetate. (B) The light membrane fraction was analyzed by EM after thin sectioning. (C) The light membrane fraction was incubated for 60 min with cytosol and an energy regenerating system and analyzed as in B. Arrows indicate membrane-bound ribosomes. Bars, 500 nm.
We first developed an assay in which the behavior of membranes could be followed in bulk solution. The light membrane fraction was mixed with cytosol and incubated at room temperature in the presence of ATP, GTP, and an ATP-regenerating system. Then, octadecyl rhodamine was added and the stained membranes were viewed with a fluorescence microscope. An elaborate network of interconnected membrane tubules was seen (Fig. 1 B). The network could also be stained with another hydrophobic fluorescent dye (3,3′-dihexyloxacarbocyanine iodide) and was sensitive to detergents. It was not seen if the membranes were omitted, if energy was depleted, or if the reaction mixture was kept on ice (data not shown). The network was very delicate; it was disrupted by repeated pipetting up and down or by centrifugation (data not shown).
When the light membrane fraction was extensively washed with buffer and incubated in the absence of cytosol, the membranes fused to form large vesicles rather than a tubular network (Fig. 1 C). This process was also temperature- and energy-dependent (data not shown), and will be referred to as the basic fusion reaction. Washing of the membranes with buffers containing high salt concentrations did not abolish their basic fusion activity (data not shown), indicating that the fusion machinery is tightly bound to the membranes. Small amounts of cytosol, e.g., the residual cytosol after washing the light membranes only once with buffer, were sufficient to obtain at least some network formation. Cytosol had to be present during the fusion reaction for networks to be formed; it was without effect if added to the large vesicles that had been formed in the basic fusion reaction (data not shown).
To better visualize the different membrane structures, we allowed the membranes in the reaction mixture to settle onto the glass surface of a 10-well microscope slide. After washing away unbound membranes, the attached structures were stained with octadecyl rhodamine and visualized with a fluorescence microscope. The network that formed when membranes and cytosol were present was seen as a polygonal structure with tubules several micrometers long connected by three-way junctions (Fig. 1 E). A very similar network was seen with an unfractionated egg extract after settling onto a glass surface, even though in bulk solution it was difficult to visualize, possibly because of the presence of other stained membranes that did not form networks (data not shown). Only small membrane structures, likely small vesicles, were seen when the membranes were allowed to settle onto a glass surface without having been incubated at elevated temperature (Fig. 1 D). When membranes were incubated in the absence of cytosol and allowed to settle onto a glass surface, large spherical vesicles with a diameter of up to a few micrometers were seen (Fig. 1 F), in agreement with the results obtained in bulk solution (Fig. 1 C).
The network was also examined by EM. When allowed to settle onto an electron microscope grid and negatively stained, the membrane tubules had a diameter of ~100 nm (Fig. 2 A). The distance between the two membrane surfaces is about the same as observed in previous in vitro experiments and in ER membranes of mammalian cells (Dabora and Sheetz 1988; Alberts et al. 1994; Allan and Vale 1994). Given the size of the original vesicles, a 10-μm tubule must have been generated by the fusion of >100 membrane vesicles. Similarly, spheres with a diameter of 2 μm must have been generated by fusion of at least 100 membrane vesicles. Since negative staining did not allow us to visualize details of the tubular network, we also used thin sectioning EM. Although this technique did not preserve large areas of the network, tubular structures could be seen when the incubation was carried out at room temperature, but not when performed on ice (Fig. 2C vs. B). The tubules were studded with ribosomes, suggesting that they represent rough ER (Fig. 2 C). Cryo-EM confirmed that the tubules have membrane-bound ribosomes and indicated that they do not contain any obvious coat structure (data not shown).
The Network Contains ER Membranes
To further test whether the network is formed from ER membranes, we determined if it contained ER proteins. Immunofluorescence indicated that the network contains the rough ER protein TRAPα (Fig. 3 A) (Gorlich et al. 1990; Vogel et al. 1990). When the antibody was presaturated with the peptide it was raised against, this fluorescence was eliminated (Fig. 3 B). Staining was also observed with fluorescently labeled ConA, a lectin that binds carbohydrate chains on ER proteins (Fig. 3 C). In contrast, WGA, a lectin that binds to glycoproteins of the Golgi apparatus and endosomes, did not stain the network (Fig. 3 D; see also Allan 1995). The mAb mAb414, which recognizes several nuclear pore proteins, also did not bind to the network (Fig. 3F vs. E), although in control reactions, mAb414 antibodies stained the nuclear envelope of nuclei formed in Xenopus egg extracts in the presence of sperm chromatin (Fig. 3 F, inset; Macaulay and Forbes 1996). Together with the EM results, these data show that the network is formed from ER membranes and contains little or no Golgi or nuclear pore proteins.
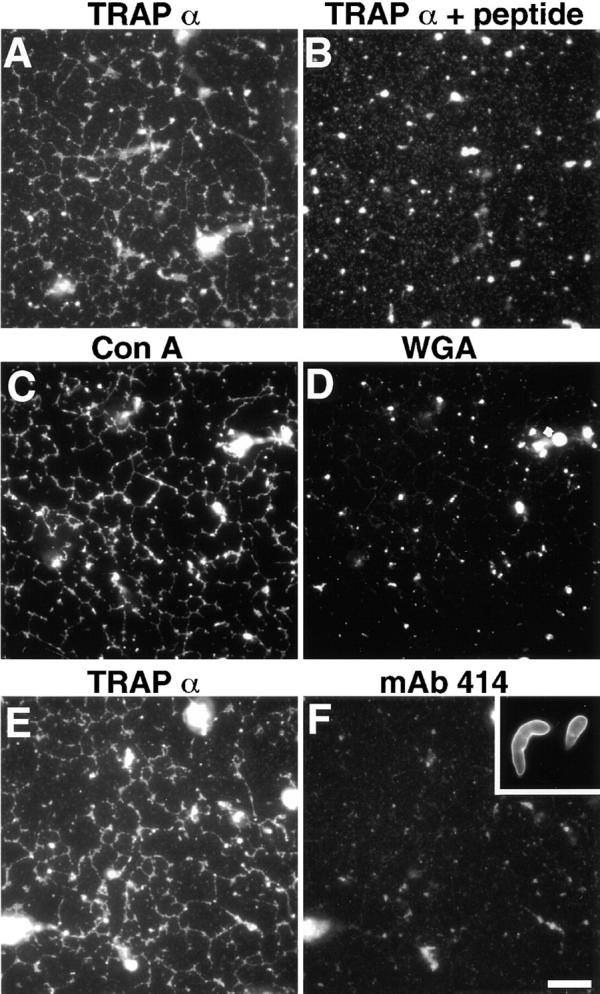
The network contains ER membranes. Membrane networks were formed and attached to the glass surface of a microscope slide. (A) After fixation, the network was incubated with an antibody against the ER marker protein TRAPα, followed by a fluorescently labeled secondary antibody. (B) The anti-TRAPα antibody was preincubated with the peptide against which it was raised and used as in A. (C and D) A membrane network was labeled with both rhodamine-labeled ConA and Oregon green–labeled WGA and analyzed with rhodamine (C) and fluorescein (D) channels. (E and F) A membrane network was labeled with both the anti-TRAPα antibody and the nuclear pore antibody mAb414. The inset in F shows staining with mAb414 of the nuclear envelope of in vitro assembled nuclei. Bar, 10 μm.
Formation of the Network Does Not Require Microtubules or Actin Filaments
Next, we tested whether cytoskeletal elements are required for ER network formation. Various inhibitors of microtubule polymerization were added to our in vitro reaction containing membranes and cytosol. After incubation on ice for 15 min to promote microtubule depolymerization, the mixture was further incubated at room temperature. Nocodazole (up to 300 μM), colchicine (up to 200 μM), or vinblastine (up to 200 μM) did not inhibit formation of the network in bulk solution or on a glass surface (Fig. 4A and Fig. B; data not shown). Identical results were obtained when unfractionated egg extract was used (Fig. 5 A). Quantitation of network formation by counting the number of three-way junctions in a given area confirmed that the drugs have no inhibitory effect (Fig. 5 A). Experiments with colchicine demonstrated that the time course of network formation did not change either (Fig. 5 B). The highest concentrations of microtubule inhibitors in our assay were much higher than commonly used to prevent the formation of microtubules. Indeed, upon addition of colchicine, no microtubules could be seen when labeling was performed with fluorescently labeled taxol (Fig. 5 D; control shown in Fig. 5 C). Similar results were obtained with the other drugs (data not shown).

Formation of the ER network does not require microtubules or actin filaments. ER formation was carried out with light membranes and cytosol in the presence of 200 μM nocodazole (A), 100 μM colchicine (B), tubulin-depleted cytosol (C), tubulin-depleted cytosol plus 200 μM vinblastine (D), or 200 μM latrunculin A (E). All pictures show surface-attached membrane networks. Bar, 10 μm.
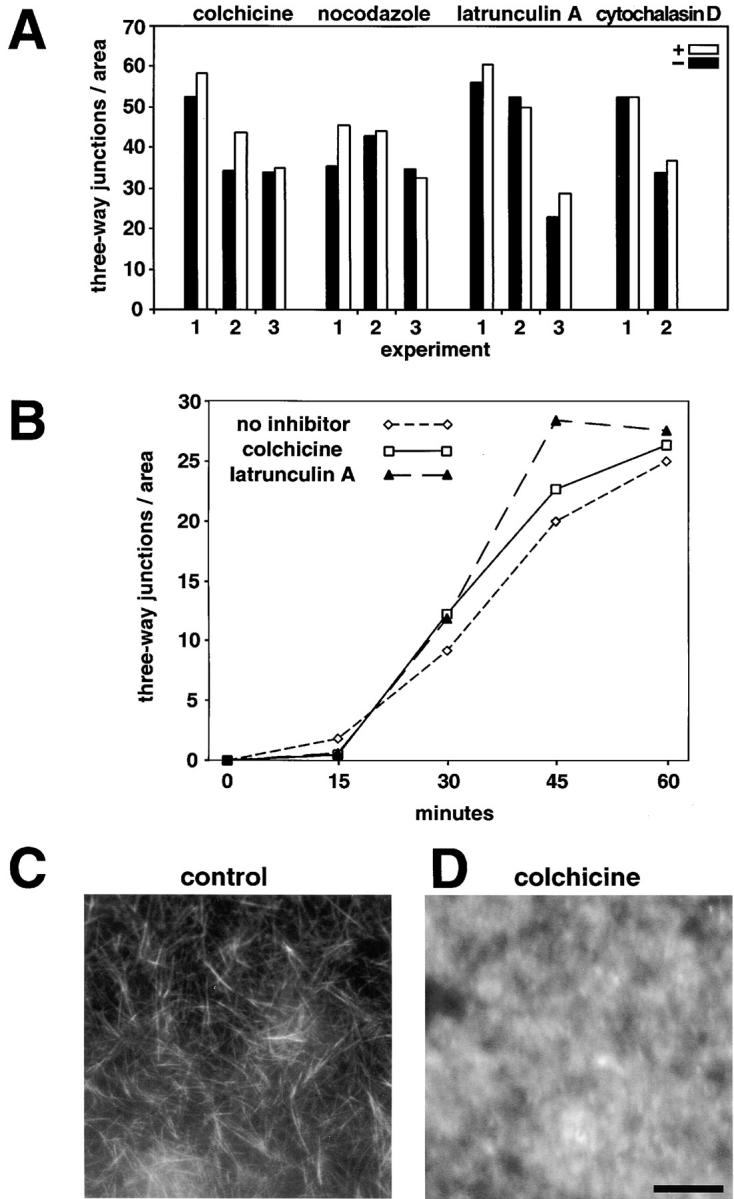
Quantitation of ER network formation in the absence of microtubules or actin filaments. (A) ER network formation was carried out for 1 h with egg extracts in the presence (black columns) or absence (grey columns) of the indicated inhibitors. Quantitation was done by counting and averaging the number of three-way junctions of surface-attached membrane networks from 10 fields (65 × 65 μm2). The numbers below the bars indicate different experiments. (B) The time course of network formation was determined in the presence or absence of the indicated inhibitors. (C) Microtubules formed in egg extract were visualized with fluorescently labeled taxol. (D) Egg extract was pretreated with 100 μM colchicine before the addition of fluorescently labeled taxol. Bar, 20 μm.
To provide further evidence that microtubules are not required for ER network formation, tubulin was depleted from the cytosol. Although the membranes contain a small amount of tubulin that is not removed by salt washing, the cytosol contributes >95% of the total tubulin in the reaction mixture (data not shown). In one set of experiments, we added short microtubules as seeds for microtubule polymerization and taxol to stabilize the microtubules, and sedimented the polymerized microtubules. Quantitative immunoblotting with an antibody to α-tubulin showed that 90–95% of the protein was removed from the cytosol (data not shown). When the depleted cytosol was mixed with washed membranes, the network was formed as efficiently as in controls (Fig. 4 C). Even when vinblastine was added to this reaction to prevent polymerization of the residual tubulin, there was no sign of a reduction in network formation (Fig. 4 D). Similar results were obtained when tubulin was removed from the cytosol by the addition of vinblastine; this drug leads to the formation of large complexes with tubulin that can be removed by centrifugation (Na and Timasheff 1982), resulting in tubulin depletion by >95% (data not shown).
Actin filaments also did not seem to be required for the formation of the ER network. Neither latrunculin A nor cytochalasin D had any detectable effect, even when used at very high concentrations (Fig. 4 E and Fig. 5 A). Quantitation of the number of three-way junctions demonstrated that neither the extent nor the kinetics of network formation were altered (Fig. 5A and Fig. B).
Previously, it had been suggested that microtubules are required for the tubular ER network formation. To reconcile this discrepancy, we performed experiments in a similar way as described in previous studies. A simple flow chamber consisting of a closely spaced microscope slide and cover glass was precoated with taxol-stabilized microtubules, and an unfractionated egg extract was flowed into the chamber. As observed by others before, when microtubules were present, a dense membrane network was seen attached to the glass surface, in contrast to the situation without microtubules (Fig. 6 A). When the nonattached membranes were recovered from the flow chamber, no difference in the extent of network formation was detected with and without microtubules (data not shown). Interestingly, when the isolated light membrane and cytosol fractions were mixed and analyzed in an analogous manner, microtubules had only little effect on the extent of the network attached to the glass surface (Fig. 6 B). These results indicate that microtubules do not affect network formation per se, but may rather stimulate the attachment of membranes from unfractionated extracts onto a glass surface.

ER network formation in the presence of microtubules. (A) ER formation was carried out for 1 h with egg extracts in flow chambers that were either precoated with microtubules (grey columns) or had no microtubules (black columns). When no microtubules were present, nocodazole was also added to the extract. Quantitation was done by counting and averaging the number of three-way junctions from 10 fields (31 × 31 μm2). The number of three-way junctions is given per 65 × 65 μm2 as in Fig. 5. (B) ER formation was carried out and quantitated as in A, but with a mixture of light membranes and cytosol instead of an unfractionated extract.
Taken together, these results lead to the surprising conclusion that an elaborate network of ER membranes can form without a cytoskeletal scaffold.
Protein Factors Are Required for Generation and Maintenance of the Network
To begin to analyze the mechanism responsible for network formation, we first asked whether the membrane and cytosol fractions could be replaced with material from other sources. Among the membranes tested, only the light membrane fraction from Xenopus eggs gave networks (Table ). These data indicate that the Xenopus egg membranes are specifically primed for network formation, consistent with their behavior in vivo during early development. In contrast, the cytosol from Xenopus eggs could be replaced with that from bovine liver, bovine pancreas, rat liver, or rabbit reticulocyte lysate; wheat germ extract and yeast cytosol were inactive (Table ). Thus, the cytosolic factor(s) appear to be common.
Table 1
ER Network Formation with Different Membranes and Cytosols
| Cytosol | Membranes | |||
|---|---|---|---|---|
| X. laevis egg | Dog pancreas | Rat liver | S. cerevisiae | |
| X. laevis egg | ++ | − | − | − |
| Cow pancreas | + | − | nd | nd |
| Cow liver | + | nd | nd | nd |
| Rat liver | + | − | − | nd |
| Rabbit reticulocyte | + | nd | nd | nd |
| Wheat germ | − | nd | nd | nd |
| S. cerevisiae | − | − | nd | − |
ER formation was carried out with the indicated combinations of membranes and cytosol. In each case, the ratio of membranes and cytosol was varied to determine the optimum. The extent of network formation is given as very efficient (++), good (+), or not at all (−). nd, not determined.
At least one of the cytosolic factors required for network formation is a protein. When the cytosol was dialyzed or gel-filtered, activity was found in the high molecular weight fraction. In addition, heat treatment of this material (5 min at 95°C) led to a drastic reduction of the activity (data not shown).
Proteins are not only required for the formation of the network, but also for its maintenance. When proteinase K was added to preformed ER networks, the diameter of the tubules dramatically increased up to a few micrometers (Fig. 7A vs. B), indicating that protease-sensitive components are required to maintain the narrow diameter of the membrane tubules.
Network Formation Requires Energy and Is Sensitive to Fusion Inhibitors
In other systems, steps preceding the actual membrane fusion reaction can be inhibited by the sulfhydryl-modifying reagent NEM and the poorly hydrolyzable GTP analogue GTPγS (Denesvre and Malhotra 1996; Novick and Zerial 1997). Therefore, we tested these reagents in our in vitro system. If the membranes were preincubated with NEM, they became inactive both in the basic fusion reaction in the absence of cytosol (data not shown), and in the network formation in its presence (Fig. 8 A). Similarly, GTPγS inhibited both the basic fusion reaction (data not shown) and network formation (Fig. 8 B). Treatment of the cytosol with NEM had no effect on network formation (data not shown). These data confirm that the machinery leading to fusion is located on the membranes and suggest that some steps in the reactions with and without cytosol are similar.

ER network formation is inhibited by NEM, GTPγS, or ATPγS. (A) The light membrane fraction was pretreated with 10 mM NEM, and after removal of unreacted NEM, used in an ER formation reaction with cytosol. (B) GTPγS was added at a concentration of 1 mM to a reaction with light membranes and cytosol. (C) ATPγS was added at a concentration of 1 mM to a basic fusion reaction with washed light membranes and buffer. (D) Basic fusion reaction without ATPγS. (E) ATPγS was added at a concentration of 1 mM to an ER network formation reaction with washed membranes and cytosol. (F) ER formation reaction without ATPγS. All pictures show membranes in bulk solution. Bar, 20 μm.
Energy is required for both the basic fusion reaction in the absence of cytosol and for network formation. Addition of an energy regenerating system with ATP and GTP gave optimal results for the network formation, whereas the basic fusion reaction was almost as efficient with 1 mM GTP alone as with a complete energy regenerating system (data not shown). Although we cannot exclude that ATP is also required because some GTP could have been converted into ATP, the poorly hydrolyzable analogue ATPγS did not inhibit the basic fusion reaction (Fig. 8C and Fig. D). In contrast, network formation in the presence of cytosol required ATP and was inhibited by ATPγS (Fig. 8E and Fig. F). Interestingly, ATPγS blocked network formation at a stage distinct from that seen in the presence of GTPγS. While in the presence of GTPγS, the membranes appeared as a homogeneously stained area, essentially like at the beginning of the reaction, with ATPγS the vesicles appeared to cluster (Fig. 8A vs. E). Other ATP analogues (AMP-PNP and AMP-PCP) had similar, but weaker effects (data not shown). Taken together, these results suggest that network formation includes an ATP-dependent step that is not required for the basic fusion reaction.
An Intermediate Stage in Network Formation
The apparent clustering of vesicles in the presence of ATPγS raised the possibility that this may represent an intermediate stage in network formation, similar to the intermediates of docked or tethered membrane vesicles described for other fusion reactions (Ungermann et al. 1998; Christoforidis et al. 1999). Indeed, when the network formation reaction was viewed at early timepoints, the vesicles also appeared to be clustered. In a time course experiment, the clusters were first seen after 5–10 min of incubation (Fig. 9). With time, most of the clusters disappeared, and membrane tubules appeared that eventually formed a large network (Fig. 9). These data suggest that vesicle clustering is an intermediate stage, reached in an energy-requiring and NEM- and GTPγS-sensitive reaction, and that further progression towards a network requires an ATPγS-inhibitable step.
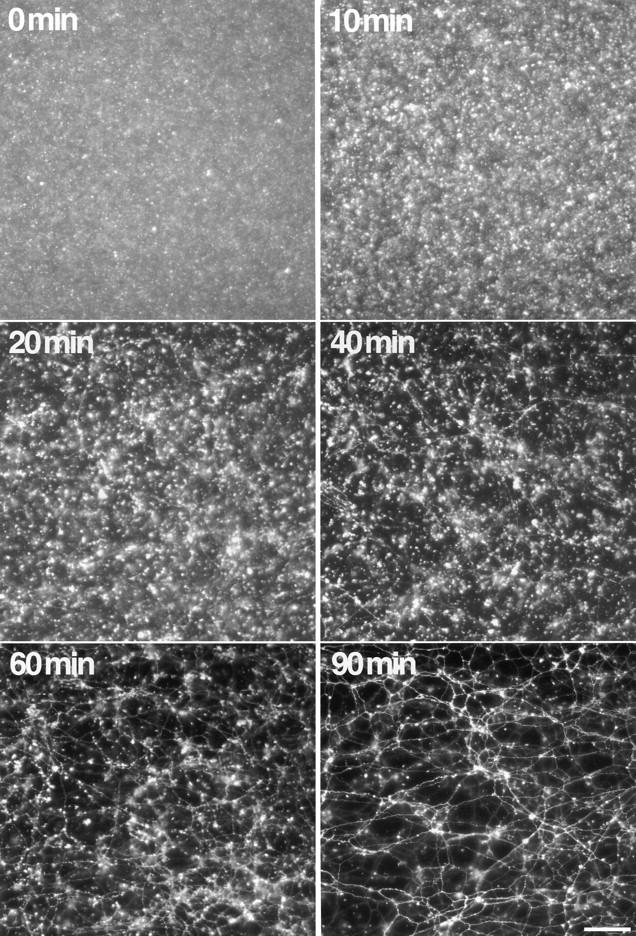
Time course of ER network formation. An ER formation reaction with light membranes and cytosol was incubated for the indicated time periods and analyzed in solution. Note the early appearance of small aggregated membrane structures (10 min), followed by the formation of membrane tubules (20–60 min) that eventually form a dense network (90 min). Bar, 40 μm.
The Network Is Sensitive to High Cytosolic Ca2+ Concentrations
The ER network in mammalian cells has been reported to disassemble in the presence of high cytosolic Ca2+ concentrations (Koch et al. 1988; Subramanian and Meyer 1997). We confirmed these observations with BHK cells, which stably express a fusion of the ER protein Sec61β to the green fluorescent protein. The ER network disintegrated and formed round vesicles when treated with the Ca2+ ionophores ionomycin or A23187 in the presence of high extracellular Ca2+ concentrations (Fig. 10A vs. B; data not shown). The ER network formed in vitro behaved similarly. When Ca2+ was added to a preformed network, the membrane tubules disintegrated, were dilated, or converted into vesicles that were often still attached to each other (Fig. 10C vs. D). Although the Ca2+ concentrations required (1–2 mM) were much higher than those normally occurring in the cytosol, the similarity of the effects argues that the in vitro system reflects the behavior of the ER in vivo.

The ER network is sensitive to high cytosolic Ca2+ concentrations. (A) A BHK cell line that stably expresses a fusion of the ER protein Sec61β to the green fluorescent protein was treated with 10 μM of the Ca2+ ionophore ionomycin for 10 min at 37°C. (B) Untreated cells are shown. (C) 2 mM Ca2+ was added with the staining solution to membrane networks preformed in vitro. Images were taken in bulk solution after 15 min. Arrows indicate large membrane vesicles. (D) A control without the addition of Ca2+ is shown. Bars: (A and B) 20 μm; (C and D) 40 μm.
High cytosolic Ca2+ concentrations also inhibited the de novo formation of the network. At 200 μM Ca2+, only a few tubules were seen and instead membrane clusters formed. After longer incubations (1.5 h), larger vesicles were also seen (Fig. 11A and Fig. B). Similar effects were observed when Ca2+ was released from the interior of the vesicles by the addition of ionomycin or A23187 (Fig. 11 C; data not shown). The inhibitory effect of ionomycin could be reversed by the addition of the Ca2+ chelator EGTA (Fig. 11 D), indicating that ionomycin was indeed acting by increasing the cytosolic Ca2+ concentration. Similarly, inhibition of the Ca2+ pumps in the membrane vesicles by thapsigargin (25 μM), which is also expected to increase the cytosolic Ca2+ concentration, inhibited network formation and caused the appearance of membrane clusters (data not shown). It should be noted that neither the addition of high Ca2+ concentrations nor of Ca2+ ionophores or Ca2+ pump inhibitors prevented the formation of large vesicles in the absence of cytosol (data not shown). Thus, ER network formation, but not the basic fusion reaction, requires a Ca2+-sensitive step.
Similarities between Network and Nuclear Envelope Formation
Both the unfractionated Xenopus egg extract and a mixture of membranes and cytosol prepared from it are capable of forming nuclei when chromatin or DNA are present (Lohka and Masui 1983; Newport 1987). When sperm chromatin was added to a mixture of light membranes and cytosol, small nuclei with a cylindrical shape and a continuous inner and outer nuclear membrane were formed (Fig. 12 D). Thus, the same fractions that give rise to ER networks form nuclear envelopes when chromatin is added. However, the formation of large round nuclei requires the additional presence of a heavy membrane fraction (Vigers and Lohka 1991; Drummond et al. 1999; and data not shown).
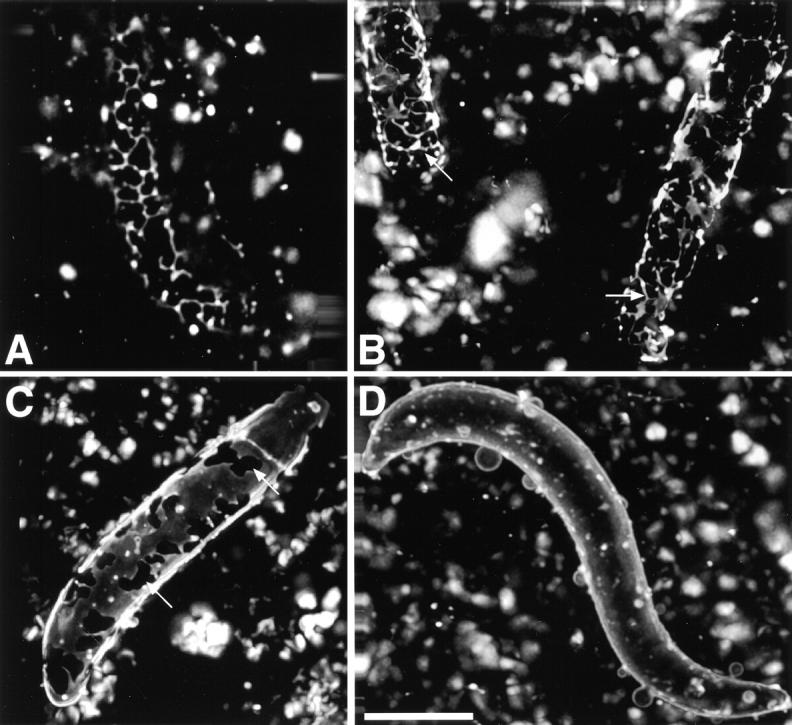
Different stages of nuclear envelope formation. Xenopus sperm was added to a mixture of light membranes and cytosol and the formation of a nuclear envelope around the chromatin was followed over time after staining with a hydrophobic, fluorescent dye with a fluorescence microscope (A and B, 10 min; C, 30 min; D, 60 min). In B–D, images from several focal planes were taken, deconvolved, and a three-dimensional picture was calculated. Note the network of membrane tubules and tubules that connect small patches of membranes on the chromatin surface after short incubations (A and arrows in B). Arrows in C point to areas on the chromatin surface that are not yet covered by the nuclear envelope. D shows a completely enclosed nuclear envelope. Bar, 10 μm.
When the formation of the nuclear envelope was followed over time, at early timepoints a network of tubular structures was seen on the chromatin surface, similar to the tubules in ER networks, as well as small flat patches (Fig. 12A and Fig. B), which have been described before (Wiese et al. 1997). At later timepoints, the chromatin-bound membrane patches became larger and eventually completely enclosed the chromatin (Fig. 12C and Fig. D).
Other data also indicate similarities between ER network and nuclear envelope formation. In agreement with results in the literature (Boman et al. 1992; Newport and Dunphy 1992), GTPγS or treatment of nuclear membranes with NEM inhibited the fusion of chromatin-bound vesicles, whereas treatment of the cytosol with NEM did not (data not shown). As in the ER network formation system, high cytosolic Ca2+ concentrations are inhibitory, although in this case, the effect may be due to changes of the chromatin (Sullivan et al. 1993; Marshall et al. 1997).
Further similarities are borne out by experiments with Ca2+ chelators. Nuclear envelope formation was maximally inhibited by chelators with a binding constant of ~1 μM, and less by chelators with higher or lower binding constants (Sullivan et al. 1993). Similarly, in the ER formation system, 5,5′-dibromo BAPTA, a Ca2+ chelator with a binding constant of ~1 μM (Sullivan et al. 1993), had the maximal inhibitory effect on ER network formation. At a concentration of 2 mM, only a few tubules were seen after 45 min, and instead abundant clusters of membranes were formed (Fig. 13 A). Even after 90 min, the network was only partially formed (Fig. 13 B). As in the nuclei formation system, BAPTA, which has a higher affinity for Ca2+, had a weaker effect. After 45 min the network was less dense and membrane clusters were seen, but after 90 min the network looked normal (Fig. 13C and Fig. D). 5-nitro BAPTA (binding constant ~20 μM) and 5,5′-dimethyl BAPTA (binding constant ~40 nM) had effects similar to BAPTA, with 5,5′-dimethyl BAPTA being less inhibitory than 5-nitro BAPTA. As noted before (Fig. 11 D), EGTA (binding constant ~120 nM) had only a marginal inhibitory effect.
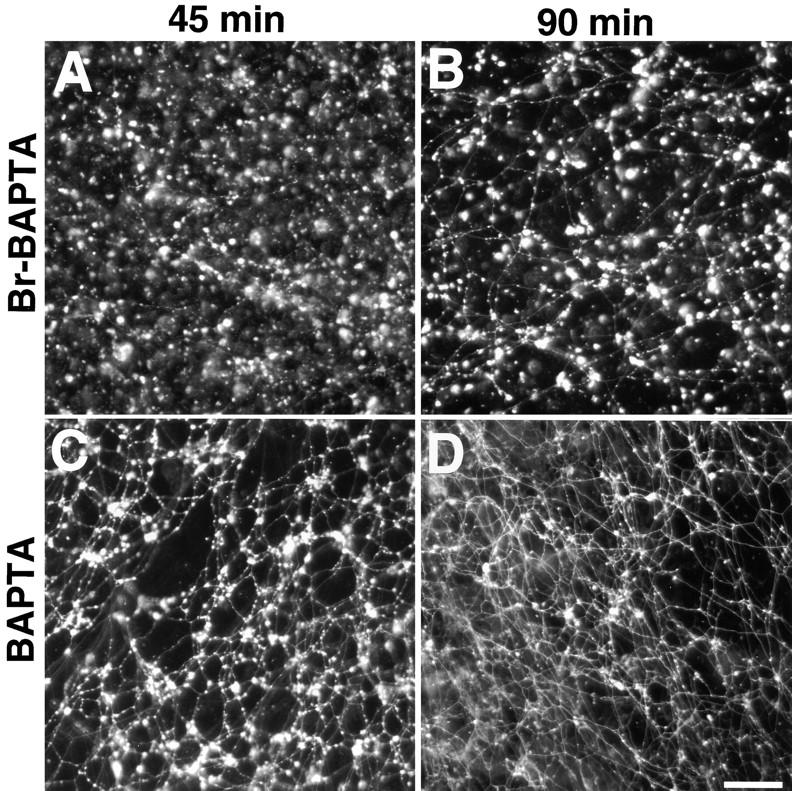
Effects of Ca2+ chelators on ER network formation. 2 mM 5,5′-dibromo BAPTA (A and B) or 2 mM BAPTA (C and D) were added to an ER formation reaction and incubated for 45 or 90 min. Bar, 40 μm.
Finally, both ER network and nuclear envelope formation only occur in interphase extracts. Mitotic egg extracts were generated by the addition of a cyclin B1 mutant protein that is made nondegradable by deletion of its destruction box (cyclin B1 Δ90); this leads to constitutive activation of the mitotic CDC2 kinase (Murray et al. 1989). These extracts formed much fewer ER networks than interphase extracts, as determined by the number of three-way junctions per given area (Fig. 14). The mitotic state of the extract was confirmed by the increase of the histone H1 kinase activity by a factor of 20–30 compared with an interphase extract, and by the fact that no nuclei were formed when chromatin was added (data not shown). Taken together, these results suggest that the ER and nuclear envelope form in a related process.
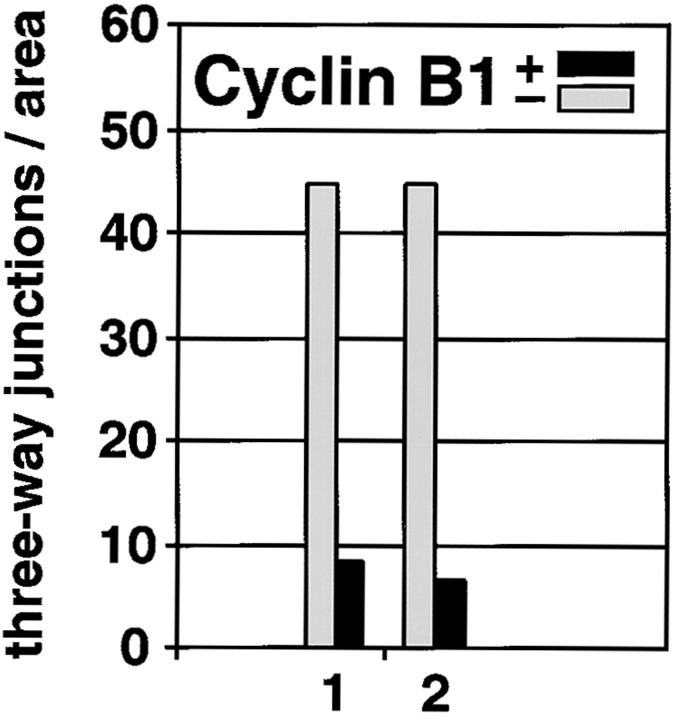
ER network formation is strongly reduced in mitotic extracts. Formation of the ER network in interphase extract (− cyclin B1, gray columns) and in a mitotic extract, obtained by addition of nondegradable cyclin B1 Δ90 (+ cyclin B1, black columns), was quantitated by counting the number of three-way junctions as in Fig. 5 A. Shown are results from two experiments.
Discussion
We have established a system from Xenopus eggs in which the reticular network of the ER is formed in vitro. Our data lead to the surprising conclusion that an ER network can form independently of microtubules or actin filaments. Previously, a direct role for microtubules in the formation of the ER network was proposed based on studies of the interaction between the ER and microtubules in cells and in vitro (Dabora and Sheetz 1988; Lee and Chen 1988; Allan and Vale 1991; Waterman-Storer et al. 1995). The design of previous in vitro assays allowed the observation of only a subset of membrane tubules that are attached or close to the glass surface of a flow chamber. With an unfractionated Xenopus egg extract, we could reproduce the stimulatory effect of surface-bound microtubules on the formation of attached membrane networks in flow chambers, but our data also indicated that microtubules have no effect in bulk solution or in a fractionated system. Thus, the tethering of membrane tubules to the glass surface, but not their generation per se, appears to be stimulated by the microtubules. From the data in the literature it is clear that microtubules can serve to pull membrane tubules out of surface-attached membranes. Whether both microtubule-dependent and -independent mechanisms operate in vivo for the de novo formation of the tubular ER network is unclear, but our data show that an elaborate membrane network can form without microtubules.
Although not essential for the de novo formation, the cytoskeleton appears to play an important role in distributing the ER network throughout the cell (Terasaki et al. 1986; Waterman-Storer and Salmon 1998). In mammalian cells, attachment of membranes to microtubules or motors may be required to move the ER network into the periphery of the cell, explaining why membrane tubules are often aligned with microtubules. In plant or yeast cells, actin filaments may play an analogous role (Terasaki 1990). Golgi membrane tubules and networks have been reported to form de novo by both microtubule-dependent and -independent mechanisms, although it is unclear if and how they act together (Cooper et al. 1990; Allan and Vale 1991; Cluett et al. 1993; de Figueiredo et al. 1998; Fullerton et al. 1998). In any case, microtubules are required to localize the Golgi apparatus in the cell (Rogalski and Singer 1984).
If the ER membrane network is not formed along a cytoskeletal scaffold, how is it generated? An important hint to the answer is that, in the absence of cytosol, the membranes can fuse, but form large spherical vesicles rather than networks. Furthermore, cytosol has to be present during the fusion reaction to mediate tubular network formation. When cytosol is added to the large vesicles formed in a basic fusion reaction, networks are not formed. Since spheres are possibly the thermodynamically most stable result of fusion, one or more cytosolic factors could modify this default reaction to convert it into a fusion reaction that results in networks. At least one of the cytosolic factors is a protein. Both network formation and the basic fusion reaction could be inhibited by GTPγS and by treatment of the membranes with NEM, suggesting that a similar fusion machinery is used. NEM and GTPγS are inhibitors of steps that in other systems precede the actual fusion reaction. Known targets are the NEM-sensitive fusion protein (NSF) (or related proteins, such as p97) and rab proteins (Rothman 1994; Denesvre and Malhotra 1996; Novick and Zerial 1997), but we were unable to demonstrate that these proteins play a role in our system. The activity of NEM-treated membranes could not be restored by the addition of mammalian NSF or Xenopus p97, and the addition of a dominant-negative NSF protein (Colombo et al. 1996) or the extraction of the membranes with mammalian GTP dissociation inhibitor had no effect (data not shown).
Network formation may initially proceed similarly to the basic fusion reaction, but must then diverge. Our data show that during network formation the vesicles first form cluster-like structures in a reaction that can be inhibited by NEM and GTPγS. This may correspond to the tethering or docking of membrane vesicles that have been described as early steps in other fusion reactions (Ungermann et al. 1998; Christoforidis et al. 1999; Pfeffer 1999). To proceed with network formation, an ATPγS-sensitive step is required. High cytosolic Ca2+ concentrations appear to interfere at this step as well, explaining why they do not affect the basic fusion reaction. Our data with various Ca2+ chelators also suggest that Ca2+ fluctuations of ~1 μM may be important for network formation. A chelator with a binding constant in this range had a stronger inhibitory effect than chelators with lower or higher binding constants. In other systems, similar observations were explained by the fact that cytosolic Ca2+ gradients can be most effectively dispersed by chelators with a binding constant in the range of the average Ca2+ concentration in the gradient (Speksnijder et al. 1989; Sullivan et al. 1993). It is thus tempting to speculate that a Ca2+-dependent step regulates the fusion event that leads to network formation. A regulatory role for Ca2+ has been established in many other fusion reactions, such as in controlling the fusion of secretory vesicles and of vacuoles (Rothman 1994; Goda and Sudhof 1997; Peters and Mayer 1998). Once established, the ER network still requires a protein to maintain the narrow diameter of the membrane tubules and is sensitive to high cytosolic Ca2+ concentrations both in vivo and in vitro.
The exact mechanism by which a tubular membrane network is generated without a cytoskeleton remains mysterious. Several models of how the shape of membranes can be determined have been described. A number of mechanisms are based on changes in the preferred curvature of the lipid bilayer (Sheetz and Singer 1974; Mui et al. 1995; Chou et al. 1997). If small vesicles maintain their high curvature while they fuse, a sphere cannot be formed and membrane tubules may result instead. Alternatively, a higher curvature may be generated in vesicles after their fusion. Interestingly, the formation of membrane tubules from Golgi stacks, both in vivo and in vitro, is sensitive to inhibitors of phospholipase A2 (de Figueiredo et al. 1998, de Figueiredo et al. 1999). Phospholipase A2 could be directly involved in the formation of membrane tubules by locally generating cone-shaped lyso-phospholipids in the outer leaflet (by cleaving off the C2 fatty acid from phospholipids), which in turn could increase the curvature of the lipid bilayer and thereby induce tubule formation. Enzymatically induced changes in bilayer curvature have also been proposed for the budding of endocytic vesicles and for the vesiculation of Golgi membranes (Schmidt et al. 1999; Weigert et al. 1999). Another mechanism could involve the binding of large protein assemblies to the membrane, which would impose their structure onto the membrane, similar to a role of clathrin and the coat protein complexes COP I and II in endocytosis and vesicle budding (Schekman and Orci 1996). Compelling examples for such a mechanism come from experiments in which dynamin and amphiphysin, proteins involved in endocytosis, were added alone or together to liposomes and shown to induce the formation of membrane tubules with characteristic diameters and protein coat structures (Sweitzer and Hinshaw 1998; Takei et al. 1998, Takei et al. 1999; Stowell et al. 1999). The dilation of the narrow membrane tubules by proteinase K could be readily explained by the disruption of such a protein coat, but we have not been able to visualize a coat by EM. Yet another mechanism would be analogous to that proposed for the fenestration of Golgi membranes (Rothman and Warren 1994); the fusion of small vesicles by a machinery on their cytosolic face may be followed by a periplasmic fusion process caused by a machinery located on the lumenal face of the membrane, resulting in ring structures or tubules. Whatever the mechanism by which tubules are generated, the formation of three-way junctions may require additional fusion events between them.
Consistent with the fact that the outer nuclear membrane and the ER form a continuous membrane, our results indicate that the in vitro formation of the two structures shares similarities. Both processes were inhibited by NEM, GTPγS, and high Ca2+ concentrations, and were affected in a similar manner by the various Ca2+ chelators. Our data also suggest that the nuclear envelope forms via ER-like tubular intermediates bound to chromatin, similar to observations in mammalian cells (J. Ellenberg, personal communication). The formation of chromatin-bound membrane sheets and of an intact nuclear envelope require cytosolic factors, and in the absence of cytosol, chromatin-bound vesicles fuse to form large vesicles with diameters of up to a few micrometers (Boman et al. 1992). Again, these observations are reminiscent of those in the ER system. In mitotic extracts neither nuclei nor ER tubules were formed. Although the disassembly of the nuclear membrane during mitosis in vertebrate cells is well established, the behavior of the ER is less clear. Some data suggest that the ER network is at least partially disassembled (Warren 1993), whereas more recent results in tissue culture cells argue that the ER stays intact as a continuous network (Ellenberg et al. 1997). Our data and those of others would suggest that the ER is at least not formed during mitosis (Allan and Vale 1991).
Acknowledgments
We would like to thank Tanya Civco, Judy Chou, Maria Ericsson, Julie Huang, Tim Mitchison, Jan-Michael Peters, Melissa Rolls, Pascal Stein, Todd Stukenberg, and Sidney Whiteheart for help with experimental techniques and for materials, and Jean-Francois Menetret and Chris Akey for cryo-EM. We thank Kent Matlack, Kathrin Plath, Will Prinz, and Melissa Rolls for critical reading of the manuscript.
T.A. Rapoport is a Howard Hughes Medical Institute Investigator.
Footnotes
Abbreviations used in this paper: BAPTA, 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; ConA, concanavalin A; NEM, N-ethylmaleimide; TRAPα, translocon-associated protein α.
References
- Alberts B., Bray D., Lewis J., Raff M., Roberts K., Watson J.D. Molecular Biology of the Cell Garland Publishing, Inc. NewYork. 577 pp1994. [Google Scholar]
- Allan V. Protein phosphatase 1 regulates the cytoplasmic dynein-driven formation of endoplasmic reticulum networks in vitro. J. Cell Biol. 1995;128:879–891. [Europe PMC free article] [Abstract] [Google Scholar]
- Allan V., Vale R. Movement of membrane tubules along microtubules in vitroevidence for specialized sites of motor attachment. J. Cell Sci. 1994;107:1885–1897. [Abstract] [Google Scholar]
- Allan V.J. Organelle motility and membrane network formation in metaphase and interphase cell-free extracts. Methods Enzymol. 1998;298:339–353. [Abstract] [Google Scholar]
- Allan V.J., Vale R.D. Cell cycle control of microtubule-based membrane transport and tubule formation in vitro. J. Cell Biol. 1991;113:347–359. [Europe PMC free article] [Abstract] [Google Scholar]
- Boman A.L., Delannoy M.R., Wilson K.L. GTP hydrolysis is required for vesicle fusion during nuclear envelope assembly in vitro. J. Cell Biol. 1992;116:281–294. [Europe PMC free article] [Abstract] [Google Scholar]
- Chou T., Jaric M.V., Siggia E.D. Electrostatics of lipid bilayer bending. Biophys. J. 1997;72:2042–2055. [Europe PMC free article] [Abstract] [Google Scholar]
- Christoforidis S., McBride H.M., Burgoyne R.D., Zerial M. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 1999;397:621–625. [Abstract] [Google Scholar]
- Cluett E.B., Wood S.A., Banta M., Brown W.J. Tubulation of Golgi membranes in vivo and in vitro in the absence of brefeldin A. J. Cell Biol. 1993;120:15–24. [Europe PMC free article] [Abstract] [Google Scholar]
- Colombo M.I., Taddese M., Whiteheart S.W., Stahl P.D. A possible predocking attachment site for N-ethylmaleimide-sensitive fusion protein. Insights from in vitro endosome fusion. J. Biol. Chem. 1996;271:18810–18816. [Abstract] [Google Scholar]
- Cooper M.S., Cornell-Bell A.H., Chernjavsky A., Dani J.W., Smith S.J. Tubulovesicular processes emerge from trans-Golgi cisternae, extend along microtubules, and interlink adjacent trans-golgi elements into a reticulum. Cell. 1990;61:135–145. [Abstract] [Google Scholar]
- Dabora S.L., Sheetz M.P. The microtubule-dependent formation of a tubulovesicular network with characteristics of the ER from cultured cell extracts. Cell. 1988;54:27–35. [Abstract] [Google Scholar]
- Davis L.I., Blobel G. Identification and characterization of a nuclear pore complex protein. Cell. 1986;45:699–709. [Abstract] [Google Scholar]
- de Figueiredo P., Drecktrah D., Katzenellenbogen J.A., Strang M., Brown W.J. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc. Natl. Acad. Sci. USA. 1998;95:8642–8647. [Europe PMC free article] [Abstract] [Google Scholar]
- de Figueiredo P., Polizotto R.S., Drecktrah D., Brown W.J. Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol. Biol. Cell. 1999;10:1763–1782. [Europe PMC free article] [Abstract] [Google Scholar]
- Denesvre C., Malhotra V. Membrane fusion in organelle biogenesis. Curr. Opin. Cell Biol. 1996;8:519–523. [Abstract] [Google Scholar]
- Drummond S., Ferrigno P., Lyon C., Murphy J., Goldberg M., Allen T., Smythe C., Hutchison C.J. Temporal differences in the appearance of NEP-B78 and an LBR-like protein during Xenopus nuclear envelope reassembly reflect the ordered recruitment of functionally discrete vesicle types. J. Cell Biol. 1999;144:225–240. [Europe PMC free article] [Abstract] [Google Scholar]
- Ellenberg J., Siggia E.D., Moreira J.E., Smith C.L., Presley J.F., Worman H.J., Lippincott-Schwartz J. Nuclear membrane dynamics and reassembly in living cellstargeting of an inner nuclear membrane protein in interphase and mitosis. J. Cell Biol. 1997;138:1193–1206. [Europe PMC free article] [Abstract] [Google Scholar]
- Fullerton A.T., Bau M.Y., Conrad P.A., Bloom G.S. In vitro reconstitution of microtubule plus end-directed, GTPγS-sensitive motility of Golgi membranes. Mol. Biol. Cell. 1998;9:2699–2714. [Europe PMC free article] [Abstract] [Google Scholar]
- Goda Y., Sudhof T.C. Calcium regulation of neurotransmitter releasereliably unreliable? Curr. Opin. Cell Biol. 1997;9:513–518. [Abstract] [Google Scholar]
- Gorlich D., Prehn S., Hartmann E., Herz J., Otto A., Kraft R., Wiedmann M., Knespel S., Dobberstein B., Rapoport T.A. The signal sequence receptor has a second subunit and is part of a translocation complex in the endoplasmic reticulum as probed by bifunctional reagents. J. Cell Biol. 1990;111:2283–2294. [Europe PMC free article] [Abstract] [Google Scholar]
- Koch G.L., Booth C., Wooding F.B. Dissociation and re-assembly of the endoplasmic reticulum in live cells. J. Cell Sci. 1988;91:511–522. [Abstract] [Google Scholar]
- Lee C., Chen L.B. Dynamic behavior of endoplasmic reticulum in living cells. Cell. 1988;54:37–46. [Abstract] [Google Scholar]
- Lee C., Ferguson M., Chen L.B. Construction of the endoplasmic reticulum. J. Cell Biol. 1989;109:2045–2055. [Europe PMC free article] [Abstract] [Google Scholar]
- Liebe S., Menzel D. Actomyosin-based motility of endoplasmic reticulum and chloroplasts in Vallisneria mesophyll cells. Biol. Cell. 1995;85:207–222. [Abstract] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J.G., Schweizer A., Berger E.G., Hauri H.P., Yuan L.C., Klausner R.D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. [Abstract] [Google Scholar]
- Lohka M.J., Masui Y. Formation in vitro of sperm pronuclei and mitotic chromosomes induced by amphibian ooplasmic components. Science. 1983;220:719–721. [Abstract] [Google Scholar]
- Macaulay C., Forbes D.J. Assembly of the nuclear porebiochemically distinct steps revealed with NEM, GTPγS, and BAPTA. J. Cell Biol. 1996;132:5–20. [Europe PMC free article] [Abstract] [Google Scholar]
- Marshall I.C., Gant T.M., Wilson K.L. Ionophore-releasable lumenal Ca2+ stores are not required for nuclear envelope assembly or nuclear protein import in Xenopus egg extracts. Cell Calcium. 1997;21:151–161. [Abstract] [Google Scholar]
- Mui B.L., Dobereiner H.G., Madden T.D., Cullis P.R. Influence of transbilayer area asymmetry on the morphology of large unilamellar vesicles. Biophys. J. 1995;69:930–941. [Europe PMC free article] [Abstract] [Google Scholar]
- Murray A.W. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [Abstract] [Google Scholar]
- Murray A.W., Solomon M.J., Kirschner M.W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. [Abstract] [Google Scholar]
- Na G.C., Timasheff S.N. In vitro vinblastine-induced tubulin paracrystals. J. Biol. Chem. 1982;257:10387–10391. [Abstract] [Google Scholar]
- Newmeyer D.D., Wilson K.L. Egg extracts for nuclear import and nuclear assembly reactions. Methods Cell Biol. 1991;36:607–634. [Abstract] [Google Scholar]
- Newport J. Nuclear reconstitution in vitrostages of assembly around protein-free DNA. Cell. 1987;48:205–217. [Abstract] [Google Scholar]
- Newport J., Dunphy W. Characterization of the membrane binding and fusion events during nuclear envelope assembly using purified components. J. Cell Biol. 1992;116:295–306. [Europe PMC free article] [Abstract] [Google Scholar]
- Novick P., Zerial M. The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 1997;9:496–504. [Abstract] [Google Scholar]
- Panzner S., Dreier L., Hartmann E., Kostka S., Rapoport T.A. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell. 1995;81:561–570. [Abstract] [Google Scholar]
- Peters C., Mayer A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature. 1998;396:575–580. [Abstract] [Google Scholar]
- Pfeffer S.R. Transport-vesicle targetingtethers before SNAREs. Nature Cell Biol. 1999;1:E17–E22. [Abstract] [Google Scholar]
- Prehn S., Herz J., Hartmann E., Kurzchalia T.V., Frank R., Roemisch K., Dobberstein B., Rapoport T.A. Structure and biosynthesis of the signal-sequence receptor. Eur. J. Biochem. 1990;188:439–445. [Abstract] [Google Scholar]
- Rogalski A.A., Singer S.J. Associations of elements of the Golgi apparatus with microtubules. J. Cell Biol. 1984;99:1092–1100. [Europe PMC free article] [Abstract] [Google Scholar]
- Rolls M.M., Stein P.A., Taylor S.S., Ha E., McKeon F., Rapoport T.A. A visual screen of a GFP-fusion library identifies a new type of nuclear envelope membrane protein. J. Cell Biol. 1999;146:29–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Rothman J.E. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. [Abstract] [Google Scholar]
- Rothman J.E., Warren G. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 1994;4:220–233. [Abstract] [Google Scholar]
- Schekman R., Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. [Abstract] [Google Scholar]
- Schmidt A., Wolde M., Thiele C., Fest W., Kratzin H., Podtelejnikov A.V., Witke W., Huttner W.B., Soling H.D. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. [Abstract] [Google Scholar]
- Sheetz M.P., Singer S.J. Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc. Natl. Acad. Sci. USA. 1974;71:4457–4461. [Europe PMC free article] [Abstract] [Google Scholar]
- Speksnijder J.E., Miller A.L., Weisenseel M.H., Chen T.H., Jaffe L.F. Calcium buffer injections block fucoid egg development by facilitating calcium diffusion. Proc. Natl. Acad. Sci. USA. 1989;86:6607–6611. [Europe PMC free article] [Abstract] [Google Scholar]
- Steffen W., Karki S., Vaughan K.T., Vallee R.B., Holzbaur E.L., Weiss D.G., Kuznetsov S.A. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell. 1997;8:2077–2088. [Europe PMC free article] [Abstract] [Google Scholar]
- Stowell M.H.B., Marks B., Wigge P., McMahon H.T. Nucleotide-dependent conformational changes in dynaminevidence for a mechanochemical molecular spring. Nat. Cell Biol. 1999;1:27–32. [Abstract] [Google Scholar]
- Stukenberg P.T., Lustig K.D., McGarry T.J., King R.W., Kuang J., Kirschner M.W. Systematic identification of mitotic phosphoproteins. Curr. Biol. 1997;7:338–348. [Abstract] [Google Scholar]
- Subramanian K., Meyer T. Calcium-induced restructuring of nuclear envelope and endoplasmic reticulum calcium stores. Cell. 1997;89:963–971. [Abstract] [Google Scholar]
- Sullivan K.M., Busa W.B., Wilson K.L. Calcium mobilization is required for nuclear vesicle fusion in vitroimplications for membrane traffic and IP3 receptor function. Cell. 1993;73:1411–1422. [Abstract] [Google Scholar]
- Sweitzer S.M., Hinshaw J.E. Dynamin undergoes a GTP-dependent conformational change causing vesiculation. Cell. 1998;93:1021–1029. [Abstract] [Google Scholar]
- Takei K., Haucke V., Slepnev V., Farsad K., Salazar M., Chen H., De Camilli P. Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell. 1998;94:131–141. [Abstract] [Google Scholar]
- Takei K., Slepnev V.I., Haucke V., De Camilli P. Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1999;1:33–39. [Abstract] [Google Scholar]
- Terasaki M. Recent progress on structural interactions of the endoplasmic reticulum. Cell Motil. Cytoskelet. 1990;15:71–75. [Abstract] [Google Scholar]
- Terasaki M., Chen L.B., Fujiwara K. Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol. 1986;103:1557–1568. [Europe PMC free article] [Abstract] [Google Scholar]
- Ungermann C., Sato K., Wickner W. Defining the functions of trans-SNARE pairs. Nature. 1998;396:543–548. [Abstract] [Google Scholar]
- Vogel F., Hartmann E., Gorlich D., Rapoport T.A. Segregation of the signal sequence receptor protein in the rough endoplasmic reticulum membrane. Eur. J. Cell Biol. 1990;53:197–202. [Abstract] [Google Scholar]
- Vigers G.P., Lohka M.J. A distinct vesicle population targets membranes and pore complexes to the nuclear envelope in Xenopus eggs. J. Cell Biol. 1991;112:545–556. [Europe PMC free article] [Abstract] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. [Abstract] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu. Rev. Biochem. 1993;62:323–348. [Abstract] [Google Scholar]
- Waterman-Storer C.M., Salmon E.D. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol. 1998;8:798–806. [Abstract] [Google Scholar]
- Waterman-Storer C.M., Gregory J., Parsons S.F., Salmon E.D. Membrane/microtubule tip attachment complexes (TACs) allow the assembly dynamics of plus ends to push and pull membranes into tubulovesicular networks in interphase Xenopus egg extracts. J. Cell Biol. 1995;130:1161–1169. [Europe PMC free article] [Abstract] [Google Scholar]
- Weigert R., Silletta M.G., Spano S., Turacchio G., Cericola C., Colanzi A., Senatore S., Mancini R., Polishchuk E.V., Salmona M. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. [Abstract] [Google Scholar]
- Wiese C., Goldberg M.W., Allen T.D., Wilson K.L. Nuclear envelope assembly in Xenopus extracts visualized by scanning EM reveals a transport-dependent ‘envelope smoothing’ event. J. Cell Sci. 1997;110:1489–1502. [Abstract] [Google Scholar]
Articles from The Journal of Cell Biology are provided here courtesy of The Rockefeller University Press
Full text links
Read article at publisher's site: https://doi.org/10.1083/jcb.148.5.883
Read article for free, from open access legal sources, via Unpaywall:
https://rupress.org/jcb/article-pdf/148/5/883/1290203/9909132.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/158642725
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1083/jcb.148.5.883
Article citations
The endoplasmic reticulum as an active liquid network.
Proc Natl Acad Sci U S A, 121(42):e2409755121, 11 Oct 2024
Cited by: 0 articles | PMID: 39392663 | PMCID: PMC11494354
Elucidating the Morphology of the Endoplasmic Reticulum: Puzzles and Perspectives.
ACS Nano, 17(13):11957-11968, 28 Jun 2023
Cited by: 2 articles | PMID: 37377213 | PMCID: PMC10339789
Review Free full text in Europe PMC
A tubule-sheet continuum model for the mechanism of nuclear envelope assembly.
Dev Cell, 58(10):847-865.e10, 24 Apr 2023
Cited by: 4 articles | PMID: 37098350 | PMCID: PMC10205699
The interconnection of endoplasmic reticulum and microtubule and its implication in Hereditary Spastic Paraplegia.
Comput Struct Biotechnol J, 21:1670-1677, 20 Feb 2023
Cited by: 2 articles | PMID: 36860342 | PMCID: PMC9968982
Review Free full text in Europe PMC
A role for endoplasmic reticulum dynamics in the cellular distribution of microtubules.
Proc Natl Acad Sci U S A, 119(15):e2104309119, 04 Apr 2022
Cited by: 13 articles | PMID: 35377783 | PMCID: PMC9169640
Go to all (130) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Reconstitution of brefeldin A-induced golgi tubulation and fusion with the endoplasmic reticulum in semi-intact chinese hamster ovary cells.
Mol Biol Cell, 11(9):3073-3087, 01 Sep 2000
Cited by: 25 articles | PMID: 10982401 | PMCID: PMC14976
GTP hydrolysis is required for vesicle fusion during nuclear envelope assembly in vitro.
J Cell Biol, 116(2):281-294, 01 Jan 1992
Cited by: 66 articles | PMID: 1730756 | PMCID: PMC2289297
Assembly of the nuclear pore: biochemically distinct steps revealed with NEM, GTP gamma S, and BAPTA.
J Cell Biol, 132(1-2):5-20, 01 Jan 1996
Cited by: 106 articles | PMID: 8567730 | PMCID: PMC2120707
Reconstituting the reticular ER network - mechanistic implications and open questions.
J Cell Sci, 132(4):jcs227611, 22 Jan 2019
Cited by: 35 articles | PMID: 30670475
Review