Abstract
Free full text

TNF-α stimulates activation of pro-MMP2 in human skin through NF-κB mediated induction of MT1-MMP
SUMMARY
Tumor necrosis factor-alpha (TNF-α) is an important mediator during the inflammatory phase of wound healing. Excessive amounts of pro-inflammatory cytokines such as TNF-α are associated with inflammatory diseases including chronic wounds. Matrix metalloproteinases (MMPs) are involved in matrix re-modeling during wound healing, angiogenesis and tumor metastasis. As with proinflammatory cytokines, high levels of MMPs have been found in inflammatory states such as chronic wounds. In this report we relate these two phenomena. TNF-α stimulates secretion of active MMP-2, a type IV collagenase, in organ-cultured full-thickness human skin. This suggests a mechanism whereby excess inflammation affects normal wound healing.
To investigate this observation at the cellular and molecular levels, we examined TNF-α mediated activation of pro-MMP-2, induction of MT1-MMP, and the intracellular signaling pathways that regulate the proteinase in isolated human dermal fibroblasts. We found that TNF-α substantially promoted activation of pro-MMP-2 in dermal fibroblasts embedded in type-I collagen. In marked contrast, collagen or TNF-α individually had little influence on the fibroblast-mediated pro-MMP-2 activation. One well-characterized mechanism for pro-MMP-2 activation is through a membrane type matrix metalloproteinase, such as MT1-MMP. We report that TNF-α significantly induced MT1-MMP at the mRNA and protein levels when the dermal fibroblasts were grown in collagen. Although the intracellular signaling pathway regulating mt1-mmp gene expression is still obscure, both TNF-α and collagen activate the NF-κB pathway. In this report we provide three sets of evidence to support a hypothesis that activation of NF-κB is essential to induce MT1-MMP expression in fibroblasts after TNF-α exposure. First, SN50, a peptide inhibitor for NF-κB nuclear translocation, simultaneously blocked the TNF-α and collagen mediated MT1-MMP induction and pro-MMP-2 activation. Secondly, TNF-α induced IκB to breakdown in fibroblasts within the collagen lattice, a critical step leading to NF-κB activation. Lastly, a consensus binding site for p65 NF-κB (TGGAGCTTCC) was found in the 5′-flanking region of human mt1-mmp gene.
Based on these results and previous reports, we propose a model to explain TNF-α activation of MMP-2 in human skin. Activation of NF-κB signaling in fibroblasts embedded in collagen induces mt1-mmp gene expression, which subsequently activates the pro-MMP-2. The findings provide a specific mechanism whereby TNF-α may affect matrix remodeling during wound healing and other physiological and pathological processes.
INTRODUCTION
Healing of wounds after trauma is a complex process relying on the coordinated action of distinct tissues, cells and mediators (Clark, 1997; Martin, 1997). The mechanisms whereby these processes are regulated are outlined but not fully understood. Although the complete roles of inflammatory cells such as macrophages and their secreted cytokines are not delineated, evidence suggests that these cells are important for wound healing and that they function primarily through the production of soluble factors including TNF-α (Leibovitch, 1987). While this cytokine synthesis appears necessary for wounds to heal, excessive amounts of inflammatory cytokines are exhibited in non-healing wounds such as ulcers and/or inflammatory diseases (Cooney et al., 1997; Garner et al., 1993; Trengove et al., 2000). Animal experiments have shown differing roles for a cytokine depending on the dosage. For example, high concentrations of TNF-α impair angiogenesis (Fajardo et al., 1992). In support of this conclusion, we recently reported an association of elevated levels of IL-8 in slow healing wounds and IL-8 induced decreases in keratinocyte replication and fibroblast reorganization of collagen (Iocono et al., 2000). Taken together, these data suggest that, while normal wound healing requires some involvement of inflammatory cytokines abnormal wound healing may result from increased amounts of these mediators.
Selective breakdown of the extracellular matrix (ECM) is necessary during tissue repair, morphogenesis and angiogenesis (Birkedal-Hansen, 1995; Parks, 1999; Werb and Chin, 1998). Excessive destruction of ECM is associated with pathological processes such as rheumatoid arthritis, osteoarthritis, autoimmune skin blistering diseases and tumor invasion (Kahari and Saarialho-Kere, 1999). Matrix metalloproteinases are a family of related zinc-containing proteinases that have the ability to degrade most extracellular matrix (Birkedal-Hansen et al., 1993). MMPs are upregulated or activated during the early phases of wound healing in a spatial and temporal manner (Arumugam et al., 1999; Mohan et al., 1998; Moses et al., 1996). On the other hand, we and others have documented that high level expression or activation of MMPs is associated with impaired wound healing (Wysocki et al., 1999; Wysocki et al., 1993; Bullen et al., 1995). Little is known of the regulators of induction and activation of the MMP in human tissue during wound healing and tumor metastasis.
Among the MMP family there are two unique members, MMP-2 (gelatinase A) and MMP-9 (gelatinase B), both of which contain fibronectin-like domains for collagen binding (reviewed by Yu, 1995). Collectively named as type IV collagenases, these two MMPs can also cleave type I, V, VII and XI collagen and laminin. This ability suggests that type IV collagenases are involved in basement membrane remodeling, an activity that ultimately regulates cell migration and proliferation during cancer cell invasion and wound healing (Birkedal-Hansen et al., 1993). MMP-2 is secreted into the tissue in a latent form, pro-MMP-2. Accumulated evidence demonstrates that activation of this latent enzyme is controlled by cleavage of the N-terminal prodomain. This is coordinated by the membrane type metalloproteinases (MT-MMP) in participation with the tissue inhibitor of the metalloproteinases (TIMP) (reviewed by Murphy et al., 1999). Although many factors including cytokines, growth factors and extracellular matrix proteins have been reported to promote expression of MT1-MMP, it is not known whether these factors work coordinately or antagonistically, in human tissue (Migita et al., 1996; Haas et al., 1998). Moreover, the intracellular signaling pathway that regulates MT1-MMP expression is not characterized.
To investigate the role of inflammatory cytokines on MMP expression and activation in human tissue, we established an MMP induction and activation profile using human skin in organ culture. Then, we studied the cytokine mediated proteinase responsiveness at the cellular level by examining MMP induction and activation in isolated human dermal fibroblasts. Next, we analyzed the intracellular signaling pathway leading to the transcriptional control of the MMP gene. We report that TNF-α promotes activation of pro-MMP-2 in organ-cultured human skin. TNF-α also mediated activation of pro-MMP-2 in isolated dermal fibroblasts, but only when the cells were embedded in type I collagen. This suggests that matrix and cytokine signals in the tissue environment are important to regulators of a tissue remodeling proteinases. In addition, we provide evidence that the stimulation of NF-κB signal transduction pathway is essential for TNF-α to induce MT1-MMP by the fibroblasts in a collagen lattice. Thus, matrix and cytokine signals may coordinately regulate tissue remodeling by the summation of distinct signals within a common activation pathway.
MATERIALS AND METHODS
Materials and reagents
Vitrogen was purchased from COHESION Technologies (Palo Alto, CA, USA). It is purified, pepsin-solubilized bovine dermal 99.9% pure collagen and is 95–98% type I collagen, the remainder being type III collagen. TNF-α and other cytokines were purchased from R&D Systems (Minneapolis, MN, USA). The antibodies against MMP-2 (ab809), TIMP-2 (mAb3310), MT1-MMP (ab815) were purchased from Chemicon International (Temecula, CA, USA). The antibodies against IκB-α (sc-847) were purchased from Santa Cruz (Santa Cruz, CA, USA). The Immobilon-P was purchased from Millipore (Bedford, MA, USA). The Enhanced Chemiluminescence (ECL) was from Amersham Pharmacia (England). The gelatin was purchased from Sigma (St Louis, MO, USA). Gelatin-Sepharose 4B was purchased from Pharmacia (Uppsala, Sweden). The AMV reverse transcriptase, dNTPs, random hexamers and Rnasin were purchased from Promega (Madison, WI, USA) and the Taq DNA polymerase from Fisher (Pittsburgh, PA, USA). The peptide inhibitor for NF-κB, SN50, and the control mutant peptide, SN50M, were from Calbiochem (La Jolla, CA, USA). Collagenase II was from Worthington Biochemical (Lakewood, NJ, USA). The oligodeoxynucleotide primers were synthesized at the USC/Norris Comprehensive Cancer Center. The protein concentration was determined using the bicinchoninic acid kit from Sigma (St Louis, MO, USA).
Organ culture of human skin and cytokine stimulation
Normal human skin was obtained from patients undergoing reconstructive or aesthetic plastic surgery (USC IRB #999061). The full thickness skin was decontaminated by incubation in DMEM supplemented with 200 i.u./ml penicillin G sodium, 200 U/ml streptomycin sulfate and 0.5 µg/ml amphotericin B at 4°C overnight. The skin was cut into 0.5×0.5 cm pieces and incubated in DMEM at 37°C with 5% CO2 for 8 hours. The medium was replaced three times during this incubation. The explants were stimulated with TNF-α at 10 ng/ml in 2 ml of DMEM for 64 hours at 37°C in 5% CO2. The conditioned media were collected for gelatinolytic zymogram assay. The total RNA was extracted from the skin tissue for RT-PCR analysis as described below.
Preparation of human dermal fibroblasts
Dermal fibroblasts were isolated from normal human skin discarded after reconstructive or aesthetic surgical procedures (Garner et al., 1993; Tuan et al., 1994). Briefly, the skin was minced, followed by incubation with collagenase (1 mg/ml in DMEM) for 1–2 hours at 37°C. Then collagenase was removed by washing with DMEM. The isolated cells were allowed to attach on plastic flasks and cultured in DMEM with 10% FBS at 37°C with 5% CO2. After three passages the fibroblasts were used for experimentation. Three independent strains of normal human fibroblasts were used in this study. There were no identified differences of MMP-2 activation in the responses to cytokines.
Preparation of fibroblast-populated collagen lattices
Attached (Bell et al., 1979) and floating (Ehrlich and Rajaratnam, 1990) collagen lattices were used in this study. The lattices were fabricated by mixing 0.3 ml of 10× MEM, 0.3 ml of 0.1 NaOH, 0.7 ml of DMEM, 1.0 ml of cell suspension and 2.4 ml vitrogen (2.9 mg/ml). The final concentration of collagen was 1.9 mg/ml and cell density was 0.5×106 cells/cm3. A 0.3 ml sample of this suspension was applied to each well of a 48-well plate. Plates were incubated at 37°C for 40 minutes to allow the collagen to polymerize. To prepare the floating lattices, the lattice was released from the well by circumscribing the gel with a spatula. 1 ml of DMEM with the factors indicated for each experiment was then added. The lattice was cultured at 37°C with 5% CO2. Conditioned medium was sampled at the indicated times.
Gelatinolytic zymography
Conditioned media from the organ culture or collagen lattice were analyzed for gelatin degradation activity by SDS-PAGE under non-reducing conditions. The gel contained 5.8 mg/ml gelatin and 10% acrylamide (Heussen and Dowdle, #261). Electrophoresis was carried out at 4°C. After a brief wash with water, the SDS in the acrylamide gel was extracted by incubation with 2% Triton X-100. Gelatinolytic activities were developed in a buffer containing 5 mM CaCl2, 150 mM NaCl and 50 mM Tris at 37°C for 16 hours or for the time indicated in the figure legend. The gelatinolytic activities were visualized by staining with Coommasie Blue R-250. The ratio of active 62 kDa MMP-2 in the total MMP-2 was determined using scanning densitometry with NIH Image analysis software.
Western blot
Samples for the western blot were prepared differently for each individual protein. For the MT1-MMP, the lattices or monolayer cells were extracted with buffer containing 1% Triton X-100, 150 mM NaCl, 50 mM Tris, pH 7.5, and 1 mM PMSF. The cell debris was removed by centrifugation at 14,000 g. The supernatant was subjected to SDS-PAGE. The MMP-2 enzyme was enriched by incubation of 500 µl conditioned medium with 30 µl gelatin-conjugated Sepharose 4B. The sepharose was washed with buffer containing 400 mM NaCl, 50 mM Tris, pH 7.5, and the bound protein was eluted with 1× SDS-sample buffer. IκBα was prepared from the cytoplasmic fractions. The lattices were washed with PBS three times and homogenized in a buffer containing 2 mM MgCl2, 4 mM CaCl2, 10 mM Tris, pH 7.5, and 0.4% NP-40. The samples were centrifuged at 14,000 g for 20 minutes and the supernatant was collected as the cytoplasmic fraction. All samples were resolved by reducing SDS-PAGE and transferred to Immobolin-P. Filters were blocked with 5% non-fat milk in TBS (150 mM NaCl, 50 mM Tris, pH 7.5) for 2 hours at room temperature, then incubated overnight at 4°C with individual antibodies in TBS + 0.05% Tween-20 (TBST). The polyclonal anti-MT1-MMP and anti-MMP-2 were used at 1:5000 dilution, the monoclonal anti-TIMP-2 at 1:4000 dilution, or the polyclonal anti-IκBα at 1:1000 dilution. The blot was incubated for 2 hours with a horseradish peroxidase-conjugated goat anti-rabbit antibody at 1:5000 dilution or a rabbit anti-mouse antibody at 1:2500 dilution in TBST and visualized with enhanced chemiluminescence.
Reverse transcriptase polymerase chain reaction (RT-PCR)
The intact skin was cultured for 46 hours with TNF-α at 10 ng/ml and then washed with PBS. The total RNA was extracted with Trizol Reagent (Gibco BRL). Fibroblast-populated collagen lattices (2×105 cells per lattice) were treated with 10 ng/l TNF-α for 26 hours and the total RNA was extracted with a buffer containing 4 M guanidium isothiocyate, 25 mM sodium citrate, 0.5% laurocylsarcosine and 0.7% β-mercaptoethanol. Reverse transcription was carried out in 50 µl reactions with 4 µg RNA and 2 µg random deoxynucleotide hexamers according to the manufacturer’s instructions (Fisher). The reaction was terminated by incubation of the samples at 94°C for 3 minutes. Specific cDNAs for uPA, PAI-1, TIMP-1, TIMP-2, MT1-MMP, MMP-2, β-actin and GAPDH were amplified with the ROBOCYCLER (Strategen, USA). The optimal PCR results for these genes were obtained at an annealing temperature of 60°C with 35 cycles. The PCR products were resolved by agarose gel electrophoresis followed by staining with ethidium bromide. The PCR products were recorded by digital camera and the relative densities of the band was quantitated by NIH Image analysis software. The sequence information used to design the PCR primers used in this study was obtained from previous publications. The predicted PCR product size for β-actin is 548 bp. The predicted PCR product size for TIMP-1 and TIMP-2 are 404 bp and 332 bp, respectively (Fortunato et al., 1998). The predicted PCR product size for PAI-1 and uPA is 263 bp and 474 bp, respectively (Reno, 1998). The predicted PCR product size for MMP-2 and GAPDH is 480 bp and 459 bp, respectively (Yammamoto et al., 1999). The predicted PCR product size for human MT1-MMP is 548 bp (Forsyth et al., 1999).
Treatment of the collagen lattice with NF-κB inhibitor
The peptide inhibitor for NF-κB, SN50, and the control mutant peptide, SN50M, were dissolved in DMSO followed by dilution with DMEM. These peptides were added during casting of the lattices. The final concentrations of these peptides were 0, 50 and 100 µg/ml. The lattices were or were not stimulated by 10 ng/ml TNF-α. The conditioned media were harvested after 46 hours and tested for MMP-2 using zymography. The cell lysates from the lattice were subjected to western blot analysis for MT1-MMP.
RESULTS
Activation of pro-MMP-2 in organ-cultured human full-thickness skin is elevated in response to TNF-α
Initially, we wanted to determine the effect of cytokines on MMP induction/activation in intact skin. Adult human full-thickness normal skin was cultured in DMEM with 10 ng/ml TNF-α (n=3). After 72 hours the conditioned media were analyzed by gelatinolytic zymography. As shown in Fig. 1A, a 72 kDa form of MMP was the predominant gelatinolytic MMP released by the full-thickness skin. Upon TNF-α treatment, an additional 62 kDa band appeared. Western blot analysis showed that the 72 kDa and 62 kDa proteinase activities were pro-MMP2 and the active enzyme, respectively (Fig. 1B). The organ culture time-course experiment demonstrated further that TNF-α did not affect the constitutive secretion of the 72 kDa MMP-2 (Fig. 1C). The 62 kDa MMP-2 did not appear in the medium until after culturing for 48–72 hours and a substantial amount of the active form accumulated after 96 hours. This time-course data matched well with the clinical study of burn wounds, which showed the presence of active MMP-2 on day 4 after the trauma (Young and Grinnell, 1994).
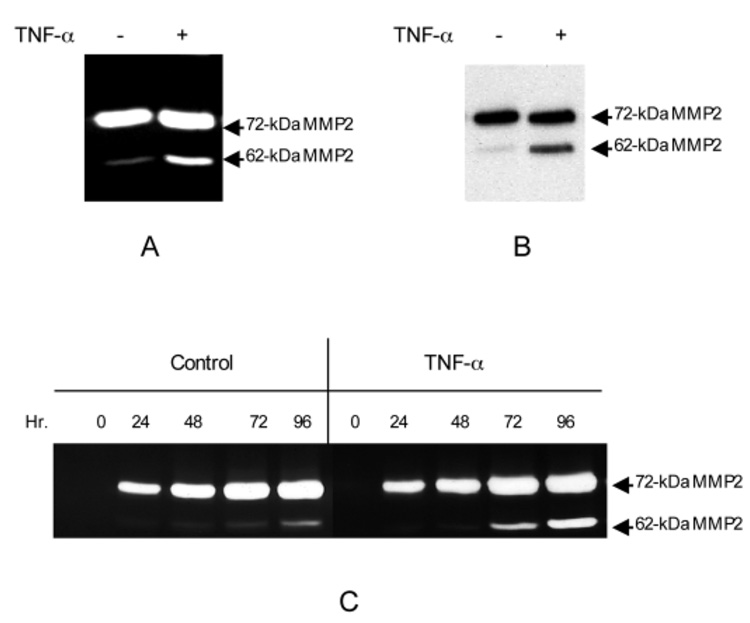
TNF-α stimulates secretion of active MMP-2 from the organ-cultured human skin. The biopsies of full-thickness human skin were processed as described in Materials and Methods. The biopsies (0.5×0.5 cm) were floated in 2 ml DMEM with or without 10 ng/ml TNF-α. After culture for 64 hours at 37°C with 5% CO2 the conditioned media were collected for gelatinolytic activity assay and biopsies were processed for RT-PCR analysis as shown in Fig. 5. (A) The gelatinolytic activities from the conditioned media were examined by zymogram. Arrows indicate the 72 kDa latent and 62 kDa active MMP-2. (B) The identity of the gelatinolytic activity was confirmed by western blot with polyclonal anti-MMP-2, which recognizes both 72 and 62 kDa MMP-2. The gelatinase activities from the conditioned media were enriched by gelatin-Sepharose-4B. The bound protein was resolved by a reducing SDS-PAGE followed by a western blot. (C) The time course of TNF-α induced activation of pro-MMP-2 in human skin organ culture. The conditioned medium was sampled at the indicated time points and assayed by gelatinolytic zymogram. The skins from three independent donors were analyzed and gave similar results.
TNF-α stimulates activation of pro-MMP-2 by fibroblasts in collagen lattices
To further understand the cellular basis of the MMP activation in the human skin we isolated the dermal fibroblasts from normal adult skin and examined the MMP induction and activation. We grew the fibroblasts in type-I collagen as a tensioned lattice in order to simulate the collagen-rich skin environment. The lattice was stimulated by TNF-α at 0, 0.5, 5 and 50 ng/ml (Fig. 2A). The conditioned media were examined for gelatinolytic activity after a 68 hour incubation. Substantial 72 kDa gelatinolytic activities were constitutively expressed in the conditioned media regardless of TNF-α. However, the 62 kDa, the active form of MMP-2, was increased by TNF-α in a dose-dependent manner. The 0.5 ng/ml dose of TNF-α induced a modest amount of 62 kDa MMP-2. At the 50 ng/ml dose of TNF-α approximately 45% of the MMP-2 was in the active form. A western blot confirmed the identity of the 62 kDa and 72 kDa gelatinolytic activities to be the active enzyme and the zymogen of MMP-2, respectively (Fig. 2B).
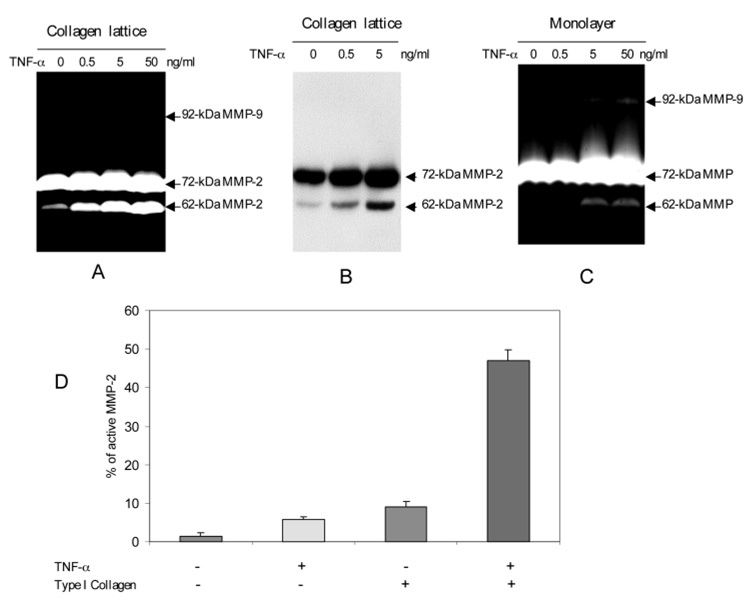
TNF-α stimulates activation of pro-MMP-2 by human dermal fibroblasts embedded in collagen lattice. Primary human dermal fibroblasts were isolated from the normal human skin and the early passage cells were used for experiments. The fibroblasts were plated either as monolayer or embedded in collagen lattices at the same cell density and were stimulated by TNF-α at the indicated concentration. After 68 hours the conditioned media were analyzed by gelatinolytic zymogram. (A) The conditioned media from fibroblasts embedded in collagen lattice were analyzed for gelatinolytic activities. The zymogram was developed for 16 hours as standard procedure. (B) Identity of MMP-2 was confirmed by western blot with anti-MMP-2 antibodies. The conditioned medium was enriched for gelatinase by binding to gelatin-conjugated Sepharose. The bound protein was resolved by SDS-PAGE followed by western blot. (C) The conditioned media from fibroblasts grown in monolayer were assayed for gelatinolytic activities. This zymogram was developed for 40 hours in order to reveal the weak 62 kDa MMP-2. (D) The relative efficacy of collagen and TNF-α on activation of pro-MMP-2 was determined by densitometric scanning of the 72 and the 62 kDa MMP-2. The percentage of the 62 kDa MMP-2 at 0 and 50 ng/ml TNF-α were plotted. These data are the average of three independent experiments.
The specific role of collagen in TNF-α induced MMP-2 activation was then addressed. We compared the TNF-α responsiveness of the dermal fibroblasts residing in a collagen lattice to those plated in a monolayer on plastic. Our results showed that TNF-α mediated activation of pro-MMP-2 was significantly weaker in the monolayer-cultured fibroblasts (Fig. 2C). In order to visualize the weaker signal of the 62 kDa MMP-2 derived from the monolayer culture, an extended development time of 40 hours rather than the standard 16 hours was needed. We quantitated the percentage of active 62 kDa MMP-2 and latent 72 kDa MMP-2 using densitometry scanning to compare the role of TNF-α, collagen and their combination on pro-MMP-2 activation. Collagen and TNF-α individually activated MMP-2 minimally, to approximately 8–10% of active MMP-2 (Fig. 2D). However, the combination of TNF-α and collagen stimulated the activation of a much greater percentage of pro-MMP-2, an average of 45% in the active form (P<0.009).
TNF-α activates pro-MMP-2 in a time-dependent manner
Clinical investigations on human incisional wounds and animal studies both documented a temporal pattern of MMP-2 expression (Arumugam et al., 1999; Moses et al., 1996; Young and Grinnell, 1994). To test whether isolated fibroblasts responded in a similar manner, we performed a time-course experiment. Dermal fibroblasts were grown in a collagen lattice or in monolayer and stimulated with TNF-α at 10 ng/ml. The resulting conditioned media were sampled at the indicated time points. The 72 kDa pro-MMP-2 was significantly increased at 20 hours and neither TNF-α nor collagen had an effect on the constitutive expression (Fig. 3). The initial detection of active 62 kDa MMP-2 from fibroblasts embedded in collagen was 29 hours after TNF-α exposure. A substantial amount of the 62 kDa MMP-2 was detected 46 hours after TNF-α stimulation in the lattice. Again, a minor activation of pro-MMP-2 was observed in the collagen lattice in the absence of the cytokine. Similarly, TNF-α slightly activated the pro-MMP-2 on the monolayer-cultured fibroblasts. This time-course experiment of TNF-α mediated activation of pro-MMP-2 matches temporally with the clinical observations demonstrating the sequential events of expression and activation of the proteinase.
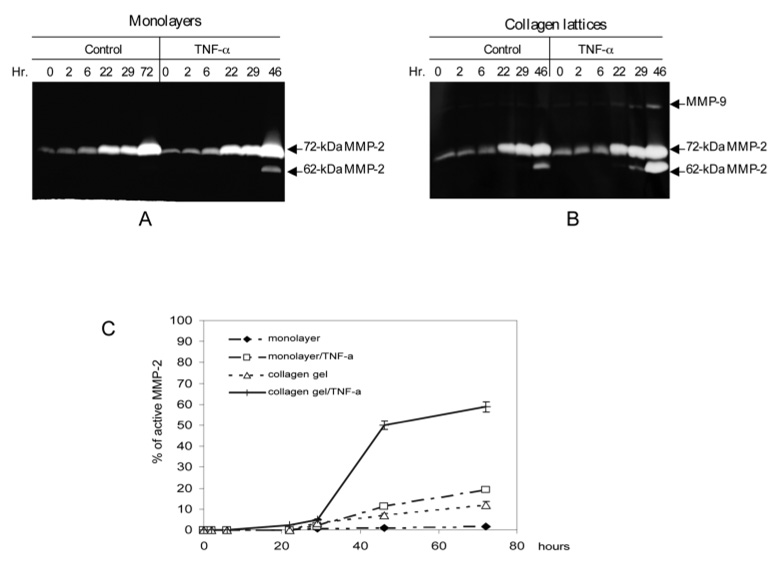
The time-course study of TNF-α and collagen on the activation of pro-MMP-2. The early passages of human dermal fibroblasts were seeded as monolayers or cast into collagen lattices and stimulated with or without 10 ng/ml TNF-α. (A) The monolayer fibroblasts were stimulated or not with TNF-α and conditioned medium was sampled at the indicated time points. Gelatinolytic activities were examined by zymogram. (B) Fibroblasts embedded in collagen lattices were stimulated with or without TNF-α. (C) The relative amount of the 62 kDa MMP-2 from A and B was determined by densitometric scanning. The percentage of the 62 kDa active MMP-2 from was plotted against the time. These data are the average of three independent experiments.
MTI-MMP is induced by TNF-α from fibroblasts cultured in collagen lattice
Activation of pro-MMP-2 is controlled by MT-MMPs including MT1-MMP in participation with the tissue inhibitor metalloproteinase, TIMP-2 (Sato et al., 1994). We speculated that the TNF-α mediated MMP-2 activation in human skin and fibroblast-populated collagen lattices may be through the upregulation of MT1-MMP. To test this hypothesis, we compared the ability of TNF-α stimulated fibroblasts in a collagen lattice versus those in monolayer to produce MT1-MMP protein. The western blot analysis showed that a small amount of 65 kDa MT1-MMP was transiently expressed in the monolayer culture without TNF-α stimulation (Fig. 4A). Stimulation of the monolayer with TNF-α increased the 65 kDa MT1-MMP protein minimally. Fibroblasts embedded in the collagen lattice with no TNF-α stimulation showed an increase in the 65 kDa MT1-MMP versus the monolayer with a minimal increase of the 63 kDa protein, the putative active form of MT1-MMP (Fig. 4B). However, the protein levels of both forms of MT1-MMP decreased quickly. In contrast, stimulation of the collagen lattice cells with TNF-α substantially increased the levels of both 65 kDa and 63 kDa MT1-MMP proteins. This TNF-α and collagen mediated synergistic induction of MT1-MMP correlated well with the synergistic activation of pro-MMP-2, as shown in Fig 2 and Fig 3.
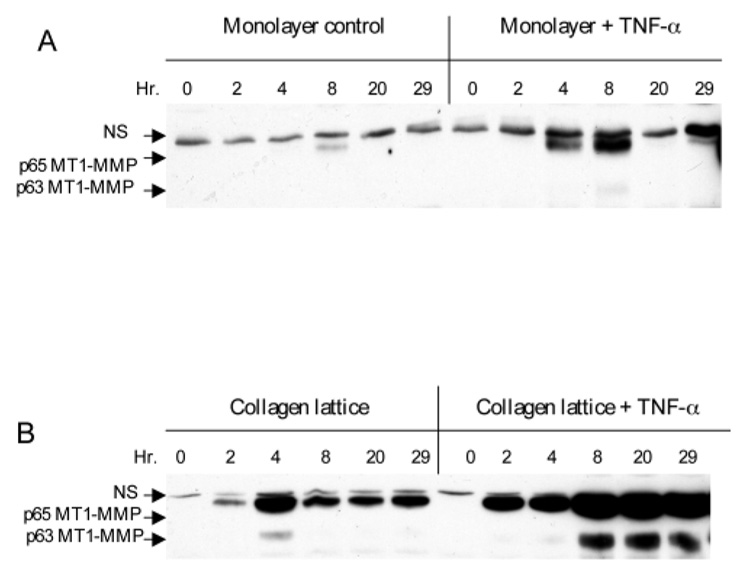
TNF-α and collagen synergistically induce MT1-MMP protein. The dermal fibroblasts were grown either as monolayer (A) or in collagen lattice (B). The cells were stimulated or not with 10 ng/ml TNF-α. Monolayer and lattice were collected at the indicated time and cell lysates were subjected to western blot with anti-MT1-MMP antibodies. Two forms of MT1-MMP of molecular mass 65 and 63 kDa were detected. A nonspecific band (NS) is indicated.
TNF-α upregulates MT1-MMP mRNA in organ-cultured human skin and dermal fibroblasts in a collagen environment
We then asked whether TNF-α elevated the mRNA level of MT1-MMP in organ-cultured human skin tissue. Total RNA was extracted from the skin and mRNA of human MT1-MMP and β-actin were measured by RT-PCR analysis. The results showed a very low basal level of MT1-MMP mRNA at basal state and that was significantly increased after TNF-α treatment (Fig. 5A). In contrast, TNF-α had no effect on the β-actin mRNA level.
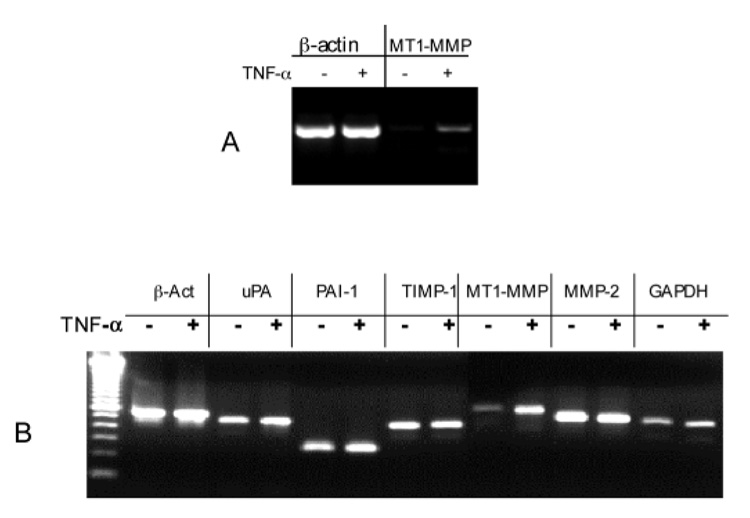
TNF-α increases MT1-MMP mRNA in human skin and the dermal fibroblasts within collagen lattice. (A) Normal adult human skin was cultured in DMEM and stimulated with or without 10 ng/ml TNF-α as described in Fig. 1. The total RNA from the skin piece was extracted. The mRNA levels of human MT1-MMP and β-actin were measured by RT-PCR. One third of the PCR product for β-actin and the whole product for MT1-MMP were resolved by agarose gel and stained by ethidium bromide. (B) Human dermal fibroblasts were embedded in collagen lattice. The lattices were stimulated with TNF-α at 10 ng/ml or not for 24 hours. The mRNA levels of human β-actin, MT1-MMP, MMP-2, uPA, PAI-1, TIMP-1 and GAPDH were analyzed by RT-PCR. The size of each PCR product matches the predicted size (see Materials and Methods).
To confirm this observation at the cellular level, the fibroblasts were embedded in collagen and stimulated with TNF-α. The mRNA of several wound healing related genes were measured. The mRNA levels of MT1-MMP were increased fourfold upon TNF-α stimulation (Fig. 5B). In contrast, mRNA levels of MMP-2 and of two control genes, β-actin and GAPDH, were unchanged upon TNF-α treatment. The constant high level of MMP-2 mRNA matched well with the constitutively expressed latent MMP-2. In addition, the mRNA levels of the urokinase-type plasminogen activator (uPA), its inhibitor PAI-1, and the inhibitor for MMP-9 TIMP-1 were unchanged by TNF-α treatment (Fig. 5B). Thus, TNF-α specifically increased the mRNA level of MT1-MMP in the fibroblast populated collagen lattice. Together, these data suggest that the TNF-α mediated activation of pro-MMP-2 in the skin and in the fibroblasts is likely through induction of MT1-MMP.
SN50, a NF-κB inhibitor, blocks the TNF-α induced MT1-MMP expression and pro-MMP-2 activation
Since both TNF-α and collagen activate NF-κB signaling in dermal fibroblasts (Xu et al., 1998), activation of this pathway might explain the synergistic induction of MT1-MMP by TNF-α and collagen. We utilized a synthesized peptide inhibitor for NF-κB, SN50, to investigate this possibility. The inhibitor has a NF-κB nuclear translocation motif flanked by a signaling peptide that facilitates membrane permeability (Lin et al., 1995). The inhibitor blocks TNF-α induced NF-κB translocation into the nucleus. A control mutant peptide, SN50M, in which two positively charged amino acid residues are replaced, has no effect on this signaling.
Dermal fibroblasts were embedded in collagen and either the inhibitor or the control peptide was added at three dosages (0, 50 and 100 µg/ml). The lattices were stimulated or not with TNF-α at 10 ng/ml for 72 hours. The conditioned media were analyzed for gelatinolytic activities and the lattice was examined for MT1-MMP protein (Fig. 6). The results showed that SN50 blocked both collagen and TNF-α mediated activation of pro-MMP-2. At 50 µg/ml of SN50, the intermediate 64 kDa MMP-2 was accumulated and at 100 mg/ml the formation of 62 kDa MMP-2 was completed blocked by the inhibitor. As expected, the expression of MT1-MMP protein was similarly inhibited by SN50. In contrast, the control peptide SN50M had little effect on either MT1-MMP protein expression or pro-MMP-2 activation. These results support our hypothesis that activation of the NF-κB signaling pathway leads to the induction of MT1-MMP and subsequently activates pro-MMP-2.
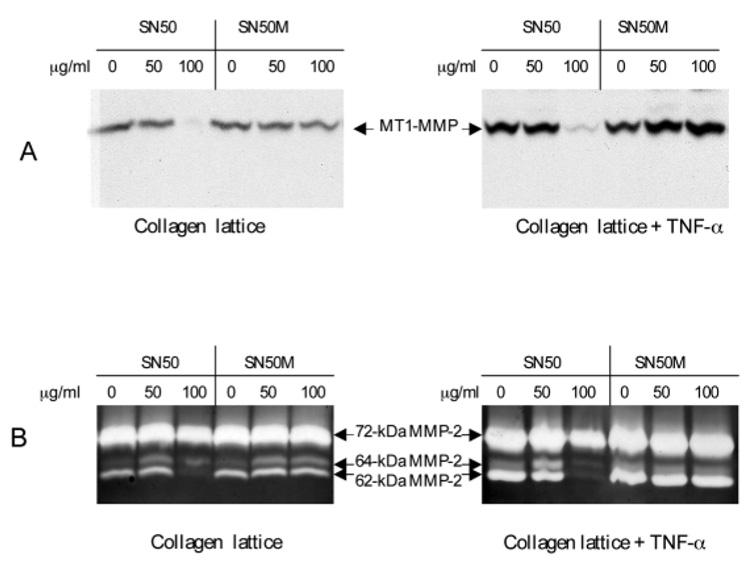
NF-κB inhibitor, SN50, blocks TNF-α and collagen mediated MT1-MMP expression and pro-MMP-2 activation. The dermal fibroblasts were embedded in collagen lattice. The cell-permeable peptide inhibitor for NF-κB, SN50 and the control mutant peptide, SN50M, were added to the lattices at the indicated concentrations followed by stimulation or not with TNF-α at 10 ng/ml in DMEM. Conditioned media and lattices were collected after culture for 48 hours. (A) Cell lysates from the treated lattices were subjected to western blotting with anti-MT1-MMP. (B) Conditioned medium was analyzed by zymogram. The latent, an intermediate and the active MMP-2 with molecular mass 72, 64 and 62 kDa, respectively, are indicated.
TNF-α stimulates IκB breakdown in fibroblast populated collagen lattices
IκB sequesters NF-κB in the cytoplasmic compartment by binding to its inhibitory subunit (Ghosh et al., 1998). TNF-α can induce the degradation of IκB via a sophisticated cascade pathway. The breakdown of IκB leads to the release of NF-κB, which can then translocate into the nucleus and turn on specific genes. We examined the kinetics of IκBα breakdown in the fibroblast-populated collagen lattices. Our results showed that in the absence of TNF-α there was a modest breakdown of the 37 kDa IκBα protein that began 30 minutes after culturing in collagen. However, IκBα protein quickly recovered to the basal level by the 60 minutes (Fig. 7). Stimulation of the lattices by TNF-α led to greater IκBα breakdown and prolonged the time of recovery for the IκBα protein from 30 minutes to 90 minutes. These results provide additional support for the hypothesis that NF-κB is involved in activation pathway of pro-MMP2.
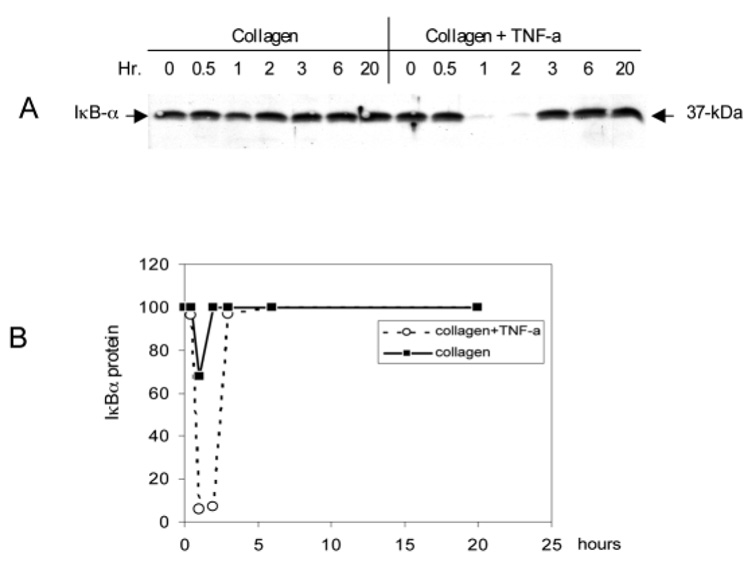
TNF-α stimulates the breakdown of IκB in fibroblasts populated in collagen lattices. Activation of the NF-κB pathway was monitored by the breakdown of IκBα. The fibroblasts were embedded in collagen and stimulated with or without 10 ng/ml TNF-α. The lattices were collected at the indicated time and the cytosolic fractions were subjected to western blot with anti-IκBα antibodies. (A) A representative western blot of two independent experiments is shown. (B) The IκBα protein level from the cytosolic fraction was determined by densitometric scanning.
A consensus NF-κB response element is identified in the promoter of human mt1-mmp gene
Although TNF-α can trigger different signal transduction pathways, most agree that the NF-κB cascade is important for pro-inflammatory activities. The end point of this pathway is NF-κB binding to the 10 bp consensus site in the promoter region to initiate transcription of the target gene. The DNA sequence information of the 5′-flanking region of the human mt1-mmp gene was recently available in GenBank (Lohi et al., 2000). We searched for potential transcription factor binding sites in the promoter of human mt1-mmp. We noticed that a consensus p65 NF-κB binding site, TTCTGGAGCTTCC, is located 1142 bp upstream of a transcription start site (MatIspector 2.2). Similarly, a consensus NF-κB binding site was also previously identified in the promoter of mouse mt1-mmp (Haas et al., 1999). Direct evidence for the role of these cis-elements in response to TNF-α mediated MT1-MMP induction needs to be further elucidated by a transcription reporter assay and electrophoretic mobility-shift assay. However, identification of the potential NF-κB binding site in the promoter of human mt1-mmp gene provides additional supports for our proposed model.
DISCUSSION
The complex interactions between cells, cytokines and proteinases that result in wound healing, tumor invasion or organ fibrosis are not fully understood. This report addresses one aspect of these interactions by determining the cellular and molecular basis for the effects of pro-inflammatory cytokines on an ECM remodeling enzyme. At the first stage of the investigation we identified a profile of cytokine-mediated MMP induction/activation from organ-cultured human skin. We then dissected these profiles by isolating a defined skin cell type and documented the intracellular molecular pathway that regulates the induction of the MMP. Our report of TNF-α induced MMP-2 activation in human skin may link the TNF-α mediated angiogenesis with the MMPs involved in wound healing or cancer cell invasion. The finding that TNF-α regulates a type IV gelatinase may also explain the clinical observation that higher levels of TNF-α have been associated with ulcers and low levels of TNF-α have been linked to hypertrophic scars (Cooney et al., 1997; Garner et al., 1993; Trengove et al., 2000; Kitzis et al., 1999). Equally important, we demonstrated that the matrix environment in which the cells reside is required for TNF-α mediated pro-MMP-2 activation. This finding is important as a reminder that the matrix environment in which a cell resides is essential to understanding its behavior.
These results will help us to understand how multiple factors coordinately regulate MMPs. Previous reports have documented that TNF-α could activate the pro-MMP-2 activation in synovial fibroblasts and that collagen could stimulate rat endothelial cells to activate the pro-MMP-2 (Migita et al., 1996; Haas et al., 1998; Thompson et al., 1994). Although we confirmed these results, we found that TNF-α and collagen individually were only moderately active in MMP-2 activation by human dermal fibroblasts. In contrast, coordinated exposure of TNF-α and collagen results in a much greater activation of the pro-MMP-2. This increased activation is reflected by the upregulation of MT1-MMP expression from TNF-α treated fibroblasts embedded in collagen. However, it is important to realize that this pattern of MMP activation is not universal. The ability of collagen to affect the activation of pro-MMP-2 is specific since TNF-α mediated regulation of MMP-9 is not affected by collagen (Y.-P. Han et al., unpublished).
Our data presented here provide further support for the theory that TNF-α mediated pro-MMP-2 activation is through induction of MT1-MMP. First, the data from the experiment of human skin organ culture demonstrated that the TNF-α mediated pro-MMP-2 activation correlated well with the cytokine induced MT1-MMP mRNA level. Secondly, at the cellular level, both the TNF-α dose-dependent and time-course experiments resulted in a strong quantitative and temporal relationship between MT1-MMP protein level and activation of pro-MMP-2. Thirdly, at the molecular level, MT1-MMP expression and MMP-2 activation were blocked simultaneously by the inhibitor for NF-κB in a dose-dependent manner.
The signal pathway that regulates MT1-MMP expression is not fully delineated. Our observation that the NF-κB inhibitor blocked MT1-MMP expression in the lattice led us to search for a potential NF-κB response element in the promoter region of the human mt1-mmp gene. Indeed, scanning the sequence of 5′-flanking region of the human mt1-mmp we identified a potential p65 NF-κB binding site. To support our hypothesis that activation of the NF-κB signaling pathway induced mt1-mmp gene expression, we measured the TNF-α mediated IκB breakdown in the lattice. NF-κB can be activated in cells by a variety of stimuli ranging from inflammatory cytokines to bacterial and viral products. NF-κB activation is achieved through the signal-induced proteolytic breakdown of IκB in the cytoplasm (Mercurio and Manning, 1999). We believe the mechanism for collagen mediated pro-MMP-2 activation is through the activation of NF-κB. The reason for this conclusion is that the collagen mediated induction of MT1-MMP and subsequent MMP-2 activation was blocked by the NF-κB inhibitor. Other investigators have also reported that collagen could stimulate NF-κB activation in human dermal fibroblasts, which supports our hypothesis (Xu et al., 1998).
A model for the cytokine-mediated activation of pro-MMP-2 in human tissue is outlined here, based on our and other’s findings (Fig. 8). Binding of TNF-α and collagen to their respective receptors triggers the signaling pathway that leads to NF-κB activation via the breakdown of its inhibitory subunit IκB. Translocation of NF-κB into the nucleus and binding to the consensus site at the promoter of the mt1-mmp gene initiates the transcription. Accumulated MT1-MMP protein on the plasma membrane thus cleaves the N-terminal pre-domain of pro-MMP-2 and generates the 62 kDa active enzyme.
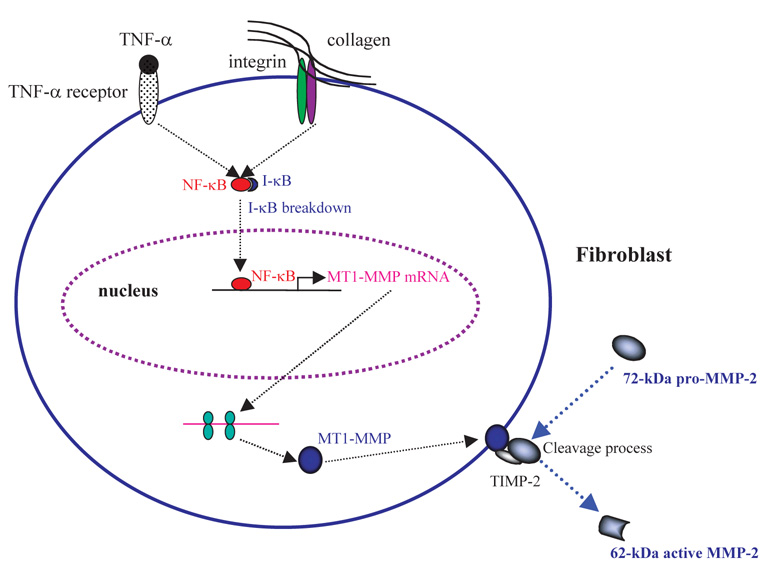
Mechanism of the TNF-α and collagen mediated activation of pro-MMP-9 in human dermal fibroblasts. Based on our results and those of others, we propose the following pathway for pro-MMP-2 activation, showing the role of TNF-α and the interplay between molecules involved in the pro-MMP-2 activation cascade. Binding of TNF-α and type I collagen to their receptors activates the NF-κB signaling pathway by inducing the breakdown of IκB, the cellular inhibitor of NF-κB. NK-κB then translocates from the cytosolic compartment into the nucleus. Binding of NF-κB to the NF-κB responsive cis-element in the promoter of the mt1-mmp gene triggers the transcription of mt1-mmp. Elevated levels of MT1-MMP mRNA generate MT1-MMP protein, which then translocates to the plasma membrane and forms a functional complex with TIMP-2. In contrast, mmp-2 gene transcription is constitutively active in fibroblasts and pro-MMP-2 accumulates in the extracellular environment. Increased MT1-MMP protein on the cellular membrane promotes cleavage of the N-terminal pro-domain of the 72 kDa pro-MMP-2, increasing the amount of the 62 kDa active form of MMP-2.
This study provides the first evidence that an inflammatory cytokine regulates MMP activation in human skin. Inflammation is found during matrix remodeling in many clinical situations, including wound healing and cancer metastasis. Because basement membrane components, type IV collagen and laminin, are potential substrates for MMP-2, activation of a type IV collagenase by this cytokine may provide a mechanistic explanation for the role of TNF-α during tissue remodeling. Since high levels of TNF-α are found in chronic wounds, TNF-α mediated activation of pro-MMP suggests a mechanism for the destructive role of excessive inflammation on tissue. Accordingly, the observation that high levels of active MMP-2 are found in chronic wounds could be explained by our proposed linkage of TNF-α to a molecular pathway for pro-MMP-2 activation. Similarly, inflammation has long been linked to angiogenesis dependent metastasis where ECM remodeling, especially the controlled breakdown of basement membrane, is essential. TNF-α may play a similar role during this process.
Acknowledgments
We thank Dr Susan Downey for supplying of the human skin discarded after reconstructive procedures. Tuan and Wu are supported by NIH grant GM55081. Han, Hughes and Garner are supported by NIH grant GM 50967. We also thank Drs Nimni and Han for sharing equipment.
REFERENCES
- Arumugam S, Jang YC, Chen-Jensen C, Gibran NS, Isik FF. Temporal activity of plasminogen activators and matrix metalloproteinases during cutaneous wound repair. Surgery. 1999;125:587–593. [Abstract] [Google Scholar]
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA. 1979;76:1274–1278. [Europe PMC free article] [Abstract] [Google Scholar]
- Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr. Opin. Cell Biol. 1995;7:728–735. [Abstract] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit. Rev. Oral Biol. Med. 1993;4:197–250. [Abstract] [Google Scholar]
- Brenner CA, Daniel SL, Adler RR. Cytokines: A Practical Approach. Oxford: IRL Press; 1991. Cytokine MAPPing: observation and quantification of cytokine mRNA in small numbers of cells using the polymerase chain reaction. [Google Scholar]
- Bullen EC, Longaker MT, Updike DL, Benton R, Ladin D, Hou Z, Howard EW. Tissue inhibitor of metalloproteinases-1 is decreased and activated gelatinases are increased in chronic wounds. J. Invest. Dermatol. 1995;104:236–240. [Abstract] [Google Scholar]
- Clark RAF. The Molecular and Cellular Biology of Wound Repair. New York: Plenum Press; 1997. [Google Scholar]
- Cooney R, Iocono J, Maish G, Smith JS, Ehrlich P. Tumor necrosis factor mediates impaired wound healing in chronic abdominal sepsis. J. Trauma. 1997;42:415–420. [Abstract] [Google Scholar]
- Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue Cell. 1990;22:407–417. [Abstract] [Google Scholar]
- Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am. J. Pathol. 1992;140:539–544. [Europe PMC free article] [Abstract] [Google Scholar]
- Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br. J. Cancer. 1999;79:1828–1835. [Europe PMC free article] [Abstract] [Google Scholar]
- Fortunato SJ, Menon R, Lombardi SJ. The effect of transforming growth factor and interleukin-10 on interleukin-8 release by human amniochorion may regulate histologic chorioamnionitis. Am. J. Obstet. Gynecol. 1998;179:794–799. [Abstract] [Google Scholar]
- Garner WL, Karmiol S, Rodriguez JL, Smith DJ, Jr, Phan SH. Phenotypic differences in cytokine responsiveness of hypertrophic scar versus normal dermal fibroblasts. J. Invest. Dermatol. 1993;101:875–879. [Abstract] [Google Scholar]
- Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. [Abstract] [Google Scholar]
- Haas TL, Davis SJ, Madri JA. Three-dimensional type I collagen lattices induce coordinate expression of matrix metalloproteinases MT1-MMP and MMP-2 in microvascular endothelial cells. J. Biol. Chem. 1998;273:3604–3610. [Abstract] [Google Scholar]
- Haas TL, Stitelman D, Davis SJ, Apte SS, Madri JA. Egr-1 mediates extracellular matrix-driven transcription of membrane type 1 matrix metalloproteinase in endothelium. J. Biol. Chem. 1999;274:22679–22685. [Abstract] [Google Scholar]
- Iocono J, Colleran K, Remick D, Gilespie B, Ehrlich H, Garner W. Interleukin-8 levels and activity in delayed-healing human thermal wounds. Wound Repair Regen. 2000;8:216–225. [Abstract] [Google Scholar]
- Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann. Med. 1999;31:34–45. [Abstract] [Google Scholar]
- Kitzis V, Engrav LH, Quinn LS. Transient exposure to tumor necrosis factor-alpha inhibits collagen accumulation by cultured hypertrophic scar fibroblasts. J. Surg. Res. 1999;87:134–141. [Abstract] [Google Scholar]
- Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. [Abstract] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 1995;270:14255–14258. [Abstract] [Google Scholar]
- Lohi J, Lehti K, Valtanen H, Parks WC, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242:75–86. [Abstract] [Google Scholar]
- Madtes DK, Raines EW, Sakariassen KS, Assoian RK, Sporn MB, Bell GI, Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988;53:285–293. [Abstract] [Google Scholar]
- Martin P. Wound healing – aiming for perfect skin regeneration. Science. 1997;276:75–81. [Abstract] [Google Scholar]
- Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr. Opin. Cell Biol. 1999;11:226–232. [Abstract] [Google Scholar]
- Migita K, Eguchi K, Kawabe Y, Ichinose Y, Tsukada T, Aoyagi T, Nakamura H, Nagataki S. TNF-alpha-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunology. 1996;89:553–557. [Abstract] [Google Scholar]
- Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J. Biol. Chem. 1998;273:25903–25914. [Abstract] [Google Scholar]
- Moses MA, Marikovsky M, Harper JW, Vogt P, Eriksson E, Klagsbrun M, Langer R. Temporal study of the activity of matrix metalloproteinases and their endogenous inhibitors during wound healing. J. Cell Biochem. 1996;60:379–386. [Abstract] [Google Scholar]
- Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J. Mechanisms for pro matrix metalloproteinase activation. APMIS. 1999;107:38–44. [Abstract] [Google Scholar]
- Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. [Abstract] [Google Scholar]
- Reno C, Boykiw R, Martinez ML, Hart DA. Temporal alterations in mRNA levels for proteinases and inhibitors and their potential regulators in the healing medial collateral ligament. Biochem. Biophys. Res. Commun. 1998;252:757–763. [Abstract] [Google Scholar]
- Salomon GD, Kasid A, Cromack DT, Director E, Talbot TL, Sank A, Norton JA. The local effects of cachectin/tumor necrosis factor on wound healing. Ann. Surg. 1991;214:175–180. [Abstract] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells [see comments] Nature. 1994;370:61–65. [Abstract] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C. Macrophages and angiogenesis. J. Leuko. Biol. 1994;55:410–422. [Abstract] [Google Scholar]
- Thompson EW, Yu M, Bueno J, Jin L, Maiti SN, Palao-Marco FL, Pulyaeva H, Tamborlane JW, Tirgari R, Wapnir I, et al. Collagen induced MMP-2 activation in human breast cancer. Breast Cancer Res. Treat. 1994;31:357–370. [Abstract] [Google Scholar]
- Trengove NJ, Bielefeldt-Ohmann H, Stacey MC. Mitogenic activity and cytokine levels in non-healing and healing chronic leg ulcers. Wound Repair Regen. 2000;8:13–25. [Abstract] [Google Scholar]
- Tuan TL, Keller LC, Sun D, Nimni ME, Cheung D. Dermal fibroblasts activate keratinocyte outgrowth on collagen gels. J. Cell Sci. 1994;107:2285–2289. [Abstract] [Google Scholar]
- Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann. NY Acad. Sci. 1998;857:110–118. [Abstract] [Google Scholar]
- Wysocki AB, Kusakabe AO, Chang S, Tuan TL. Temporal expression of urokinase plasminogen activator, plasminogen activator inhibitor and gelatinase-B in chronic wound fluid switches from a chronic to acute wound profile with progression to healing. Wound Repair Regen. 1999;7:154–165. [Abstract] [Google Scholar]
- Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J. Invest. Dermatol. 1993;101:64–68. [Abstract] [Google Scholar]
- Xu J, Zutter MM, Santoro SA, Clark RA. A three-dimensional collagen lattice activates NF-kappaB in human fibroblasts: role in integrin alpha2 gene expression and tissue remodeling. J. Cell Biol. 1998;140:709–719. [Europe PMC free article] [Abstract] [Google Scholar]
- Yammamoto H, Itoh F, Adachi Y, Fukushima H, Itoh H, Sasaki S, Hinoda Y, Imai K. Messenger RNA expression of matrix metalloproteinase and tissue inhibitors of metalloproteinase in human hepatocellular carcinoma. Jpn J. Clin. Oncol. 1999;29:58–62. [Abstract] [Google Scholar]
- Young PK, Grinnell F. Metalloproteinase activation cascade after burn injury: a longitudinal analysis of the human wound environment. J. Invest. Dermatol. 1994;103:660–664. [Abstract] [Google Scholar]
- Yu AE, Murphy AN, Stetler-Stevenson WG. 72-kDa gelatinase (gelatinase A): structure, activation, regulation, and substrate specificity. In: Parks WC, editor. Matrix Metalloproteinases. New York: Academic Press; 1998. pp. 85–113. [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1242/jcs.114.1.131
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2435089
Free after 6 months at jcs.biologists.org
http://jcs.biologists.org/cgi/reprint/114/1/131.pdf
Free to read at jcs.biologists.org
http://jcs.biologists.org/cgi/content/abstract/114/1/131
Free after 6 months at jcs.biologists.org
http://jcs.biologists.org/cgi/content/full/114/1/131
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/104889295
Article citations
Stromal softness confines pancreatic cancer growth through lysosomal-cathepsin mediated YAP1 degradation.
Cell Mol Life Sci, 81(1):442, 26 Oct 2024
Cited by: 0 articles | PMID: 39460766 | PMCID: PMC11512982
Antioxidant and Anti-Inflammatory Properties of Hydrolyzed Royal Jelly Peptide in Human Dermal Fibroblasts: Implications for Skin Health and Care Applications.
Bioengineering (Basel), 11(5):496, 16 May 2024
Cited by: 0 articles | PMID: 38790362 | PMCID: PMC11118532
GATA4 regulates the transcription of MMP9 to suppress the invasion and migration of breast cancer cells via HDAC1-mediated p65 deacetylation.
Cell Death Dis, 15(4):289, 23 Apr 2024
Cited by: 1 article | PMID: 38653973 | PMCID: PMC11039647
Redefining metalloproteases specificity through network proteolysis.
Trends Mol Med, 30(2):147-163, 29 Nov 2023
Cited by: 1 article | PMID: 38036391
Review
Human Probiotic Lactobacillus paracasei-Derived Extracellular Vesicles Improve Tumor Necrosis Factor-α-Induced Inflammatory Phenotypes in Human Skin.
Cells, 12(24):2789, 07 Dec 2023
Cited by: 2 articles | PMID: 38132109 | PMCID: PMC10741892
Go to all (219) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Collagen-induced proMMP-2 activation by MT1-MMP in human dermal fibroblasts and the possible role of alpha2beta1 integrins.
Eur J Cell Biol, 80(1):68-77, 01 Jan 2001
Cited by: 62 articles | PMID: 11211937
Role of PKC-α in NF-κB-MT1-MMP-mediated activation of proMMP-2 by TNF-α in pulmonary artery smooth muscle cells.
J Biochem, 153(3):289-302, 24 Dec 2012
Cited by: 5 articles | PMID: 23266860
Osteopontin induces nuclear factor kappa B-mediated promatrix metalloproteinase-2 activation through I kappa B alpha /IKK signaling pathways, and curcumin (diferulolylmethane) down-regulates these pathways.
J Biol Chem, 278(16):14487-14497, 07 Dec 2002
Cited by: 159 articles | PMID: 12473670
The Role of Membrane-Type 1 Matrix Metalloproteinase-Substrate Interactions in Pathogenesis.
Int J Mol Sci, 24(3):2183, 22 Jan 2023
Cited by: 2 articles | PMID: 36768503 | PMCID: PMC9917210
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIGMS NIH HHS (6)
Grant ID: GM55081
Grant ID: R01 GM055081
Grant ID: R29 GM050967
Grant ID: GM 50967
Grant ID: R29 GM050967-05
Grant ID: R01 GM050967




