Abstract
Background
Hereditary pancreatitis (HP) is a rare autosomal dominant disorder with variable expression and an overall lifetime penetrance of 80%. We hypothesised that (1) monozygotic twins within similar environments would develop the typical signs of HP at a similar age, and (2) if penetrance were due to modifier genes or environment, all twin pairs would be concordant for expression of HP.Aim
Identify monozygotic twins with HP and determine the penetrance, concordance, and age of onset of symptoms.Methods
Twins from HP kindreds were identified from the Midwest Multicenter Pancreatic Study group database, referrals, and literature searches. Each twin set was assessed for phenotypic expression, concordance, and difference in age of phenotypic onset of pancreatitis. The difference in onset of symptoms for symptomatic affected non-twin sibling pairs as well as non-twin pairs that were mutation, sex, and age matched were calculated as two comparison groups.Results
Seven of 11 monozygotic pairs identified were suitable for evaluation and four were concordant for pancreatitis. Forty eight affected sibling pairs and 33 pairs of mutation, sex, and age matched (cationic trypsinogen R122H (30 pairs) and N29I (three pairs)) subjects were identified for comparison groups. The median (quartiles Q1, Q3) difference in the age of phenotypic onset in the concordant twins was 1 (0, 2.4) years, 2 (1, 6) for the affected siblings, and 7 (2, 15) years in the comparison control group. Three of the seven sets of twins (43%) were discordant for phenotypic expression of pancreatitis. The overall penetrance in the seven pairs of monozygotic twins was 78.6%.Conclusions
Genetic and/or environmental factors contribute to expression and age of onset of HP. Nuclear genes or general environmental factors alone cannot explain the 80% penetrance. Determining the mechanism of non-penetrance may help in developing a strategy to prevent the phenotypic expression of pancreatitis in individuals with an underlying genetic predisposition.Free full text

Expression and penetrance of the hereditary pancreatitis phenotype in monozygotic twins
Abstract
BACKGROUND—Hereditary pancreatitis (HP) is a rare autosomal dominant disorder with variable expression and an overall lifetime penetrance of 80%. We hypothesised that (1) monozygotic twins within similar environments would develop the typical signs of HP at a similar age, and (2) if penetrance were due to modifier genes or environment, all twin pairs would be concordant for expression of HP.
AIM—Identify monozygotic twins with HP and determine the penetrance, concordance, and age of onset of symptoms.
METHODS—Twins from HP kindreds were identified from the Midwest Multicenter Pancreatic Study group database, referrals, and literature searches. Each twin set was assessed for phenotypic expression, concordance, and difference in age of phenotypic onset of pancreatitis. The difference in onset of symptoms for symptomatic affected non-twin sibling pairs as well as non-twin pairs that were mutation, sex, and age matched were calculated as two comparison groups.
RESULTS—Seven of 11 monozygotic pairs identified were suitable for evaluation and four were concordant for pancreatitis. Forty eight affected sibling pairs and 33 pairs of mutation, sex, and age matched (cationic trypsinogen R122H (30 pairs) and N29I (three pairs)) subjects were identified for comparison groups. The median (quartiles Q1, Q3) difference in the age of phenotypic onset in the concordant twins was 1 (0, 2.4) years, 2 (1, 6) for the affected siblings, and 7 (2, 15) years in the comparison control group. Three of the seven sets of twins (43%) were discordant for phenotypic expression of pancreatitis. The overall penetrance in the seven pairs of monozygotic twins was 78.6%.
CONCLUSIONS—Genetic and/or environmental factors contribute to expression and age of onset of HP. Nuclear genes or general environmental factors alone cannot explain the 80% penetrance. Determining the mechanism of non-penetrance may help in developing a strategy to prevent the phenotypic expression of pancreatitis in individuals with an underlying genetic predisposition.
Keywords: hereditary pancreatitis; genetic; twins; penetrance; trypsinogen
Full Text
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COMFORT MW, STEINBERG AG. Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology. 1952 May;21(1):54–63. [Abstract] [Google Scholar]
- Sossenheimer MJ, Aston CE, Preston RA, Gates LK, Jr, Ulrich CD, Martin SP, Zhang Y, Gorry MC, Ehrlich GD, Whitcomb DC. Clinical characteristics of hereditary pancreatitis in a large family, based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG) Am J Gastroenterol. 1997 Jul;92(7):1113–1116. [Abstract] [Google Scholar]
- Sibert JR. Hereditary pancreatitis in England and Wales. J Med Genet. 1978 Jun;15(3):189–201. [Europe PMC free article] [Abstract] [Google Scholar]
- Whitcomb DC. Genetic predispositions to acute and chronic pancreatitis. Med Clin North Am. 2000 May;84(3):531–vii. [Abstract] [Google Scholar]
- Whitcomb DC, Gorry MC, Preston RA, Furey W, Sossenheimer MJ, Ulrich CD, Martin SP, Gates LK, Jr, Amann ST, Toskes PP, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996 Oct;14(2):141–145. [Abstract] [Google Scholar]
- Gorry MC, Gabbaizedeh D, Furey W, Gates LK, Jr, Preston RA, Aston CE, Zhang Y, Ulrich C, Ehrlich GD, Whitcomb DC. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997 Oct;113(4):1063–1068. [Abstract] [Google Scholar]
- Witt H, Luck W, Becker M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology. 1999 Jul;117(1):7–10. [Abstract] [Google Scholar]
- Dasouki MJ, Cogan J, Summar ML, Neblitt W, 3rd, Foroud T, Koller D, Phillips JA., 3rd Heterogeneity in hereditary pancreatitis. Am J Med Genet. 1998 Apr 28;77(1):47–53. [Abstract] [Google Scholar]
- Kattwinkel J, Lapey A, Di Sant'Agnese PA, Edwards WA. Hereditary pancreatitis: three new kindreds and a critical review of the literature. Pediatrics. 1973 Jan;51(1):55–69. [Abstract] [Google Scholar]
- Le Bodic L, Schnee M, Georgelin T, Soulard F, Ferec C, Bignon JD, Sagniez M. An exceptional genealogy for hereditary chronic pancreatitis. Dig Dis Sci. 1996 Jul;41(7):1504–1510. [Abstract] [Google Scholar]
- Mathew P, Wyllie R, Van Lente F, Steffen RM, Kay MH. Antioxidants in hereditary pancreatitis. Am J Gastroenterol. 1996 Aug;91(8):1558–1562. [Abstract] [Google Scholar]
- Segal I, Gut A, Schofield D, Shiel N, Braganza JM. Micronutrient antioxidant status in black South Africans with chronic pancreatitis: opportunity for prophylaxis. Clin Chim Acta. 1995 Jul 31;239(1):71–79. [Abstract] [Google Scholar]
- Perrault J. Hereditary pancreatitis. Gastroenterol Clin North Am. 1994 Dec;23(4):743–752. [Abstract] [Google Scholar]
- Whitcomb DC, Preston RA, Aston CE, Sossenheimer MJ, Barua PS, Zhang Y, Wong-Chong A, White GJ, Wood PG, Gates LK, Jr, et al. A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996 Jun;110(6):1975–1980. [Abstract] [Google Scholar]
- BEALL JH, Jr, BELL JW, JESSEPH JE, NYHUS LM. Fatal acute hemorrhagic pancreatitis occurring simultaneously in identical twins. Gastroenterology. 1960 Aug;39:215–218. [Abstract] [Google Scholar]
- Freud E, Barak R, Ziv N, Leiser A, Dinari G, Mor C, Zer M. Familial chronic recurrent pancreatitis in identical twins. Case report and review of the literature. Arch Surg. 1992 Sep;127(9):1125–1128. [Abstract] [Google Scholar]
- Henderson J, Ingram D, House T. Acute pancreatitis in identical twins. Med J Aust. 1982 May 15;1(10):432–434. [Abstract] [Google Scholar]
- Pandya A, Blanton SH, Landa B, Javaheri R, Melvin E, Nance WE, Markello T. Linkage studies in a large kindred with hereditary pancreatitis confirms mapping of the gene to a 16-cM region on 7q. Genomics. 1996 Dec 1;38(2):227–230. [Abstract] [Google Scholar]
- Le Bodic L, Bignon JD, Raguénès O, Mercier B, Georgelin T, Schnee M, Soulard F, Gagne K, Bonneville F, Muller JY, et al. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996 Apr;5(4):549–554. [Abstract] [Google Scholar]
- Gress TM, Micha AE, Lacher U, Adler G. Diagnose einer "hereditären Pankreatitis" durch Nachweis der Mutation im kationischen Trypsinogen-Gen. Dtsch Med Wochenschr. 1998 Apr 9;123(15):453–456. [Abstract] [Google Scholar]
- Teich N, Mössner J, Keim V. Mutations of the cationic trypsinogen in hereditary pancreatitis. Hum Mutat. 1998;12(1):39–43. [Abstract] [Google Scholar]
- Nishimori I, Kamakura M, Fujikawa-Adachi K, Morita M, Onishi S, Yokoyama K, Makino I, Ishida H, Yamamoto M, Watanabe S, et al. Mutations in exons 2 and 3 of the cationic trypsinogen gene in Japanese families with hereditary pancreatitis. Gut. 1999 Feb;44(2):259–263. [Europe PMC free article] [Abstract] [Google Scholar]
- Turker MS, Bestor TH. Formation of methylation patterns in the mammalian genome. Mutat Res. 1997 Apr;386(2):119–130. [Abstract] [Google Scholar]
- Yoder JA, Bestor TH. Genetic analysis of genomic methylation patterns in plants and mammals. Biol Chem. 1996 Oct;377(10):605–610. [Abstract] [Google Scholar]
- Lalande M. Parental imprinting and human disease. Annu Rev Genet. 1996;30:173–195. [Abstract] [Google Scholar]
Figures and Tables
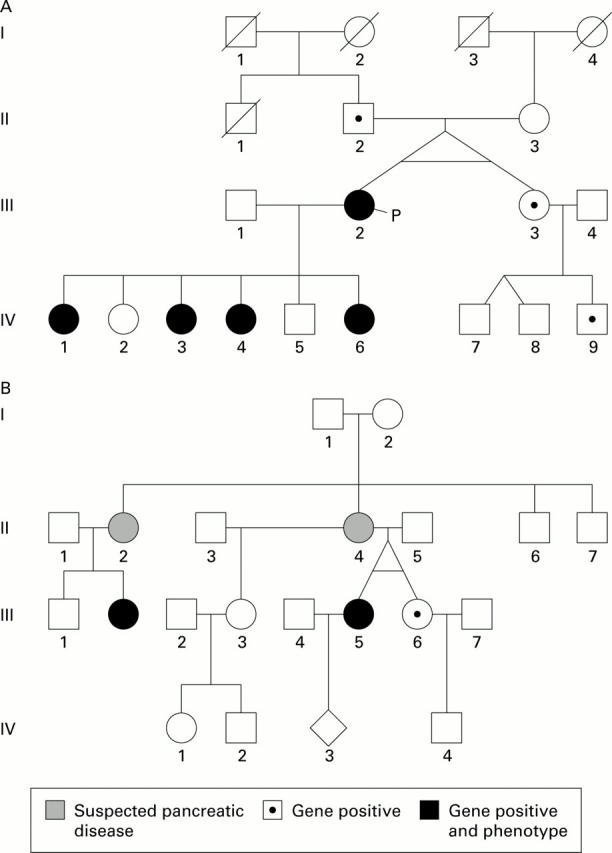
A and B represent kindreds "A" and "G", respectively. In both of these families the twins were raised in the same environment, attended the same college, and lived together until their 20s. Despite identical genes and very similar environments, only one twin of each pair developed phenotypic hereditary pancreatitis.
Articles from Gut are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/gut.48.4.542
Read article for free, from open access legal sources, via Unpaywall:
https://gut.bmj.com/content/gutjnl/48/4/542.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1136/gut.48.4.542
Article citations
The Role of Gut Microbiota and Genetic Susceptibility in the Pathogenesis of Pancreatitis.
Gut Liver, 16(5):686-696, 16 Dec 2021
Cited by: 6 articles | PMID: 34911043 | PMCID: PMC9474482
Review Free full text in Europe PMC
Impact of hereditary pancreatitis on patients and their families.
J Genet Couns, 29(6):971-982, 05 Feb 2020
Cited by: 6 articles | PMID: 32026589 | PMCID: PMC7747647
Genetic predisposition in pancreatitis.
Curr Opin Pediatr, 30(5):660-664, 01 Oct 2018
Cited by: 3 articles | PMID: 30015686
Review
PRSS1 (R122H) mutation in an Indian family with low penetrance is associated with pancreatitis phenotype.
Indian J Gastroenterol, 37(1):67-69, 01 Jan 2018
Cited by: 2 articles | PMID: 29476405
Hereditary Pancreatitis Associated With the N29T Mutation of the PRSS1 Gene in a Brazilian Family: A Case-Control Study.
Medicine (Baltimore), 94(37):e1508, 01 Sep 2015
Cited by: 5 articles | PMID: 26376395 | PMCID: PMC4635809
Go to all (32) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A 93 year old man with the PRSS1 R122H mutation, low SPINK1 expression, and no pancreatitis: insights into phenotypic non-penetrance.
Gut, 55(5):728-731, 14 Dec 2005
Cited by: 28 articles | PMID: 16354799 | PMCID: PMC1856140
The variable phenotype of the p.A16V mutation of cationic trypsinogen (PRSS1) in pancreatitis families.
Gut, 59(3):357-363, 01 Dec 2009
Cited by: 28 articles | PMID: 19951905
Neuroanatomic variation in monozygotic twin pairs discordant for the narrow phenotype for autism.
Am J Psychiatry, 161(3):539-546, 01 Mar 2004
Cited by: 71 articles | PMID: 14992981
[Hereditary pancreatitis].
Ugeskr Laeger, 165(5):447-451, 01 Jan 2003
Cited by: 1 article | PMID: 12599840
Review
Funding
Funders who supported this work.
NIAAA NIH HHS (1)
Grant ID: AA10885
NIDDK NIH HHS (1)
Grant ID: DK51954




