Abstract
Free full text

Requirement for p27KIP1 in Retinoblastoma Protein-Mediated Senescence
Abstract
In vivo and in vitro evidence indicate that cells do not divide indefinitely but instead stop growing and undergo a process termed cellular proliferative senescence. Very little is known about how senescence occurs, but there are several indications that the retinoblastoma protein (pRb) is involved, the most striking being that reintroduction of RB into RB−/− tumor cell lines induces senescence. In investigating the mechanism by which pRb induces senescence, we have found that pRb causes a posttranscriptional accumulation of the cyclin-dependent kinase inhibitor p27KIP1 that is accompanied by an increase in p27KIP1 specifically bound to cyclin E and a concomitant decrease in cyclin E-associated kinase activity. In contrast, pRb-related proteins p107 and p130, which also decrease cyclin E-kinase activity, do not cause an accumulation of p27KIP1 and induce senescence poorly. In addition, the use of pRb proteins mutated in the pocket domain demonstrates that pRb upregulation of p27KIP1 and senescence induction do not require the interaction of pRb with E2F. Furthermore, ectopic expression of p21CIP1 or p27KIP1 induces senescence but not the morphology change associated with pRb-mediated senescence, uncoupling senescence from the morphological transformation. Finally, the ability of pRb to maintain cell cycle arrest and induce senescence is reversibly abrogated by ablation of p27KIP1 expression. These findings suggest that prolonged cell cycle arrest through the persistent and specific inhibition of cdk2 activity by p27KIP1 is critical for pRb-induced senescence.
Instead of having an infinite capacity to proliferate, eukaryotic cells are thought to have a limited replicative lifespan. They undergo a terminal arrest of cellular division and take on an altered cellular state, termed senescence (6, 21). Cellular senescence was first observed by Hayflick and Moorhead in human fibroblasts passaged in culture and has since been seen to occur upon passage of many cell types from various species and upon oncogenic stimuli (25, 38, 45, 48, 52, 69). Importantly, senescence is believed to be tumor suppressive, as bypass of senescence leads to immortalization and, potentially, oncogenic transformation.
Senescent cells share several basic characteristics. One of these is stringency in human cells—while rodent cells often spontaneously immortalize, human cells do not (6, 21). Also, though senescent cells continue to be metabolically active, they undergo cell cycle arrest in the G1 phase that is irreversible in that they can not be mitogen stimulated to reenter S phase (6, 21). In addition, cells that have senesced exhibit changes in the expression of proteins that regulate cell cycle and take on an altered cellular morphology (5, 21, 52). Recently, the triggering of senescence has been associated with telomere shortening, and senescent cells, in contrast to immortal cells, exhibit decreased telomerase activity (31, 63, 66, 67). In fact, in transformed cells telomerase activation was found to be sufficient to allow cells to escape from senescent crisis (16, 23). However, the mechanism by which senescence occurs is relatively unclear, though evidence suggests that the retinoblastoma protein (pRb), a potent tumor suppressor, may play a role (32).
pRb, the product of the RB1 gene, is a 105-kDa phosphoprotein that regulates cell cycle progression from the G1 to S phase by reversibly inhibiting E2F-mediated transcription of genes required for S-phase entry (19, 47, 58, 65). pRb releases E2F when the former is phosphorylated and inactivated by the cyclin D-cdk4-cdk6 kinase in G1 and the cyclin E-cdk2 kinase at the G1-S boundary (4, 28, 33). The activity of these kinase complexes is negatively regulated by cyclin-dependent kinase inhibitors. Members of the INK4 family (p15, p16, p18, and p19) inhibit D-type cyclins, while CIP/KIP family members (p21, p27, and p57) inhibit E- and A-type cyclins (36, 51). In almost all human cancers, either RB or components of its regulatory pathway are mutated, suggesting that loss of pRb function is critical for oncogenesis.
In addition, the p53 gene, another potent tumor suppressor, is also found to be mutated or deleted in most human tumors (29). The primary anti-oncogenic function of p53 may be its rapid upregulation and subsequent induction of cell cycle arrest and apoptosis upon detection of DNA damage signals (20, 34, 50). An important mediator of p53-induced cell cycle arrest is its transcriptional target, the cyclin-dependent kinase inhibitor p21CIP1 (20). Many oncogenic, proliferation-promoting events have been demonstrated to induce p53-dependent apoptosis, suggesting that in cancer cells, selective loss of p53 protects them from programmed cell death (55).
Ample evidence implicates a major role for tumor suppressors in cellular senescence (6, 21). However, recent findings indicate that pRb may be a crucial regulator of certain forms of senescent cell cycle exit in human cells, while p53 may be less critical. p53 and p21 levels are often seen to increase in senescent human diploid fibroblasts (2, 3, 38, 48, 69). Nevertheless, it has been observed that bypass of replicative senescence by human diploid fibroblasts did not require p53 inactivation, though this immortalization did occur with the introduction of the pRb-inactivating viral oncoprotein E7 in combination with increased telomerase activity (32). Similarly, in human cells p53 was found to be dispensable in oncogenic Ras-induced senescence, while E1A—which inactivates and sequesters pRb—blocked the senescence triggered by oncogenic Ras (48). Also, the reestablishment of the pRb pathway by the readdition of p16INK4a in cells mutated for p16INK4a led to senescence (15). Finally, reintroduction of pRb into RB−/− tumor cell lines induced senescence, even in cells that did not contain wild-type p53 (67). Characteristic of senescence, the state induced by pRb was found to be irreversible—the inactivation of pRb in senescent cells led to the abortive reentry of these cells into S phase and their subsequent apoptotic death (58, 67).
With pRb clearly implicated in senescence, we wanted to determine its unique role in the senescence process and establish the mechanism by which pRb-mediated senescence occurs. We have found that, even in the absence of an intact p53 pathway, pRb is able to induce senescence, causing an accumulation of p27KIP1 in senescent cells. The posttranscriptional upregulation of p27KIP1 by pRb is critical to the ability of pRb to maintain cellular arrest but is not sufficient for the singular function of pRb to promote the morphological change associated with senescent cells.
MATERIALS AND METHODS
Cell culture, plasmids, and transfection.
The human osteosarcoma cell line SAOS-2, subclone 2.4, was used for these studies (28). The cells were maintained in Dulbecco's modified Eagle's medium (Gibco/BRL) supplemented with 15% heat-inactivated fetal bovine serum and penicillin-streptomycin in a 5% CO2 incubator at 37°C. The pRb expression plasmid was constructed in the pSVE vector (28, 57). pCMVHA-p107 and pCMVHA-p130 were gifts from Jim DeCaprio and have been described previously (61, 71). Made in the pSG5L vector (Stratagene), pRbΔ651, pRbΔ657, and pRbΔ663 were constructed as described previously and were generous gifts of William Kaelin (46). pCMVp16 has been described before (27). pCMVCdk2NFG was a gift from Sander Van der Heuvel (1993). For puromycin selection, the vector pBabepuro was used (41).
SAOS-2 cells were transfected at 80% confluency with the indicated plasmids on 10- and 6-cm plates using 2× Bes-buffered saline and calcium phosphate (9, 28). DNA precipitates were removed as described previously (35). Six-well plates with SAOS-2 cells at 80% confluency were transfected using the Fugene 6 reagent (Gibco/BRL).
Immunoblotting and immunoprecipitation.
Expression of transfected and endogenous proteins was detected by immunoblotting and metabolic labeling. Cells were lysed in 100 μl of ELB (50 mM HEPES [pH 7.2], 250 mM NaCl, 2 mM EDTA, 0.1% NP-40, 1 mM dithiothreitol) plus protease and phosphatase inhibitors (1 μg of aprotinin per ml, 1 μg of leupeptin per ml, 100 μg of Pefabloc, 4 mM sodium orthovanadate, 2 mM sodium PPi) per 10-cm plate. For metabolic labeling, cells were incubated in methionine-free media with 15% dialyzed fetal bovine serum for 45 min, labeled for 4 h (cyclin E immunoprecipitation), 1.5 h (pulse-chase-p27 immunoprecipitation), or 1 h (p27 immunoprecipitation) with 500 μCi of Expre-35S35S (NEN) in 2 ml of methionine-free media plus 15% dialyzed fetal bovine serum, and then lysed in 1 ml of ELB plus inhibitors. For the p27 immunoprecipitation, harvested lysates were first boiled at 100°C for 5 min. Protein concentrations in cell lysates were determined by Bio-Rad protein assay.
For immunoblotting, 30 to 50 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose by standard procedures. Antibodies used for immunoblotting include the following: anti-pRb Ab-5 (Oncogene Sciences), antihemagglutinin (Babco), anti-p27KIP1 K25020 (Transduction Laboratories), and anti-PAI-1 Ab-1 (Calbiochem). Anti-cyclin A H-432, anti-cyclin E HE12, and anti-Cdk2 M2 were all from Santa Cruz. Anti-p16INK4a monoclonal antibodies JC2 and JC8 were the gifts of Ed Harlow and Jim Koh; anti-p21CIP1 monoclonal antibodies CP74 and CP36 were the gifts of Ed Harlow and Brian Dynlacht. Proteins were detected using horseradish peroxidase-conjugated donkey anti-mouse or anti-rabbit antibodies (Jackson Immunosciences).
Immunoprecipitations were performed using 100 to 150 μg of cell lysate. Lysates were immunoprecipitated with anti-p27KIP1 K25020 (Transduction Laboratories) for 2 h at 4°C, 30 μl of protein A beads was added for 1 h, and then the beads were washed four times with ELB and separated by SDS-PAGE. Cyclin E and cyclin A2 were immunoprecipitated with agarose-conjugated antibodies HE111 AC and BF683 AC, respectively (Santa Cruz), for 1 h with CL-4B beads, washed four times with ELB, and separated by SDS-PAGE. To detect cyclin E kinase activity, immunoprecipitations were carried out as described above and then were subjected to an in vitro kinase assay essentially as described previously with slight modifications (35). The kinase reaction was stopped with 12 μl of 6× SDS sample buffer. Prior to autoradiography the gel was stained for 5 min with Coomassie brillant blue to ensure an equal amount of histone H1 and then was destained overnight and dried for 1 h.
Cell cycle analysis.
Determination of the percentage of cells in G1 phase for the indicated plasmids was done using fluorescence-activated cell sorting (FACS) as described previously (71). For 10-cm or 6-well plates, 5 or 0.5 μg, respectively, of the expression plasmid for the B-cell surface marker CD20, pCMVCD20 (62), was cotransfected with 10 or 0.5 μg, respectively, of the indicated plasmids. Forty-eight hours posttransfection the cells were rinsed off the plates with phosphate-buffered saline (PBS) plus 0.1% EDTA, pelleted, and stained with 20 μl of fluorescein isothiocyanate (FITC)-conjugated CD20 (Pharmingen). Cells were fixed with 90% ethanol and left at 4°C for 1 h to several days. Prior to FACS analysis cells were stained with 20 μg of propidium iodide (PI) per ml and treated with 200 μg of RNase A per ml for 30 min at room temperature in the dark. Flow cytometric analysis was performed on a Becton Dickinson machine, with the intensity of PI and FITC measured by CellQuest and the cell cycle analysis done using ModFit (Becton Dickinson).
To analyze the number of cells actively synthesizing DNA in S phase, SAOS-2 cells on 6-cm plates at 80% confluency were cotransfected with 0.5 μg of pBabepuro and 5 μg of the indicated plasmid. Twenty-four hours after transfection cells were split to coverslips in a 24-well plate, put in selective media 24 h after being split, and measured for their ability to incorporate bromodeoxyuridine (BrdU). The BrdU labeling reagent was added to a final concentration of 10 mM 5 or 10 days after transfection. Coverslips were fixed in a solution containing 70% ethanol and 50 mM glycine (pH 2.0) at −20°C and incubated for 1 h at 37°C with a BrdU monoclonal antibody from the 5′-bromo-2′-deoxyuridine labeling and detection kit II (Roche Molecular Biochemicals). Cells were then washed three times in PBS plus 0.1% bovine serum albumin (BSA) and incubated with FITC-conjugated anti-mouse (BrdU kit; Roche) for 30 min at 37°C and counterstained with Hoechst stain. After being washed as described above, coverslips were mounted in Fluoromount G. At least 300 cells were counted using a Leica microscope.
Immunofluorescence.
On 6-well plates, SAOS-2 cells at 80% confluency were transfected with the indicated plasmids using the Fugene 6 reagent. Cells were split to coverslips 24 h after transfection and at the indicated time were fixed for 5 min in methanol followed by 2 min in acetone at −20°C. Coverslips were then washed three times in PBS plus 0.1% BSA, incubated in primary antibody for 1 h at 37°C, washed in PBS plus 0.1% BSA again, and then incubated in secondary antibody for 30 min at 37°C. Primary antibodies used for staining were monoclonal anti-p27 K25050 (1:100 dilution) (Transduction Laboratories), goat polyclonal anti-cyclin E C-19 (1:50 dilution) (Santa Cruz), rabbit polyclonal anti-cdk2 M2 (1:50 dilution) (Santa Cruz), and monoclonal anti-α-tubulin (1:1,000 dilution) (Sigma). Secondary antibodies consisted of Oregon green-conjugated anti-rabbit (1:1,000 dilution) (Molecular Probes), Cy5-conjugated goat anti-rabbit (1:250 dilution) (Amersham Pharmacia Biotech), Cy3-conjugated goat anti-mouse (1:100 dilution), and FITC-conjugated donkey anti-goat (1:50 dilution) (Jackson Immunosciences). Since both the p27KIP1 and α-tubulin antibodies were from the same host, the antibody staining was done serially. First cells were incubated with anti-p27KIP1 antibody, then with rabbit anti-mouse immunoglobidin G antibody (1:100), and then Oregon green-conjugated anti-rabbit antibody. Following p27KIP1 staining, α-tubulin staining was performed as described above. After staining, coverslips were mounted using Fluoromount G and visualized using a Leica microscope with Sony digital imaging (p27 single staining) or a Delta Vision deconvolution microscope (coimmunostaining).
SA–β-gal assay.
For the senescence-associated β-galactosidase (SA–β-gal) assay, SAOS-2 cells were cotransfected with the indicated plasmids and pBabepuro and selected 24 h posttransfection. The cells were maintained in selective media for 10 days, and the SA–β-gal assay was performed as described previously (17). All photography of SA–β-gal-positive cells was done on a Leica microscope with Sony digital imaging.
Antisense oligonucleotide treatment.
SAOS-2 cells at 80% confluency were transfected on 6-well plates using the Fugene 6 reagent with 0.5 to 1 μg of the indicated plasmids. Twenty-four hours after transfection the cells were split to another 6-well plate and coverslips in a 24-well plate. Twenty-four hours after the cells were split, they were transfected using the Fugene 6 transfection reagent with p27 antisense (p27AS) or p27 mismatch (p27C) oligonucleotides. Six-well plates were transfected with 1 μg of the oligonucleotides (to 0.5 μg of DNA), and 24-well plates were transfected with 0.3 μg of the oligonucleotides (to 0.1 μg of DNA). The oligonucleotides were constructed to base pairs 306 to 320 of murine KIP1 as follows: p27AS, UGG CUC UCC UGC GCC, and p27C, UCC CUU UGG CGC GCC (13; Biosource International-Keystone). For immunoblotting, immunofluorescence, and FACS, 10 h after oligonucleotide treatment cells were harvested and extracts were prepared for immunoblotting or harvested cells were fixed and PI stained for FACS as described previously. For BrdU and SA–β-gal assays, cells were transfected every 48 h with p27C and p27AS oligonucleotides for the indicated time. We found that the cells did not take up the oligonucleotides as well in selective medium, so the selective medium was replaced with nonselective medium immediately prior to transfection of the oligonucleotides. Cells were then placed under selection again 10 to 18 h after oligonucleotide transfection. After the indicated incubation time, the SA–β-gal or BrdU assay was performed as previously described.
Northern blotting.
Total RNA was isolated from subconfluent cultures using Trizol (Gibco/BRL). Ten micrograms of RNA was resolved by electrophoresis, transferred to Hybond-N+ membranes, and probed according to standard procedures. The 500-bp DNA probe was constructed to the human p27KIP1 gene and labeled with [32α]dCTP using the High Prime DNA labeling kit (Roche Molecular Biochemicals).
RESULTS
pRb acutely increases p27KIP1 to inhibit cyclin E-associated kinase activity.
To determine the distinct role of pRb in senescence, we chose to employ the osteosarcoma tumor cell line SAOS-2, which is mutated for both p53 and RB. Past work has indicated that pRb causes an acute G1-S cell cycle arrest in SAOS-2 cells and senescence (22, 28, 47, 58, 67); hence, prolonged cellular arrest by pRb may be important in the maintenance of the senescent phenotype. In order to further understand the nature of the G1 arrest imposed by pRb, we investigated changes to cell cycle proteins upon reintroduction of RB into SAOS-2 cells.
Forty-eight hours after transfection of SAOS-2 cells with RB, when these cells exhibit a G1 cell cycle arrest, lysates from RB-transfected cells were compared to lysates of cells transfected with empty vector by immunoblotting. We saw a striking upregulation of p27KIP1, while there were no changes in the level of cyclin E, cyclin A, or cdk2 (Fig. (Fig.1A).1A). p21CIP1 expression, which is at nearly undetectable levels in these cells due to lack of p53, did not change, but there was a small increase in p16INK4a levels, which are already high, consistent with pRb loss and the observed lack of cdk4-cdk6 activity in these cells (1, 43, 49, 68). Because the endogenous expression of p16INK4a in SAOS-2 cells is sufficient to inactivate most of the endogenous cdk4-cdk6, one would predict the increase in p16INK4a would not have any consequence on cell cycle. Indeed, this has been observed by ourselves and others (39 and data not shown). However, increased expression of p27KIP1 could well be responsible for G1 arrest by inhibiting cdk2 activity.
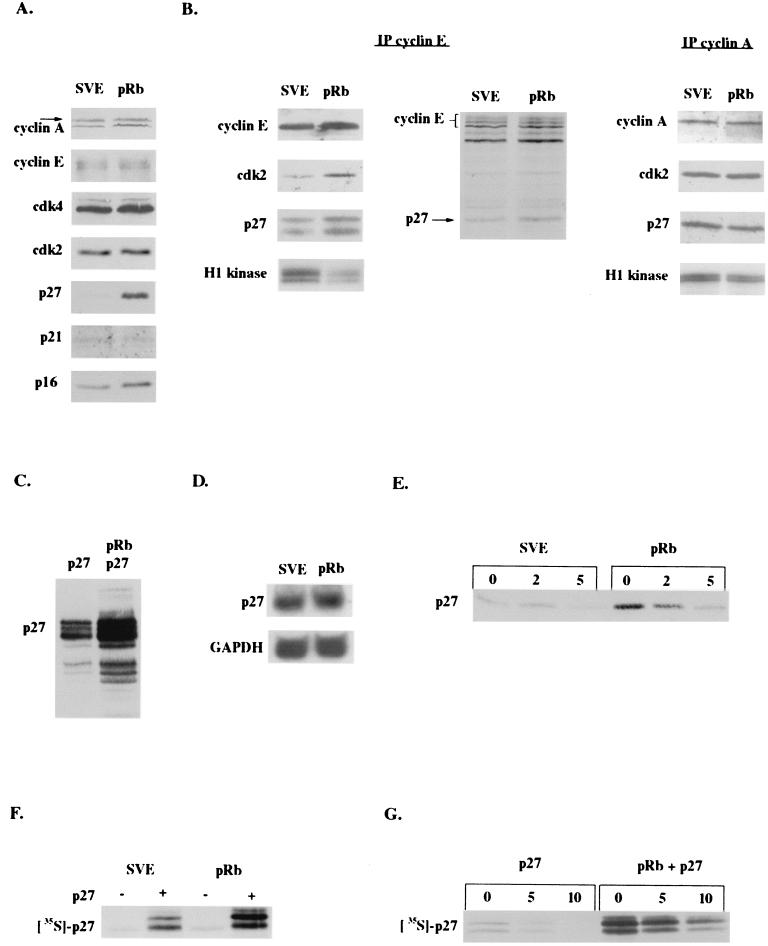
pRb increases p27KIP1 levels posttranscriptionally. The SAOS-2 human osteosarcoma cell line was transfected with empty vector (pSVE) or pRb expression plasmids and cell lysates obtained 48 to 72 h posttransfection. (A) Immunoblot of cell cycle proteins. (B) Immunoblot and in vitro kinase assay of cyclin E (left panel) and cyclin A (right panel) immunoprecipitations (IP). Cells labeled for 4 h with 35S-methionine were immunoprecipitated with anti-cyclin E and visualized by SDS-PAGE (middle panel). (C) Immunoblot of cells cotransfected with a p27KIP1 expression vector. (D) Northern blot of vector and pRb-transfected cells. (E) Transfected cells were treated with cycloheximide for the indicated times, and cell lysates were immunoblotted for p27KIP1 to determine its half-life. (F) Cells were cotransfected with vector or p27KIP1 and labeled for 1 h with 35S-methionine, and lysates were obtained and boiled at 100°C and immunoprecipitated with anti-p27KIP1. (G) Cells cotransfected with p27KIP1 were labeled for 1.5 h with 35S-methionine and chased with unlabeled methionine. Lysates were then harvested at the indicated timepoints and immunoprecipitated with anti-p27KIP1.
To determine the effect of increased p27KIP1 expression on the kinase complexes it regulates, we immunoprecipitated cyclin E from lysates prepared 48 h after cells were transfected with RB. We found that compared to cells transfected with vector, RB-transfected cells displayed increased p27KIP1 binding to cyclin E but not cyclin A (Fig. (Fig.1B).1B). In addition, there was more cdk2 bound to cyclin E, perhaps due to the increased G1 fraction in the presence of pRb. We confirmed this result by immunoprecipitating cyclin E from RB-transfected cells that had been metabolically labeled with 35S-methionine. Again, we observed more p27KIP1 in complex with cyclin E in the presence of pRb (Fig. (Fig.1B).1B). Finally, we looked at the effect of increased p27KIP1-cyclin E binding on cyclin E-cdk2 activity. We found that in the presence of pRb, the ability of the cyclin E-cdk2 complex to phosphorylate histone H1 in vitro was significantly reduced compared to that in cells without pRb. There was a comparatively small decrease in cyclin A-associated kinase activity, perhaps due to cyclin A-cdc2 activity in the population of cells still remaining in the G2-M phase of the cell cycle. Hence, the primary consequence of the pRb-mediated increase in p27KIP1 levels appears to be to bind and inhibit cyclin E-cdk2 complexes.
pRb increases p27KIP1 levels posttranscriptionally.
With the increase in p27KIP1 levels implicated in pRb-mediated cell cycle arrest, we wanted to investigate how pRb was regulating p27KIP1 expression. Interestingly, cotransfection of RB with p27KIP1 resulted in increased expression of the exogenously introduced p27KIP1, suggesting that pRb regulation of p27KIP1 may be posttranscriptional (Fig. (Fig.1C).1C). We confirmed this by performing a Northern blotting of cells transfected with RB either with or without p27KIP1, demonstrating that pRb did not increase either endogenous (Fig. (Fig.1D)1D) or exogenous (data not shown) p27KIP1 levels transcriptionally.
Much work has shown that p27KIP1 can be regulated posttranslationally through degradation (7, 42, 54, 60), so we investigated this possibility. Using cycloheximide to block new protein synthesis, we determined the half-life of p27KIP1 levels in cells with or without exogenous RB. We found that endogenous p27KIP1 has a half-life of approximately 2.5 h in SAOS-2 cells, but despite its upregulation in the presence of pRb its half-life was increased only marginally (Fig. (Fig.1E).1E). The results were similar for exogenous p27KIP1 in the presence of RB (data not shown). These results suggested that pRb regulation of p27KIP1 might occur primarily at the level of protein synthesis; however, it was also possible that cycloheximide was allowing the degradation of a posttranslational regulator of p27KIP1 that may have a shorter half-life than p27KIP1 itself. To differentiate between the two possibilities, we metabolically labeled cells transfected with RB both with and without exogenous p27KIP1. Cells cotransfected with RB, labeled for 1 h with 35S-methionine, and immunoprecipitated for p27KIP1 displayed higher p27KIP1 levels than those cells not transfected with RB (Fig. (Fig.1F).1F). Similarly, cells cotransfected with or without RB and p27KIP1, labeled for 1.5 h with 35S-methionine, and then chased with unlabeled methionine demonstrated again that there was more incorporation of 35S-methionine into p27KIP1 in cells cotransfected with RB, suggesting that pRb expression increased the synthesis rate of p27KIP1 (Fig. (Fig.1G).1G). In addition, in agreement with the cycloheximide results, the chase of labeled cells with excess unlabeled methionine indicated that there was no significant increase in the half-life of p27KIP1 in the presence of pRb (Fig. (Fig.1G).1G). Altogether the data indicate that pRb initially upregulates p27KIP1 primarily at the level of protein synthesis, consistent with the translational regulation of p27KIP1 described previously (26).
pRb, but not p107 or p130, induces senescence and p27KIP1 accumulation.
The pRb-like proteins p107 and p130 are thought to have functions in the cell that are both overlapping with and distinct from those of pRb. Others have shown that p107 and p130 can acutely arrest SAOS-2 cells in G1 phase in a manner comparable to that of pRb but do not comparably induce the pRb-mediated morphology change, termed flat-cell formation, after prolonged expression (28, 46). In addition, both p107 and p130 can directly inhibit cdk2 in a manner similar to that of p27KIP1 (8, 13, 70). Hence, we wanted to determine whether senescence was a unique function of pRb and if the increase in p27KIP1 levels played a role in maintaining this terminally arrested phenotype. To do this we investigated the ability of ectopically expressed p107 and p130 to induce senescence in SAOS-2 cells.
To assay the proliferative capacity of cells at the time of senescent arrest, SAOS-2 cells were transfected with RB and HA-tagged p107 or 130, put under puromycin selection 24 to 36 h later, and stained for BrdU incorporation at 5 days posttransfection, when flat cells first begin to appear. As was seen 2 days after transfection (11, 28, 46, 71), p107 and p130 did maintain the cells in an arrested state, decreasing the number of cells in S phase as effectively as pRb (Fig. (Fig.2A).2A). We then looked at the ability of p107 and p130 to induce senescence by staining puromycin-selected cells 10 days after transfection for a senescence marker—SA–β-gal activity. Consistent with previous results, we found that p107 and p130 induced flat cells poorly compared to pRb (46) and further demonstrated very reduced or no ability to induce SA–β-gal in these cells (Fig. (Fig.2B).2B). Thus, cell cycle arrest is not enough to induce the morphology change and senescence; this process seems to be a specific function of pRb.
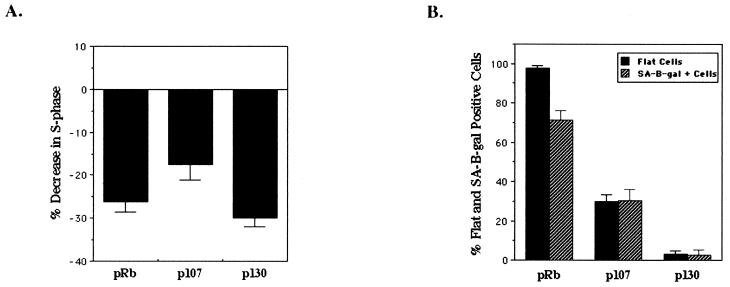
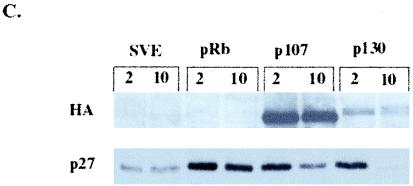
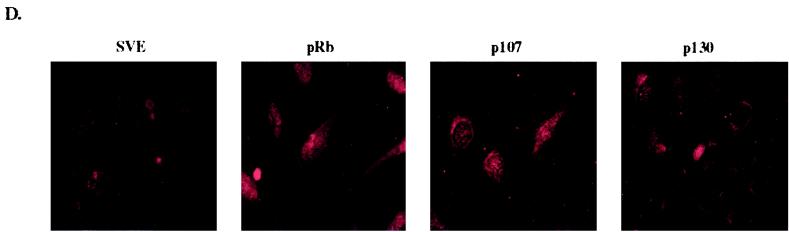
pRb-mediated senescence is linked to p27KIP1 accumulation. SAOS-2 cells were transfected with empty vector (pSVE), RB, HA-p107, or HA-p130 expression plasmids and pBabe-puro to select transfected cells by puromycin drug resistance. (A) Forty-eight hours after transfection cells were placed under puromycin selection. Five days posttransfection cells were labeled with BrdU and immunostained with an anti-BrdU antibody (Roche), and BrdU-positive cells were counted (at least 250 cells). The number of BrdU-positive cells is represented as a decrease in BrdU-positive cells compared to vector-transfected cells and represents the average of at least three experiments. (B) Forty-eight hours posttransfection cells were selected with puromycin and then 10 days after transfection were stained for SA–β-gal activity and flat and SA–β-gal-positive cells were counted. Percent flat and SA–β-gal-positive cells indicates the number of flat cells or SA–β-gal-positive cells divided by the total number of cells counted (at least 100 cells) and represents at least five independent experiments. (C) Immunoblot of cells 2 and 10 days posttransfection. (D) p27KIP1 expression in senescent cells. Ten days after transfection puromycin-selected cells were immunostained with anti-p27KIP1.
Next, we wanted to determine if ectopic expression of p107 and p130 led to an increase in p27KIP1 levels. SAOS-2 cells transfected with RB, HA-p107, and HA-p130 were harvested at both 2 and 10 days posttransfection to look at acute and persistent induction of p27KIP1 by immunoblotting. Interestingly, all the pocket proteins increased p27KIP1 expression transiently, perhaps indicating an involvement of p27KIP1 in normal cell cycle arrest, but only pRb maintained high levels of p27KIP1 at 10 days, correlating elevated p27KIP1 expression with senescence (Fig. (Fig.2C).2C). In contrast, compared to vector-transfected cells at 10 days, p107 only slightly increased p27KIP1 levels while p130 appeared to repress p27KIP1 expression. To confirm this result on a cellular level, cells transfected with the pocket proteins and put under selection were stained for p27KIP1 expression after 10 days, enabling us to look at the level of p27KIP1 expression in individual cells by immunofluorescence. While p27KIP1 was highly expressed nuclearly and cytoplasmically in RB-transfected cells, p27KIP1 staining was comparatively much less in p107-transfected cells and was nearly absent in vector- and p130-transfected cells at 10 days (Fig. (Fig.2D).2D). In addition, both the immunoblot and immunofluorescence results suggest a direct correlation between high levels of p27KIP1 expression and number of flat cells, possibly indicating that maintenance of p27KIP1 expression is linked to the morphology change.
pRb upregulation of p27KIP1 is not E2F-dependent.
pRb is thought to enforce cell cycle arrest through its interaction with E2F by repressing E2F-mediated transcription of target genes whose expression is required for S-phase entry. In agreement with this, it was shown that mutation of the gene encoding pRb in the pocket domain abolished its ability to stably bind E2F, repress transcription, and induce an acute G1 growth arrest (46). To determine if the pRb-E2F interaction was involved in pRb's regulation of p27KIP1, we employed these pRb pocket mutants. With a flexible linker sequence, NAAIRS, inserted at the first amino acid indicated by the mutant name, HA-pRbΔ651 and HA-pRbΔ657 bind E2F in solution but not on DNA, while HA-pRbΔ663 does not bind E2F either in solution or on DNA (46). By FACS analysis we confirmed that transiently at 48 h pRb induced a significant G1 arrest, while the non-E2F-regulating pRb mutants, pRbΔ651, pRbΔ657, and pRbΔ663, did not (Fig. (Fig.3A)3A) (46).
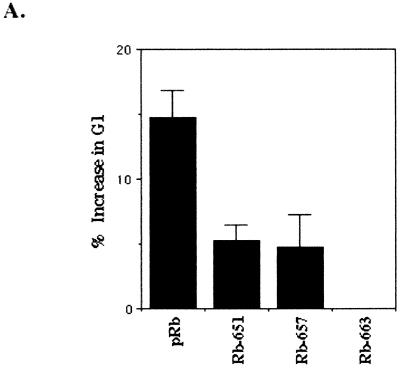
E2F repression is not required for pRb upregulation of p27KIP1. SAOS-2 cells were transiently transfected with empty vector (pSVE), RB, HA-pRbΔ651, HA-pRbΔ657, or HA-pRbΔ663. (A) Cells were cotransfected with an expression vector for the CD20 cell surface protein. Forty-eight hours posttransfection, cells were harvested, fixed, incubated with FITC-conjugated anti-CD20 to identify transfected cells, and stained with PI to determine DNA content. Cell cycle analysis was then performed by FACS, with 10,000 CD20-positive events counted. Results represent the percent increase of cells in the G1 phase over vector-transfected cells and are the average of at least two independent experiments. (B) Immunoblot of transfected cells 48 h posttransfection. (C) Immunoblot and in vitro kinase assay of lysates immunoprecipitated with anti-cyclin E 48 h after transfection. (D) Immunofluorescence analysis of cyclin E, cdk2, and p27KIP1 in transfected cells. Cells transfected with vector, RB, or HA-pRbΔ663 were coimmunostained with cyclin E (green), cdk2 (blue), and p27KIP1 (red) antibodies 48 h posttransfection.
Next, to investigate whether the pRb-E2F interaction was required for pRb-mediated upregulation of p27KIP1, we performed an immunoblotting of lysates from cells harvested 48 h after transfection with pRb and the pRb pocket mutants. We found that, like wild-type pRb, pRbΔ651, pRbΔ657, and pRbΔ663 also induced a transient increase in p27KIP1 levels, indicating that pRb does not have to interact with E2F in order to regulate p27KIP1 expression (Fig. (Fig.3B).3B). However, the pRb pocket mutants also displayed elevated cdk2 levels compared to those of control and wild-type pRb-transfected cells. Thus, this paradoxical increase in p27KIP1 levels in the absence of a G1 arrest suggested that p27KIP1 may not be sufficient to inhibit cdk2 activity in cells expressing the pRb mutants. To investigate this possibility we examined levels of p27KIP1 and cdk2 complexed with cyclin E by immunoprecipitating cyclin E from lysates of cells transfected with wild-type and mutant pRb. As with wild-type pRb, the transient increase in p27KIP1 levels produced by the pRb pocket mutants also led to an increase in p27KIP1 bound to cyclin E (Fig. (Fig.3C).3C). Strikingly, there was more cdk2 bound to cyclin E in pRbΔ651, pRbΔ657, and most notably in pRbΔ663 transfectants, correlating well with the inability of these proteins to induce a G1 arrest. This result implies that the higher levels of cdk2 bound to cyclin E could negate the increase in p27KIP1 levels, shifting the equilibrium back towards cellular proliferation. In agreement with this, when lysates transiently transfected with the pRb pocket mutants were assayed in vitro for their cyclin E-associated kinase activity, we found that cells transfected with the pRb pocket domain mutants had higher cyclin E-associated kinase activity than that in pRb-transfected cells (Fig. (Fig.3C).3C). The cyclin E, cdk2, p27KIP1 interaction was further investigated in vivo by coimmunostaining. Compared to vector-transfected cells, both pRb and pRbΔ663 upregulated p27KIP1; however, in RB-transfected cells p27KIP1 primarily colocalized with cdk2 (purplish hue), while pRbΔ663 transfectants displayed a preponderance of non-p27KIP1-associated cdk2 (Fig. (Fig.33D).
pRb mutants deficient in E2F regulation induce a delayed cell cycle arrest and senescence that correlates with p27KIP1 accumulation.
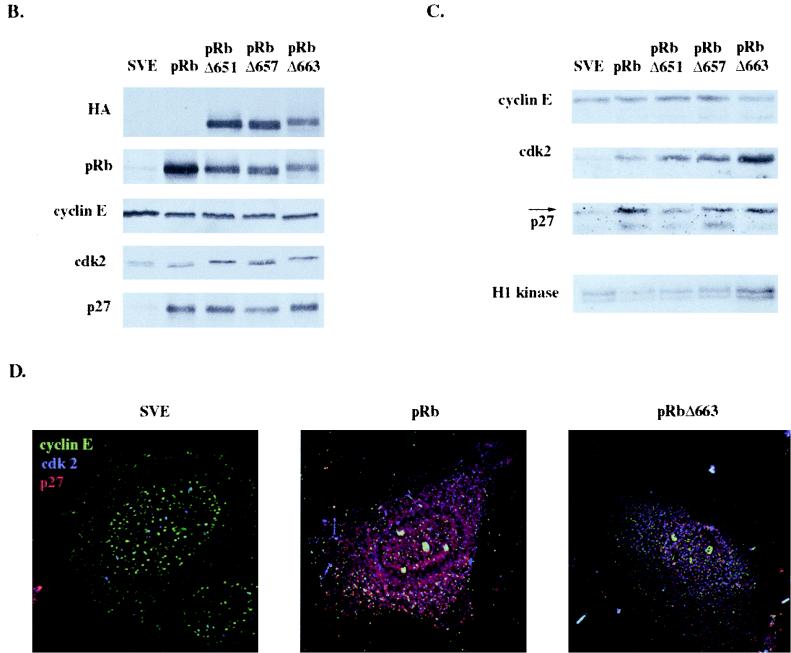
It has been reported that some of the non-E2F binding pRb mutants could cause the morphology change in SAOS-2 cells that has recently been linked to senescence and differentiation (46, 64). We wanted to determine if the flat cells produced by those mutants, pRbΔ651 and pRbΔ663, were senescent, clarifying if pRb regulation of E2F was required for pRb-mediated senescence. We determined the abilities of cells transfected with HA-pRbΔ651, HA-pRbΔ657, and HA-pRbΔ663 to incorporate BrdU compared to those of the other pocket proteins, pRb, p107, and p130. We found that by 5 days, pRbΔ651 and pRbΔ663 had achieved an arrested cellular state, decreasing the number of cells in S phase comparably to that of p107 (Fig. (Fig.4A).4A). pRbΔ657, however, was not as successful at halting cellular proliferation. When we assayed the ability of the pRb pocket domain mutants to induce senescence, pRbΔ651 and pRbΔ663, mutants that had established cell cycle arrest by 5 days, retained the ability to induce flat cells that stained positively for SA–β-gal activity (Fig. (Fig.4B).4B).
pRb pocket mutants induce senescence and p27KIP1 accumulation. SAOS-2 cells were transfected with empty vector (pSVE), RB, HA-p107, HA-p130, HA-pRbΔ651, HA-pRbΔ657, or HA-pRbΔ663 and a puromycin resistance plasmid. (A) Cells were puromycin selected 48 h after transfection and at 5 days posttransfection were labeled with BrdU and stained with anti-BrdU, and BrdU-positive cells were counted (at least 250 cells). Results represent the decrease in BrdU-positive cells compared to vector-transfected cells and are the average of at least three experiments. (B) Ten days after transfection puromycin-selected cells were stained for SA–β-gal activity and flat cells (solid bars) and SA–β-gal-positive cells (hatched bars) were counted. Results are the number of flat or SA–β-gal-positive cells divided by the total number of cells counted (at least 100 cells) and represent the average of at least three experiments. (C) Immunoblot at 10 days of lysates from cells transfected with the indicated plasmids and puromycin selected. (D) Ten days posttransfection cyclin E immunoprecipitation and in vitro kinase assay of cells transfected with the indicated plasmids and puromycin selected. (E) Coimmunostaining of vector-, RB-, or HA-pRbΔ663-transfected cells with cyclin E (green), cdk2 (blue), and p27KIP1 (red) antibodies 10 days posttransfection. Yellow staining in RB-transfected cells indicates colocalization of cyclin E and p27KIP1.
We were interested in how these pRb pocket mutants that were unable to bind E2F and transiently arrest cells were eventually able to arrest cells and induce senescence. Hypothesizing that p27KIP1 or other cell cycle proteins regulated by it may be involved, we performed immunoblotting of lysates from cells transfected with pRb, HA-p130, and the pRb pocket domain mutants 10 days posttransfection. Strikingly, the expression of p27KIP1 paralleled the ability of the pocket proteins to induce senescence (Fig. (Fig.4C).4C). pRb, pRbΔ651, and pRbΔ663—all of which induced senescence—also led to p27KIP1 accumulation, while p130 and pRbΔ657 produced few senescent cells and did not provide p27KIP1 accumulation. In addition, at 10 days pRb and p130 had altered the expression patterns of cyclin E and cyclin A2, while the pRb pocket domain mutants had not, perhaps reflecting E2F-mediated regulation of the cyclin promoters. As was seen at 2 days (Fig. (Fig.3B),3B), expression of the pRb pocket domain mutants continued to be accompanied by higher levels of cdk2 protein, but the elevated expression of p27KIP1 by pRbΔ651 and pRbΔ663 likely prevented the activity of this kinase by 10 days after transfection.
To confirm this hypothesis we investigated cyclin E kinase activity and changes to the cyclin E kinase complex at 10 days. When the in vitro kinase activity of cyclin E was assayed at 10 days, lysates from cells transfected with the senescence-proficient pRb pocket domain mutants, pRbΔ651 and pRbΔ663, had significantly reduced cyclin E kinase activity compared to cells transfected with vector or the senescence-deficient mutant, pRbΔ657 (Fig. (Fig.4D).4D). This suggests that increase of p27KIP1 by pRbΔ651 and pRbΔ663 is sufficient for the establishment of G1 arrest as a result of inhibition of cyclin E-cdk2 activity. Interestingly, p107- and p130-transfected cells, despite an inability to maintain p27KIP1 expression, also had low cyclin E-associated kinase activity, consistent with their ability to act as bona fide cdk2 inhibitors (8, 70). This result could indicate that the specific inhibition of cdk2 by p27KIP1 may be important for senescence or, alternatively, that p27KIP1 may play some other role in senescence distinct from cyclin E regulation. In addition, coimmunostaining of cells transfected with vector, RB, or HA-pRbΔ663 at 10 days indicated that though pRbΔ663-transfected cells still expressed higher levels of cdk2 than RB-transfected cells, the majority of cdk2 and cyclin E now colocalized with p27KIP1 (Fig. (Fig.44E).
CIP1/KIP1 inhibitors induce senescence but not flat cells.
The observation that the cell cycle arrest produced by a nonphosphorylatable pRb mutant can still be bypassed by cyclin E overexpression indicates that cyclin E must have a pRb-independent role in cell cycle progression (10, 37). This, in fact, underscores the requirement for a mechanism to inhibit cyclin E activity independent of the pRb-E2F interaction in senescent cells. With a strong correlation between p27KIP1 accumulation and senescence, we wanted to determine if p27KIP1 played a causative role in senescence downstream of pRb.
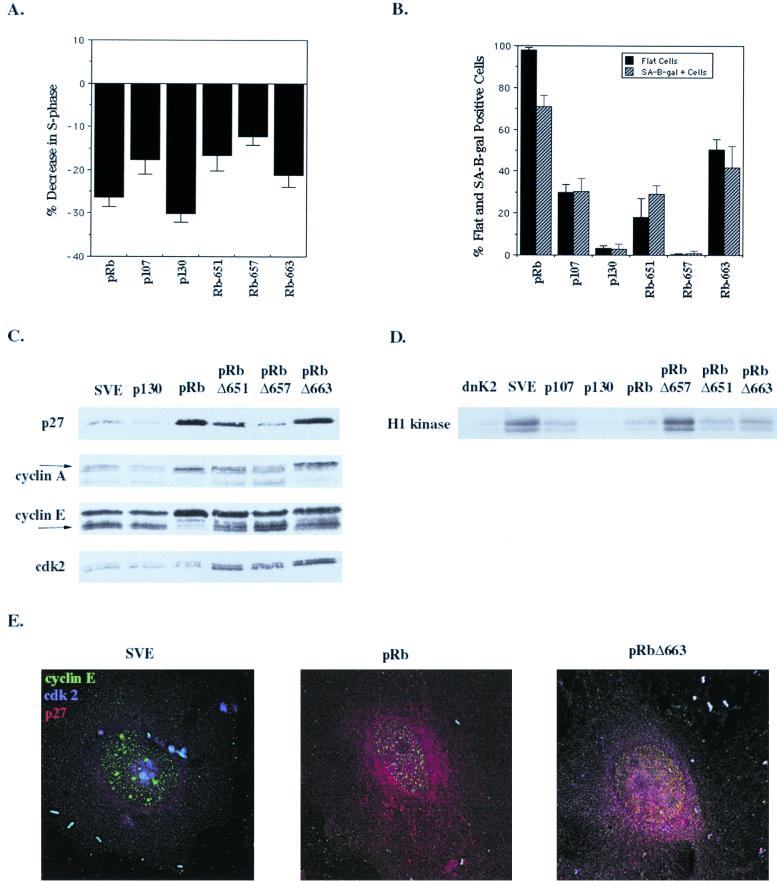
As previously observed, we found that even in the absence of pRb, p21CIP1, p27KIP1, and a dominant-negative cdk2 mutant (Cdk2NFG) induced a transient and prolonged G1 cell cycle arrest, decreasing the number of cells in S phase (24, 44, 59, 62, and data not shown). To determine if the increase in p27KIP1 levels in senescent cells was simply correlative or if p27KIP1 played an active role in promoting senescence, we tested its ability to produce SA–β-gal-positive cells. Unexpectedly, at 10 days posttransfection p21CIP1, p27KIP1, and dnCdk2 all produced SA–β-gal-positive cells while p16INK4a did not (Fig. (Fig.5A).5A). This demonstrates that the inhibitors of cdk2 can induce senescence even in the absence of pRb and p53. In addition, all the inhibitors of cdk2 augmented the ability of pRb to promote senescence. The observation that dnCdk2 induced senescence suggested that inhibition of cdk2 could trigger the senescent process. This, though, is inconsistent with the inability of p107 and p130 to induce senescence despite their inhibition cyclinE-cdk2 activity at 10 days. This contradiction was resolved by the fact that p27KIP1 levels were strikingly elevated in dnCdk2-transfected cells, even above the levels in pRb-transfected cells (Fig. (Fig.5B),5B), strongly supporting the hypothesis that p27KIP1 accumulation is a requirement for senescence induction.
p27KIP1 induces senescence. SAOS-2 cells were transfected with empty vector (pSVE), RB, p16INK4a, p21CIP1, p27KIP1, and dominant-negative Cdk2 (dnCdk2 or dnK2) expression vectors. (A) Cell cycle inhibitors were cotransfected with empty vector (solid bars) or with RB (hatched bars) and with pBabe-puro, selected with puromycin 24 h after transfection, and then stained for SA–β-gal activity 10 days posttransfection. (B) p27KIP1 immunoblot at 10 days of lysates from cells transfected with the indicated plasmids. (C) Phenotype of pRb and p27KIP1 SA–β-gal-positive cells 10 days posttransfection. (D) PAI-1 immunoblot of cells transfected with the indicated plasmids 10 days after transfection. (E) Effect of pRb and p27KIP1 on microtubulin in senescent cells. Ten days posttransfection cells were coimmunostained with p27KIP1 (green) and α-tubulin (red) antibodies.
Interestingly, the SA–β-gal-positive cells induced by p21CIP1, p27KIP1, and dnCdk2 expression were phenotypically different from pRb-induced SA–β-gal-positive cells. While pRb produced enlarged, elongated, flattened cells typically seen in senescence, the inhibitors of cdk2 all produced slightly bigger rounded cells that were SA–β-gal positive (Fig. (Fig.5C).5C). This morphological difference between the pRb and p27KIP1 senescent cells uncouples senescence from the specific morphology change induced by pRb and reconfirms that flat-cell formation is a function unique to pRb. However, when the cdk2 inhibitors and pRb were coexpressed, the pRb-mediated senescent morphology change took precedence over that of p27KIP1 (data not shown), demonstrating that pRb flat-cell formation occurs through a separate yet dominant pathway. To confirm that the SA–β-gal-positive cells produced by p27KIP1 were truly senescent, levels of an additional marker of senescence, the plasminogen activator type-1 (PAI-1), were determined. After 10 days, increased PAI-1 protein expression was observed by immunoblotting in lysates from cells transfected with either pRb or p27KIP1, consistent with the establishment of senescence (Fig. (Fig.55D).
Finally, we investigated the physical difference between the pRb and p27KIP1 senescent morphology change. We have seen that treatment of pRb flat cells with taxol, but not nocodazole, leads to the loss of the elongated cellular phenotype—the cells take on the small, rounded appearance of p27KIP1 senescent cells (unpublished data). This suggests that continued microtubule depolymerization is required for maintenance of the flat-cell phenotype. To determine if microtubule alteration was occurring in pRb-mediated flat cells but not in p27KIP1 senescent cells, we coimmunostained the cells at 10 days for α-tubulin and p27KIP1 expression. α-Tubulin had clearly undergone significant reorganization in pRb-transfected cells compared to wild-type cells and p27KIP1 senescent cells (Fig. (Fig.5E).5E). Thus, the phenotypic change caused by pRb expression in these cells, while perhaps contributing to the senescent phenotype, is not required for senescence and is separable from p27KIP1 induction.
p27KIP1 is required for pRb-mediated senescence.
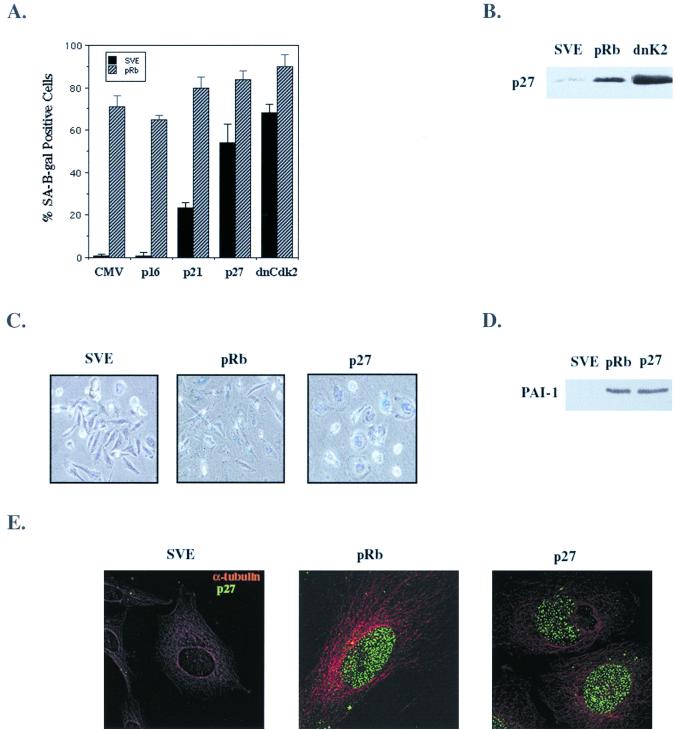
With p27KIP1 implicated in a causative role in senescence, we wanted to determine if its accumulation was necessary for pRb-mediated senescence. To eliminate p27KIP1 expression from SAOS-2 cells, we chose to use p27KIP1 antisense oligonucleotides previously described (12). The efficacy of the p27 antisense oligonucleotides in reducing pRb upregulation of p27KIP1 was assessed by immunoblot and immunofluorescence. p27 antisense oligonucleotides significantly reduced the pRb-induced increase in p27KIP1 levels, while the control oligonucleotide had no effect (Fig. (Fig.6A).6A). Similarly, staining of oligonucleotide-treated cells for p27KIP1 demonstrated a sharp decrease in p27KIP1 expression in pRb-transfected cells incubated with the p27 antisense oligonucleotides, confirming their specific effect in inhibiting p27KIP1 expression (Fig. (Fig.6B).6B).
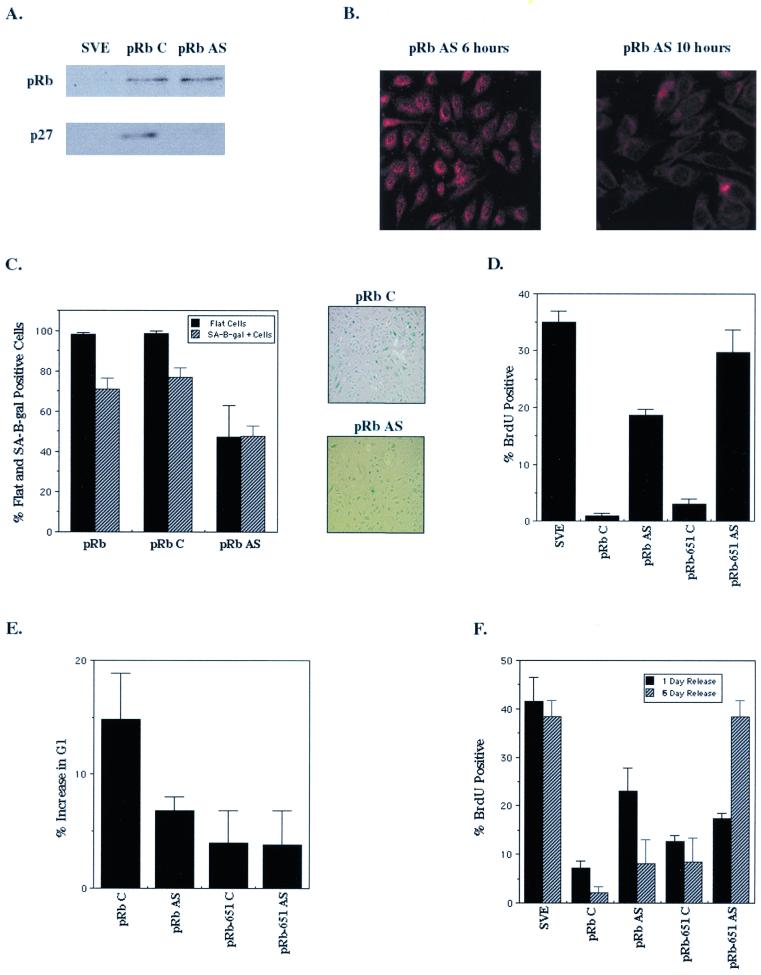
p27KIP1 is required for pRb-mediated senescence. SAOS-2 cells were transfected with RB and 24 h later were treated with p27KIP1 mismatch (C) or p27KIP1 antisense (AS) oligonucleotides. (A) Immunoblot of RB-transfected cell lysates 24 h after p27 antisense treatment. (B) p27KIP1 immunostaining of RB-transfected cells 6 h (left panel) and 10 h (right panel) after treatment with p27 antisense oligonucleotides. (C) Effect of loss of p27KIP1 expression on pRb-induced senescence. Starting 24 h posttransfection, cells were treated every 48 h with p27KIP1 mismatch (pRb C) or p27 antisense (pRb AS) oligonucleotides over a period of 10 days. After 10 days cells were stained for SA–β-gal activity, and flat cells (solid bars) and SA–β-gal-positive cells (hatched bars) were counted as previously described. p27KIP1 mismatch (top panel) and antisense (bottom panel) oligonucleotide-treated cells were stained for SA–β-gal activity at 10 days. (D) BrdU incorporation of vector (pSVE)- or RB- and HA-pRbΔ651-transfected cells treated every 48 h over a period of 10 days with mismatch or p27 antisense oligonucleotides. Results are the average of at least three experiments. (E) Cells were cotransfected with RB or HA-pRbΔ651 and CD20 expression plasmids and treated 24 h later with p27 mismatch or p27 antisense oligonucleotides, and FACS analysis was performed as previously described 24 h after oligonucleotide treatment. (F) RB-transfected cells were treated with p27 mismatch and antisense oligonucleotides at 2 and 4 days posttransfection, released from oligonucleotide treatment for 1 or 6 days, and then assayed for BrdU incorporation. Results are the average of at least two independent experiments.
The effect of loss of p27KIP1 expression on pRb-mediated senescence was studied by treating cells every 48 h with either control or p27 antisense oligonucleotides following transfection with pRb. While the control oligonucleotides had no effect, p27 antisense treatment of pRb-transfected cells decreased the number of SA–β-gal-positive cells by about 40% (Fig. (Fig.6C).6C). In addition, we saw with p27 antisense treatment a decrease in flat cells formed; instead, there were several small colonies of cells (Fig. (Fig.6C).6C). Finally, on those plates of pRb-transfected cells incubated with the p27 antisense oligonucleotides there were fewer cells overall, suggesting a loss of growth arrest in antisense-treated cultures which would cause a loss of the cotransfected drug selection marker (Fig. (Fig.66C).
To examine the role of p27KIP1 in the long-term growth arrest initiated by pRb, the effect of p27 antisense treatment on cell proliferation was determined. Cells were transfected with RB or HA-pRbΔ651, treated with oligonucleotides as described previously, and assayed for their ability to incorporate BrdU at 10 days. A significant portion of the pRb-transfected cells treated with p27 antisense oligonucleotides were in S phase in contrast to those treated with control oligonucleotides, implying that pRb requires high expression of p27KIP1 to maintain G1 arrest (Fig. (Fig.6D).6D). The inability of pRbΔ651 to bind E2F led to an exacerbation of the p27 antisense effect (Fig. (Fig.6D)—antisense-treated6D)—antisense-treated cells were proliferating at nearly normal levels, suggesting that the wild-type pRb-E2F complex may have an antiproliferative effect even in the absence of p27KIP1. Thus, without p27KIP1 induction and without the ability to bind E2F, pRbΔ651 had no means by which to arrest cells, intimating that E2F regulation and p27KIP1 induction by pRb collaborate to arrest cells in G1 phase.
To determine if increased p27KIP1 expression was necessary for acute as well as senescent cell cycle arrest in response to pRb, we analyzed the cell cycle profiles of p27 antisense-treated cells. Two days after transfection with a pRb-encoding plasmid, FACS analysis indicated that, concurrent with the loss of p27KIP1 expression 10 h after p27 antisense treatment, pRb-mediated G1 arrest was significantly antagonized (Fig. (Fig.6E).6E). As expected, the reduced ability of pRbΔ651 to arrest cells was little affected by the p27 antisense oligonucleotide. So, though repression of E2F is clearly important for immediate cell cycle arrest, p27KIP1 likely also contributes to the loss of proliferative capacity that occurs soon after pRb expression in these cells (12, 46).
We next asked if the effect of p27 antisense treatment on immediate and prolonged arrest was reversible. Cells were transfected with RB or HA-pRbΔ651 and treated 2 and 4 days posttransfection with control and p27 antisense oligonucleotides. Then oligonucleotide treatment was halted, and the cells were assayed for BrdU incorporation both 1 day (5 days posttransfection) and 5 days (10 days posttransfection) after the last oligonucleotide treatment. Twenty-four hours after release from p27 antisense treatment, pRb-mediated senescent arrest was still compromised; however, 6 days after p27 antisense treatment was halted, pRb had reestablished cellular arrest (Fig. (Fig.6F)6F) in contrast to pRb-transfected cells treated for 10 days with the p27 antisense oligonucleotide (Fig. (Fig.6D).6D). This recovery of cell cycle arrest by pRb after 5 days of release appeared to occur without any apparent loss in cell number or change in cellular morphology, as the cells were flat like their control oligonucleotide-treated counterparts (data not shown).
p27KIP1 partially rescues the ability of pRb pocket mutants to induce senescence.
With the indication that p27KIP1 was both necessary and sufficient for senescence, one prediction would be that supplying exogenous p27KIP1 to cells transfected with pocket proteins that have diminished or no ability to induce senescence would rescue their ability to do so. To test this hypothesis we cotransfected p27KIP1 with HA-p107, HA-p130, RB, and the pRb pocket domain mutants. Exogenous expression of p27KIP1 had no effect on the ability of p107 or p130 to form flat cells but slightly augmented the incidence of p107 senescent cells (Fig. (Fig.7A7A and B). This result again demonstrates that p27KIP1 has no ability on its own to induce the flat-cell phenotype, but instead its expression is linked to senescence. In addition, there was an increase in senescent cells when p27KIP1 was overexpressed with p130, but these senescent cells were phenotypically identical to the senescent cells induced by p27KIP1 alone, not pRb-mediated flat cells (Fig. (Fig.7B).7B). Thus, p130, which is unable to induce flat cells, showed no benefit from p27KIP1 overexpression and instead inhibited the ability of p27KIP1 to induce senescence itself (Fig. (Fig.7B).7B). Interestingly, these results seemed to correlate with the expression levels of cotransfected p27KIP1—p107 did not induce p27KIP1 levels above that of cells transfected with p27KIP1 alone while, similar to its effect on endogenous p27KIP1 at 10 days, p130 appeared to repress exogenous p27KIP1 expression (Fig. (Fig.7C).7C).
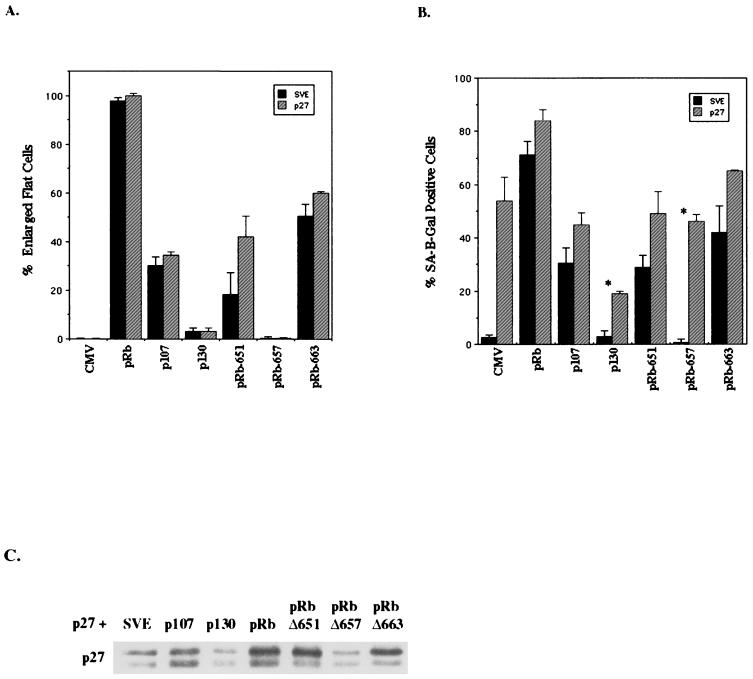
p27KIP1 partially rescues the ability of pRb pocket mutants to induce flat cells and senescence. SAOS-2 cells were cotransfected with p27KIP1 and RB, HA-p107, HA-p130, HA-pRbΔ651, HA-pRbΔ657, or HA-pRbΔ663 and a puromycin resistance plasmid. (A) Ten days posttransfection the number of pRb-phenotypic flat cells per total cells counted (at least 100 cells) for vector (CMV) or each protein alone (solid bars) or cotransfected with p27KIP1 (hatched bars). Results represent the average of three independent experiments. (B) Ten days posttransfection cells were stained for SA–β-gal activity and the SA–β-gal-positive cells per total cells were counted (at least 100 cells) for vector (CMV) or each protein alone (solid bars) or cotransfected with p27KIP1 (hatched bars). Results are the average of three independent experiments. An asterisk indicates p27 phenotypic senescent cells. (C) p27KIP1 immunoblot of cotransfected lysates at 10 days posttransfection with vector (pSVE) or the indicated expression plasmid.
Despite the lack of effect on p107 and p130, it was possible that cotransfection of p27KIP1 with the pRb pocket domain mutants would allow them to arrest cells acutely, enabling them to induce flat and senescent cells at levels of wild-type pRb. Indeed, coexpression of p27KIP1 with pRbΔ651 significantly increased its flat cell and senescence-inducing abilities, suggesting that acute G1 cell cycle arrest did allow pRbΔ651 to form more flat cells (Fig. (Fig.7A7A and B). While cotransfection of p27KIP1 with pRbΔ663 only slightly increased the number of flat cells, perhaps indicating the upper limit of this mutant's ability to produce flat cells, it more dramatically increased the number of pRbΔ663 senescent cells. However, in a complete lack of rescue, exogenous p27KIP1 did not enable pRbΔ657 to form flat, senescent cells. Instead, like p27KIP1 with p130, there were many p27KIP1 phenotypic senescent cells. In summary, these data suggest that flat-cell induction by pRb is compromised or absent in the pocket domain mutants used here regardless of the proliferative status of the cell. Thus, pRb-mediated arrest and senescence in SAOS-2 cells may arise from three collaborative but distinct processes—E2F regulation, p27KIP1 induction, and the morphological change.
DISCUSSION
Reintroduction or reactivation of pRb in human tumor cell lines that lack functional pRb often results in senescence. In this study we have investigated the contribution of pRb to senescence by reintroducing RB into an osteosarcoma tumor cell line mutated for both RB and p53. In doing so we examined the transient and prolonged effects of pRb on cell cycle protein levels and activities, cellular proliferation, and cellular morphology and the importance of these changes in cellular function to senescence. We found that soon after pRb expression, p27KIP1 synthesis increased in an E2F-independent manner, cyclin E-cdk2 kinase activity decreased, and the cells arrested in the G1 phase. These properties persisted upon prolonged pRb expression and progression into the senescent state, suggesting that they are important in the senescence process.
Most significantly, we found that only pRb and not p107 or p130 could induce sustained p27KIP1 synthesis and senescence, despite the fact that p107 and p130 can cause cell cycle arrest through E2F repression and cdk2 inhibition (11, 53, 71). Indeed, recent evidence points to p107 and p130 being the primary regulators of cellular proliferation through E2F-dependent mechanisms. p130 was seen to be the predominant pocket protein bound to E2F target gene promoters in G0 and early G1, while p107 dominated at late G1 and S phase (30, 56). Further, mouse embryo fibroblasts (MEFs) from RB−/− mice exhibit normal cell cycle regulation and few E2F target gene alterations, suggesting that E2F regulatory functions may be adequately performed by p107 and p130 in RB−/− MEFs (30). Given this ability of p107 and p130 to control cell cycle through E2F association at least as well as pRb, it is interesting that p107 and p130 induce senescence poorly or not at all in comparison to pRb. This strongly indicates that pocket protein-mediated E2F repression and subsequent cell cycle arrest are not enough to initiate a senescent phenotype; rather, a specific function of pRb is required.
Despite the importance of E2F regulation in pocket protein-mediated cell cycle arrest, we found senescence induction to best correlate with p27KIP1 induction. Wild-type pRb and senescence-competent mutants all induced persistent upregulation of p27KIP1, whether or not they were able to block the cell cycle through interaction with E2F. Further, ectopic expression of p27KIP1 could induce aspects of senescence on its own, and ablation of p27KIP1 expression prevented senescence induction by wild-type pRb. Thus, even in the absence of pRb and p53, p27KIP1 can cause SAOS-2 cells to enter the senescence pathway. Indeed, p27KIP1- or p21CIP1-mediated inhibition of cdk2 appears to be specifically required for senescence induction. Loss of cdk2 activity at the hands of p130 does not lead to senescence, and instead we observed an inhibition of both p27KIP1 expression and senescence in the presence of p130. Together, these data argue that p27KIP1 (or p27KIP1-cdk2 complexes) may play an active role in the senescence program rather than passively allowing cell cycle exit to occur as a consequence of lack of kinase activity. This E2F-independent induction of p27KIP1 and senescence by pRb may in part explain the prevalence of RB mutation in cancer. Perhaps tumor cells selectively inactivate pRb to prevent its initiation of a senescence program upon oncogenic stimuli or cellular exhaustion of proliferative capacity.
Although the evidence outlined above demonstrates mechanistic differences in p27KIP1 induction and E2F regulation by pRb, it is important to note that these functions likely collaborate in cell cycle arrest. For example, higher levels of cdk2 were found after expression of senescence-competent, E2F nonbinding pRb mutants, suggesting that the level of cyclin E-cdk2 complex might be regulated by E2F and thus affect the ability of p27KIP1 to effect cell cycle arrest. Further, wild-type pRb-mediated arrest was attenuated by inhibition of p27KIP1 expression despite the retention of an E2F binding domain, suggesting that E2F regulation and cdk2 inhibition must both occur to achieve cell cycle arrest. Indeed, the fact that an active cyclin E-cdk2 kinase complex can clearly bypass pRb-mediated cell cycle arrest potentially explains the requirement for the blocking of both E2F and cyclin E proliferative pathways for complete cell cycle arrest (10, 37). Exactly how pRb leads to increased p27KIP1 synthesis is under study but may be related to a recently described mechanism of p27KIP1 translational control (40). This translational regulation of p27KIP1 expression is mediated by a 5′ U-rich element which could explain how pRb regulates endogenous p27KIP1 levels (40). However, this element does not appear to be in the p27KIP1 construct used in these studies, suggesting that another regulatory mechanism may be in place. Still, the lack of this particular U-rich element does not preclude the possibility that a related sequence exists in the construct that would result in translational regulation of p27KIP1 in a manner similar to the mechanism previously described.
The two collaborative functions of pRb described above are apparently augmented by a third function that results in the unique morphological alteration elicited by pRb. Although the flat-cell phenotype is similar to that seen in many senescent cell systems, pRb's induction of this phenotype is now clearly shown to be a consequence neither of senescence induction nor of combined E2F repression and cdk2 inhibition. First, despite a slight change in morphology, CIP/KIP inhibition of cdk2 did not lead to the formation of the enlarged, flattened cells seen in pRb senescent cells, although two markers of senescence, SA–β-gal activity and induction of plasminogen activator inhibitor, were efficiently induced. Second, p130 was found to be completely unable to induce flat cells despite its block of E2F and cdk2 activity. These observations are reminiscent of those reported recently that unlink phenotypic senescence and cell cycle arrest in human diploid fibroblasts (18). However, these data do not preclude the possibility that p27KIP1 expression is necessary but not sufficient for the flat-cell phenotype, nor do they argue against a role for the cytoskeletal alterations in augmenting p27KIP1 expression. Indeed, we found a tight correlation between p27KIP1 induction and flat-cell formation by pRb mutants, and the observation that the small number of flat cells induced by p107 express the highest levels of p27KIP1 suggests that these responses to pocket protein expression are related. How and why cells undergo this pRb-dependent, extensive morphology change involving, as we found, at least microtubule reorganization is unknown but currently being studied and is likely to contribute significantly to pRb's ability to induce permanent cell cycle exit.
Altogether our data suggest the model of pRb-induced senescence shown in Fig. Fig.8.8. With the proper senescence-promoting stimulus, pRb represses E2F transcriptional activity to initiate an acute cell cycle arrest. In a non-E2F-dependent manner, pRb increases p27KIP1 levels posttranscriptionally, leading to an accumulation of p27KIP1, and it is in this way that pRb pocket domain mutants unable to stably interact with E2F induce growth arrest and senescence. The increased levels of p27KIP1 inhibit cyclin E-cdk2 kinase activity, triggering a prolonged G1 arrest—a function that exogenous expression of p21CIP1 and p27KIP1 (or dominant-negative cdk2) duplicates. It is this persistent inhibition of cyclin E kinase activity specifically by p27KIP1 that results in the formation of small, round senescent cells. However, it is a unique function of pRb to induce an extensive cellular morphology alteration, producing enlarged, flattened cells that are senescent. Changes in cytoskeletal signaling in these flat cells may in fact maintain the irreversible cell cycle exit and high levels of p27KIP1 expression.
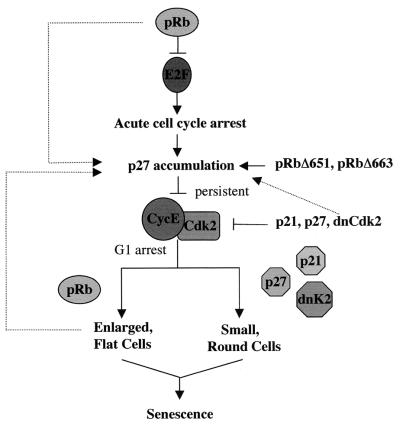
Model for pRb-mediated senescence. pRb represses E2F-mediated transcription of S-phase genes, inducing an acute cell cycle arrest. In a non-E2F-dependent manner, pRb upregulates p27KIP1 expression, leading to an accumulation of p27KIP1 levels and a persistent inhibition of cyclin E-cdk2 kinase activity. The specific inhibition of cyclin E kinase activity by the CIP/KIP inhibitors triggers senescence, but it is a unique function of pRb to induce the morphology change associated with senescent cells that appears to strongly correlate with levels of p27KIP1 expression.
This model reinforces the idea that the p53/p21 pathway is not crucial for cellular senescence in human cells and that pRb can achieve this antioncogenic cellular state at least in SAOS-2 cells through p27KIP1 induction. However, the observation of an increase in p53 activity and a requirement for p21CIP1 in senescent primary human cells (3, 18) and in p53-positive human tumor cells (15) suggests that the pRb-p27KIP1 pathway may be subordinated by the existence of an intact p53 pathway or may be specific to SAOS-2 cells or certain cell types. We believe that the pRb-p27KIP1 pathway is a general response to senescence stimuli in the absence of p53 for two reasons. First, we have observed that the simultaneous blocking of p53 function and reestablishment of the pRb pathway by the reintroduction of p16INK4a into U20S cells, an osteosarcoma cell line that is wild type for pRb and p53, result in the upregulation of p27KIP1 instead of p21CIP1 in these senescent cells (data not shown). Second, our preliminary data indicate that this pRb-p27KIP1 senescence-inducing mechanism occurs in other cell types shown to be susceptible to pRb-mediated senescence (67). Additionally, evidence is accumulating for p27KIP1-dependent, p53-independent mechanisms of senescence in normal cells. For example, recent work with MEFs has shown that inhibition of phosphoinositide 3-kinase leads to a senescence that is associated with an elevation of p27KIP1 levels but not p53, p19ARF, p16INK4a, or p21CIP1 levels (14). Thus, certain physiological stimuli, tissue contexts, or intracellular environments may modulate the pathways by which cells enter senescence in an effort to avoid tumorigenesis. The prevalence of disruption of the pRb pathway in tumors is likely to partially result from pRb's critical role in cell cycle exit programs such as the one described here. The mechanism through which pRb augments p27KIP1 synthesis and the specific role of p27KIP1-mediated cdk2 inhibition in senescence remain to be elucidated but promise to yield significant clues to an important tumor-suppressive process.
ACKNOWLEDGMENTS
We thank Peter Sicinski and David Fisher for careful reading of the manuscript.
K.A. is an MPM Scholar. This work was supported by Research Project Grant 95-013-06-CCG from the American Cancer Society.
REFERENCES
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.21.11.3616-3631.2001
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc86983?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/mcb.21.11.3616-3631.2001
Article citations
Awakening of Dormant Breast Cancer Cells in the Bone Marrow.
Cancers (Basel), 15(11):3021, 01 Jun 2023
Cited by: 2 articles | PMID: 37296983 | PMCID: PMC10252003
Review Free full text in Europe PMC
Friend or Foe: Regulation, Downstream Effectors of RRAD in Cancer.
Biomolecules, 13(3):477, 05 Mar 2023
Cited by: 6 articles | PMID: 36979412 | PMCID: PMC10046484
Review Free full text in Europe PMC
Insights into the role of senescence in tumor dormancy: mechanisms and applications.
Cancer Metastasis Rev, 42(1):19-35, 21 Jan 2023
Cited by: 10 articles | PMID: 36681750
Review
Genetic Drivers of Ileal Neuroendocrine Tumors.
Cancers (Basel), 13(20):5070, 10 Oct 2021
Cited by: 5 articles | PMID: 34680217 | PMCID: PMC8533727
Review Free full text in Europe PMC
RAS specific protease induces irreversible growth arrest via p27 in several KRAS mutant colorectal cancer cell lines.
Sci Rep, 11(1):17925, 09 Sep 2021
Cited by: 6 articles | PMID: 34504197 | PMCID: PMC8429734
Go to all (104) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Bcl-2 retards cell cycle entry through p27(Kip1), pRB relative p130, and altered E2F regulation.
Mol Cell Biol, 20(13):4745-4753, 01 Jul 2000
Cited by: 94 articles | PMID: 10848600 | PMCID: PMC85901
Molecular mechanisms underlying interferon-alpha-induced G0/G1 arrest: CKI-mediated regulation of G1 Cdk-complexes and activation of pocket proteins.
Oncogene, 18(18):2798-2810, 01 May 1999
Cited by: 94 articles | PMID: 10362250
Cyclin E and c-Myc promote cell proliferation in the presence of p16INK4a and of hypophosphorylated retinoblastoma family proteins.
EMBO J, 16(17):5322-5333, 01 Sep 1997
Cited by: 135 articles | PMID: 9311992 | PMCID: PMC1170164
Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation.
Crit Rev Biochem Mol Biol, 31(3):237-271, 01 Jun 1996
Cited by: 74 articles | PMID: 8817077
Review




