Abstract
Free full text

Antibody targeting studies in a transgenic murine model of spontaneous colorectal tumors
Abstract
Monoclonal antibodies (mAbs) have been used to treat malignancies in humans with varying degrees of success. Progress has been hindered by the lack of suitable animal models, which would ideally consist of immunocompetent animals that are tolerant to tumor-associated antigens. Suitable models would allow the study and optimization of anti-tumor immunotherapy. We describe a murine model for the study of immunotherapy in colorectal cancers. Carcinoembryonic antigen (CEA) is a cell-surface glycoprotein that is expressed on normal human intestinal epithelium and that is overexpressed in intestinal tumors. Mice that are transgenic for the human CEA gene (CEA.Tg) were crossed with multiple intestinal neoplasia (MIN) mice. MIN mice carry a germline APC mutation and are prone to the development of intestinal adenomas. The offspring from the MIN × CEA.Tg cross developed intestinal adenomas that were shown by immunohistochemistry to overexpress CEA. Pharmacokinetic studies by using 125I-labeled anti-CEA mAb PR1A3 showed rapid localization of antibody to tissues expressing CEA, especially the gastrointestinal tract. Macroscopic and microscopic radioautographic analysis of the gastrointestinal tracts from MIN/CEA.Tg mice indicated that PR1A3 targeted and was retained in tumors at levels higher than in areas of normal gut. These results demonstrate the utility of the MIN/CEA.Tg mouse as a model for the study of anti-CEA immunotherapy and, furthermore, demonstrate the efficiency of tumor localization by PR1A3.
Colorectal cancer (CRC) is the third most common cause of cancer-related death in the Western World (1). The disease is characterized by development of a tumor in the large bowel that then spreads throughout the body. Although surgery is the mainstay of treatment for the primary tumor, treatment of the metastases requires some form of adjuvant therapy such as radio-immunotherapy (RAIT). This therapy depends on the use of systemically administered mAbs that are specific for tumor-associated antigens (TAAs) to deliver conjugated radioisotopes to the tumor cells.
Carcinoembryonic antigen (CEA) is a member of the Ig superfamily and is composed of seven domains linked to the cell membrane through a glycosylphosphatidylinositol anchor (2). CEA is normally expressed in a variety of glandular epithelial tissues (such as the gastrointestinal, respiratory, and urogenital tracts) where it appears to be localized to the apical surface of the cells (3). In tumors arising from these tissues, there is an increase in level of CEA within the cells (together with secretion of the protein), and the expression extends from the apical cell membrane to include the whole of the cell surface (3). These changes make CEA a potentially useful TAA for targeting with RAIT.
PR1A3 is a murine IgG1k mAb that is specific for the membrane bound form of CEA (4). Unlike some other anti-CEA antibodies, it will not detect the soluble form of CEA or other members of the CEA family (5). PR1A3 has been extensively used in humans as a sensitive and specific radioimmunoscintigraphic guide for localizing CEA-bearing metastatic deposits from colorectal carcinomas (6). In addition, it shows a high frequency of immunohistochemical binding to colorectal tumors regardless of their degree of differentiation (4). PR1A3 is thus considered an attractive candidate for use in anti-CEA RAIT in colorectal cancers.
To evaluate PR1A3 as a potential tool in RAIT, an animal model would be of great value. A suitable animal model not only would allow testing of the efficacy of the mAb as an anti-tumor treatment but also would allow study of the mode of action of the antibody. To date, most preclinical studies of CRC have been performed by using human tumor xenografts implanted into immunodeficient mice (7, 8). Such models are not a good parallel to spontaneous tumorgenesis and also do not allow determination of the toxicity of RAIT on either the immune system or tissues that would normally endogenously express the target antigen.
Mice have no direct CEA counterpart and are not immunologically tolerant to the molecule (9). As a consequence, RAIT studies in immunocompetent mice implanted with syngeneic tumor transfected with CEA have been hampered by the induction of anti-CEA antibodies in their nontolerant host (10). Eades-Perner and colleagues developed a transgenic mouse that carries the human CEA (CEA.Tg) and is driven by its own promoter (11). Such mice express CEA in a tissue-specific fashion similar to that reported in humans(11). In contrast to nontransgenic mice, tumor implant studies in CEA.Tg mice have indicated that they are immunologically tolerant to CEA (12).
A further development of the CEA.Tg model would be to induce neoplastic changes within both CEA-positive and CEA-negative tissues, then test the ability of therapies to treat such tumors in immunocompetent animals against the background of “normal” CEA expression. This approach would provide a more realistic indication of their potential to treat gastrointestinal cancer in humans. Several natural mutant mice have been identified that are prone to tumors in specific organs (13, 14). The MIN (multiple intestinal neoplasia) mouse has a non-sense mutation in the APC (adenomatous polyposis coli) gene that results in the formation of multiple intestinal adenomas from birth through the first three months of development (15). Thompson and colleagues crossed the CEA.Tg with the MIN mouse to generate a line that spontaneously develops human CEA-expressing adenomas in the intestine (16). In this report we further characterize the phenotype of the MIN/CEA.Tg mouse and confirm the validity of the model for preclinical assessment of CRC-directed therapies by radio-biodistribution studies with the anti-CEA mAb PR1A3.
Materials and Methods
Animals.
Human CEA transgenic (CEA.Tg; C57BL/6, H-2b) heterozygous mice, were obtained in 1995 from J. Thompson (University of Freiburg, Germany). MIN (C57BL/6J-APCMIN/+) heterozygous mice were imported in 1992 from A. Moser (University of Wisconsin). Both colonies were bred and maintained by back-crossing with a colony of C57BL/6J mice (ICRF Biological Resources, Clare Hall, South Mimms, U.K.). Mice with spontaneously arising CEA-expressing intestinal adenomas were derived by breeding the CEA.Tg with the MIN mice. All mice were housed and maintained in negative pressure isolators. Experiments were conducted on 10- to 12-wk-old mice in full accordance with the U.K. Home Office Animal (Scientific Procedures) Act 1986.
Genotyping.
DNA was extracted from CEA.Tg × MIN F1 mice tail-snips, by using a DNeasy Tissue kit (Qiagen, Germany) according to the manufacturer's instructions. PCR was used to identify mice carrying the CEA transgene and/or the APC mutation. The general PCR conditions including primer pairs and amplification have been described previously for detection of the CEA transgene (16) and APC mutation (17).
Radioiodination and Biodistribution Studies.
For biodistribution studies, PR1A3, a murine (m) IgG1k mAb against CEA (4), was used. HMFG1, a murine IgG1 mAb against MUC1, was used as an isotype-matched control (18). Antibodies were radiolabeled with 125I by using the Bolton and Hunter reagent (Amersham Pharmacia Nycomed) as follows. Antibodies were transferred into 0.1 M Hepes buffer (pH 8.0) and concentrated by ultracentrifugation (Amicon) to concentrations of 5–10 mg/ml. Twenty microliters (3.7 MBq) of N-succimidyl-3-(4-hydroxy-3-[125I]iodophenyl) propionate (Amersham Pharmacia Nycomed) was dried in the bottom of a 1.5-ml polypropylene tube in a Speed-vac, and 50 μg of antibody was added. After mixing and incubation for 10 min, the reaction was quenched by addition of 0.2 ml of 0.2 M glycine in 0.1 M Hepes, and the labeled antibodies were purified by gel-filtration on PD-10 Sephadex columns (Amersham Pharmacia Nycomed) using PBS containing 0.5% Tween 20. The immunoreactivity of the radiolabeled PR1A3 antibody was confirmed to be greater than 90% in a cell binding assay as previously described (19). For biodistribution analysis, mice were injected i.v. via the tail vein with 2 μCi (50 μl) of radiolabeled murine antibody. After injection, groups of mice (n = 4/group) were killed at 24 and 48 h, the intestines were resected, and the contents were removed. The tissue samples were counted on an automated gamma-counter (LKB Ultragamma), together with a dilution of the injected material, and the proportion of the injected dose (ID) present was calculated.
Digital Autoradiography.
Tissue was fixed overnight in neutral-buffered formalin, pinned onto a board, and covered with Saran Wrap (Dow). The sample was covered with a multipurpose Cyclone storage phosphor screen (Packard) and placed in an x-ray film cassette overnight at room temperature. The latent image generated on the screen was read by using the Cyclone Phosphor imaging system and analyzed by using the proprietary integral optiquant software.
Emulsion-Dipped Autoradiography.
Samples were fixed in neutral-buffered formalin and embedded in paraffin wax by using standard procedures (20). Sections were cut at 5 μm onto slides, dewaxed, dipped in photographic emulsion (Ilford K5), dried, and exposed at 4°C. After 2 wk, sections were dipped in photographic developer (Kodak D19) and counterstained by Giemsa's method. Sections were examined with a reflected light microscope under dark-field conditions allowing individual autoradiographic silver grains to be seen as white dots.
Immunohistochemistry.
Mouse tissue sections were examined for the presence of CEA by using a horseradish peroxidase-conjugated rabbit anti-human CEA polyclonal Ab (Code No. P0167, Dako). Mouse tissues were snap-frozen in liquid nitrogen at the time of mouse sacrifice, and 5- to 6-μm-thick sections were subsequently cut and fixed in acetone. Endogenous peroxidase activity and nonspecific antibody binding were prevented by incubating the sections for 15 min in 0.5% hydrogen peroxide/methanol (vol/vol) and normal swine serum (NSS, 1:5 dilution with Tris-buffered saline; Dako), respectively. Sections were incubated for 1 h in primary antibody diluted in NSS. Labeling was detected with diaminobenzidine substrate solution (Sigma), and sections were counter stained with Mayer's hematoxylin.
Statistical Analysis.
Group means were compared by using Student's two-tailed, unpaired t test. Probability (P) values less than 0.05 were interpreted as significant. All statistics were performed by using Microsoft excel.
Results
Human CEA Transgenic Expression in CEA.Tg/MIN Mice.
Mice carrying the APC MIN mutation develop adenomas from birth, and, at around 3 mo, adenomas and carcinomas can be observed in the small and large intestine (15). Various tissues of the MIN and MIN/CEA.Tg mice were examined histologically for CEA expression by using a rabbit anti-CEA polyclonal antiserum. No CEA expression was detected in the control nontransgenic MIN mice. In MIN mice carrying the CEA transgene, tissue expression of CEA was similar to that reported previously in CEA.Tg mice (11), with the TAA being localized in the gastrointestinal tract but not expressed in other organs examined, including liver, kidney, and pancreas. Positive CEA staining was seen in the colon, caecum, and small and large intestine, with the majority of crypt luminal epithelial cells lining the crypts showing mainly apical staining. In the small intestine, as previously reported (11), the crypt cells were positive, but the cells within the villi were negative for CEA expression. In adenomas resected from both the small and large intestines from MIN/CEA.Tg mice, immunohistochemistry showed very strong CEA expression in these structures relative to surrounding tissue (Fig. (Fig.11 A and B), with a more diffuse pattern of staining. All tumors removed from MIN mice labeled with the anti-CEA antiserum stained negative for CEA (data not shown).
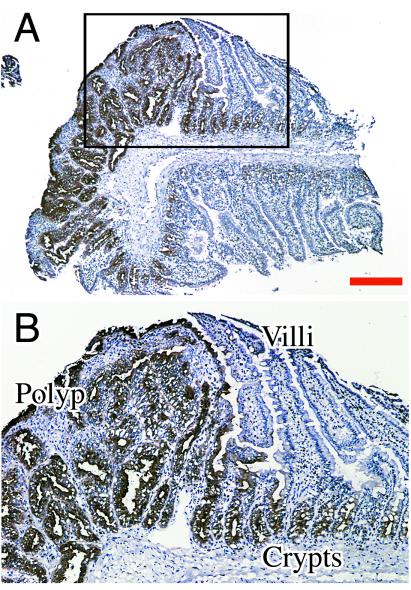
Analysis of CEA expression by immunohistochemistry. Cryostat sections of resected tissue were stained directly with a rabbit anti-CEA polyclonal-horseradish peroxidase conjugate and counterstained with Mayer's hematoxylin. (A) small intestinal adenoma from MIN/CEA.Tg mouse; (B) high power view of region boxed in previous section. Bar = 250 μm for A.
Biodistribution Studies in MIN and MIN/CEA.Tg Mice Using the Anti-CEA Antibody PR1A3.
MIN mice carrying the CEA transgene thus appear to have a CEA expression pattern in the gastrointestinal tract comparable to that observed in some human colorectal cancers (4). By using a radioiodinated anti-CEA mAb PR1A3, the MIN/CEA.Tg mouse was evaluated as a suitable preclinical model for antibody-targeted therapy. In a series of studies, adult (8 to 10 wk) MIN and MIN/CEA.Tg mice received one i.v. injection of either radiolabeled PR1A3 or the radiolabeled isotype-matched control mAb. At sample points (24 h and 48 h) postinjection, blood and tissues were removed, and antibody uptake was measured.
In MIN mice, the two antibodies demonstrated a similar pattern of biodistribution. Blood clearance was relatively slow, and the only tissues showing significant amounts of antibody uptake were the excretory organs, all of which showed a pattern of declining radioactivity with time. For example at 48 h, the mean uptakes in the liver, intestines, and kidney were 2.5, 2.1, and 1.3%ID for PR1A3, and 3.0, 1.8, and 0.6%ID for the isotype-matched control. In the MIN/CEA.Tg animals, significant (P < 0.01) differences were observed in the biodistribution of the two antibodies in the intestines, with uptake of PR1A3 being up to twice that of the isotype control. A comparison of the biodistribution of PR1A3 in MIN and MIN/CEA.Tg mice also highlighted differences, most notably in the intestine. At all time points, greater uptake of PR1A3 was seen in the intestines of MIN/CEA.Tg mice compared with nontransgenic littermates. Intestinal uptake of the mAbs at 24 and 48 h postinjection are shown in Fig. Fig.2.2.
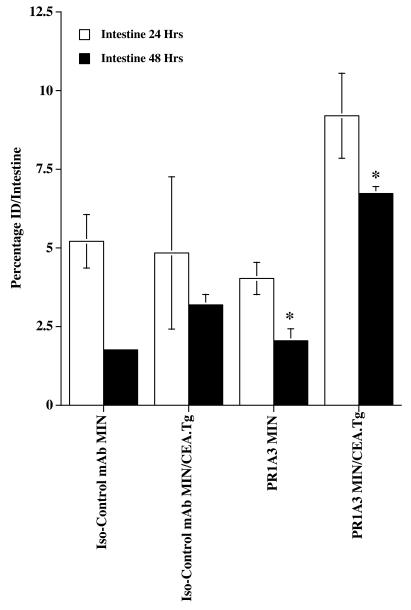
PR1A3 can detect in vivo expression of CEA in MIN/CEA.Tg mice. MIN and MIN/CEA.Tg mice were injected i.v. with 125I-labeled antibody, either PR1A3 or isotype-matched control. Organs, including the intestine, were resected at 24 and 48 h time points, and antibody uptake was determined. Data are mean percentage ID per intestine ± SEM (n = 3–4 per group). Results shown are representative of two separate experiments. * indicates a statistically significant difference (P < 0.01) between PR1A3 uptake in MIN and MIN/CEA.Tg intestine at the 48 h sample point. A statistically significant difference (P < 0.01) between PR1A3 and an isotype-matched control mAb uptake in MIN/CEA.Tg intestine at 48 h was found.
Autoradiography in Adenomas from Mice Injected with 125I-Labeled PR1A3.
Macroautoradiographical analysis of MIN/CEA.Tg mice was conducted to determine the localization of labeled antibody in the intestine. Mice were injected i.v. with either 125I-radiolabeled PR1A3 or isotype control mAb. Forty eight hours later, intestines were resected and placed on phosphor-imaging screens in x-ray cassettes. Imaging results show that tumors present in the intestines of MIN/CEA.Tg mice injected with PR1A3 were readily visualized (Fig. (Fig.33D). Quantitative analysis indicated that PR1A3 uptake in adenomas was up to twice that in surrounding intestinal tissue. In contrast, no uptake into tumors could be imaged in the intestines of MIN/CEA.Tg injected with the isotype control mAb (Fig. (Fig.33B). Similarly, no foci were seen in the intestines of nontransgenic MIN injected with either PR1A3 or the isotype control mAb (data not shown). To refine the macroautoradiography findings, we performed dipped emulsion autoradiography on sections of intestinal adenomas 48 h after injection of either PR1A3 or the isotype control mAb (Fig. (Fig.4).4). Microautoradiography confirmed that PR1A3 was targeting the tumors within the intestines of MIN/CEA.Tg mice. Analysis of sections from these mice revealed a high density of silver grains within the tumor microenvironment coincident with mAb uptake (Fig. (Fig.44 E–H). No specific silver grain accumulation was seen in intestinal sections from MIN/CEA.Tg mice injected with the isotype control mAb (Fig. (Fig.44 A–D).
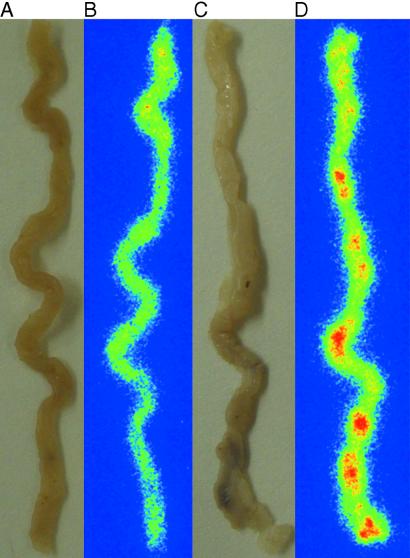
PR1A3 preferentially targets CEA within intestinal adenomas. MIN/CEA.Tg mice were injected i.v. with 125I-labeled antibodies, and, after 48 h, intestines were removed. Autoradiographs were developed and analyzed, as described in Materials and Methods. Small intestines are from MIN/CEA.Tg mice injected with either, isotype-matched control mAb (A, photograph; B, autoradiograph) or PR1A3 (C, photograph; D, autoradiograph).
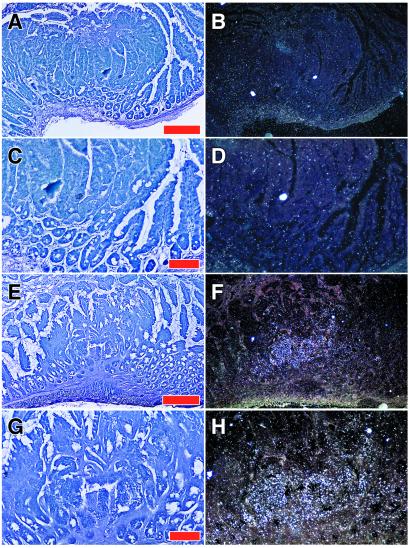
Microautoradiographical analysis of PR1A3 targeting within the tumor microenvironment. Localization of radiolabeled mAb in small intestinal adenomas was determined by dipped emulsion autoradiography, as described in Materials and Methods. (A–D) Min/CEA mouse injected with 125I-labeled isotype-matched control mAb. (A and B) Low power view of sectioned adenoma stained with Giemsa shown in bright (A) or reflected light dark-field illumination (B) revealing autoradiographic silver grains in white. (C and D) Higher magnification of one region demonstrating the absence of specific labeling. (E–H) Min/CEA mouse treated with 125I-labeled PR1A3. (E and F) Low power view showing a cluster of silver grains within an adenoma and not in flanking normal epithelium. (G and H) Higher magnification of the labeled region revealing that silver grains are concentrated over the adenomatous epithelium. Bars = 250 μm for A and E, and 100 μm for C and G.
Discussion
Numerous potential cancer therapies are being developed that require preclinical assessment before being selected to enter clinical trials (21). Unfortunately, many current cancer models show limitations, being based either on murine model antigens (22), or requiring implantation of xenografts into immunodeficient mice (23). To optimize the screening of reagents intended for human use, animal models should resemble the human situation as closely as possible. Animals models should ideally possess a viable immune system and develop spontaneous tumors that express relevant TAAs that are found in human tumors. Here, we describe a radiolabeled antibody-targeting study in a mouse model for colorectal cancer that fulfills these criteria. Mice transgenic for human CEA (11) were mated with a mutant mouse line that develops spontaneous intestinal adenomatous polyps (15). A quarter of the offspring from this cross developed intestinal adenomas that, on histological examination, were found to express high levels of CEA. Because most colorectal tumors express high levels of CEA, it has been proposed that the molecule may contribute to malignancy although the role of this molecule in tumor development is not known.
Antibodies are increasingly emerging as beneficial adjuncts to cancer therapy. Some success has been achieved with naked mAb therapy in CRC; for instance, patients with Dukes' C colorectal cancer receiving adjuvant treatment with the murine mAb 17-1A (anti-Ep-CAM) showed an apparent 30% improvement in overall survival and an equivalent reduction in distant metastatic recurrence (24). Radiolabeled anti-CEA mAbs have shown excellent targeting in patients with CEA positive tumors (8, 25). For instance, technetium-99m radioimmunoscintigraphic studies performed in CRC patients with the murine anti-CEA mAb PR1A3 have reported a 94% accuracy, with a negative predictive value of 100% (6).
To validate the MIN/CEA.Tg as a suitable preclinical model for human CRC, we carried out a series of experiments by using the anti-CEA mAb PR1A3. Radiolabeled antibody studies in MIN/CEA.Tg mice showed that PR1A3 was able to locate specifically the CEA-expressing tissues of the intestine. Macroautoradiographical analysis of MIN/CEA.Tg intestines indicated that PR1A3 was preferentially taken-up and retained within the tumors, compared with the surrounding tissue. An average ratio of 1.6:1 was noted when the uptake within a tumor was compared with that in an adjacent section of intestine. These results are comparable to the differences seen in uptake between malignant and normal tissues in moderately differentiated CRC patients imaged with 99m-Tc-labeled PR1A3 (6). Radiolabeled mAbs that bind to shed CEA have, in some patients, shown false-positive uptake in normal lymph nodes because of binding to sequestered antigen (26, 27). PR1A3 avoids this problem because it binds only to cell-associated CEA. In this study, no uptake into lymph nodes was observed on microautoradiographic analysis. The fact that PR1A3 was able to target preferentially CEA in neoplastic tissues may reflect a different expression pattern of the molecule within the tumor microenvironment. Furthermore, it gives credence to the idea that this antibody may be a suitable candidate not only for imaging but also for targeted therapy.
Characterization of the MIN/CEA.Tg mouse indicates that it will be a useful preclinical model for CRC. Some of the most striking antibody therapy results in the clinic have been obtained in patients with minimal residual disease (24). To observe a response (determined either by increased survival or reduced numbers of adenomas), direct antibody therapy with PR1A3 may be best administered to younger MIN mice. Such studies may include higher and repeated doses of radiolabeled mPR1A3 or involve mAb combined with a chemotherapeutic agent. The anti-erbB-2 mAb Herceptin, delivered with doxorubicin or paclitaxel, has shown promising results both in animal models (28) and in humans (29). Such strategies in the MIN/CEA model may help to identify the optimal approach to mAB therapy of colorectal cancer.
Acknowledgments
We thank Gillian Lewis [Imperial Cancer Research Fund (ICRF) Hybridoma Development Unit, London, U.K.] for antibody preparation; Gary Martin and Sandra Peak (ICRF Biological Services Unit, London, U.K.) for animal husbandry; Toby Hunt, Jan Longcroft, and Rosemary Jeffery (ICRF In Situ Hybridization Service, London, U.K.) for autoradiography of tissue sections; Alice Lee-MacAry and Catherine Townshend [Imperial Cancer Research Technology (ICRT) Applied Development Laboratory, London, U.K.) for proofreading and transgenic genotyping; and David Ellison for radiolabeling and sample counting. We are grateful to Drs. John Thompson and Wolfgang Zimmermann (University of Freiburg, Germany) for counsel and kindly providing the CEA.Tg mouse. These studies were financially supported by Antisoma Plc (London, U.K.).
Abbreviations
| CEA | carcinoembryonic antigen |
| CRC | colorectal cancer |
| MIN | multiple intestinal neoplasia |
| RAIT | radio-immunotherapy |
| TAA | tumor-associated antigen |
| APC | adenomatous polyposis coli |
| ID | injected dose |
References
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.181353498
Read article for free, from open access legal sources, via Unpaywall:
https://www.pnas.org/doi/pdf/10.1073/pnas.181353498
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines.
Oncoimmunology, 6(3):e1277306, 11 Jan 2017
Cited by: 136 articles | PMID: 28405498 | PMCID: PMC5384349
Relevance, advantages and limitations of animal models used in the development of monoclonal antibodies for cancer treatment.
Crit Rev Oncol Hematol, 62(1):34-42, 02 Jan 2007
Cited by: 45 articles | PMID: 17197192
Review
Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors.
Cancer, 109(2):170-179, 01 Jan 2007
Cited by: 153 articles | PMID: 17154393
Review
Expression profiling of genes and proteins in HaCaT keratinocytes: proliferating versus differentiated state.
J Cell Biochem, 93(5):1048-1062, 01 Nov 2004
Cited by: 15 articles | PMID: 15389883
Immunoprevention of colorectal cancer: a future possibility?
Gastroenterol Clin North Am, 31(4):1001-1014, 01 Dec 2002
Cited by: 3 articles | PMID: 12489274
Review
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Evaluation of a transgenic mouse model for anti-human CEA radioimmunotherapeutics.
J Nucl Med, 43(10):1368-1376, 01 Oct 2002
Cited by: 12 articles | PMID: 12368376
Vaccine-based therapy directed against carcinoembryonic antigen demonstrates antitumor activity on spontaneous intestinal tumors in the absence of autoimmunity.
Cancer Res, 62(23):6944-6951, 01 Dec 2002
Cited by: 64 articles | PMID: 12460911
Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice.
Cancer Res, 64(10):3668-3678, 01 May 2004
Cited by: 45 articles | PMID: 15150127
Apc mice: models, modifiers and mutants.
Pathol Res Pract, 204(7):479-490, 05 Jun 2008
Cited by: 100 articles | PMID: 18538487
Review





