Abstract
Free full text

The HLA Class I A Locus Affects Susceptibility to Type 1 Diabetes
Abstract
Human leukocyte antigen A (HLA-A) genotypes were determined for samples from 283 multiplex, Caucasian, type 1 diabetes families from the Human Biological Data Interchange (HBDI) using an immobilized probe assay. Distribution of HLA-A alleles transmitted to patients was significantly different from that in affected family-based controls (AFBAC) (p = 0.004). Transmission disequilibrium test (TDT) analysis revealed differential transmission of several HLA-A alleles from parents to affected offspring. HLA class II DRB1 and DQB1 loci were also typed, allowing assignment of HLA-A alleles to haplotypes and calculation of linkage disequilibrium values. Some of the apparent effects of HLA-A alleles on type 1 diabetes susceptibility were attributable to linkage disequilibrium with DR and DQ alleles, although others were not. The differences in frequencies between patients and controls of alleles A*0101, A*2402, and A*3002 could not be explained by linkage disequilibrium alone. Our results suggest an important role for class I antigens in modulating susceptibility to type 1 diabetes.
INTRODUCTION
Type 1 diabetes, formerly called insulin-dependent diabetes mellitus (IDDM), is an autoimmune disease characterized by the destruction of insulin producing beta cells in the pancreas [1]. Although multiple genes have been implicated, human leukocyte antigen (HLA) region genes are the major type 1 diabetes susceptibility markers known to date, and, under a multiplicative model of multi-locus disease risk, constitute ~53% [2] of the type 1 diabetes genetic component [3, 4]. It is widely recognized that the major component of HLA susceptibility to type 1 diabetes involves DRB1, DQA1, and DQB1 genes [2, 5-8]. Recent studies have suggested that genes in the HLA region other than DR and DQ also contribute to type 1 diabetes susceptibility [9-11].
The association of HLA class I polymorphism with the age at onset of type 1 diabetes has been previously reported [12-15]. Because class II molecules are involved in the initial antigen presentation event and in triggering the autoimmune response, they may affect the initial events in the development of type 1 diabetes. On the other hand, HLA class I molecules, given their role in target-cell recognition by CD8+ cytotoxic T lymphocytes (CTL), play a role in the ongoing immune response, and, therefore, may affect the rate of pancreatic beta-cell destruction. This hypothesis may help explain the observed associations of HLA class I with type 1 diabetes, particularly the association of HLA-A*2402 with early disease onset [13, 15]. A*24 has been reported to be associated both with rapid disease progression [16, 17] and with total beta-cell destruction [18]. The data presented here are consistent with a role for A*2402 in type 1 diabetes susceptibility and also implicate the alleles A*0101, and A*3002.
The evaluation of a potential role for HLA-A alleles in type 1 diabetes requires examining patterns of linkage disequilibrium with other known risk alleles in the HLA region. The Human Biological Data Interchange (HBDI) families, which we have typed for all class II alleles and for HLA-B alleles, thus allow determination of unambiguous haplotypes. These families represent a highly informative set of samples with which to assess HLA-A associations with type 1 diabetes.
MATERIALS AND METHODS
Study Cohort
DNA samples from 283 Caucasian, multiplex families were obtained from the collection of the Human Biological Data Interchange (HBDI, Philadelphia, PA, USA). The HBDI is a repository for cell lines derived from type 1 diabetes families and was established in part for the purpose of mapping type 1 diabetes-associated genes by linkage analysis. Most of the HBDI families are nuclear families with unaffected parents and at least two affected siblings (multiplex) from the United States. Only two affected siblings from each family have been included in this analysis. Controls are taken both from the families themselves (see “Genotyping Methods”) and from the Caucasian family collection of the Centre d’Etude du Polymorphisme Humain (CEPH).
Genotyping Methods
Molecular HLA typing data were generated using a modification of the previously described polymerase chain reaction (PCR) sequence-specific oligonucleotide probe (SSOP) method [19]. Six additional oligonucleotide probes were added to the assay to give allele-level resolution for the 56 alleles reported as of 1996 [20]. Control haplotypes (n = 399) were determined based on the affected family-based control (AFBAC) method [21].
Statistical Analysis: Transmission Disequilibrium Test
The transmission disequilibrium test (TDT), originally developed by Spielman et al. [22], is for biallelic markers. When the marker is multi-allelic the contributions of the two parents are not independent. For example, a T1DM child whose mother’s genotype is DR3/DR4 and whose father’s genotype is DR4/DR1, who inherited the DR4 allele from the father, would be more likely to have the DR3 than the DR4 chromosome transmitted from the mother, because DR3/DR4 has a higher penetrance than DR4/DR4. We must, therefore, consider the genotypes of both parents. The Tmhet statistic developed by Kaplan et al. [23] addresses this issue and, under the null hypothesis of no association, has a Chi-square distribution with m – 1 degrees of freedom (m = number of alleles).
Expected allele frequencies were computed, given linkage disequilibrium and haplotype relative penetrances. The null hypothesis (H0) in this case is that HLA-A allele frequencies will differ between patients and controls due to linkage disequilibrium between HLA-A and DRB1-DQB1, and due to chance (sampling), consistent with a neutral role for HLA-A polymorphism relative to disease predisposition.
Under H0 the expected allele frequencies at HLA-A can be computed using the equation derived by Thomson [24]:
Dij denotes pairwise linkage disequilibrium between the ith DR-DQ haplotype and the jth HLA-A allele in the control sample, q denotes frequency in patients, and p denotes frequency in controls. This method relies on sampling estimates of pairwise linkage disequilibrium between HLA-A and DRB1-DQB1 and on the patient and control frequencies derived from the samples under study. Thus, a sampling error will be associated with the computed value for expected HLA-A allele frequencies. The larger the control sample the smaller this error will be. This has been taken into account in the statistical tests carried out:
For any individual Ai where Np is the patient sample size, Nc is the control sample size, qAi is the observed frequency of the ith allele in patients, and q [H6126]Ai is derived from Eq. (1).
To make this analysis more robust, we have computed expected A allele frequencies under the null hypothesis using linkage disequilibrium values and control frequencies from two sample sets: (1) derived using the AFBAC [21], and (2) from 80 CEPH samples [25]. The allele and haplotype frequencies derived by AFBAC are an unbiased estimate of the control frequencies from which the patient population originated. For multiplex families, they are computed as the sum of parental alleles or haplotypes that were not transmitted to either of the affected siblings in a pair. These frequencies are robust to population stratification. As further confirmation and as a point of reference, we have also included DR-DQ-HLA-A haplotypes derived from CEPH families. The CEPH collection is comprised of Utah Mormon, French, Amish, and Venezuelan Caucasian families and has been utilized extensively for mapping and allele frequency determination. The CEPH families were not ascertained based on disease status; therefore, they are considered representative of the general Caucasian population. In a previous study, we compared HLA-DRB1-DQB1 haplotype frequencies in the CEPH with those in AFBAC from a 180-family subset of the HBDI families, and found the two to be nearly indistinguishable [2]. Finding a genetic association in the HBDI patient sample independently with the CEPH control set and with the AFBAC gives greater confidence that the association is neither spurious nor due to population stratification.
Homozygous Parent TDT
An alternative approach to address linkage disequilibrium within the HLA region entails using homozygote parents matched for DR-DQ and assessing by TDT a bias in transmission of HLA-A alleles. Whereas this type of analysis has the advantage of addressing the confounding effects of linkage disequilibrium with the DR- and DQ-encoding loci, the high degree of polymorphism in the HLA class II region greatly decreases the informative sample size. In our case, only 55 of 566 parents are homozygous for DR-DQ. Analyses based on such a small sample size are bound to have low statistical power and are likely to yield inconclusive results.
RESULTS
Table 1 illustrates allele frequencies in affected individuals and in AFBAC from the set of 283 HBDI families. We present Chi-square tests and p values from comparing patient and control frequencies as well as from a multiallelic TDT. Results from both methods are very similar (Table 1).
TABLE 1
Multialleleic TDT and AFBAC association test between A alleles and type 1 diabetes in 283 affected sibling pairs
| AFBAC association test | Multiallelic TDTa | ||||||
|---|---|---|---|---|---|---|---|
HLA-A allele allele | AFBAC frequency | Transmitted frequency frequency | Odds ratio | Chi-square | p Value | (TDT) Tmhet | p Value |
| 0101 | 15.0% | 20.5% | 1.46 | 3.82 | 0.05 | 7.23 | 0.007 |
| 0201 | 30.3% | 31.3% | 1.05 | 0.07 | 0.01 | ||
| 0202 | 0.0% | 0.5% | 2.11 | ||||
| 0205 | 0.5% | 1.1% | 2.13 | 0.88 | 0.09 | ||
| 0206 | 0.0% | 0.4% | 1.41 | ||||
| 0301 | 13.8% | 10.1% | 0.70 | 2.78 | 0.10 | 3.77 | 0.052 |
| 0302 | 0.3% | 0.3% | 1.06 | 0.00 | |||
| 1101 | 6.5% | 3.1% | 0.46 | 6.09 | 0.014 | 7.36 | 0.007 |
| 2301 | 1.5% | 1.0% | 0.64 | 0.56 | 0.01 | ||
| 2402 | 7.3% | 11.4% | 1.64 | 4.12 | 0.042 | 4.55 | 0.033 |
| 2403 | 0.0% | 0.4% | 1.41 | ||||
| 2501 | 2.3% | 1.6% | 0.70 | 0.56 | 0.47 | ||
| 2601 | 2.5% | 3.7% | 1.50 | 1.06 | 0.71 | ||
| 2608 | 0.0% | 0.2% | 0.70 | ||||
| 2901 | 0.5% | 0.4% | 0.70 | 0.12 | |||
| 2902 | 3.3% | 2.7% | 0.81 | 0.30 | 0.50 | ||
| 3001 | 1.3% | 0.5% | 0.42 | 1.48 | 0.50 | ||
| 3002 | 0.8% | 2.4% | 3.23 | 3.65 | 0.06 | 4.26 | 0.039 |
| 3005 | 0.3% | 0.0% | 0.00 | 1.42 | |||
| 3101 | 2.0% | 2.8% | 1.42 | 0.64 | 0.14 | ||
| 3201 | 4.3% | 1.9% | 0.45 | 4.33 | 0.04 | 3.13 | 0.077 |
| 3301 | 0.8% | 0.1% | 0.12 | 2.84 | 0.09 | ||
| 3303 | 0.3% | 0.1% | 0.35 | 0.40 | |||
| 3402 | 0.3% | 0.0% | 0.00 | 1.42 | |||
| 3601 | 0.3% | 0.0% | 0.00 | 1.42 | |||
| 6601 | 0.5% | 0.1% | 0.18 | 1.54 | |||
| 6602 | 0.0% | 0.2% | 0.70 | ||||
| 68011 | 1.5% | 0.4% | 0.23 | 3.74 | 0.05 | 1.00 | |
| 68012 | 2.5% | 2.4% | 0.95 | 0.01 | 0.01 | ||
| 6802 | 0.8% | 0.9% | 1.18 | 0.05 | 0.11 | ||
| 6901 | 0.5% | 0.0% | 0.00 | 2.84 | |||
| 7401 | 0.3% | 0.0% | 0.00 | 1.42 | |||
| NEW | 0.3% | 0.0% | 0.00 | 1.42 | |||
| n | 399 | 566b | Total | 55.29 | 0.004 | Total 35.41 | 0.005 |
Abbreviations: AFBAC = affected family-based controls; HLA = human leukocyte antigen; TDT = transmission disequilibrium test.
Six alleles (A*0101, A*0301, A*1101, A*2402, A*3002, and A*3201) revealed significant or nearly significant (p < 0.1 or less) deviations from values expected under the null hypothesis by both the AFBAC and TDT methods. A seventh allele, A*68011, may be protective, but due to the very low frequencies of this allele, further examination of this potential effect requires analysis of larger data sets. The differences in allele frequencies between controls and cases for these alleles could be due exclusively to the strong linkage disequilibrium between HLA class I and class II loci. To address this issue, we have first computed Dij and normalized linkage disequilibrium in the AFBAC (Table 2). The strongest linkage disequilibrium observed in both samples is between the well-known high risk haplotype, DRB1*0301-DQB1*02 and A*0101, which explains why A*0101 is more common among patients than among controls. The two other apparently predisposing alleles, A*2402 and A*3002, indicated no significant linkage disequilibrium to any particular DR-DQ haplotype. Of the apparently protective alleles, A*0301 demonstrated very strong linkage disequilibrium with DRB1*1501-DQB1*0602, the DR-DQ haplotype associated in most Caucasian populations with dominant protection from disease. Although linkage disequilibrium was not significant for the other two apparently protective alleles, they both revealed nonsignificant trends toward linkage disequilibrium with protective DR-DR haplotypes, A*3201 with DRB1*1101-DQB1*0301, and A*1101 with both DRB1*1501-DQB1*0602 and DRB1*0701-DQB1*02 (not shown).
TABLE 2
Normalized linkage disequilibrium between DRB1-DQB1 haplotypes and A alleles in AFBAC (n = 399)
| Haplotype | ||||||
|---|---|---|---|---|---|---|
| DRB1 | DQB1 | HLA-A | Dij | D′ | χ 2 | P Value |
| 0101 | 0501 | 0301 | 0.0149 | 0.223 | 10.24 | 0.001 |
| 0301 | 02 | 0101 | 0.0380 | 0.491 | 51.07 | 8.9 × 10−13 |
| 0301 | 02 | 0201 | −0.0152 | − 0.549 | 4.99 | 0.025 |
| 0401 | 0301 | 0201 | 0.0152 | 0.435 | 8.65 | 0.003 |
| 0404 | 0302 | 3101 | 0.0097 | 0.520 | 36.13 | 1.8 × 10−9 |
| 0701 | 02 | 2301 | 0.0038 | 0.262 | 3.97 | 0.046 |
| 0701 | 02 | 2902 | 0.0160 | 0.473 | 30.86 | 2.8 × 10−8 |
| 0701 | 02 | 3001 | 0.0042 | 0.367 | 6.05 | 0.014 |
| 1501 | 0602 | 0301 | 0.0245 | 0.215 | 16.40 | 5.1 × 10−5 |
| 1501 | 0602 | 2501 | 0.0065 | 0.248 | 4.50 | 0.034 |
| 1502 | 0601 | 0101 | 0.0043 | 0.705 | 7.55 | 0.006 |
Only haplotypes that occurred at least three times in the combined data set and exhibited significant linkage disequilibrium are listed.
Abbreviations: AFBAC = affected family-based controls; HLA = human leukocyte antigen.
We computed, based on observed linkage disequilibrium patterns, the expected A allele frequencies under the null hypothesis that the A locus is neutral relative to type 1 diabetes susceptibility using both the AFBAC and CEPH controls for reference. The results, adjusted for linkage disequilibrium with DR-DQ haplotypes, are presented in Table 3. Two HLA-A alleles (A*0101 and A*3002) differ significantly from both the AFBAC and CEPH expected frequencies. A*2402 differs significantly from the AFBAC and reveals a trend versus the CEPH. These three alleles and their effects are discussed individually below. We note that the allele A*3201 remains significantly protective versus the AFBAC after the adjustment for linkage disequilibrium; however, the result for this low frequency allele was not replicated compared with the CEPH controls. We also note that the common allele A*0201 demonstrates a slight, but not significant, trend towards disease predisposition after the adjustment for linkage disequilibrium when compared with the AF-BAC. Determining whether or not these observations represent true effects will require additional studies. For all alleles, the formal possibility cannot be excluded that some nearby locus in very strong linkage disequilibrium (stronger than that with DR-DQ) with the specific HLA-A alleles may be responsible for the observed HLA-A association.
TABLE 3
Observed and expected HLA-A allele frequencies under the hypothesis that differences between cases and controls are due exclusively to linkage disequilibrium with DRB1-DQB1
| Controls | |||||
|---|---|---|---|---|---|
| Patients | AFBAC (n = 399) | CEPH (n = 160) | |||
| HLA-A allele | Observed frequency | Expected frequency | p Value | Expected frequency | p Value |
| 0101 | 20.60% | 29.12% | 0.007 | 36.20% | 0.0001 |
| 0201 | 31.00% | 26.19% | n.s. | 27.30% | n.s. |
| 0202 | 0.60% | 0.00% | n.s. | 0.00% | n.s. |
| 0205 | 1.00% | 0.00% | 0.043 | 0.90% | n.s. |
| 0206 | 0.40% | 0.00% | n.s. | 0.00% | n.s. |
| 0301 | 10.20% | 11.96% | n.s. | 6.50% | n.s. |
| 1101 | 3.10% | 2.93% | n.s. | 1.80% | n.s. |
| 2301 | 1.00% | 1.40% | n.s. | 0.40% | n.s. |
| 2402 | 11.80% | 7.20% | 0.026 | 7.10% | 0.11 |
| 2403 | 0.40% | 0.00% | n.s. | 0.00% | n.s. |
| 2501 | 1.70% | 2.05% | n.s. | 3.70% | n.s. |
| 2601 | 3.30% | 2.27% | n.s. | 1.20% | n.s. |
| 2608 | 0.20% | 0.00% | n.s. | 0.00% | n.s. |
| 2901 | 0.20% | 0.00% | n.s. | 0.00% | n.s. |
| 2902 | 2.70% | 2.91% | n.s. | 3.70% | n.s. |
| 3001 | 0.60% | 1.19% | n.s. | 0.90% | n.s. |
| 3002 | 2.50% | 0.76% | 0.046 | 0.00% | 0.05 |
| 3101 | 3.00% | 1.84% | n.s. | 2.90% | n.s. |
| 3201 | 2.00% | 4.87% | 0.018 | 1.60% | n.s. |
| 3303 | 0.10% | 0.00% | n.s. | 0.00% | n.s. |
| 6601 | 0.10% | 0.00% | n.s. | 0.00% | n.s. |
| 6602 | 0.20% | 0.00% | n.s. | 0.00% | n.s. |
| 68011 | 0.40% | 1.64% | 0.045 | 1.50% | n.s. |
| 68012 | 2.00% | 2.27% | n.s. | 0.90% | n.s. |
| Other | 1.20% | 1.40% | n.s. | 3.50% | |
Observed values are compared to both AFBAC and unrelated controls from CEPH founders.
Abbreviations: AFBAC = affected family-based controls; CEPH = Centre d’Etude du Polymorphisme Humain; HLA = human leukocyte antigen.
To examine the effects of the HLA-A locus on age of T1DM onset, we computed expected allele frequencies under H0, splitting patients into three categories: age of onset > 0 and ≤ 5 years old, age of onset > 5 and ≤ 12 years old, and age of onset > 12 years. An odds ratio (OR) was computed using expected (based on AFBAC allele frequencies and patterns of linkage disequilibrium) and observed frequencies (OR = obs freq (1 – exp freq)/[exp freq (1 – obs freq)]). Figure 1 illustrates the odds ratios for alleles A*0101 and A*2402 in the three different age of onset categories with the corresponding 95% confidence intervals. Odds ratios thus estimated were significantly different among age of onset ranges only for allele A*2402, confirming that the disease susceptibility role of this allele is most marked among younger age of onset patients.
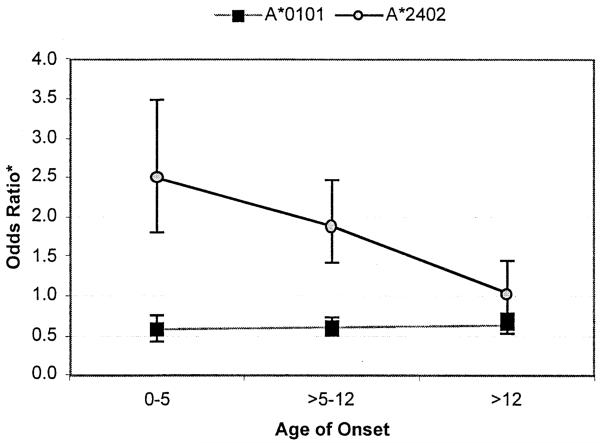
Odds ratio, calculated based on observed versus expected frequencies given linkage disequilibrium with DR-DQ, and 95% confidence intervals for alleles A*0101 and A*2401 in three different age of onset categories (age of onset > 0 and ≤ 5 years old, age of onset > 5 and ≤ 12 years old, and age of onset > 12 years old).
A*0101
The frequency of A*0101 is higher in patients than in controls; however, this increase is due to the strong linkage disequilibrium of this allele with the highly susceptible haplotype DRB1*0301-DQB1*02. These alleles are part of an extended haplotype, which spans approximately 3 Mb and exhibits strong linkage disequilibrium from HLA-A centromeric to DP: HLA - A*0101 - B*0801 - DRB1*0301 - DQA1*0501 - DQB1*02-DPB1*0101. Previous studies have indicated that B8-DR3 haplotypes have a lower risk for type 1 diabetes that do other DR3 haplotypes, such as B18-DR3 haplotypes [26].
In fact, the odds ratios for DR4 and DR3 haplotypes with A*0101 appear to be lower (if not significantly so) than those for DR3 and DR4 haplotypes not coupled to A*0101: DR4-A*0101, OR 6.4 (95%CI, 1.4–28.1), DR4-A*not0101, OR 7.1 (95%CI, 4.7–10.6), DR3-A*0101, OR 4.3 (95%CI, 2.2–8.4), DR3-A*not0101, OR 5.4 (95%CI, 3.1–9.3). Further, in this sample set, A*0101 appears to be associated with older age of onset among DR3 haplotypes [15]. Thus, although A*0101 is increased among patients versus controls in the data that are unadjusted for linkage disequilibrium, in fact, A*0101 may confer protection from type 1 diabetes.
A*2402
A*2402 (or the serologic type A*24) association with type 1 diabetes has been noted elsewhere. We have previously reported that A*2402 has a significant effect on the age of onset distribution of DR-DQ haplotypes: A*2402 occurs at a higher frequency among young age of onset patients in this cohort [15]. Nakanishi and coworkers [18, 27] have demonstrated the presence of subtle, but definite residual beta-cell function in Japanese patients with type 1 diabetes of long duration. In their studies, 90%–95% of patients with little or no residual beta-cell activity were positive for HLA-A24, whereas only 44%–53% of patients with residual beta-cell function had this serotype. Beta-cell function was measured by C-peptide immunoreactivity; residual activity was defined as > 0.033 nM. A role for A*24 in disease susceptibility in the Finnish population was reported by Fennessy et al. [28], although age of onset distribution was not reported. Recently, Bugawan et al. [29] have also observed a significant association of A*2402 with type 1 diabetes among Filipino patients in a case-control study.
In the HBDI data corrected for linkage disequilibrium (Table 3), the overall difference between the expected and observed allele frequencies for A*2402 is statistically significant when AFBAC are used for comparison, and reveals a trend when CEPH controls are used. However, when we looked at the subset of patients age of onset of 12 years or younger, the observed allele frequency of A*2402 is 13.87%, which is significantly higher than the expected frequency of 7.3% for A*2402 under the null hypothesis (Chi-square 7.84, p < 0.005). In patients with age of onset greater than 12 years, the observed frequency is only 7.5%, which does not differ significantly from the expected frequency. These data are consistent with an association of A*2402 with early age of onset.
A*3002
The allele A*3002 is infrequent in the Caucasian population with a frequency of 0.0076 in our AFBAC population and 0.0 in the CEPH families. The frequency of alleles transmitted to affected children for A*3002 is 0.025, which is a 3.29-fold increase over the AFBAC frequency and represents the largest fold-increase in these data. The finding of a significant excess of such a low frequency allele in patients versus two different control data sets is suggestive of a strong predisposing effect. Most of the transmitted A*3002 alleles (85%) are found on DR3 haplotypes, suggesting that the apparent susceptibility effect might be due to linkage disequilibrium with the high-risk DRB1*0301-DQB1*02 haplotype. However, only one of three A*3002 alleles present in the AFBACs was on a DR3 haplotype, inconsistent with strong linkage disequilibrium between A*3002 and DRB1*0301. Our data suggest that DR3 haplotypes that include A*3002 may be higher risk than other DR3 haplotypes. This observation was supported by data from an additional set of Caucasian T1DM families in which the frequency of the A*3002 allele was higher (manuscript in preparation). The A*3002 allele may itself be highly predisposing; alternatively, the A*3002 allele might simply be a marker for another predisposing locus on the extended haplotype. Our current data set cannot distinguish between these two possibilities. Examination of the transmitted DR3-A*3002 haplotypes revealed that they all also contain the allele B*1801, and most (78%) contain the allele DPB1*0202 (data not shown). Previous reports have suggested that DPB1*0202 increases the risk of DR3 haplotypes [11, 30].
The transmitted A*3002-B*1801-DR3 haplotypes that do not carry DPB1*0202 have instead DPB1*0301, which has been reported to have a positive association with type 1 diabetes [2, 9, 11]. Sorting out loci in these extended haplotypes that are actually predisposing and those that are only marking other predisposing loci on the haplotype will require analysis of much larger data sets and/or analysis of data sets in which the rare alleles DPB1*0202 and A*3002 are more frequent.
DISCUSSION
HLA class I molecules play a key role in recognition of target cells by cytotoxic T cells. Type 1 diabetes is a disease characterized by autoimmune destruction of pancreatic beta cells. Although the observed associations between HLA-A and type 1 diabetes are, as expected, modest, the data are consistent with a role for this class I molecule in destruction of pancreatic cells.
The strong contributions of the HLA class II loci to disease risk and the strong linkage disequilibrium in the HLA region can confound analyses of disease risk for other loci in the region if these factors are not properly accounted for. In these data, we found that simply comparing frequencies for a given allele in patients and controls, or in transmitted versus not transmitted haplotypes, may misrepresent the true susceptibility effect of that allele. For example, correction of our data for linkage disequilibrium with predisposing and protective HLA class II alleles revealed that the observed predisposing effect in the unadjusted data for A*0101 was the opposite of the protective association seen after the correction.
The need for comprehensive, molecular typing of the HLA class I loci to reveal disease susceptibility effects is exemplified by the data for the allele A*3002. If the data for A*3002 are combined with the data for its serologic equivalents A*3001 and A*3005, no difference is seen in transmitted versus AFBAC frequencies. In fact, the data for A*3001 alone suggest that it may be somewhat protective. Thus, the predisposing effect of A*3002 was apparent only because the typing system allowed allele-level identification. A*3002 is carried by an extended haplotype (A*3002-B*1801-DRB1*0301-DQA1*0501-DQB1*02-DPB1*0202) that appears to confer higher risk than other DR3 haplotypes, consistent with the idea that specific combinations of alleles may determine the risk associated with a given HLA haplotype.
Analysis of the specific allele and haplotype disease associations in different populations, defined by DNA-based typing, should help establish which alleles are truly functionally involved in susceptibility to and proression of type 1 diabetes. HLA region-based susceptibility to type 1 diabetes results from the effects of alleles at multiple genetic loci within the HLA region. Genetic susceptibility to T1DM is not limited to the genes encoding HLA-DR and -DQ molecules, although those loci have the strongest effect. The data presented here argue for a role of the HLA-A locus in disease risk.
ACKNOWLEDGMENTS
This work was supported by an ADA Career Development Award to J.A.N. and by NIH grants DK46626 and AI29049 to H.E.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1016/s0198-8859(02)00421-4
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4049513?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/s0198-8859(02)00421-4
Article citations
Fifty years of HLA-associated type 1 diabetes risk: history, current knowledge, and future directions.
Front Immunol, 15:1457213, 12 Sep 2024
Cited by: 0 articles | PMID: 39328411 | PMCID: PMC11424550
Review Free full text in Europe PMC
Differences in F pocket impact on HLA I genetic associations with autoimmune diabetes.
Front Immunol, 15:1342335, 25 Mar 2024
Cited by: 0 articles | PMID: 38596688 | PMCID: PMC11003304
HLA alleles measured from COVID-19 patient transcriptomes reveal associations with disease prognosis in a New York cohort.
PeerJ, 9:e12368, 15 Oct 2021
Cited by: 10 articles | PMID: 34722002 | PMCID: PMC8522641
Clinical features, epidemiology, autoantibody status, HLA haplotypes and genetic mechanisms of type 1 diabetes mellitus among children in Qatar.
Sci Rep, 11(1):18887, 23 Sep 2021
Cited by: 11 articles | PMID: 34556755 | PMCID: PMC8460652
HLA genetic polymorphism in patients with Coronavirus Disease 2019 in Midwestern United States.
HLA, 98(4):370-379, 10 Aug 2021
Cited by: 15 articles | PMID: 34338446 | PMCID: PMC8429120
Go to all (84) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
HLA-encoded susceptibility to insulin-dependent diabetes mellitus is determined by DR and DQ genes as well as their linkage disequilibria in a Chinese population.
Hum Immunol, 44(4):210-219, 01 Dec 1995
Cited by: 41 articles | PMID: 8770634
The association of specific HLA class I and II alleles with type 1 diabetes among Filipinos.
Tissue Antigens, 59(6):452-469, 01 Jun 2002
Cited by: 44 articles | PMID: 12445315
Family-based association of HLA class II alleles and haplotypes with type I diabetes in Brazilians reveals some characteristics of a highly diversified population.
Hum Immunol, 62(11):1226-1233, 01 Nov 2001
Cited by: 13 articles | PMID: 11704284
Susceptibility to type I diabetes: HLA-DQ and DR revisited.
Immunol Today, 17(7):323-329, 01 Jul 1996
Cited by: 200 articles | PMID: 8763818
Review
Funding
Funders who supported this work.
NIAID NIH HHS (1)
Grant ID: AI29049
NIDDK NIH HHS (2)
Grant ID: R01 DK061722
Grant ID: DK 46626





