Abstract
Background
Acute hematogenous osteomyelitis (AHO) occurs primarily in children and is believed to evolve from bacteremia followed by localization of infection to the metaphysis of bones. Currently, there is no consensus on the route and duration of antimicrobial therapy to treat AHO.Methods
We conducted a systematic review of a short versus long course of treatment for AHO due primarily to Staphylococcus aureus in children aged 3 months to 16 years. We searched Medline, Embase and the Cochrane trials registry for controlled trials. Clinical cure rate at 6 months was the primary outcome variable, and groups receiving less than 7 days of intravenous therapy were compared with groups receiving one week or longer of intravenous antimicrobials.Results
12 eligible prospective studies, one of which was randomized, were identified. The overall cure rate at 6 months for the short course of intravenous therapy was 95.2% (95% CI = 90.4 - 97.7) compared to 98.8% (95% CI = 93.6, 99.8) for the longer course of therapy. There was no significant difference in the duration of oral therapy between the two groups.Conclusions
Given the potential increased morbidity and cost associated with longer courses of intravenous therapy, this finding should be confirmed through a randomized controlled equivalence trialFree full text

Shorter courses of parenteral antibiotic therapy do not appear to influence response rates for children with acute hematogenous osteomyelitis: a systematic review
Associated Data
Abstract
Background
Acute hematogenous osteomyelitis (AHO) occurs primarily in children and is believed to evolve from bacteremia followed by localization of infection to the metaphysis of bones. Currently, there is no consensus on the route and duration of antimicrobial therapy to treat AHO.
Methods
We conducted a systematic review of a short versus long course of treatment for AHO due primarily to Staphylococcus aureus in children aged 3 months to 16 years. We searched Medline, Embase and the Cochrane trials registry for controlled trials. Clinical cure rate at 6 months was the primary outcome variable, and groups receiving less than 7 days of intravenous therapy were compared with groups receiving one week or longer of intravenous antimicrobials.
Results
12 eligible prospective studies, one of which was randomized, were identified. The overall cure rate at 6 months for the short course of intravenous therapy was 95.2% (95% CI = 90.4, 97.7) compared to 98.8% (95% CI = 93.6, 99.8) for the longer course of therapy. There was no significant difference in the duration of oral therapy between the two groups.
Conclusions
Given the potential increased morbidity and cost associated with longer courses of intravenous therapy, this finding should be confirmed through a randomized controlled equivalence trial.
Background
Acute hematogenous osteomyelitis (AHO) occurs mainly in children and is more common in males [1-3]. AHO is believed to evolve from bacteremia followed by localization of infection to the metaphysis of bones. Successful treatment of AHO is crucial for the prevention of morbidity and functional loss of the affected limb.
Currently, there is no consensus on the route and duration of antimicrobial therapy to treat AHO. The suggested duration of intravenous therapy ranges from 3 days to 4 to 6 weeks. One general pediatric textbook describes 7 days of parenteral therapy as standard, [4] whereas a pediatric infectious diseases textbook states that the usual duration of therapy is 4 to 8 weeks with a change to oral medication permissible when signs of local inflammation has resolved [5]. A standard orthopedic textbook suggests intravenous therapy for 5 days followed by oral therapy for 4 to 6 weeks for "typical" cases [6].
Given the paucity of randomized controlled trial evidence to guide clinicians in the management of children with AHO, they have typically relied on data presented in observational studies. The vast majority of these studies have used a cohort design. Here, a group of children diagnosed with AHO are identified and treated using antibiotic therapy. The results are usually reported as successful response rates following a specified period of treatment (e.g., 14 days).
Two studies, each including over 100 children, suggest at least 3 weeks of intravenous antimicrobial therapy for the treatment of AHO in children. In these two studies, success rates of 82% and 81% were observed in children treated with intravenous antibiotics for less than 21 days [7,8]. There is, however, wide variation between studies regarding failure rates according to treatment duration. A series of smaller studies quoting 1 to 52 days of parenteral antimicrobial therapy have reported success rates ranging from 81% to 100% [9-25]. The aim of our systematic review was to determine whether short courses (less than 1 week) of parenteral antimicrobial therapy show equivalent cure rates compared with longer courses (greater than 1 week) in children with AHO caused primarily by Staphylococcus aureus.
Methods
Search strategy
Medline and EMBASE were searched electronically to help identify relevant literature from January 1966 to April 2001. The Cochrane Controlled Trials Register, Cochrane Library was searched from 1981 to July 2000. There were no restrictions on language or publication status. The literature was monitored throughout the course of the project by periodically re-running the search in Medline and screening newly posted citations. (Please see additional file 1)
Inclusion criteria
Studies were included if they involved children between 3 months and 16 years of age. The clinical criteria used for the diagnosis of AHO was: (a) positive culture of Staphylococcus aureus from bone or periosteum; or (b) clinical signs of osteomyelitis and a concurrent positive blood culture; or (c) clinical signs and a compatible radiological study (nuclear scan or radiography). Clinical signs were to include swelling, warmth, tenderness and decreased ability to weight bear.
The study design had to meet 3 eligibility criteria: 1) the study had to be prospective cohort; 2) the intervention had to identify the antimicrobial and its route (parenteral or oral) and duration of therapy; and 3) the outcome after an average of 6 months of follow up had to be stated or inferable as clinical cure, failure or relapse.
Study selection
Titles and abstracts of each citation were downloaded to a reference database. Two authors (NLS, AH) independently screened each citation to determine whether to retain it. Potentially relevant citations were retrieved and then subjected to a relevance assessment using our eligibility criteria. Open consensus was used to settle differences.
Data abstraction
Once a study met the inclusion criteria, two members of the research team (NLS; AH) independently abstracted data using structured data abstraction forms. We captured information about the report (e.g., language of publication), design (e.g., cohort), population (e.g., diagnosis), intervention (e.g., type of antibiotic), and primary outcome (e.g., response rates). If more than one cohort was present in a study, data from each cohort was abstracted separately. Disagreements were resolved by consensus.
Data analysis
Treatment of seven days or less of parenteral therapy was considered short course. In many institutions the, typical time required for defervescence, observation, and arrangement of follow-up is about seven days. We thought that this was a practical point at which to dichotomize for the purposes of analysis.
When all children are cured, normal theory based confidence intervals for the proportion cured are unavailable, and when cure rates are close to 100%, normal theory based confidence intervals may provide poor approximations. To overcome these problems, Wilson score confidence intervals were computed [26]. Under the hypothesis of homogeneity of cure rates across cohorts, a fixed-effect estimate of the overall cure rate is provided by the total number of cured patients divided by the total number of patients. The same estimate is provided by an intercept-only logistic regression model. To test the homogeneity of cure rates, the residual deviance from the logistic regression model was compared to a chi-square distribution with degrees of freedom given by the number of cohorts minus one.
Cohorts with different treatments within the same study were analyzed as separate parallel groups. The difference between the overall cure rate under short- and long-term parenteral antimicrobial therapy was assessed using a z-test. Using the same methods, we performed a sub-group analysis comparing overall cure rates for beta-lactams and macrolides. The difference in mean length of oral therapy for cohorts that had short- and long-term parenteral antimicrobial therapy was assessed using a t-test. Unlike parallel-arm studies, where selective publication of statistically significant results may produce publication bias, quite different publication issues may apply to single-arm cohort studies. The funnel plot, often used to assess publication bias, is therefore not relevant in this review.
Results
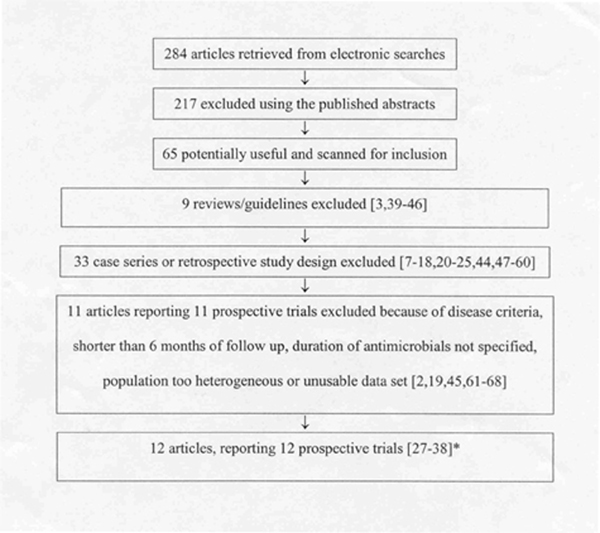
A flow diagram of the search results is illustrated in Figure Figure1.1. Two hundred and eighty-four articles were identified initially. Of these, 57 articles were considered potentially relevant. Of these, 34 were small or mixed case series or had a retrospective study design. Of the remaining 23, 11 studies that appeared to be prospective cohorts had either unusable data, short follow-up or the duration of antimicrobial could not be discerned from the data presented (Table (Table1).1). Of the remaining 12 studies, 11 that were of a prospective design were included in the systematic review [27-37]. In some cases, only a subset of patients were eligible for inclusion (see notes in Table Table2).2). We excluded one study of 25 children [38] as it was a different study architecture. It is inappropriate to combine estimates of effectiveness (i.e., cure rates) from different study types.
Table 1
Studies which were excluded and the principle reason for exclusion
| Study | Reason For Exclusion |
| Unkila-Kallio 1993 | Mainly epidemiologic data. Appears to be the cohort from which Peltola 1997 is drawn. |
| Dones 1994 | Small prospective cohort of 4 patients evaluating teicoplanin. |
| Nelson 1982 | Study addressed bactericidal titers. Follow-up did not extend to 6 months although reported cure rate was 95% with an average duration of 5 days of intravenous antimicrobials. |
| Beauvais 1981 | Addressed the use of pristinamycin orally. Data was not usable because group could not be reliably separated for route and type of antimicrobial. |
| Fleming 1970 | No follow-up data available. Trial focussed on microbiology and side effects of treatment. |
| Nussinovitch 1997 | Data on osteomyelitis cases alone could not be extracted. Maximum follow-up was about 4 months. Cure appeared to be 95% with mean duration of IV antibiotics of 13.7 days. |
| Walker 1973 | Follow-up was 4 months. Mean duration of intravenous antimicrobials in 14 children was 4.21 days (range 2–21 days) and cure was 100%. |
| Bandelon 1988 | Group of osteomyelitis and septic arthritis could not be separated with respect to length of therapy or etiology. |
| Kulhanjianv 1989 | Only 3 cases of osteomyelitis due to S. aureus. |
| Aronoff 1986 | Of 9 children only 1 child had osteomyelitis alone. No follow-up. |
| Learmonth 1984 | Combined patients with septic arthritis and osteomyelitis. Could not extract data with respect to S. aureus alone. |
Table 2
Description of studies and the associated cure rates
| Author | Publication year | Sample size1 | S. aureus positive culture (%) | Parenteral (IV) antimicrobial | Days of IV treatment Mean (range) | Oral antimicrobial | Days of oral treatment Mean (range) | Cure rate % (CI) |
| Long Course | ||||||||
| Kolyvas2 | 1980 | 5 | 50.03 | Cephradine +/- Ampicillin | 28 (28–28) | Cephradine or Cloxacillin | 18 (14–35) | 100 (67, 100) |
| Tetzlaff | 1978 | 18 | 100.0 | Methicillin or Cefazolin | 8 (7–13) | Cephalexin or Penicillin V | 20 (13–42) | 94 (77, 100) |
| Bryson | 1979 | 18 | 100.0 | Oxacillin or Methicillin | 7 (5–14) | Dicloxacillin | N/A (28–56) | 100 (89, 100) |
| Rodriguez | 1977 | 21 | 90.5 | Clindamycin4 | 25 (21–28) | Clindamycin | 42 (42–42) | 100 (91, 100) |
| Prober | 1979 | 22 | 100.0 | Nafcillin or Methicillin | 145 (4–28) | Dicloxacillin | 283 (N/A) | 100 (91, 100) |
| Short Course | ||||||||
| Kolyvas2 | 1980 | 5 | 50.03 | 3 (N/A) | Cephradine or Cloxacillin | 423 (39–60)3 | 100 (67, 100) | |
| Refass6 | 1989 | 6 | 98.9 | Cloxacillin | N/A (5–6) | Flucloxacillin | N/A (7–200)3 | 100 (72, 100) |
| Freij | 1987 | 8 | 100.0 | Imipenem + Cilastatin | 5.5 (4–9) | Cefaclor or Dicloxacillin or Cephalexin or Bacampicillin or Penicillin V | 15 (13–19) | 88 (55, 100) |
| Feigin7 | 1975 | 11 | 72.7 | Clindamycin | 6 (3–13) | Clindamycin | 425 (N/A) | 91 (65, 100) |
| Geddes8 | 1977 | 18 | 65.03 | Clindamycin | 63 (N/A) | Clindamycin | 903(N/A) | 94 (77, 100) |
| Cole9 | 1982 | 48 | 94.5 | Cloxacillin or Cephalothin | 3 (2–5) | Cloxacillin or Cephalexin | 39 (37–40) | 92 (81, 97) |
| Peltola | 1997 | 50 | 100.0 | Cephradine or Cephalothin or Clindamycin | 4 (4–6) | Cefadroxil or Clindamycin | 19 (17–19) | 100 (96, 100) |
1 Includes cases that were due to Staphylococcus aureus and those who were culture negative 2 Included only the cases that had osteomyelitis alone or had contiguous arthritis and osteomyelitis. 3 Estimated value. 4 Clindamycin is the only macrolide studied – all other antimicrobials mentioned are beta-lactams. 5 Median reported. 6 Only the 6 patients who clearly met the diagnostic criteria were included. 7 Data abstracted from the 11 cases of osteomyelitis (8 of whom had S. aureus). 8 Only data for the 18 children with acute osteomyelitis from the study were included. Some of these may only have received oral medication. 9 Data from the 48 patients for whom there was follow-up of greater than 6 months.
The range of duration of parenteral antimicrobial therapy was 3 to 28 days. Table Table22 shows details of the 12 cohort studies that were used in the analysis. Figure Figure22 graphically illustrates the cure rate versus the duration of parenteral antimicrobial therapy for these cohorts. (Figure (Figure22)
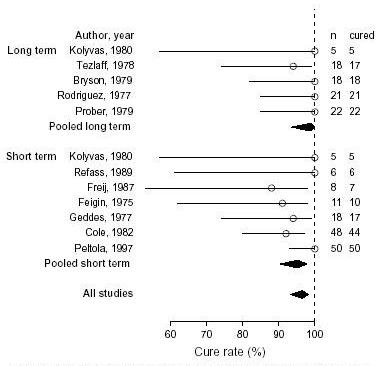
Cure rate versus the duration of parenteral antimicrobial therapy Long-term parenteral antimicrobials is defined as greater than or equal to 7 days. Short-term parenteral antimicrobials is defined as less than 7 days
The 7 cohorts that had less than 7 days of intravenous therapy (n = 146) had a pooled cure rate of 95.2% (95% confidence interval: 90.4, 97.7) [28-32,35,37]. There was no significant heterogeneity among their cure rates (chi-square= 8.2 on 6 df, p= 0.224), indicating that the variation in cure rates could be attributed to sampling error.
The 5 cohorts who had a duration of intravenous therapy of 7 days or longer (n = 84) had a pooled cure rate of 98.8% (95% confidence interval; 93.6, 99.8) [27,31,33,34,36]. There was no significant heterogeneity among these cure rates (chi-square = 3.1 on 4 df, p = 0.537). Pooling all of the cohorts regardless of duration of intravenous therapy, there was no significant heterogeneity among cure rates (chi-square = 13.7 on 11 df, p = 0.248). The fixed effects model gave a pooled cure rate of 96.5% (95% confidence interval; 93.3, 98.2). There was no significant difference in the cure rate between the two groups (z-test p-value 0.838).
Comparing beta-lactams and macrolides, there was no significant heterogeneity within either group (beta-lactams; chi square = 7.3 on 7 df, p = 0.394. macrolides; chi square = 5.2 on 4 df, p= 0.159). The pooled cure rate for beta-lactams was 95.4% (95% confidence interval; 90.3, 97.9) under a fixed effects model. The pooled cure rate for macrolides was 98.0% (95% confidence interval; 93.0, 99.4). There was no significant difference in the cure rate between the two groups (z-test p-value 0.286).
Since the total duration of antimicrobial therapy (i.e., parenteral plus oral) can affect the cure rate, we also looked at the duration of oral therapy. For the cohorts that had less than 1 week of parenteral therapy, the range of duration of oral therapy was 15 to 39 days with a mean of 32 days [28-31,35,37]. We could not reliably determine the mean number of days of oral antimicrobials from one study [32]. For the cohorts that were given a longer duration of intravenous therapy, the range of duration of oral therapy was 18 to 56 days with a mean of 33 days [27,31,33,34,36]. There was no significant difference in the duration of oral therapy between the two groups (t-test p-value 0.888).
Among the group who had short-term antimicrobials, the percentage of children that had surgery ranged from 30% to 'routine' or probably over 90% [28-31,35,37]. Two authors did not state the percentage of children who required surgery [29,32]. Among the long-term group, the range is from 50 to 90% [27,31,33,34,36]. It was not stated explicitly in most cases whether these procedures were for diagnostic or therapeutic purposes.
The number of children with septic arthritis in the short course group ranged from 0 to 40% [29,31,35,37]. In three studies the exact number could be deduced, [28,30,32] whereas in the 5 cohorts who were given greater than 7 days of therapy, 0% to 20% of children had concomitant septic arthritis [27,31,33,34,36].
Discussion
The epidemiology of childhood AHO has changed since routine childhood immunization against Haemophilus influenzae type b was introduced in the early 1990s [39]. Currently, the most common causative organism of childhood AHO is Staphylococcus aureus. For AHO therapy to be successful, levels of antimicrobial agents must be maintained sufficiently high over time to eliminate the bacteria from the site of infection. Pharmacologic evidence for adequate concentrations of oral antimicrobial agents in affected tissues supports the use of oral agents within a management scheme of 'step-down therapy.' [34,36] The benefits of shorter courses of parenteral antimicrobial therapy before switching to oral therapy include decreased costs and morbidity. There still exists, however, a dilemma with respect to the optimal duration of parenteral therapy.
Our systematic review of cohort studies using a minimum follow up of 6 months found a pooled cure rate of 95.2% (95% CI = 90.4 – 97.7) with 7 days or less of parenteral therapy. Other cohorts that had short follow-up (therefore not included in our review) reported treating children with less than 7 days of parenteral antimicrobial reported cure rates of greater than 90%; [40,41]. In one other cohort (not suitable for inclusion in our analysis due to unextractable data, heterogeneous group and a follow up period of 4 months), a 95% cure rate was observed with 14 days of parenteral antimicrobial therapy [42]. Many other prospective studies could not be included in the systematic review for a variety of reasons: lack of standardized definitions, lack of retrievable treatment information or short follow-up periods [2,19,40-49].
The small sample size used (mean of 24 patients; range 5 to 50 patients) is of concern although we gained statistical strength by pooling across studies. Thus, individual studies have wide confidence intervals, which are narrowed using the systematic review approach, providing a more precise estimate of the "true" cure rates (Figure (Figure1).1). The range of duration of oral antimicrobial agents was also wide, implying that there may have been other clinical factors that were implicit in the decision of total length of therapy. Other clinical parameters, such as the extent of bone involvement, suspicion of septic arthritis and the course of the illness before treatment, may also have influenced treatment duration, but were not reported. Although the studies were prospective cohorts with respect to length of therapy they may be inherently biased in their selection criteria.
We did not assess the quality of reports of the included studies. Quality assessment is an important part of a systematic review, particularly if a validated approach is used [50]. We are unaware of any published assessment measure for cohort studies and elected to forgo the pitfalls of developing one ourselves [51].
Clinical heterogeneity in the data from older studies can be partly attributed to the multiple pathogens that were responsible for the clinical syndrome. Only one study was published after routine childhood immunization for Haemophilus influenzae type b was introduced in the early 90's [35]. We included children who had had disease due to Staphylococcus aureus as well as those who were culture negative because in many cases this reflects the reality of clinical practice. Studies that deal only with staphylococcal disease or that were published after 1990 from areas where there is universal immunization against Haemophilus influenzae type b are most likely to best represent the child who currently presents with AHO due to methicillin sensitive Staphylococcus aureus (MSSA).
Our review had several limitations. We narrowed our focus to cohort studies. Results from randomized controlled trials may provide more valid results and we are aware of the existence of at least one such study [38]. The cure rate of 91.6% in children who received short course parenteral therapy however supports the findings of this systematic review. This report however only included 12 children in that group. Combining this study with the cohort studies might introduce bias into the estimates of cure rates.
Our results indicate that cure rates are similar regardless of whether children with AHO are treated for a shorter or longer time period. There are several potential practical advantages of the shorter course of therapy; shorter hospital stay; decreased morbidity from intravenous lines and more cost effective. We believe that the results from this systematic review warrant serious consideration be given to conducting a randomized controlled trial. Such a study could be developed to demonstrate equivalence between two durations of antibiotic therapy: a shorter course (i.e., 3–5 days) compared to a more "standard" course of 14 to 21 days. This would only be relevant if cure rates were not equivalent. The results from such a study could be used to better inform clinicians as to the management of children with AHO in the future.
Competing interests
None declared.
Authors' contributions
NLS conceived of the study, participated in study design, reviewed trials for inclusion, abstracted data, participated in data analysis, and drafted the first manuscripts.
AH participated in initial study design, reviewed trials for inclusion, abstracted data, participated in data analysis, contributed to writing of the manuscript.
NJB planned and directed the statistical analyses, and participated in the drafting and revision of the manuscript.
IG extracted data from trials, carried out statistical analyses, and participated in the drafting and revision of the manuscript.
MS participated in the development of the literature search, interpretation of the results and preparation of the manuscript.
DM supervised the systematic review and advised on methods issues.
All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Contains= database names, dates searched, and search strings for the electronic search strategies.
Acknowledgements
We would like to thank Dr. Barbara Law and for helpful comments on an earlier draft of this manuscript.
References
- Krogstad P, Smith AL. Steomyelitis and septic arthritis. In: Feigin R, Cherry J, editor. In Textbook of pediatric infectious diseases. Philadelphia: W.B. Saunders Company; 1998. pp. 683–698. [Google Scholar]
- Unkila Kallio L, Kallio MJT, Peltola H. Acute hematogenous osteomyelitis in children in Finland. Ann Med. 1993;25:545–549. [Abstract] [Google Scholar]
- Nade S. Acute hematogenous Osteomyelitis in Infancy and Childhood. J Bone Joint Surg Br. 1983;65B:109–119. [Abstract] [Google Scholar]
- Nelson J. Osteomyelitis and Suppurative Arthritis. In: Behrman R.E., Kliegman R.M., Jenson H.B., editor. In Nelson Textbook of Pediatrics. Philadelphia: W.B. Saunders Company; 2000. pp. 776–780. [Google Scholar]
- Gutierrez KM. Osteomyelitis. In: Long SS, Pickering L.K., Prober C.G., editor. In Principles and Practice of Pediatric Infectious Diseases. New York: Churchill Livingston Inc.;; 1997. pp. 528–537. [Google Scholar]
- Morrissy RT. Bone and joint sepsis. In: Morrissy R, Weinstein SL, editor. In Lovell and Winter's Pediatric Orthopedics. Philadelphia: Lippincott Williams & Wilkins;; 2001. pp. 459–503. [Google Scholar]
- Dich VQ, Nelson JD, Haltalin KC. Osteomyelitis in infants and children: a review of 163 cases. Am J Dis Child. 1975;129:1273–1278. [Abstract] [Google Scholar]
- Blockey NJ, Watson JT. Acute osteomyelitis in children. J Bone Joint Surg Br. 1970;52B:77–87. [Abstract] [Google Scholar]
- Jacobs RF, Augustine RA, Aronson J, McCarthy RE, Steele RW, Yamauchi T. Timentin therapy for bone, joint, and deep soft tissue infections in children. Am J Med. 1985;79:188–191. 10.1016/0002-9343(85)90158-5. [Abstract] [CrossRef] [Google Scholar]
- Meller I, Manor Y, Bar-Ziv J, Torok G. Acute hematogenous osteomyelitis in children: long-term results of surgical treatment. Orthop Rev. 1989;18:824–831. [Abstract] [Google Scholar]
- Mollan R, Piggot J. Acute osteomyelitis in children. J Bone Joint Surg Br. 1977;9B:2–7. [Abstract] [Google Scholar]
- Bamberger T, Gugler E. [Acute osteomyelitis in childhood. A follow-up of predominantly conservatively treated children]. [German]. Schweiz Med Wochenschr. 1983;113:1219–1228. [Abstract] [Google Scholar]
- Rud B, Halken S, Damholt V. Hematogenous osteomyelitis in children. Acto Orthop Scand. 1986;57:440–443. [Abstract] [Google Scholar]
- Syrogiannopoulos GA, Nelson JD. Duration of antimicrobial therapy for acute suppurative osteoarticular infections. Lancet. 1988:37–40. 10.1016/S0140-6736(88)91013-6. [Abstract] [CrossRef] [Google Scholar]
- LaMont RL, Anderson PA, Dajani AS, Thirumoorthi MC. Acute hematogenous osteomyelitis in children. J Pediatr Orthop. 1987;7:579–583. [Abstract] [Google Scholar]
- Kandel SN, Mankin HJ. Pyogenic abscess of the long bones in children. Clin Orthop. 1973;96:108–117. [Abstract] [Google Scholar]
- Karwowska A, Davies HD, Jadavji T. Epidemiology and outcome of osteomyelitis in the era of sequential intravenous-oral therapy. Pediatr Infect Dis J. 1998;17:1021–1026. 10.1097/00006454-199811000-00012. [Abstract] [CrossRef] [Google Scholar]
- Petersen S, Knudsen FU, Anderson EA, Egebald M. Acute haematogenous osteomyelitis and septic arthritis. Acto Orthop Scand. 1980;51:451–457. [Abstract] [Google Scholar]
- Dones P, Scarlata F, Di Gangi M. Effectiveness of teicoplanin as a monotherapy in the treatment of coagulase-positive Staphylococcus aureus in osteomyelitis. Mediterranean J of Infect & Parasitic Dis. 1994;9:99–100. [Google Scholar]
- Arango JL, Trujillo H, Worren D, Uribe A, Agudelo NH, de Vidal EL. Effectiveness of two new cephalosporins, cephazolin and cephapirin, administered intermittently in acute and chronic osteomyelitis in children. J Int Med Res. 1976;4:183–194. [Abstract] [Google Scholar]
- Sadat-Ali M. Manage of acute osteomyelitis in children–should it be conservative? Indian J Med Sci. 1992;46:297–300. [Abstract] [Google Scholar]
- Highland TR, Lamont RL. Osteomyelitis of the pelvis in children. J Bone Joint Surg Am. 1983;65-A:230–234. [Abstract] [Google Scholar]
- Geissler WB, Purvis JM. Hematogenous osteomyelitis and septic arthritis in children: a ten year review. J Miss State Med Assoc. 1989;30:71–74. [Abstract] [Google Scholar]
- Green JH. Cloxacillin in treatment of acute osteomyelitis. BMJ. 1967;2:414–416. [Europe PMC free article] [Abstract] [Google Scholar]
- Lane-O'Kelly A, Moloney AC. Acute haematogenous osteomyelitis – evaluation of management in the 1990s. Ir J Med Sci. 1995;164:285–288. [Abstract] [Google Scholar]
- Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E. [Abstract] [CrossRef] [Google Scholar]
- Bryson YJ, Connor JD, LeClerc M, Giammona ST. High-dose oral dicloxacillin treatment of acute staphylococcal osteomyelitis in children. J Pediatr. 1979;94:673–675. [Abstract] [Google Scholar]
- Cole WG, Dalziel RE, Leitl S. Treatment of acute osteomyelitis in childhood. J Bone Joint Surg Br. 1982;64:218–223. [Abstract] [Google Scholar]
- Feigin RD, Pickering LK, Anderson D, Keeney RE, Shackleford PG. Clindamycin treatment of osteomyelitis and septic arthritis in children. Pediatrics. 1975;55:213–223. [Abstract] [Google Scholar]
- Freij BJ, Kusmiesz H, Shelton S, Nelson JD. Imipenem and cilastatin in acute osteomyelitis and suppurative arthritis. Am J Dis Child. 1987;141:335–342. [Abstract] [Google Scholar]
- Kolyvas E, Ahronheim G, Marks MI. Oral antibiotic therapy of skeletal infections in children. Pediatrics. 1980;65:867–871. [Abstract] [Google Scholar]
- Refass A, Harouchi A, Fehri M, El Andaloussi M, Bellamine A, Merini MF. The treatment of acute osteomyelitis in children by monoantibiotic therapy with flucloxacillin. Medecine et Maladies Infectieuses. 1989;19:96–100. [Google Scholar]
- Rodriguez W, Ross S, Khan W, McKay D, Moskowitz P. Clindamycin in the treatment of osteomyelitis in children. Am J Dis Child. 2000;131:1088–1093. [Abstract] [Google Scholar]
- Tetzlaff TR, McCracken GH, Nelson JD. Oral antibiotic therapy for skeletal infections in children. J Pediatr. 1978;92:485–490. [Abstract] [Google Scholar]
- Peltola H, Unkila Kallio L, Kallio MT, Aalto K, Anttolainen I, Fagerholm R, et al. Simplified treatment of acute staphylococcal osteomyelitis of childhood. Pediatrics. 1997;99:846–850. [Abstract] [Google Scholar]
- Prober CG, Yeager AS. Use of the serum bactericidal titer to assess the adequacy of oral antibiotic therapy in the treatment of acute hematogenous osteomyelitis. J Pediatr. 1979;95:131–135. [Abstract] [Google Scholar]
- Geddes AM, Dwyer NStJ, Ball AP, Amos RS. Clindamycin in bone and joint infections. J Antimicrob Chemother. 1977;3:501–507. [Abstract] [Google Scholar]
- Kaplan SL, Mason EOJ, Feigin RD. Clindamycin versus nafcillin or methicillin in the treatment of Staphylococcus aureus osteomyelitis in children. South Med J. 1982;75:138–142. [Abstract] [Google Scholar]
- Howard AW, Viskontas D, Sabbagh C. Reduction in osteomyelitis and septic arthritis related to Haemophilus influenzae type B vaccination. J Pediatr Orthop. 1999;19:705–709. 10.1097/00004694-199911000-00003. [Abstract] [CrossRef] [Google Scholar]
- Nelson JD, Bucholz RW, Kunmiesz H, Shelton S. Benefits and risks of sequential parenteral-oral cephalosporin therapy for suppurative bone and joint infections. J Pediatr Orthop. 1982;2:255–262. [Abstract] [Google Scholar]
- Walker SH. Staphylococcal osteomyelitis in children: success with cephaloridine-cephalexin therapy. Clin Pediatr (Phila) 1973;12:98–100. [Abstract] [Google Scholar]
- Nussinovitch M, Shalit I, Einhorn M, Keren G, Rachmel A, Asia A, et al. Amoxicillin-clavulanate versus standard antibiotic therapy for the treatment of septic arthritis and osteomyelitis. Pediatrics & Related Topics. 1997;36:73–82. [Google Scholar]
- Beauvais P, Filipe G, Berniere J, Carlioz H. Oral pristinamycinum therapy for bone and joint infections in children. A report of 50 cases. Arch Fr Pediatr. 1981;38:489–493. [Abstract] [Google Scholar]
- Fleming PC, Huda SS, Bobechko WP. Cephaloridine and the penicillins in the treatment of staphylococcal osteomyelitis and arthritis. Postgrad Med J. 1970:89–93. [Abstract] [Google Scholar]
- Badelon O, Bingen E, Sauzeau C, Lambert-Zechovsky N, de Ribier A, Bensahel H. [Choice of first-line antibiotic therapy in the treatment of bone and joint infections in children]. [French]. Pathol Biol (Paris) 1988;36:746–749. [Abstract] [Google Scholar]
- Kulhanjian J, Dunphy MG, Hamstra S, Levernier K, Rankin M, Petru A, et al. Randomized comparative study of ampicillin/sulbactam vs. ceftriaxone for treatment of soft tissue and skeletal infections in children. Pediatr Infect Dis J. 1989;8:605–610. [Abstract] [Google Scholar]
- Learmonth ID, Dall G, Pollock DJ. Acute osteomyelitis and septic arthritis in children. A simple approach to treatment. South African Medical Journal. 1984;65:117–120. [Abstract] [Google Scholar]
- Aronoff SC, Scoles PV, Makley JT, Jacobs MR, Blumer JL, Kalamchi A. Efficacy and safety of sequential treatment with parenteral sulbactam/ampicillin and oral sultamicillin for skeletal infections in children. Rev Infect Dis. 1986;8:S639–S643. [Abstract] [Google Scholar]
- Unkila-Kallio L, Kallio MJ, Eskola J, Peltola H. Serum C-reactive protein, erythrocyte sedimentation rate, and white blood cell count in acute hematogenous osteomyelitis of children. Pediatrics. 1994;93:59–62. [Abstract] [Google Scholar]
- Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, et al. Assessing the quality of randomized controlled trials: Implications for the conduct of meta-analyses. Health Technol Assess. 1999;3:1–98. [Abstract] [Google Scholar]
- Marshall M, Lockwood A, Bradley C, Adams CE, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomized controlled trials of treatments for schizophrenia. British Journal of Psychiatry. 2000;176:249–252. 10.1192/bjp.176.3.249. [Abstract] [CrossRef] [Google Scholar]
- Al-Harby S. Acute hematogenous osteomyelitis of childhood in Saudi Arabia. Why does treatment fail? Saudi Med J. 1997;18:148–150. [Google Scholar]
- Babaiantz P. [Acute osteomyelitis in infants and children. Follow-up study of 25 cases]. [French]. Schweiz Rundsch Med Prax. 1975;64:706–720. [Abstract] [Google Scholar]
- Dagan R, Phillip M, Watemberg NM, Kassis I. Outpatient treatment of serious community-acquired pediatric infections using once daily intramuscular ceftriaxone. Pediatr Infect Dis J. 1987;6:1080–1084. [Abstract] [Google Scholar]
- Dirschl DR. Acute pyogenic osteomyelitis in children. Orthop Rev. 1994;23:305–312. [Abstract] [Google Scholar]
- Hoffman EB, Knudsen CJ, Paterson MP. Acute osteomyelitis and septic arthritis in children: A spectrum of disease. Pediatr Surg Int. 1990;5:382–386. [Google Scholar]
- Jackson MA, Burry VF, Olson LC. Pyogenic arthritis associated with adjacent osteomyelitis: identification of the sequelae-prone child. Pediatr Infect Dis J. 1992;11:9–13. [Abstract] [Google Scholar]
- Braun HS, Ender A, Friedel B. [Follow-up studies in infantile osteomyelitis]. [German]. Beitr zur Orthop Traumatol. 1984;31:305–313. [Abstract] [Google Scholar]
- Aigner RM, Fueger GF, Vejda M. Follow-up of osteomyelitis of infants with systemic serum parameters and bone scintigraphy. Nucl Med (Stuttg) 1996;35:116–121. [Abstract] [Google Scholar]
- Anderson JR, Scobie WG, Watt B. The treatment of acute osteomyelitis in children: A 10-year experience. J Antimicrob Chemother. 1981;7:43–50. [Abstract] [Google Scholar]
- Craigen MAC, Watters J, Hackett JS. The changing epidemiology of osteomyelitis in children. J Bone Joint Surg Br. 1992;74:541–545. [Abstract] [Google Scholar]
- Fink CW, Nelson JD. Septic arthritis and osteomyelitis in children. Clin Rheum Dis. 1986;12:423–435. [Abstract] [Google Scholar]
- François P, Sarlangue J, Grimprel E, Carrieré JC, Garnier JM, De , et al. Epidemiology and bacteriological diagnosis of osteoarticular infections in children. A multicentric study (I). Medecine et Maladies Infectieuses. 1992;22:758–762. [Google Scholar]
- Gillespie WJ, Mayo KM. The management of acute haematogenous osteomyelitis in the antibiotic era. A study of the outcome. J Bone Joint Surg Br. 1981;63:126–131. [Abstract] [Google Scholar]
- Roine I, Arguedas A, Faingezicht I, Rodriguez F. Early detection of sequela-prone osteomyelitis in children with use of simple clinical and laboratory criteria. Clin Infect Dis. 1997;24:849–853. [Abstract] [Google Scholar]
- Scott RJ, Christofersen MR, Robertson WW, Jr, Davidson RS, Rankin L, Drummond DS. Acute osteomyelitis in children: a review of 116 cases. J Pediatr Orthop. 1990;10:649–652. [Abstract] [Google Scholar]
- Van Brederode NE, Ponsen RG. Acute haematogenic osteomyelitis. Ned Tijdschr Geneeskd. 1980;124:453–455. [Abstract] [Google Scholar]
- Vaughan PA, Newman NM, Rosman MA. Acute hematogenous osteomyelitis in children. J Pediatr Orthop. 1987;7:652–655. [Abstract] [Google Scholar]
- Anderson JR, Orr JD, Maclean DA, Scobie WG. Acute haematogenous osteitis. Archives of Disease in Childhood. 1980;55:953–957. [Europe PMC free article] [Abstract] [Google Scholar]
- Faden H, Grossi M. Acute osteomyelitis in children. Reassessment of etiologic agents and their clinical characteristics. Am J Dis Child. 1991;145:65–69. [Abstract] [Google Scholar]
- Gillespie WJ. Late recurrence following acute haematogenous osteomyelitis. New Zealand Medical Journal. 1975;82:304–305. [Abstract] [Google Scholar]
- Trujillo H, Alvarez RM, Rodriguez AB, Roldán RF, Warren DS, Gil HL, et al. La rifampicina y las penicinas en el tratamiento de las osteomielitis hematogenas de los niño. Antioquia Medica. 1974;24:443–452. [Google Scholar]
Articles from BMC Infectious Diseases are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1471-2334-2-16
Read article for free, from open access legal sources, via Unpaywall:
https://bmcinfectdis.biomedcentral.com/track/pdf/10.1186/1471-2334-2-16
Citations & impact
Impact metrics
Citations of article over time
Article citations
Delayed Diagnosis of Pediatric Sternoclavicular Joint Infections and Clavicular Osteomyelitis During the COVID-19 Pandemic: A Report of 3 Cases.
J Am Acad Orthop Surg Glob Res Rev, 6(9):e21.00302, 27 Sep 2022
Cited by: 0 articles | PMID: 36166203 | PMCID: PMC9519139
Is Early Surgical Intervention Necessary for Acute Neonatal Humeral Epiphyseal Osteomyelitis: A Retrospective Study of 31 Patients.
Children (Basel), 9(4):527, 07 Apr 2022
Cited by: 1 article | PMID: 35455571 | PMCID: PMC9028880
A Rare Presentation of Brodie Abscess in the Clavicle.
J Am Acad Orthop Surg Glob Res Rev, 5(4), 13 Apr 2021
Cited by: 0 articles | PMID: 33848278 | PMCID: PMC8049390
Prediction of Adverse Outcomes in Pediatric Acute Hematogenous Osteomyelitis.
Clin Infect Dis, 71(9):e454-e464, 01 Dec 2020
Cited by: 11 articles | PMID: 32129457 | PMCID: PMC7904074
Oral Flucloxacillin for Treating Osteomyelitis: A Narrative Review of Clinical Practice.
J Bone Jt Infect, 5(1):16-24, 01 Jan 2020
Cited by: 3 articles | PMID: 32117685 | PMCID: PMC7045523
Review Free full text in Europe PMC
Go to all (48) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Daptomycin for Pediatric Gram-Positive Acute Hematogenous Osteomyelitis.
Pediatr Infect Dis J, 39(9):814-823, 01 Sep 2020
Cited by: 4 articles | PMID: 32639465
Early Transition to Oral Antimicrobial Therapy Among Children With Staphylococcus aureus Bacteremia and Acute Hematogenous Osteomyelitis.
Pediatr Infect Dis J, 41(9):690-695, 13 Jun 2022
Cited by: 1 article | PMID: 35703303
[Intravenous antibiotic therapy for acute hematogenous osteomyelitis in children: short versus long course].
Arch Pediatr, 20(5):464-469, 06 Apr 2013
Cited by: 1 article | PMID: 23566577
Review
Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis.
J Infect, 54(6):539-544, 02 Jan 2007
Cited by: 43 articles | PMID: 17198732

 1,2
1,2