Abstract
Free full text

Quantitative investigation of reproduction of gonosomal condensed chromatin during trophoblast cell polyploidization and endoreduplication in the east-european field vole Microtus rossiaemeridionalis
Abstract
Simultaneous determinations of DNA content in cell nuclei and condensed chromatin bodies formed by heterochromatized regions of sex chromosomes (gonosomal chromatin bodies, GCB) have been performed in two trophoblast cell populations of the East-European field vole Microtus rossiaemeridionalis: in the proliferative population of trophoblast cells of the junctional zone of placenta and in the secondary giant trophoblast cells. One or two GCBs have been observed in trophoblast cell nuclei of all embryos studied (perhaps both male and female). In the proliferative trophoblast cell population characterized by low ploidy levels (2–16c) and in the highly polyploid population of secondary giant trophoblast cells (32–256c) the total DNA content in GCB increased proportionally to the ploidy level. In individual GCBs the DNA content also rose proportionally to the ploidy level in nuclei both with one and with two GCBs in both trophoblast cell populations. Some increase in percentage of nuclei with 2–3 GCBs was shown in nuclei of the placenta junctional zone; this may be accounted for by genome multiplication via uncompleted mitoses. In nuclei of the secondary giant trophoblast cells (16–256c) the number of GCBs did not exceed 2, and the fraction of nuclei with two GCBs did not increase, which suggests the polytene nature of sex chromosomes in these cells. In all classes of ploidy the DNA content in trophoblast cell nuclei with the single GCB was lower than in nuclei with two and more GCBs. This can indicate that the single GCB in many cases does not derive from fusion of two GCBs. The measurements in individual GCBs suggest that different heterochromatized regions of the X- and Y-chromosome may contribute in GCB formation.
Introduction
Study of genome multiplication in various trophoblast cell populations is of great importance for elucidating different mechanisms of genome reproduction responsible for performance by the trophoblast cells of their specific functions. These functions include invasion and phagocytosis of maternal tissues as well as, on the other hand, proliferation of trophoblast cell populations that do not contact directly the maternal tissues. To study behavior of chromosomes during the modified cell cycle characteristic of trophoblast cells, various chromosomal markers can be used.
One of such markers is one of two X-chromosomes, which in female individuals is genetically inactive; this provides for a dose compensation of X-linked genes in somatic cells of female (XX) and male (XY) individuals [1]. The inactivated X-chromosome is visualized in the interphase nucleus as sex chromatin or Barr body: a clump of condensed chromatin located beneath the nuclear membrane or near nucleolus [1-4]. In most studied mammalian species, inactivation ("lyonization") involves a major part of one of X-chromosomes in somatic cells of female individuals [5-7]. Nevertheless, in some species, the second X-chromosome also participates in heterochromatization of sex chromosomes. Such a regularity is observed in the species whose X-chromosome contains large blocks of structural heterochromatin [8-12]. In several vole species the structural heterochromatin is concentrated mainly in X- and Y-chromosomes [13-17]. On the other hand, in some vole species, large condensed chromatin clumps resembling sex chromatin (Barr body) are revealed in cells both of female and male individuals [8,18,19], presumably due to a significant degree of heterochromatization of both sex chromosomes.
In the East-European field vole Microtus rossiaemeridionalis (formerly Microtus subarvalis) the structural heterochromatin also is present predominantly in sex chromosomes and forms a large, telomere-localized block in the long arm of X-chromosome and practically the entire long arm of the acrocentric Y-chromosome [16,17,20,21]. Analysis of behavior of the gonosomal eu- and heterochromatin in interphase nuclei of trophoblast cells is important for study of mechanisms and role played for the inactivation of sex chromosomes in embryogenesis and embryo implantation. Besides, the bodies of the condensed chromatin formed by sex chromosomes (gonosomal chromatin bodies, GCB) in M. rossiaemeridionalis can be used to study behavior of interphase nucleus chromatin for elucidating mechanisms of cell polyploidization not only in highly polyploid nuclei, but also in nuclei of a relatively low ploidy.
Materials and Methods
Used in the work were placentae of the East-European field vole; this species was initially described as Microtus subarvalis [22], but later, according to the nomenclature codex, was changed to Microtus rossiaemeridionalis [23]. Placentae were kindly provided by Dr.E.D. Sholl. To obtain the placentae females were caged with males and the morning on which vaginal plugs were confirmed was designated by 1st day post coitum (pc).
The experiments were performed in compliance with regulations of the National Ethics Commettee under the auspice of the Russian Academy of Sciences.
The placentae were fixed with 3:1 mixture of ethanol and glacial acetic acid for 2 hour. A total of 12 embryos from 6 females were studied: 3 females each at the 10th and 14th day pc.
Permanent squash preparations were made of the main part of the material. For this purpose the fixed placentae were dehydrated in the two portions of 96% ethanol (1 hour in each portion), then incubated in one portion of 70% ethanol (2 hour); the 2–3 mm pieces of placentae were macerated for 20 min in 45% acetic acid, placed on object slides, slightly pressed and covered with coverslips. The slides were then placed on dry ice (CO2). On freezing the preparations, the coverslips were removed and the slides were air-dried. The slides were Feulgen-stained (hydrolysis in 5 N HCl for 30 min at room temperature).
A part of the fixed material was dehydrated in a graded series of ethanols and embedded in paraffin; from this material, histological sections were cut at 7 μm and Feulgen-stained. Besides, there was analyzed a collection material of placentae at the 9–17th days pc (more than 30 fetuses from 8 females), which was fixed, paraffin embedded and cut according to the procedure mentioned above; these section were stained with Boemer hematoxylin and by Feulgen reaction as described above.
The DNA content was measured using a "Videotest" image analyze system composed of an CPT 83 60 digital CCD-videocamera (Chipper, USA) installed on an EC Bimam-13 microscope and of computer IBM PC 166. Input of the image and measurement of the integral optical density characterizing the DNA amount in the nucleus and separately in GCB were performed using a Videotest-Morpho software (St. Petersburg); a possibility of measuring DNA content in hepatocyte nuclei with this image analyze system was shown earlier [24]. An objective 40 × 0.65 and an interference filter at 550 nm were used. In each placenta there were analyzed from 150 to 400 trophoblast cell nuclei of each of cell populations and 50 nuclei of fetal erythrocyte nuclei as a control of the DNA content in diploid nuclei. The following parameters were evaluated: a) DNA content per nucleus in arbitrary units (a.u.) that reflects ploidy (c); b) DNA content in GCB (in individual bodies and as the total in nuclei with 2–3 GCB, in a.u); c) ratio of the DNA content in the nucleus to the total DNA content in GCB; d) number of GCB; e) percentage of nuclei containing one and several GCB. From these data, the means and the standard errors were calculated as well as the correlation coefficient (r) between various parameters. Since the DNA content in GCB could differ depending on sex of the embryos, a part of numerical data were presented for individual embryos.
Results
At the initial period of placentation of the field vole embryo (9th day pc) the continuous layer of secondary giant trophoblast cells is present at the periphery of the ectoplacental cone; these cells invade actively decidua basalis. However, as early as at the 10th day of development, during differentiation of the placenta junctional zone and labyrinth, accumulations of trophoblast cells of the junctional zone migrate towards decidua basalis. They move across the layer of the secondary giant cells to form finally together with them the peripheral zone of the fetal part of placenta [25]. For several next days, in the depth of the placenta junctional zone and labyrinth the mitotic activity still is high, whereas the trophoblast cells of the peripheral zone undergo differentiation and completely stop mitotic divisions.
Nuclei of the secondary giant trophoblast cells and of the placenta junctional zone of all studied field vole embryos both in the squash preparations and in the Feulgen-stained sections contained one or, more seldom, 2–3 compact bodies of gonosomal chromatin. We failed to distinguish sex of the M. rossiaemeridionalis embryos by the presence of the GCB, like the sex is determined in many mammals from the presence of sex chromatin [26-31]. Analysis of the collection material of the East-European field vole (more than 30 embryos from 8 females) did not reveal placentae whose trophoblast cells were lacked of large GCB resembling morphologically the sex chromatin. Therefore, we believe that in the vole M. rossiaemeridionalis, like in M. agrestis [18,19] and M. oregoni [8], the condensed bodies of the gonosomal chromatin are present in cells of both sexes. In trophoblast cells of the placenta junctional zone, GCBs are morphologically similar to Barr bodies: they are as a rule closely adjacent to the nuclear membrane surface or lay near the nucleolus. In most nuclei, one GCB was revealed; however, nuclei with 2, more seldom 3 GCBs were present not infrequently (Fig. (Fig.11).
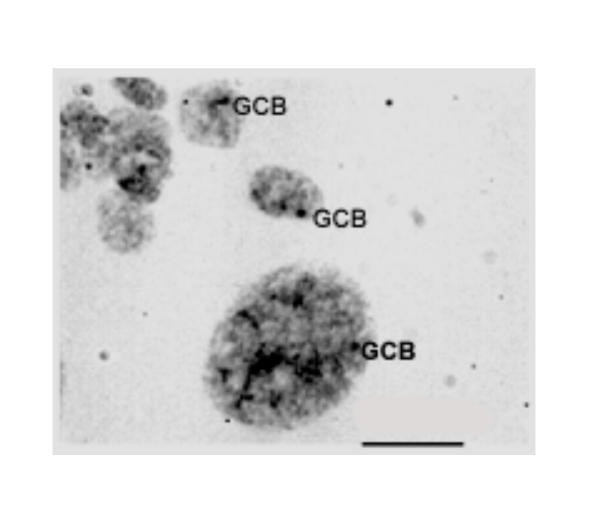
The nuclei of the trophoblast cells of the junctional zone of placenta and a secondary giant trophoblast cell (down, right) with one or two gonosomal chromatin bodies (GCBs) attached to the nuclear membrane or in contact with nucleolus (10 day pc). Feulgen staining. Bar is 15 μm.
In nuclei of secondary giant trophoblast cells, 1–2 large compact GCB contacting the nuclear membrane or nucleolus are observed (Fig. (Fig.22).
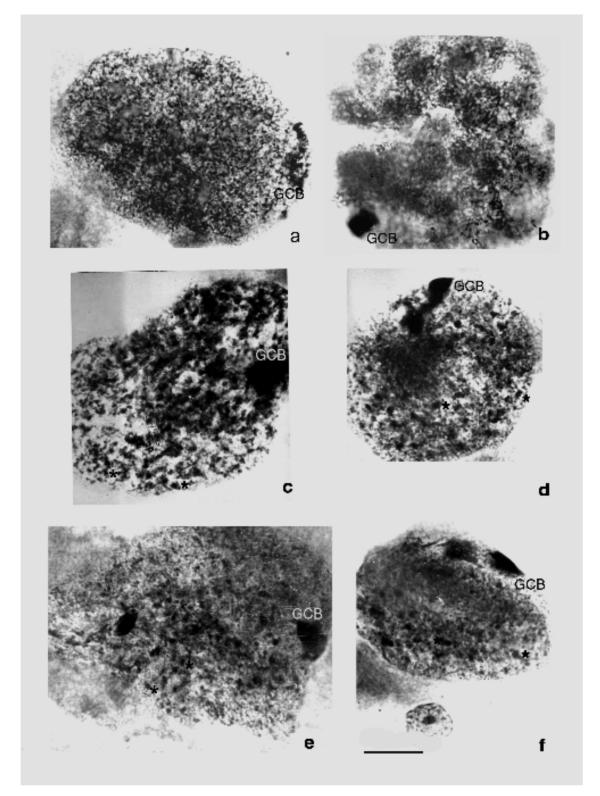
The secondary giant trophoblast cells of the field vole Microtus rossiameridionalis at 10th (a, b) and 14th (c-f) day pc. a – a nucleus of a giant trophoblast cell of network structure (endointerphase), the elongated GCB (GCB) with signs of disc patterns characteristical of polytene chromosomes; b – a nucleus of a giant trophoblast cell with bundles of polytene chromosomes (endoprophase); GCBs are different of these bundles by higher degree of condensation; c-f – nuclei of the secondary giant trophoblast cells with 1–2 GCBs and a great number of endomitotic chromosomes (asterisks). Feulgen staining. Bar is 10 μm.
It is to be noted that at early development stages (the 10th day pc), when nuclei contain strands of non-classic polytene chromosomes (endoprophase [32]), GCB are somewhat similar to them by their size, while differ by a higher condensation (Fig. (Fig.2b).2b). At the same stages, in nuclei of the reticular structure (endointerphase [32]) the heterochromatized body in some cases is non-uniform by the degree of chromatin condensation; occasionally, there was observed a banded pattern of a part of the body (Fig. (Fig.2a)2a) similar to that in polytene chromosome discs.
At the late development stages (the 14th day pc) when strands of polytene chromosomes are disintegrated presumably into endochromosomes GCBs predominantly retain their entirety: the nucleus keeps containing one-two large compact bodies (Fig. 2c,2d,2e,2f).
We measured the DNA content in the nuclei and simultaneously in their GCBs of the secondary giant trophoblast cells and in cells of the placenta junctional zone; lower ploidy levels (2–16c) characterized the latter, as compared with the secondary giant cells (32–2048c) [25,32-34].
Results of measurement of the DNA content in GCBs are presented in Figs. Figs.33,,44,,55 and in Tables Tables11,,22,,33,,4.4. The total amount of DNA in GCBs in nuclei of trophoblast cells have been found to increase proportionally with increase of the ploidy level at both studied stages of embryonal development in the both cell populations (Table (Table1,1, ,2).2). This parallel increase takes place in nuclei both with one and with 2–3 GCB. Such proportional increase of the GCB material in the course of polyploidization is also confirmed by a high positive correlation between the DNA content in the nucleus and the total DNA amount in GCB (r = 0.88 and 0.79 for cells of the junctional zone of placenta at the 10th and 14th days pc, respectively). The separate GCBs also shown the increase of the DNA content proportionally to the ploidy level (Fig. (Fig.3,3, ,4).4). The number of GCBs in nuclei of different ploidy somewhat varies. In the trophoblast cells of the placenta junctional zone, a marked increase of the fraction of 8c and 16c nuclei containing 2–3 GCBs was found (Fig. 5a,5c). By contrast, in the secondary giant trophoblast cells the portion of nuclei with 2 GCBs at the 10th day of development was lower than in the 8–16c nuclei in the placenta junctional zone. At the 14th day pc the portion of nuclei with 2 GCBs in the secondary giant trophoblast cells was markedly higher than at the 10th day pc (Fig. 5b,5d).
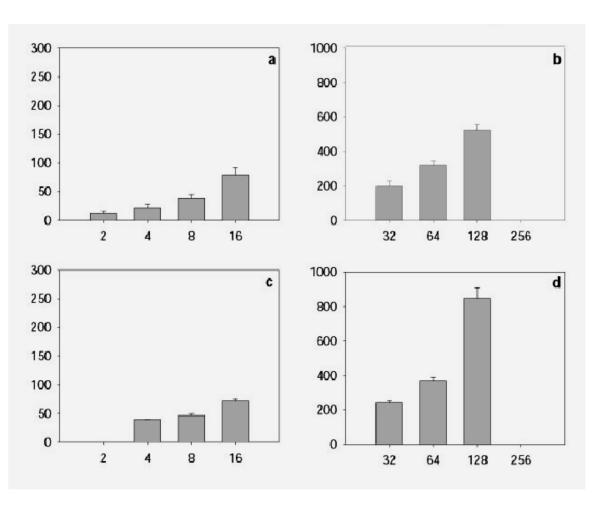
The mean DNA content in the separate GCBs in the trophoblast cells of the placenta junctional zone (a, c) and the secondary giant trophoblast cells (b, d) at the 10th (a, b) and 14th (c, d) day pc. Abscissa – ploidy, c; ordinate – the mean DNA content in the separate GCBs in each class of ploidy, arbitrary units.
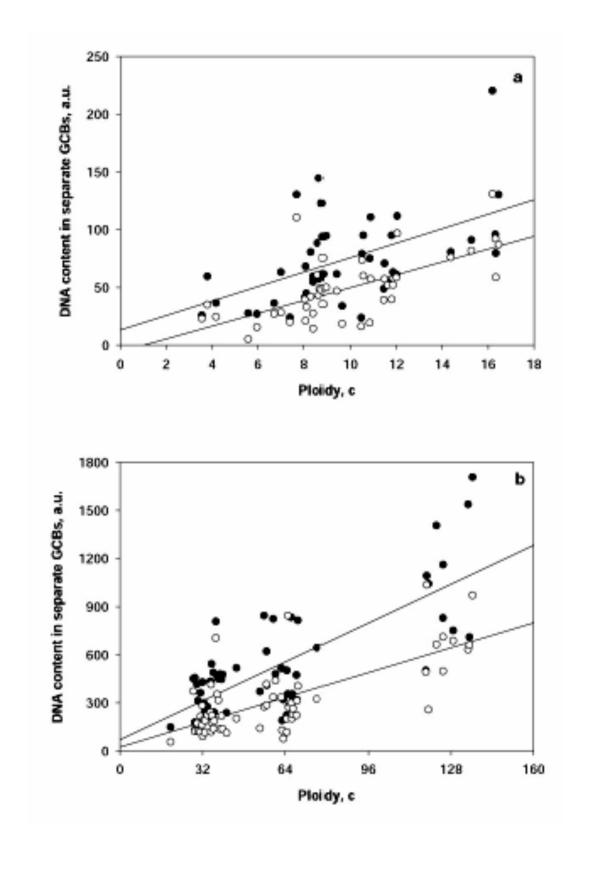
Relationship between ploidy and DNA content in the larger (black circle) and smaller (white circle) GCB in the nuclei with two GCBs in the trophoblast cells of the junctional zone of placenta (a) and seconday giant trophoblast cells (b). DNA content in separate GCBs is expressed in arbitrary units (a.u.).
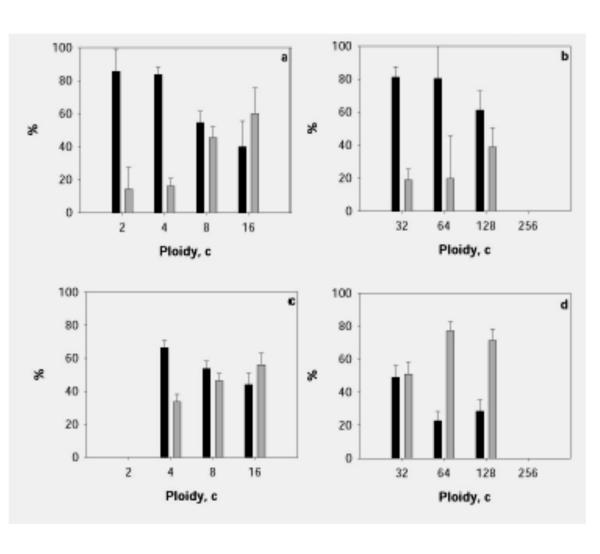
Percentage of nuclei with one and two or more GCBs in the trophoblast cells of the junctional zone of placenta (a, c) and in nuclei with one and two GCBs in giant trophoblast cells (b, d) at 10th (a, b) and 14th (c, d) day pc. Black columns – nuclei with one GCB, grey columns – nuclei with two or more GCBs.
Table 1
DNA content in the gonosomal chromatin bodies (GCB) of placental trophoblast cells at the different ploidy levels at the 10th day pc (arbitrary units).
| Cell population | Ploidy | DNA content in a GCB in the nuclei with single GCB | Total DNA content in GCBs in the nuclei with two or more GCBs | ||
| No. of embryo | No. of embryo | ||||
| 1–3 | 1–4 | 1–3 | 1–4 | ||
| Trophoblast cells of the junctional zone of placenta | 2 c | 14.9 ± 1.06 | 14.3 ± 0.4 | 24.5 ± 2.6 | - |
| 4 c | 32.0 ± 2.0 | 25.7 ± 0.9 | 44.3 ± 3.8 | 33.6 ± 2.0 | |
| 8 c | 66.2 ± 4.5 | 53.8 ± 2.0 | 83.1 ± 11.3 | 84.2 ± 2.4 | |
| 16 c | 124.9 ± 28.9 | 184.6 ± 6.1 | 182.5 ± 16.2 | 151.6 ± 6.7 | |
| Secondary giant trophoblast cells | 32 c | 291.8 ± 46.9 | 218.9 ± 4.7 | 413.0 ± 45.3 | 386.1 ± 26.5 |
| 64 c | 525.7 ± 38.1 | 426.9 ± 14.6 | 640.1 ± 58.1 | 788.3 ± 35.4 | |
| 128 c | 869.3 ± 98.9 | 182.7 ± 30.5 | 1120.5 ± 98.9 | 1296.3 ± 97.1 | |
Table 2
DNA content in the gonosomal chromatin bodies (GCB) of placental trophoblast cells at the different ploidy levels at 14th day pc (arbitrary units).
| Cell population | Ploidy | DNA content in a GCB in the nuclei with single GCB | Total DNA content in GCBs in the nuclei with two or more GCBs | ||||
| No. of embryo | No. of embryo | ||||||
| 2–1 | 2–4 | 2–2 | 2–1 | 2–4 | 2–2 | ||
| Trophoblast cells of the junctional zone of placenta | 2 c | 26.4 ± 6.1 | - | - | - | - | - |
| 4 c | 37.0 ± 3.7 | 32.1 ± 1.1 | 35.8 ± 7.2 | 68.5 ± 13.7 | 66.6 ± 3.3 | 60.4 ± 11.3 | |
| 8 c | 70.3 ± 3.3 | 78.0 ± 4.3 | 70.1 ± 10.4 | 114.9 ± 8.8 | 103.5 ± 4.6 | 105.6 ± 23.1 | |
| 16 c | 147.4 ± 13.2 | 252.0 ± 11.9 | 152.5 ± 21.2 | 165.5 ± 16.9 | 245.4 ± 11.8 | 196.6 ± 25.8 | |
| Secondary giant trophoblast cells | 32 c | 349.0 ± 29.5 | 336.1 ± 9.5 | 414.3 ± 73.4 | 505.12 ± 35.6 | 509.5 ± 22.5 | 510.5 ± 65.2 |
| 64 c | 696.1 ± 114.1 | 526.3 ± 7.2 | 820.4 ± 112.1 | 753.2 ± 55.3 | 928.0 ± 70.1 | 872.5 ± 97.5 | |
| 128 c | 1250.5 ± 290.3 | 729.1 ± 31.9 | 1504.8 ± 108.7 | 1828.8 ± 227.8 | 1283.5 ± 19.7 | 1661.5 ± 252.5 | |
| 256 c | 1875.2 ± 383.5 | - | - | - | - | - | |
Table 3
Ratio of nuclear DNA content to the total DNA content in gonosomal chromatin bodies (GCBs) in the placental trophoblast cells at 10th day pc
| Cell population | Ploidy | Nuclei with single GCB | Nuclei with two or more GCBs | ||
| No. of embryo | No. of embryo | ||||
| 1–3 | 1–4 | 1–3 | 1–4 | ||
| Trophoblast cells of the junctional zone of placenta | 2c | 23.7 ± 2.0 | 23.6 ± 2.9 | 16.0 ± 1.4 | - |
| 4c | 22.2 ± 1.1 | 25.9 ± 1.6 | 14.1 ± 1.2 | 17.6 ± 12.2 | |
| 8c | 21.3 ± 13.7 | 23.7 ± 2.0 | 16.8 ± 2.8 | 15.6 ± 11.5 | |
| 16c | 25.2 ± 4.7 | 14.5 ± 3.6 | 13.6 ± 1.6 | 13.9 ± 1.8 | |
| Secondary giant trophoblast cells | 32 c | 21.3 ± 3.7 | 22.4 ± 15.3 | 13.8 ± 1.4 | 20.1 ± 16.9 |
| 64 c | 24.6 ± 2.0 | 23.3 ± 11.5 | 20.6 ± 2.9 | 13.6 ± 11.6 | |
| 128 c | 27.1 ± 3.8 | 23.3 ± 11.5 | 20.3 ± 1.6 | 13.1 ± 11.3 | |
| 256 c | 23.2 ± 10.9 | - | - | - | |
Table 4
Ratio of nuclear DNA content to the total DNA content in gonosomal chromatin bodies (GCBs) in the placental trophoblast cells at 14th day pc.
| Cell population | Ploidy | Nuclei with single GCB | Nuclei with two or more GCBs | ||||
| No. of embryo | No. of embryo | ||||||
| 2–1 | 2–4 | 2–2 | 2–1 | 2–4 | 2–2 | ||
| Trophoblast cells of the junctional zone of placenta | 2 c | 12.4 ± 2.4 | - | - | - | - | - |
| 4 c | 25.1 ± 3.0 | 18.7 ± 1.8 | 20.7 ± 6.2 | 9.5 ± 7.1 | 11.2 ± 1.7 | 11.4 ± 1.8 | |
| 8 c | 23.2 ± 1.6 | 17.6 ± 1.7 | 17.3 ± 2.8 | 14.1 ± 3.8 | 12.7 ± 1.5 | 12.5 ± 1.7 | |
| 16 c | 17.7 ± 1.6 | 10.4 ± 1.4 | 16.0 ± 1.8 | 14.8 ± 3.1 | 10.2 ± 1.4 | 13.2 ± 1.5 | |
| Secondary giant trophoblast cells | 32 c | 17.7 ± 1.6 | 15.0 ± 1.7 | 14.8 ± 3.4 | 13.0 ± 3.4 | 10.2 ± 1.4 | 8.9 ± 1.3 |
| 64 c | 21.9 ± 3.7 | 16.6 ± 1.7 | 12.7 ± 2.3 | 16.1 ± 2.8 | 11.3 ± 1.7 | 10.3 ± 1.3 | |
| 128 c | 19.6 ± 0.2 | 30.9 ± 2.8- | 11.9 ± 0.8 | 13.9 ± 5.2 | 13.5 ± 2.0 | 11.9 ± 20.2 | |
| 256 c | - | - | - | - | 8.3 ± 1.9 | - | |
It is remarkable that the total DNA content in GCBs in nuclei with 2–3 GCBs in (junctional zone of placenta) and in nuclei with 2 GCBs (in the secondary giant trophoblast cells) in all ploidy classes, on average, exceeds the DNA content in the single body in nuclei with one GCB (Tables (Tables11 and and2).2). This regularity is revealed at both the 10th and at the 14th day pc; in the latter case, it is even more pronounced. It is also to be noted that in nuclei with two GCB these bodies often differ essentially from each other by their DNA content (Fig. (Fig.44).
The proportional rise of the DNA content in GCB during trophoblast cell polyploidization is also confirmed by the ratio of the DNA amount in the nucleus to the total DNA amount in GCB (Tables (Tables33 and and4).4). The data obtained show that this value does not change significantly throughout the series of polyploidization rounds, which indicates a multifold augmentation of the material of heterochromatized blocks of sex chromosomes. This regularity is observed both in the low-polyploid (2–16c) trophoblast cells of the placenta junctional zone and in highly polyploid (32–128c) secondary giant trophoblast cells in all studied embryos. It has turned out that in all cases the ratio of the DNA content in the nucleus to the DNA content in GCBs in total is higher in nuclei with one GCB than in nuclei containing several GCBs. The data obtained indicate that in nuclei with 2 GCBs, sex chromosomes are heterochromatized to a greater degree, which seems to be due to a more complicated, than in other mammals, character of inactivation of sex chromosomes. The DNA content in individual GCB (Fig. (Fig.3,3, ,4),4), proved a tendency for doubling in the course of consecutive polyploidization cycles: both in cell nuclei of the placenta junctional zone and in the secondary giant trophoblast cells.
Discussion
According to the data obtained, the presence of one, more seldom two GCBs is characteristic of trophoblast cell nuclei of all studied embryos of M. rossiaemeridionalis. In this aspect our data resemble results of studying interphase nuclei of M. agrestis both in embryonal cells and in definite tissues of adult females and males [9,13,18,19]. In nuclei of both male and female there were most often observed two large compact heterochromatin bodies seen as heteropicnotic areas of X- and Y-chromosomes when the cells entered mitosis. The presence of GCBs in cells of both sexes in M. rossiaemeridionalis and M. agrestis seems to be due to peculiarities of the structural heterochromatin of the vole sex chromosomes [35,36]; this also affects considerably the character of inactivation of X-chromosome in these animals [16,17].
Our data indicate that the GCB material doubles in each polyploidization cycle in the placenta trophoblast cells of M. rossiaemeridionalis. This is accompanied in the trophoblast cells of the placenta junctional zone both by a rise of the number of GCBs (the percentage of nuclei with 2–3 GCBs somewhat increases at transition from 2–4c to 8c and 16c) and by doubling of material of individual heterochromatin bodies. The rise of the GCB number at polyploidization seems to be due to the occurrence of uncompleted polyploidizing mitoses leading to an increase of the number of chromosomes [34]; such mitoses were described in trophoblast of the placenta junctional zone of this vole species [25]. The multifold augmentation of the DNA amount in individual GCBs (in nuclei both with one and with two and more GCBs) in each genome reproduction cycle seems to be due to a tendency for the close binding of sister chromatids of sex chromosomes. In several tissues of M. agrestis not only heteropicnosis of X- and Y-chromosomes was observed in mitosis, but also a longer binding of sister chromatids of sex chromosomes in metaphase; this seems to result in their delayed separation in anaphase [18]. We observed similar pictures in M. rossiaemeridionalis (M. subarvalis [25]) as well as in the present study (data not shown). It can be suggested that the longer attachment of sister chromatids in mitosis in trophoblast cells of the placenta junctional zone might be one of prerequisite for the subsequent transition of the trophoblast cells to the endoreduplication cycle.
As to the giant trophoblast cells, in which pictures of non-classic polyteny were observed, they also shown a multifold augmentation of the GCB material in nuclei both with one and with two GCBs; however, this is not accompanied by any clear-cut tendency of increase of the GCB number. Thus, these data confirm the polytene nature of GCBs in the giant trophoblast cells in M. rossiaemeridionalis, when the multiplication of the material of chromosomes occurs without increase of their number. This resembles behavior of Barr bodies in giant trophoblast cells of rats and rabbits [32,37-39].
In a number of tissues of Diptera, polytenization is accompanied by an underreduplication of heterochromatin regions [40,41]. Data of the present work suggest the complete replication of the GCB material, a major part of which consists of structural heterochromatin. In Drosophila the underreduplication is restricted to the region containing satellite DNA [40,42]; meanwhile, in the field vole M. agrestis, heterochromatin of sex chromosomes does not contain satellite DNA (Arrighi et al, 1970). This indicates the regular replication of the whole material of sex chromosomes (or of its significant part) in the giant trophoblast cells of M.rossiaemeridionalis in the course of a series of endoreduplication cycles.
It is of interest that the total DNA content in GCBs in trophoblast cells in nuclei with two GCBs is not equal to that in the single GCB, but exceeds it in all ploidy classes. This indicates that the single GCB in many cases does not seem to result from fusion of two GCBs. In this connection, it is not unlikely that in nuclei with one and with two GCBs different heterochromatized regions of both sex chromosomes may contribute in GCB formation.
The highly condensed chromatin bodies originated from two sex chromosomes were observed in the embryonic and adult tissues of M. agrestis [18]. Thus, during cleavage the heteropicnotic sex chromosomes were revealed as paired strands of different shape, whereas both at postimplantation stages and in adult tissues the gonosomal chromatin was present as two ovoid compact clumps or elongated bodies adjacent to the nuclear membrane [18].
Data about the time of replication of the material of gonosomes and the large chromocenters that they form in interphase indicate that the chromocenters may consist of both the inactivated euchromatic part of X-chromosome and structural heterochromatin regions of sex chromosomes. Euchromatin of the inactivated X-chromosome of M. agrestis is replicated later than the autosomal euchromatin, but earlier than constitutive heterochromatin of sex chromosomes [9,12,43]. A prominent chromocenter was observed in the cultured kidney epithelial cell nuclei of M. agrestis; it comprised late replicating constitutive heterochromatin and "sex chromatin" that undergoes replication later than euchromatin but earlier than constitutive heterochromatin. [9].
The peculiarities of gonosomal chromatin body formation in field voles seem to be due to the nature of heterochromatin of their sex chromosomes. Large regions of structural heterochromatin of sex chromosomes of M. agrestis differ by their composition and properties from the pericentromeric autosomal heterochromatin that exhibits the most of characteristics of the constitutive heterochromatin. In sex chromosomes of M. agrestis, 65% of heterochromatin are composed of single copies, while 34%, of repeated sequences [35]. In these regions, retrotranspozons are accumulated [36]. It has been shown that the heterochromatin regions of M. agrestis are capable for transcription on previously condensed chromosomes to the same degree as euchromatin [44]. Therefore, some authors believe that these regions by their structural organization are similar to G-discs of metaphase chromosomes and are composed mainly of middle repetitive mobile elements capable for transcription [36]. Thus, the heterochromatin regions (or, at least, their individual loci) of sex chromosomes of M. agrestis have, to a degree, properties of euchromatin.
The sex chromosomes of M. rossiaemeridionalis, like gonosomes of the sufficiently well studied M. agrestis, contain a significant part of the genome constitutive heterochromatin. The X- chromosome is the largest acrocentric of the chromosomal set about a half of its long arm is composed of structural heterochromatin. The Y-chromosome also is one of the largest acrocentrics, and practically its entire long arm is composed of structural heterochromatin [45]. This allows suggesting that the gonosomal heterochromatin and the compact bodies that it forms in M. rossiaemeridionalis are similar by their nature to those in M. agrestis. These bodies most likely can include both constitutive heterochromatin of sex chromosomes and inactivated euchromatin of one of X-chromosomes. It cannot be ruled out that the structural non-homogeneity of large GCB in the giant trophoblast cells of M. rossiaemeridionalis, in particular, the disc-like pattern in several parts of GCBs (Figure (Figure2a)2a) belongs to regions of inactivated euchromatin of X-chromosome.
The presence of two and more GCBs in trophoblast cells of M. rossiaemeridionalis can be due both to genome multiplication and to the fact that the constitutive heterochromatin and inactivated euchromatin of both sex chromosomes (XX and XY) form two, rather than one, large chromocenters. Difference between the DNA amounts in GCBs in nuclei with one and with two GCBs seems to be an indirect indication in favor of possible formation of two chromocenters from two different gonosomes. Thus, in female embryos (XX) one of two X-chromosomes may be lyonized and form a larger GCB.
The present work has revealed a peculiarity of the time of detection of GCBs in M. rossiaemeridionalis as compared with sex chromatin bodies in the rat and rabbit trophoblast. In these animals the sex chromatin bodies are observed during early embryonal development, when trophoblast cells undergo endoreduplication. At later development stages processes of chromosome replication are terminated, non-classic polytene chromosomes cease to be visualized and the nuclei acquire a reticular structure. This is accompanied by a gradual decondensation of the sex chromatin bodies [37-39]. In the trophoblast of M. rossiaemeridionalis, gonosomal heterochromatin bodies are present not only at early stages of development, but also later, up to the 17th day pc. Nuclei with non-classic polytene chromosomes, as early as at the 13–14th day pc, are transformed into nuclei containing numerous endochromosomes, probably as a result of disintegration of polytene chromosomes into oligotene fibrils. However, the disintegration, probably, does not involve heterochromatized gonosomal bodies because one or two large GCBs persists in the giant nuclei, the DNA content in separate GCBs remaining to be proportional to the ploidy level of giant nuclei. It seems likely that the presence of large blocks of constitutive heterochromatin favor a closer attachment of sister chromatids in polytene chromosomes, which prevents their separation into endochromosomes (in contrast to autosomes that do not contain the prominent blocks of heterochromatin). This suggestion is confirmed by data on a tighter and longer association of heterochromatic regions of X-chromosomes in mitosis in field voles [18,46]; this binding also seems to facilitate a close attachment of X-chromosome chromatids during endoreduplication.
Acknowledgments
The work was supported by the Russian Foundation for Basic Research (grant No. 01-04-49839). The authors are grateful to Dr. E.D. Sholl for supply of the material of field vole placentae and Dr. L.Z. Pevzner for the translation and edition of the manuscript.
References
- Lyon M. Gene action in the X-chromosome of the mouse (Mus musculus L.) Nature. 1961;190:372–373. [Abstract] [Google Scholar]
- Barr M, Bertram E. A morphological distinction between neurons of the male and female and the behaviour of the nucleolar satellite during accelerated nucleoproteid synthesis. Nature. 1949;163:676–677. [Abstract] [Google Scholar]
- Takagi N, Sugawara O, Sasaki M. Regional and temporal changes in pattern of X-chromosome replication during the early pregnancy. Chromosoma. 1982;85:275–286. [Abstract] [Google Scholar]
- Ogura H, Takada S, Mise N, Sugimoto M, Tan S, Takagi N. Translocation breakpoint possibly predisposes to nonrandom X-chromosome inactivation in mouse embryos bearing Searle's T(X;16)16H translocation. Cytogenet Cell Genet. 1998;80:173–178. [Abstract] [Google Scholar]
- Back F. The variable condition of euchromatin and heterochromatin. Intern Rev Cytol. 1976;45:25–64. [Abstract] [Google Scholar]
- Nesterova T, Zakian S. X-chromosome inactivation in mammals. Genetics. 1994;30:293–317. In Russian. [Abstract] [Google Scholar]
- Lyon M. X-chromosome inactivation: a repeat hypothesis. Cytogenet Cell Genet. 1998;80:133–137. [Abstract] [Google Scholar]
- Ohno S, Becak W, Becak L. X-autosome ratio and the behaviour pattern of individual X-chromosomes in placental mammals. Chromosoma. 1964;15:14–30. [Abstract] [Google Scholar]
- Pera F, Wolf U. Das Replication und Morphologie der X-Chromosomen waehrend der Syntheseperiode bei Microtus agrestis. Chromosoma. 1967;22:378–389. [Abstract] [Google Scholar]
- Zakharov A, Egolina N. Asynchrony of DNA replication and mitotic spiralization along heterochromatic portions of Chinese hamster chromosomes. Chromosoma. 1968;23:365–385. [Abstract] [Google Scholar]
- Zakharov A, Egolina N. Differential spiralization along mammalian mitotic chromosomes. I. BUDR-revealed differentiation in Chinese hamster chromosomes. Chromosoma. 1972;38:341–365. [Abstract] [Google Scholar]
- Schmid W, Leppert M. Rates of DNA synthesis in heterochromatic and euchromatic segments of the chromosome complements of two rodents. Cytogenetics. 1969;8:125–135. [Abstract] [Google Scholar]
- Lee J, Junis J. Constitutive heterochromatin during early embryogenesis ofMicrotus agrestis. Exp Cell Res. 1970;59:339–341. [Abstract] [Google Scholar]
- Cooper J, Hsu T. The C-band and G-band of Microtus agrestis chromosomes. Cytogenetics. 1972;11:295–304. [Abstract] [Google Scholar]
- Zakharov A, Tsvetkova T. Linear differentiation of the X-chromosomes of the field vole Microtus agrestis. Tsitologia. 1975;17:15–18. in Russian. [Abstract] [Google Scholar]
- Zakian S, Kulbakina N, Meyer M, Semenova L, Bochkarev M, Radjabli S, Serov O. Non random inactivation of the X-chromosome in interspecific hybrid voles. Genet Res. 1987;50:22–27. [Abstract] [Google Scholar]
- Zakian S, Nesterova T, Cheryakene O, Bochkarev M. Heterochromatin as a factor, affecting the inactivation of the X-chromosome in interspecific hybrid voles (Microtidae, Rodentia) Genet Res. 1991;58:105–110. [Google Scholar]
- Lee J, Junis J. A development study of constitutive heterochromatin inMicrotus agrestis. Chromosoma. 1971;32:237–250. [Abstract] [Google Scholar]
- Pera F. Structur und Position der heterochromatischen Chromosomen in Interphasekernen von Microtus agrestis. Z Zellforsch. 1969;98:421–436. [Abstract] [Google Scholar]
- Grafodatsky A, Lushnikova T, Radjabli S. Peculiarities of distribution of repeated DNA sequences of sex chromosomes of the four rodent species. Tsitologia. 1985;27:1308–1310. in Russian. [Abstract] [Google Scholar]
- Borodin P, Sablina O, Rodionova M. Pattern of X-Y chromosome pairing in microtine rodents. Hereditas. 1995;123:17–23. [Abstract] [Google Scholar]
- Meyer M, Orlov V, Sholl E. Sibling species in Microtus arvalis group (Rodentia, Cricetidae) Zool J. 1972;51:724–738. in Russian. [Google Scholar]
- Malygin V. Sistematics of common voles. Moskow, Nauka; 1983. in Russian. [Google Scholar]
- Stein G, Panteleev V, Povarkova A, Kudryavtsev B. Capacities of image analyser "Videotest" for microphotometric investigation in cytology. Tsitologia. 1998;40:913–916. in Russian. [Abstract] [Google Scholar]
- Zybina T, Zybina E, Stein G. Characteristic features of differentiation and polyploidization of trophoblast cells in the connective zone and labyrinth of placenta of the field vole, Microtus subarvalis. Tsitologia (In Russian) 1987;29:549–559. In Russian. [Google Scholar]
- Austin C, Amoroso E. Sex chromatin in early cat embryos. Exp Cell Res. 1957;13:419–421. [Abstract] [Google Scholar]
- Park W. The occurence of sex chromatin in early human and macaque embryos. J Anat. 1957;91:369–373. [Europe PMC free article] [Abstract] [Google Scholar]
- Zybina E. Sex chromatin in giant cells of trophoblast and in cells of early rabbit embryos. Fed Proc. 1965;24:868–876. [Abstract] [Google Scholar]
- Ohno S. Chromosomes and sex-linked genes. Berlin, Springer-Verlag; 1967. [Google Scholar]
- Axelson M. Sex chromatin in early pig embryos. Hereditas. 1968;60:347–354. [Google Scholar]
- Lindmark C, Melander J. Sex chromatin in pre-implantation embryos of the Syrian hamster. Hereditas. 1970;64:128–131. [Abstract] [Google Scholar]
- Zybina E, Zybina T. Polytene chromosomes in mammalian cells. Intern Rev Cytol. 1996;165:53–119. [Abstract] [Google Scholar]
- Zybina E, Kudryavtseva M, Kudryavtsev B. Morphological and cytofluorometric study of the common vole giant cells trophoblast. Tsitologia. 1975;17:254–260. in Russian. [Abstract] [Google Scholar]
- Zybina T, Zybina E, Stein G. Trophoblast Cell Invasiveness And Capability For The Cell And Genome Reproduction In Rat Placenta. EPBM. 2000;4:039–057. On line. [Abstract] [Google Scholar]
- Arrighi F, Hsu T, Saunders P, Saunders G. Localization of repetitive DNA in the chromosomes of Microtus agrestis by means of in situ hybridization. Chromosoma. 1970;32:224–236. [Abstract] [Google Scholar]
- Neitzel H, Kalscheuer V, Henschel S, Digweed M, Sperling K. Beta-heterochromatin in mammals: evidence from studies in Microtus agrestis based on the extensive accumulation of L1 and non-L1 retroposons in the heterochromatin. Cytogenet Cell Genet. 1998;80:165–172. [Abstract] [Google Scholar]
- Zybina E, Mosjan I. Sex chromatin bodies during endomitotic polyploidization of trophoblast cells. Tsitologia. 1967;9:265–272. in Russian. [Abstract] [Google Scholar]
- Zybina T, Zybina E, Kudryavtseva M, Kudryavtsev B. A cytophotometrical study of sex chromatin in the giant cell nuclei of rabbit trophoblast. Tsitologia. 1980;22:1037–1045. in Russian. [Abstract] [Google Scholar]
- Zybina E, Zybina T. Inacivated X-chromosome as an interphase chromosome marker for evaluation of mechanisms of genome multiplication in the rat and rabbit placental trophoblast cells. Trophoblast Res. 1998;11:51–63. [Google Scholar]
- Prokofieva-Belgovskaya A. Heterochromatic chromosome regions. Moskow, Nauka; 1986. in Russian. [Google Scholar]
- Zhimulev I. Polytene chromosomes: morphology and structure. Novosibirsk, Nauka; 1992. in Russian. [Google Scholar]
- Endow S, Gall J. Differential replication of satellite DNA in polyploid tissues of Drosophila virilis. Chromosoma. 1975;50:175–192. [Abstract] [Google Scholar]
- Wolf U, Flinspach G, Boehm R, Ohno S. DNS replikationmuster bei den reisen-geschlechtchromosomen von Microtus agrestis. Chromosoma. 1965;16:609–617. [Abstract] [Google Scholar]
- Sperling K, Kalscheuer V, Neitzel H. Transcriptional activity of constitutive heterochromatin in the mammal Microtus agrestis (Rodentia, Cricetidae) Exp Cell Res. 1987;173:463–472. [Abstract] [Google Scholar]
- Mazurok N, Rubtsova N, Isaenko A, Pavlova M, Slobodyanyuk S, Nesterova T, Zakian S. Comparative chromosome and mitochondrial DNA analyses and phylogenetic relationships within common voles (Microtus, Arvicolidae) Chromosome Res. 2001;9:107–120. [Abstract] [Google Scholar]
- Pera F. Arrangement of spindle apparatus in mitoses of different ploidy. Exp Cell Res. 1975;92:419–427. [Abstract] [Google Scholar]
Articles from Reproductive Biology and Endocrinology : RB&E are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1477-7827-1-32
Read article for free, from open access legal sources, via Unpaywall:
https://rbej.biomedcentral.com/track/pdf/10.1186/1477-7827-1-32
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
[Quantitative investigation of reproduction of condensed chromatin of sex chromosomes during trophoblast cell polyploidization and endoreduplication in the East European field vole Microtus rossiaemeridionalis].
Tsitologiia, 44(8):768-779, 01 Jan 2002
Cited by: 0 articles | PMID: 12506668
Whole-genome chromosome distribution during nuclear fragmentation of giant trophoblast cells of Microtus rossiaemeridionalis studied with the use of gonosomal chromatin arrangement.
Cell Biol Int, 29(12):1066-1070, 28 Nov 2005
Cited by: 10 articles | PMID: 16314124
[A study of DNA depolyploidization and depolytenization of the heterochromatized gonosomal chromatin bodies in the secondary giant trophoblast cells of the field vole Microtus rossiaemeridionalis using cytophotometry].
Tsitologiia, 47(10):866-873, 01 Jan 2005
Cited by: 0 articles | PMID: 16711385
Cell reproduction and genome multiplication in the proliferative and invasive trophoblast cell populations of mammalian placenta.
Cell Biol Int, 29(12):1071-1083, 28 Nov 2005
Cited by: 29 articles | PMID: 16316755
Review

 1
1 


