Abstract
Free full text

Human T-Cell Leukemia Virus Type 1 Envelope Glycoprotein gp46 Interacts with Cell Surface Heparan Sulfate Proteoglycans
Abstract
The major receptors required for attachment and entry of the human T-cell leukemia virus type 1 (HTLV-1) remain to be identified. Here we demonstrate that a functional, soluble form of the HTLV-1 surface envelope glycoprotein, gp46, fused to an immunoglobulin Fc region (gp46-Fc) binds to heparan sulfate proteoglycans (HSPGs) on mammalian cells. Substantial binding of gp46-Fc to HeLa and Chinese hamster ovary (CHO) K1 cells that express HSPGs was detected, whereas binding to the sister CHO lines 2244, which expresses no HSPGs, and 2241, which expresses no glycosaminoglycans (GAGs), was much reduced. Enzymatic removal of HSPGs from HeLa and CHO K1 cells also reduced gp46-Fc binding. Dextran sulfate inhibited gp46-Fc binding to HSPG-expressing cells in a dose-dependent manner, whereas chondroitin sulfate was less effective. By contrast, dextran sulfate inhibited gp46-Fc binding to GAG-negative cells such as CHO 2244, CHO 2241, and Jurkat T cells weakly or not at all. Dextran sulfate inhibited HTLV-1 envelope glycoprotein (Env)-pseudotyped virus infection of permissive, HSPG-expressing target cells and blocked syncytium formation between HTLV-1 Env-expressing cells and HSPG-expressing permissive target cells. Finally, HSPG-expressing cells were more permissive for HTLV-1 Env-pseudotyped virus infection than HSPG-negative cells. Thus, similar to other pathogenic viruses, HTLV-1 may have evolved to use HSPGs as cellular attachment receptors to facilitate its propagation.
The human T-cell leukemia virus type 1 (HTLV-1) is a human retrovirus that is the causal agent of adult T-cell leukemia and HTLV-1-associated myelopathy or tropical spastic paraparesis. The principal target cell in vivo is the CD4+ T lymphocyte, whereas the in vitro tropism spans a broad spectrum of permissive cell types. Despite a considerable effort by a number of laboratories, the cellular receptor(s) required for HTLV-1 entry into permissive cells remains elusive. Clues to the breadth of receptor expression have come from a number of studies, including analyses of syncytium formation between permissive and HTLV-infected or envelope glycoprotein (Env)-expressing cells (22, 26, 33) and infection of cells by HTLV-1 (3, 11, 20, 25, 42, 44) and by human immunodeficiency virus type 1 (HIV-1) pseudotyped with HTLV-1 Env (36, 37). Cell surface binding of HTLV-1 virions and more recently soluble, recombinant HTLV-1 surface envelope-Fc fusion proteins (16-18) has allowed a more precise analysis of cell types expressing putative receptors. It has been proposed that HTLV-1 and HTLV-2 share a receptor encoded by a gene on chromosome 17 (34, 35), although this result is now somewhat controversial (16, 36). The majority of studies aimed at characterizing receptors have been based on monoclonal antibody (MAb) inhibition of viral fusion in syncytium formation assays. In this way a number of candidate structures have been implicated in HTLV-1 infection, including vascular cell adhesion molecule and other adhesion molecules (5, 10, 38), the heat shock protein Hsc70 (31), and tetraspanin C33 (15). However, the binding pattern of soluble HTLV-1 Env and the in vitro tropism of HIV-1 pseudotyped with HTLV-1 Env are not compatible with a central role for these candidate receptors (16, 17, 37).
Proteoglycans are a group of proteins that carry sulfated polysaccharide side chains, called glycosaminoglycans (GAGs), consisting of repeating disaccharide units (1). One of their many functions is to trap soluble mediators such as cytokines and chemokines onto the solid phase via electrostatic interactions, establishing a concentration gradient for migrating leukocytes (1). A variety of microorganisms bind the GAG chains of proteoglycans, and many appear to use this association as a way to attach to target cells. Thus herpes simplex virus types 1 and 2, HIV-1, vaccinia virus, pseudorabies virus, dengue virus, and others bind highly sulfated GAG forms termed heparan sulfate proteoglycans (HSPGs), whereas others use the less densely sulfated chondroitin or dermatan sulfate (39, 41).
Here we show that HTLV-1 gp46 binds to HSPGs on cells permissive for HTLV-1 fusion and infection, that the expression of HSPGs on target cells enhances infection by HTLV-1 Env-pseudotyped viral particles, and that the soluble polyanion dextran sulfate (DexS) inhibits HTLV-1 syncytium formation and infection in HSPG-expressing cells. On this basis we propose that HTLV-1 may have evolved to use HSPGs as accessory receptors.
MATERIALS AND METHODS
Recombinant proteins, enzymes, antibodies, and soluble polyanions.
The HTLV-1 gp46-Fc fusion protein was produced and characterized as described previously (16, 17). MAb clone F58-10E4 (immunoglobulin M [IgM]) and clone F69-3G10 (IgG2b) against HSPGs were obtained from Seikagaku Corp., Tokyo, Japan. F58-10E4 recognizes a constitutively expressed epitope on HSPGs (21), whereas F69-3G10 binds an epitope exposed subsequently to heparan sulfate cleavage by heparitinase III (also known as heparan sulfate lyase [HS lyase]), which was purchased from Sigma-Aldrich (Poole, United Kingdom) and from Seikagaku Inc. Chondroitinase ABC, dextran, DexS, and chondroitin sulfates (CS) A, B, and C were from Sigma-Aldrich.
Cell culture.
Chinese hamster ovary (CHO) cell lines K1 (GAG positive; ATCC CCL-61), 2244 (GAG positive, HSPG negative; ATCC pgsD-677), and 2241 (GAG negative; ATCC pgsB-618) lines were obtained from the American Type Culture Collection and were cultured in Hams F12 modified Dulbecco's modified Eagle medium (DMEM; Gibco Lifesciences) supplemented with 5% fetal calf serum (FCS) and 1 μg of gentamicin/ml. HeLa, Jurkat, 293T, and HOS cells were obtained from Harvey Holmes at the MRC Centre for AIDS Reagents at the National Institute for Biological Standards and Controls and were cultured in DMEM (Gibco Lifesciences) supplemented with 5 to 10% FCS and 1% penicillin-streptomycin (Gibco Lifesciences).
gp46 cell binding assays.
Adherent cells (CHO and HeLa) were detached with phosphate-buffered saline (PBS) supplemented with 2 mM EDTA. Detached adherent cells and suspension Jurkat T cells were washed once in the appropriate growth medium and then were either pretreated with enzyme or were untreated prior to incubation with gp46-Fc. For enzyme treatment, 107 cells were resuspended in 200 μl of RPMI 1640 supplemented with 5% FCS and 2 mM CaCl2 and incubated for 2 h with the appropriate amount of enzyme. HeLa cells were treated with 10 mU of lyase and 1 U of chondroitinase, whereas CHO K1 cells were treated with 40 mU of lyase. The cells were pelleted at 400 × g for 5 min and resuspended in 5 ml of cold PBS supplemented with 1% bovine serum albumin (BSA) and 0.02% NaN3 (wash buffer [WB]). After one or more washes in WB, cells were resuspended at 4 × 106/ml in WB, and 50 μl per well was dispensed into U bottom 96-well microtiter plates. Dilutions in WB (in triplicate) of gp46-Fc or control supernatant or the appropriate MAb (F58-10E4 or F69-3G10) in 100 μl were added to each well, and the plates were agitated for 2 h at 4°C. After being washed twice in WB, cells were incubated with 50 μl of the appropriate anti-Ig species conjugate (Jackson Laboratories): anti-human Fc (Fab)2-phycoerythrin, anti-mouse IgM (Fab)2, or anti-mouse IgG (Fab)2 at 1/100, 1/50, and 1/100, respectively. After two further washes in WB the cells were resuspended in 200 μl of WB supplemented with 1% formaldehyde/well for 2 h at 4°C and then washed once more in WB, resuspended in 200 μl of WB, and analyzed by flow cytometry (FACScalibur; Becton Dickinson, Mountain View, Calif.). Each datum point represents the mean of three replicates derived from the mean fluorescence intensity of 104 accumulated events gated for side and forward scatter. Control values obtained by incubating cells with the fluorochrome-conjugated secondary antibody alone or an isotype-matched irrelevant IgM control antibody for F58-10E4 staining were subtracted from the test values.
Inhibition of gp46-Fc binding by soluble polyanions.
Triplicate serial dilutions of soluble polyanions or control reagents were prepared in 25 μl of WB in U bottom 96-well microtiter plates. gp46-Fc-containing supernatant or control supernatant was added (100 μl per well) and incubated for 30 min at 4°C. Twenty-five microliters of adherent cells, detached and washed as described above, or of Jurkat cells resuspended in WB at 8 × 106 cells/ml was added to each well and agitated for 2 h at 4°C. Cells were washed twice in WB and then resuspended in 50 μl of WB containing anti-human IgG-phycoerythrin conjugate at 1/100. After 30 min of agitation at 4°C the cells were washed twice in WB and then resuspended in 200 μl of WB for flow-cytometric analysis as described above.
Syncytium and syncytium inhibition assays.
HeLa cells were transfected with pHTE-1 (6) by using Fugene 6 as specified by the manufacturer (Roche, Indianapolis, Ind.) or Genejuice (Novagen, Madison, Wis.) and incubated for 18 to 24 h at 37°C. Transfected HeLa cells were detached in PBS-2 mM EDTA, washed, resuspended in growth medium, and allowed to reattach to wells of a six-well plate at 5 × 105 cells per well. Soluble polyanions were added to the HeLa cell monolayers and incubated at 37°C for 40 min. An equivalent number of untransfected HeLa target cells were then added to each well. The cells were cocultured overnight, washed, and fixed in PBS-2% formaldehyde-0.2% glutaraldehyde. Syncytium formation was quantified by counting the number of fused cells containing more than three nuclei. Syncytia in 10 low-power fields per replicate (three replicate cultures for each experiment) were counted. The means of two separate experiments are shown. Syncytium formation in CHO cells was carried out by coculturing 5 × 105 HTLV-1 Env-expressing HeLa cells, transfected as described above, with 106 K1, 2244, or 2241 cells in a six-well plate overnight; there were three replicate cultures per experiment. Syncytia were counted as described above.
HTLV-1 Env-pseudotyped virus preparation, transduction assays, and inhibition of pseudotype transduction.
Pseudotyped virus was prepared as previously described (16, 17). Briefly, 293T cells were cotransfected by using Fugene-6 with 10 μg of the Env-defective, luciferase-encoding HIV proviral clone pNL4-3.LUC.R-.E- in the presence or absence of pHTE-1 (10 μg). Sodium butyrate (20 mM) was added after 18 h, and 48 to 72 h later the viral supernatants were harvested by centrifugation at 400 × g. For transduction of CHO (K1 and 2241) cells, triplicate samples of 7 × 105 target cells in 1 ml were incubated with 1 ml of undiluted or serially diluted virus stock and plated into wells of a six-well tissue culture dish. After overnight incubation at 37°C and 5% CO2, cells were harvested, lysed, and assayed for luciferase activity in accordance with the manufacturer's instructions with the luciferase assay system (Promega, Madison, Wis.) and a Turner Designs (Sunnyvale, Calif.) TD-20/20 luminometer. For soluble polyanion inhibition experiments, 1 ml of HTLV-1 Env-pseudotyped virus was incubated with 1 ml of HOS cells (4 × 105 cells) in the presence or absence of serial dilutions of DexS or CS. After overnight culture, the cells were lysed and assayed for luciferase activity as described above.
RESULTS
HTLV-1 gp46-Fc binds to HSPG on CHO and HeLa cells.
We demonstrated previously that HTLV-1 gp46-Fc-containing supernatant binds in a specific manner to the cell surfaces of a number of immortalized cell lines derived from a variety of species and origins (16, 17). Among these lines, CHO and HeLa cells were found to bind gp46-Fc relatively strongly, suggesting that these cell types express high levels of a surface receptor for gp46. To determine whether a component of the gp46 binding was to cell surface GAGs, we compared supernatant containing HTLV-1 gp46-Fc with control supernatant for binding to wild-type CHO cells (K1) and CHO cells deficient for enzymes required to synthesize either HSPGs (2244) or all GAGs (2241). As shown in Fig. Fig.1A,1A, we confirm, using a MAb specific for HSPG (F58-10E4), that CHO K1 cells are strongly positive for HSPG expression (mean fluorescence intensity [MFI] of 1,202 ± 39), whereas CHO 2244 and 2241 are completely deficient for HSPG (MFIs of 0.8 ± 0.05 and 0.35 ± 0.3, respectively). The binding of gp46-Fc to the cell surface was detected with a human Fc-specific IgG-phycoerythrin conjugate and analyzed by flow cytometry. Robust staining of the K1 cells was observed with undiluted gp46-Fc-containing supernatant (Fig. (Fig.1B;1B; MFI of 46.5 ± 0.67), whereas 2244 and 2241 cells stained weakly (Fig. (Fig.1B;1B; MFIs of 5.4 ± 0.21 and 6.4 ± 0.27, respectively). However, despite the much-reduced gp46-Fc binding to the 2244 and 2241 cells, significant residual staining above control values was observed, demonstrating gp46-Fc binding to the cells independently of the presence of HSPGs and of GAGs in general. The levels of gp46-Fc binding to the 2244 and 2241 cell types were similar, implying that the presence of HSPGs is the dominant element for gp46 binding and that other GAGs do not play a major role. HeLa cells also expressed substantial amounts of HSPG (MFI, 408 ± 3.3), and gp46-Fc binding to HeLa cells was observed (Fig. (Fig.1B;1B; MFI, 34.3 ± 2.9).
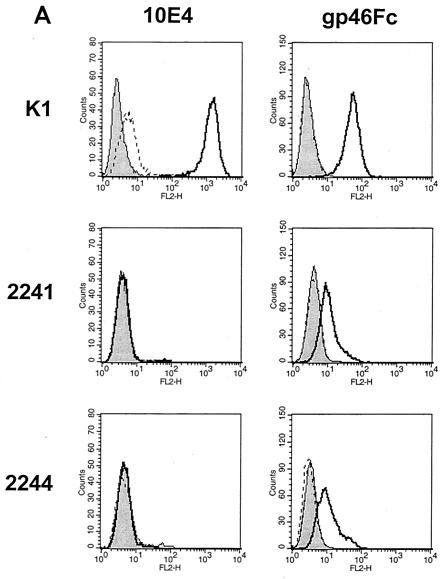
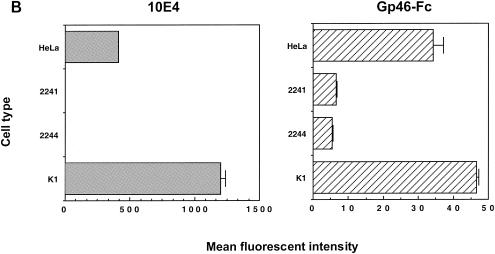
HTLV-1 gp46-Fc binds to HSPGs on CHO and HeLa cells. HeLa and CHO K1 cells and the 2241 and 2244 variants were detached, stained for 2 h at 4°C with gp46-Fc or MAb F58-10E4, specific for HSPG, washed, labeled with the appropriate secondary conjugated antibody, and fixed. Cells were analyzed by flow cytometry: 104 events gated for forward and side scatter were accumulated and analyzed in the FL2 channel. (A) Staining of CHO K1 and variants with F58-10E4 (left) and gp46-Fc (right) expressed as histograms in CellQuest. Solid curve, anti-human IgG-phycoerythrin conjugate alone; dotted curve, irrelevant IgM control antibody (anti-trinitrophenol). (B) Staining intensities (after subtraction of negative control values) for F58-10E4 (left) and gp46-Fc (right) of HeLa and CHO cells expressed as mean fluorescence intensities of 104 gated events. Each value is the mean of three replicates. Error bars represent +1 standard deviation.
Titration of gp46-Fc-containing supernatant onto the different CHO types allowed us to approach saturation at conditions approximating equilibrium (Fig. (Fig.22 and data not shown). From these extrapolated binding curves we estimated the approximate 50% binding values: with a concentration of approximately 1 μg/ml (~22 nM) in the undiluted supernatant (16), these yielded estimated dissociation constant (Kd) values of 7 nM for K1, 2 nM for 2244, and 2 nM for 2241. Although they are not precise measurements of the Kds, these extrapolations allow us to estimate an upper limit to the true affinities. These data suggest that gp46-Fc binds to at least two distinct cell surface receptor structures. The dominant binding may be to HSPGs, since the presence of HSPGs increases the maximal binding at least threefold, but residual, potentially higher-affinity binding to the other structure(s) remains when HSPGs are no longer present.
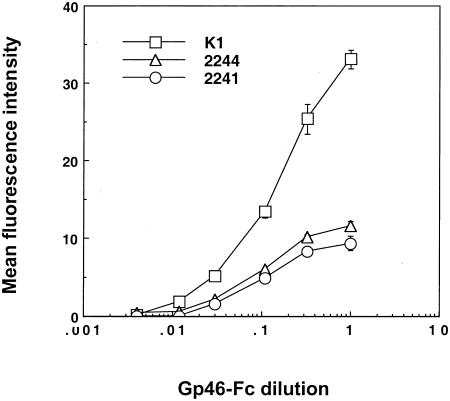
Titration of gp46-Fc onto CHO cells. CHO K1 cells and the 2241 and 2244 variants were detached, labeled with serial threefold dilutions of gp46-Fc, and stained as described above. Data are expressed as mean fluorescent intensities of 104 gated events: each datum point represents the mean of triplicate samples, with negative control values subtracted. Error bars represent ±1 standard deviation.
HS lyase treatment of cells reduces gp46-Fc binding.
HSPGs can be removed from cell surfaces with specific enzymes (heparitinase III or HS lyase). We treated HeLa and CHO K1 cells with HS lyase preparations from two commercial sources, Seikagaku Inc., which sells it as protease free, and Sigma-Aldrich and evaluated the modulation of MAb binding to HSPG and gp46-Fc binding by flow cytometry. The F58-10E4 MAb recognizes an epitope that is destroyed by HS lyase, whereas the F69-3G10 neoepitope is unmasked by HS lyase treatment. As expected, F58-10E4 binding on HeLa cells was decreased to nearly control levels by HS lyase treatment, whereas F69-3G10 staining, which was weak in the absence of lyase cleavage, increased by approximately 10-fold after cleavage (Fig. (Fig.3A).3A). gp46-Fc binding was reduced by the cleavage of HSPGs. The signal for HeLa was reduced 84% ± 6% by 1 U of the Sigma-Aldrich HS lyase (heparitinase III) and 90.6% ± 2.2% by 10 mU of HS lyase from Seikagaku Inc and was reduced 30% ± 6.5% by 1 U of chondroitinase ABC (Fig. (Fig.3B).3B). Similarly, F58-10E4 staining was abolished and gp46-Fc staining of CHO K1 was reduced by 86% ± 16.7% by 40 mU of Seikagaku HS lyase (results not shown). These data are consistent with HSPGs being the major attachment receptors for gp46 on these cell types, with some residual binding to a lyase-resistant structure(s).
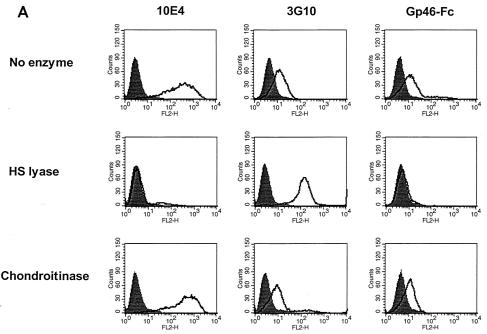
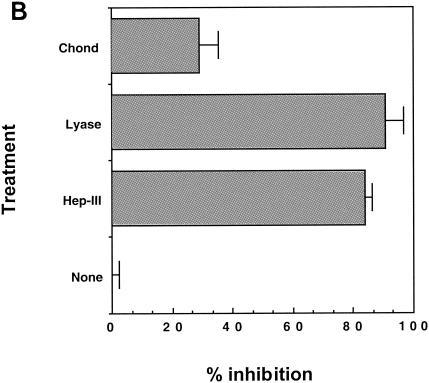
Enzymatic removal of HSPG reduces gp46-Fc binding. (A) HeLa cells were treated with HS lyase or chondroitinase ABC or not treated prior to labeling with MAb F58-10E4 (left), F69-3G10 (middle), or gp46-Fc (right). Cells were stained and fixed as described in the legend to Fig. Fig.1,1, and data are expressed as histograms in CellQuest. (B) Data from HeLa cell analysis expressed as percentages of inhibition of gp46-Fc binding normalized to 0% for gp46-Fc binding to untreated HeLa cells. Each value is the mean of triplicates, and values were derived from the mean fluorescence intensities of 104 accumulated events. Error bars represent +1 standard deviation. Chond, chondroitinase ABC; lyase, HS lyase; Hep-III, heparitinase III.
DexS inhibits gp46-Fc binding to HSPG-expressing cells.
Soluble polyanions tend to be good inhibitors of viruses that bind HSPGs, probably as a result of their interference with the electrostatic interactions facilitating virus attachment and entry. To determine whether soluble polyanions were able to interfere with gp46-Fc binding to HSPG-expressing cells, we preincubated gp46-Fc with various concentrations of DexS or CS A, B, or C prior to addition of target CHO cells. DexS strongly inhibited gp46-Fc binding to CHO cells, yielding a 50% inhibitory concentration (IC50) of 2 μg/ml (Fig. (Fig.4).4). The CS compounds were weaker inhibitors: A, B, and C gave IC50 values of >300, 200, and 50 μg/ml, respectively. Thus, consistent with the cell surface proteoglycan data implying HSPG binding by gp46-Fc, DexS, a molecule with a charge density closer to that of heparan sulfate than to those of the CS compounds, was more potent than CS by 1 to 2 orders of magnitude. At the lowest dose of DexS and CS A, a small but significant enhancement of gp46-Fc binding to HeLa cells was observed. We have seen similar enhancement of HIV Env binding to HSPGs at low DexS concentrations (unpublished results), suggesting that this represents a subtle but potentially general modulation of the electrostatic interactions between HSPGs and retroviral Envs.
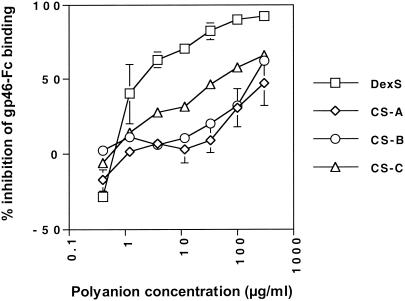
Soluble polyanions inhibit gp46-Fc binding to HeLa cells. gp46-Fc was treated with serial dilutions of DexS or CS A, B, or C for 30 min at 4°C prior to addition to HeLa cells. After 2 h at 4°C with agitation cells were washed, stained, and analyzed as described for Fig. Fig.1.1. Each datum point is the mean of triplicates representing the percentage of inhibition of gp46-Fc binding normalized to 0% for untreated gp46-Fc. Error bars represent ±1 standard deviation.
The substantial inhibition of gp46-Fc binding to GAG-expressing cells by DexS suggested that soluble polyanions might interfere with gp46-Fc binding not only to HSPGs but potentially also to non-GAG receptor structures. We had previously demonstrated that there was residual binding of gp46-Fc to CHO 2244 and 2241 cells, implying expression of a second cell surface receptor. We therefore tested the effect of DexS on gp46-Fc binding to these cells. Figure Figure5A5A shows that, whereas DexS inhibited gp46-Fc binding to HeLa and CHO K1 cells, with IC50s in this experiment of approximately 0.3 and 0.5 μg/ml, respectively, there was no inhibition of gp46-Fc binding to CHO 2244 or 2241 cells at any concentration tested. Indeed, there was some apparent enhancement of gp46-Fc binding to CHO 2241 and 2244 cells at the highest DexS concentrations, although this was not significant. Dextran did not inhibit gp46-Fc binding to any of the cell types tested. To test the effect of polyanions on gp46-Fc binding to a cell type more relevant to HTLV-1 infection in vivo, we chose to use the HSPG-negative Jurkat CD4+ T-cell line that, as we had previously demonstrated, stained strongly with gp46-Fc (16, 17). Staining of Jurkat T cells with antibody F58-10E4 demonstrated undetectable levels of HSPGs, and this was confirmed by the inability of HS lyase treatment to elicit F69-3G10 reactivity (data not shown). DexS concentrations below 100 μg/ml had no effect on gp46-Fc binding. At 300 μg/ml, inhibition of 26% compared to untreated gp46-Fc binding was observed (Fig. (Fig.5B5B).
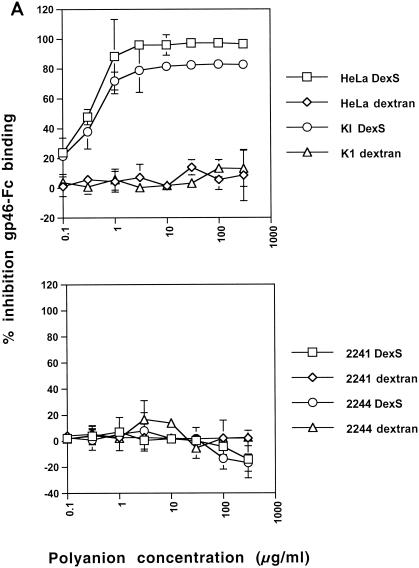
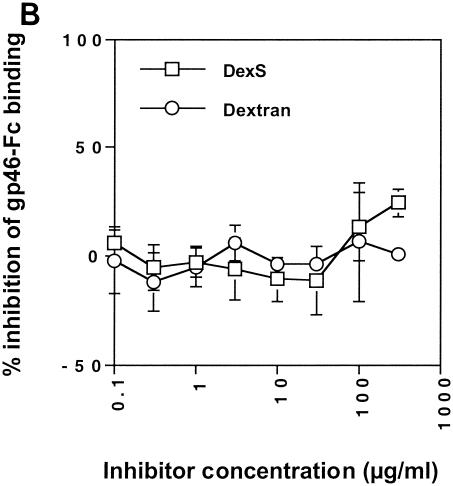
DexS inhibits HTLV-1 gp46-Fc binding to HSPG-expressing cells. (A) gp46-Fc was treated with serial dilutions of DexS or dextran for 30 min at 4°C prior to addition to HeLa or CHO K1 cells (top) or CHO 2241 or 2244 cells (bottom). (B) gp46-Fc was treated with serial dilutions of DexS or dextran for 30 min at 4°C prior to addition to Jurkat cells. After 2 h at 4°C with agitation cells were washed, stained, and analyzed as described for Fig. Fig.1.1. Each datum point is the mean of triplicate samples representing the percentage inhibition of gp46-Fc binding normalized to 0% for untreated gp46-Fc. Error bars represent ±1 standard deviation.
HTLV-1 Env-pseudotyped virus transduction is enhanced in HSPG-expressing cells.
Since HTLV-1 gp46-Fc interacts with HSPGs on cell types permissive for HTLV-1-mediated fusion and pseudotyped-virus transduction, we predicted that the presence of HSPGs on target cells would influence HTLV-1 infectivity and cell-to-cell fusion. To test this, we generated HTLV-1 Env-pseudotyped HIV-1 particles containing the firefly luciferase gene under the control of the HIV-1 long terminal repeat. These particles give rise to an abortive single cycle of virus replication in receptor-expressing cells, with a rapid and sensitive luciferase assay readout. We compared the abilities of HTLV-1 Env pseudotyped virions to transduce HSPG-expressing CHO K1 cells and HSPG-negative 2241 cells. Figure Figure66 shows titration of HTLV-1 Env pseudovirions onto CHO K1 and 2241 cells. At all concentrations of virus, there was a three- to fourfold-greater transduction of the K1 cells than the 2241 cells.
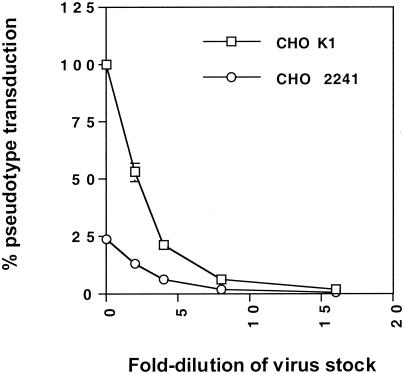
GAG expression increases HTLV-1 Env pseudotype transduction of CHO cells. Serial twofold dilutions of HTLV-1 Env-pseudotyped virus were incubated with CHO K1 or 2241 cells and then incubated for 24 h prior to lysis, addition of substrate, and measurement of luciferase activity. Results are expressed as percentages of transduction normalized to 100% for undiluted supernatant on CHO K1 cells.
The effect of HSPG expression on HTLV-1 Env function was further investigated by a fusion assay. HTLV-1 Env was expressed by transient transfection of HeLa cells, which were then cocultured with untransfected HeLa cells or untransfected CHO cells and evaluated for cell-to-cell fusion by counting the number of syncytia containing more than three nuclei. HeLa cells were used as a control target cell line for these assays, as studies have shown that HeLa cells are highly permissive for HTLV-1-mediated syncytium formation (16, 17, 26). Differences in the abilities of CHO cells to form syncytia were observed: there were approximately 20% fewer syncytia in the K1 cells than in the 2244 cells and approximately 41% fewer syncytia in the K1 cells than in the 2241 cells (Fig. (Fig.7),7), while 2241 and control HeLa cells produced similar numbers. Moreover, syncytia formed in the K1 cells were generally smaller than those in the 2244 or 2241 cells (data not shown). These results imply that expression of GAGs on the 2244 cell surface may reduce the cell-to-cell fusion efficiency of HTLV-1 Env relative to that for 2241 cells and that the greater sulfation of HSPGs over other GAGs on K1 cells may further reduce syncytium formation efficiency. However, the differences in numbers of syncytia observed between the CHO cell types were not statistically significant in this experiment, and variability among experiments prevents these differences from always being observed.
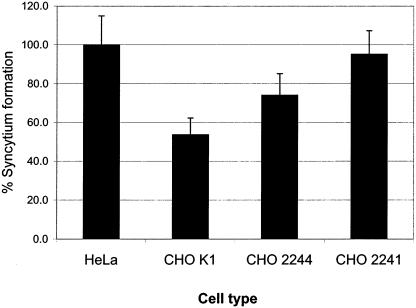
GAG expression subtly decreases HTLV-1 Env-mediated syncytium formation in CHO cells. HeLa cells transiently transfected with the HTLV-1 env gene were mixed with an equivalent number of untransfected HeLa cells (positive control normalized to 100%) or CHO K1, 2241, or 2244 cells and cocultured overnight. Syncytia in 10 low-power fields per replicate were counted, and there were three replicate cultures for each experiment. Data from a representative experiment are shown; error bars represent +1 standard deviation.
DexS inhibits HTLV-1 Env-pseudotyped virus transduction and Env-mediated syncytium formation.
The dose-dependent inhibition of gp46-Fc binding to HeLa cells by DexS suggested that this polyanion would interfere with HTLV-1 Env-mediated transduction by cell-free HTLV-1 Env-pseudotyped virus and Env-induced cell-to-cell fusion of HSPG-expressing permissive cells. Titration of DexS into the assay mixture modulated pseudotype transduction efficiency in a dose-dependent manner. At concentrations greater than 1 μg/ml, DexS potently interfered with HTLV-1 pseudovirus transduction, and inhibition was essentially complete at 10 μg/ml (Fig. (Fig.8).8). By contrast, CS A had a more subtle effect on pseudovirus transduction: 50% inhibition was not achieved even at the highest dose of 500 μg/ml. We next investigated the effect of DexS on HTLV-1 Env-induced syncytium formation. DexS at 500 μg/ml completely blocked cell-to-cell fusion, whereas CS A, B, or C at the same concentration had no obvious effect (Fig. (Fig.9).9). These data demonstrate that DexS is a potent inhibitor of HTLV-1 pseudotype transduction and Env-mediated fusion in HSPG-expressing cell types.
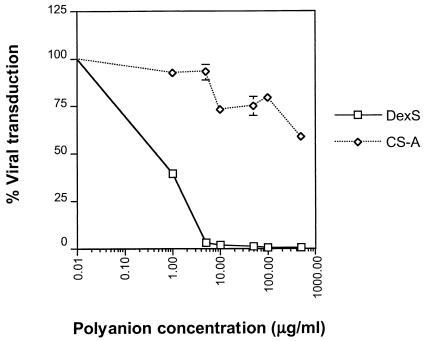
DexS inhibition of HTLV-1 Env-pseudotyped virus transduction of HeLa cells. Serial dilutions of DexS or CS A were preincubated with HTLV-1 Env-pseudotyped virus prior to plating onto HOS cells. Luciferase activity was evaluated 24 h later, and results are expressed as percentages of virus transduction normalized to 100% for the signal obtained from untreated virus. Each datum point represents the mean of triplicates, and the error bars represent ±1 standard deviation.
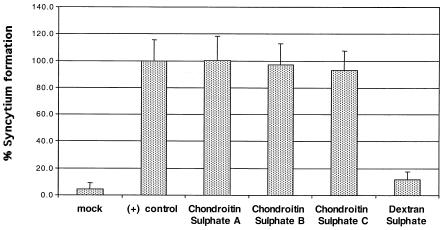
DexS inhibits HTLV-1 Env-mediated syncytium formation. HeLa cells were transfected with the HTLV-1 env gene and cocultured with an equivalent number of untransfected HeLa cells for 18 h in the presence or absence of CS A, B, or C or DexS at 500 μg/ml. After fixation, syncytia were visualized by low-power microscopy. Syncytia in 10 fields per replicate were counted, and there were three replicate cultures for each experiment. Bars represent the means of triplicate experiments, and the error bars represent +1 standard deviation.
DISCUSSION
Here we demonstrate that monomeric, soluble HTLV-1 gp46-Fc associates with cell surface HSPGs and that the soluble polyanion DexS blocks this binding to HSPG-expressing cells. These soluble HTLV-1 surface glycoprotein-cell interactions translate into a functional viral phenotype in that the expression of HSPGs on permissive cells increases HTLV-1 Env-pseudotyped virus transduction, which is inhibited by DexS. Thus, we propose that HTLV-1 can be included in the growing list of pathogens, including many viruses, that bind cell surface sulfated HSPGs and that are inhibited in their binding to HSPG-expressing cells by soluble polyanions (41).
The ability of gp46-Fc to bind target cells via HSPGs is consistent with the concept that the attachment receptor is trypsin sensitive (16, 18, 32), since the protein cores of proteoglycans are likely to be cleaved by this enzyme. Our data imply the presence on the surface of CHO and HeLa cells of a second species of receptor that binds gp46 and that may be involved in virus entry. We have estimated the combined Kd of gp46 for HSPGs and the additional cell surface receptor molecules to which it binds at approximately 7 nM but are presently unable to determine an affinity for HSPG alone. It seems likely from the cytometric analysis, however, that the HSPGs are present in greater numbers on the CHO K1 cell surface than the second structure(s) and that the above estimate may therefore represent the gp46-HSPG binding affinity. We attempted surface plasmon resonance analyses of supernatant gp46-Fc binding to polyanion (heparin)-coated sensor chips but were unable to determine affinity because of the nonspecific binding of unknown proteins in the supernatant, presumably of insect origin, to the sensor chip. Unfortunately, purification of gp46-Fc tends to aggregate it (our unpublished results), rendering other techniques for measuring affinity difficult to achieve. The residual binding of gp46-Fc to HSPG-negative CHO 2244 and 2241 cells indicates that, although the number of remaining receptors may be relatively low, based on the estimated maximum fluorescence intensity, the affinity may be relatively high.
The nearly ubiquitous expression of HSPGs on mammalian cells might shed light on some of our recent results showing that a gp46-Fc-binding receptor is widely expressed on many cell types (16, 17), some of which have been scored receptor negative by HTLV-1 Env pseudotype and syncytium formation assays (8, 26, 35, 37). We predict, therefore, that some cell types resistant to HTLV-1-mediated fusion and infection express HSPGs but have limited or no expression of the HTLV-1 entry receptor(s) (17). Alternatively, as previously suggested, some cell types may have very high levels of cell surface proteoglycans such as HSPGs and these proteoglycans may obscure or compete with the primary cell surface receptor(s) for SU binding (17). In keeping with this suggestion, recent results reveal that highly fusogenic derivatives of HTLV-1 Env induce syncytium formation in cell types previously thought to be receptor negative (19). The future use of HSPG-negative cells should facilitate the search for entry receptors in the absence of HSPG-gp46 interactions that may complicate interpretation of assays aimed at identifying receptors.
The expression of sulfated GAGs on target cells increased the interaction with gp46-Fc and the efficiency of HTLV-1 Env-pseudotyped virus transduction. By contrast, GAG expression had a subtle inhibitory effect on the efficiency with which Env-expressing HeLa cells fused with CHO cells. We suggest that this apparently conflicting result may be due to the increased repulsion between cell membranes: cell-cell fusion requires the close apposition of relatively large surfaces of cell membranes that have a naturally repulsive nature at short range resulting from their negative charges. Increasing the negative charge on one of the cell types by expression of sulfated GAGs would be expected to increase repulsion and reduce fusion efficiency. Cell-free virions would, by contrast, have a more basic envelope as a result of a minimal glycocalyx and the high density of basic Env molecules studding the surface and would therefore be attracted to the negatively charged GAG-expressing cell membrane.
The interaction of pathogens with HSPGs has been interpreted as a mechanism for semispecific attachment to the cell surface, allowing subsequent two-dimensional scanning for specific entry receptors (9, 39). However, the interpretation of our data in the context of HTLV-1 infection in vivo is complex, since the principal target cells, CD4+ T lymphocytes, express low or undetectable levels of HSPGs (4, 12, 23). Whether there is sufficient HSPG on lymphocyte surfaces to facilitate HTLV-1 infection is unclear. The situation with HTLV-1 may be partially analogous to that observed for HIV-1, in that CXCR4-using and dualtropic variants have basic Env, which associates with HSPGs on the surfaces of many permissive cell types (24, 27, 29, 46), but not the principal target cell type in vivo, primary CD4+ T cells (12). Another clue as to the role of HSPGs in viral dissemination may come from a recent study in which HSPGs on cell surfaces nonpermissive for HIV or simian immunodeficiency virus infection mediate uptake and internalization of virions into a protective intracellular environment (2). These virions can then be transmitted in trans to CD4+ T cells several days later. Whether this might be a mechanism for HTLV-1 spread within the host remains to be seen.
HTLV-1 disseminates within an infected individual principally by direct cell-to-cell spread, potentially via the formation of a type of actin-driven synapse between infected and target cells (14). The effect of HSPG expression on cell-to-cell spread of viruses is unknown, although our finding that GAG-expressing cells fused slightly less efficiently than GAG-negative cells with HTLV-1 Env-expressing HeLa cells suggests that GAG expression may be inhibitory. It remains to be seen, therefore, whether the electrostatic interactions binding HIV-1 and HTLV-1 Env to HSPGs are of overall benefit to these viruses in vivo or whether this represents a side effect of a basic envelope structure necessary for other functions, such as engaging entry receptors.
We do not know which regions of gp46 interact with HSPGs and DexS. Visual inspection of HTLV-1 gp46 sequences reveals a number of arginine and lysine residues scattered across the protein, but none clustered in such a manner as to suggest an obvious HSPG binding motif. However, it is very likely that gp46 has a complex fold (7) and that an HSPG binding site would be created from discontinuous regions of the polypeptide chain in a manner analogous to that for the basic chemokine receptor binding surface on HIV-1 gp120 (24, 28). Mapping the polyanion binding surface will therefore probably require systematic mutagenesis of the basic residues of gp46.
Inhibition of HTLV-1 fusion and Env pseudotype transduction by DexS and to a lesser extent by CS puts this virus into the category of microorganisms that are susceptible to soluble-polyanion blockade. Our data confirm and extend, by providing a mechanism, previous observations that soluble polyanions interfere with HTLV-1 infection and syncytium formation (13, 26, 30, 45). The recent demonstration that the soluble polycation Polybrene also interferes with soluble HTLV-1 Env binding and with HTLV-1 Env-pseudotyped virus transduction of cells adds weight to the idea that electrostatic interactions are involved (18). When we tested DexS for inhibition of gp46-Fc on GAG-negative cells (CHO 2244 and 2241) we saw no inhibition and only weak inhibition of gp46-Fc binding to Jurkat T cells at concentrations above 100 μg/ml. This relative insensitivity to polyanion blockade of gp46-Fc binding to HSPG-negative cell types suggests that gp46 interaction with non-HSPG receptor structures on Jurkat cells is most likely via regions of gp46 distinct from those that bind HSPGs and probably takes place predominantly via nonelectrostatic interactions. Although HTLV-1 is primarily spread by sexual contact, the precise cell types involved in viral transmission are currently obscure. If cell types expressing HSPGs, such as epithelial cells, are involved directly or indirectly in viral transmission, then soluble polyanionic compounds, some of which are more potent than DexS and which are about to enter phase III clinical trials for efficacy against HIV-1, might be effective against HTLV-1 as topically applied microbicides. Further studies should aim to elucidate the ability of polyanionic compounds such as dextrin-2-sulfate (43) and PRO2000 (40) to inhibit HTLV-1 transmission and dissemination both in vitro and in vivo.
Acknowledgments
We thank Charles Bangham and Graham Taylor for insightful discussion and Hugues Lortat-Jacob for help with the unpublished SPR studies.
This work was supported by grants from the UK Medical Research Council/Department for International Development Microbicide Development Programme (G0100137) and the American Foundation for AIDS Research (02793-30-RGM) to Q.J.S. and from The Leukemia Research Fund (LRF-9827) and Tenovus-Scotland (T98/29) to D.W.B.
REFERENCES
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.77.18.9922-9930.2003
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/77/18/9922.full.pdf
Citations & impact
Impact metrics
Article citations
The Assembly of HTLV-1-How Does It Differ from HIV-1?
Viruses, 16(10):1528, 27 Sep 2024
Cited by: 0 articles | PMID: 39459862 | PMCID: PMC11512237
Review Free full text in Europe PMC
Upregulation of Neuropilin-1 Inhibits HTLV-1 Infection.
Pathogens, 12(6):831, 15 Jun 2023
Cited by: 0 articles | PMID: 37375521 | PMCID: PMC10305173
Hepatitis E Virus Life Cycle.
Adv Exp Med Biol, 1417:141-157, 01 Jan 2023
Cited by: 1 article | PMID: 37223864
Development of a Monoclonal Antibody Targeting HTLV-1 Envelope gp46 Glycoprotein and Its Application to Near-Infrared Photoimmuno-Antimicrobial Strategy.
Viruses, 14(10):2153, 29 Sep 2022
Cited by: 2 articles | PMID: 36298708 | PMCID: PMC9608601
Safety of intraocular anti-VEGF antibody treatment under in vitro HTLV-1 infection.
Front Immunol, 13:1089286, 25 Jan 2023
Cited by: 4 articles | PMID: 36761168 | PMCID: PMC9905742
Go to all (66) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Human T-cell leukemia virus type 1 (HTLV-1) and HTLV-2 use different receptor complexes to enter T cells.
J Virol, 80(17):8291-8302, 01 Sep 2006
Cited by: 53 articles | PMID: 16912281 | PMCID: PMC1563841
Antibodies to the envelope glycoprotein of human T cell leukemia virus type 1 robustly activate cell-mediated cytotoxic responses and directly neutralize viral infectivity at multiple steps of the entry process.
J Immunol, 187(1):361-371, 06 Jun 2011
Cited by: 15 articles | PMID: 21646298
Heparan sulfate proteoglycans mediate attachment and entry of human T-cell leukemia virus type 1 virions into CD4+ T cells.
J Virol, 79(20):12692-12702, 01 Oct 2005
Cited by: 113 articles | PMID: 16188972 | PMCID: PMC1235841
Molecular aspects of HTLV-1 entry: functional domains of the HTLV-1 surface subunit (SU) and their relationships to the entry receptors.
Viruses, 3(6):794-810, 15 Jun 2011
Cited by: 47 articles | PMID: 21994754 | PMCID: PMC3185769
Review Free full text in Europe PMC





