Abstract
Free full text

Pharmacological characterization of non-NMDA subtypes of glutamate receptor in the neonatal rat hemisected spinal cord in vitro.
Abstract
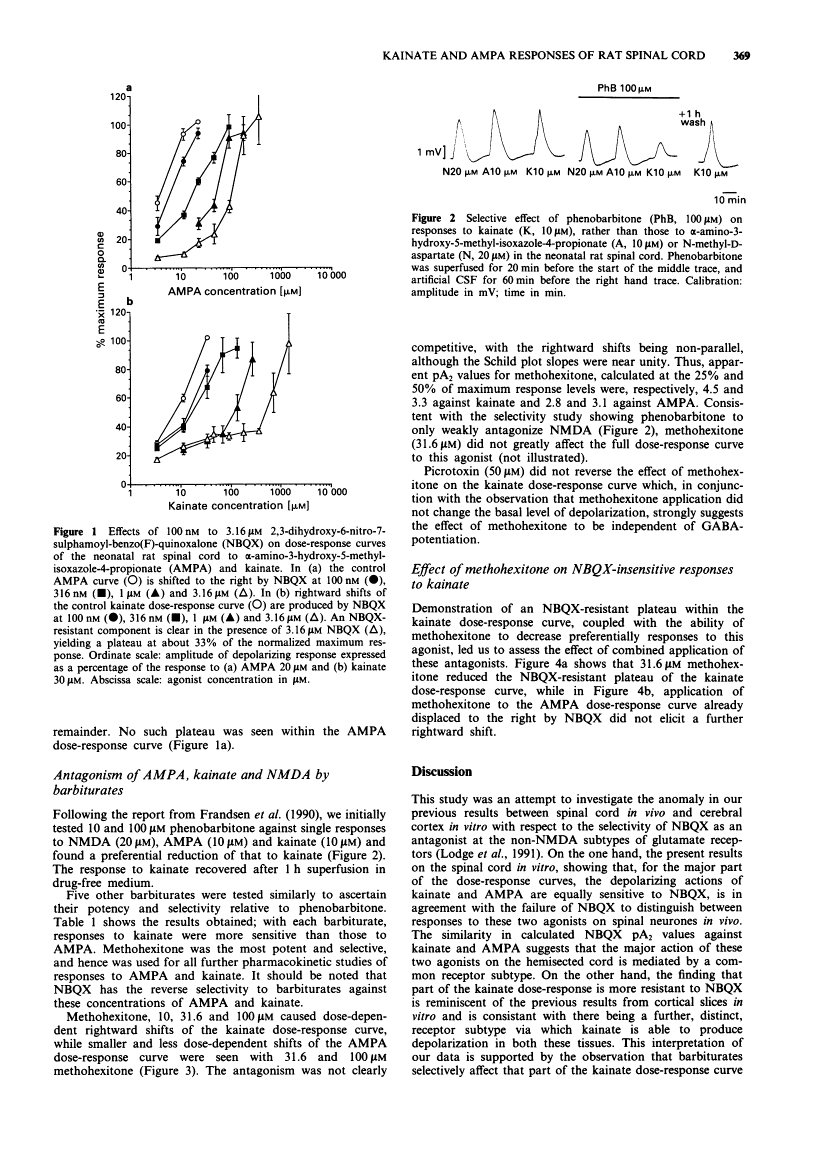
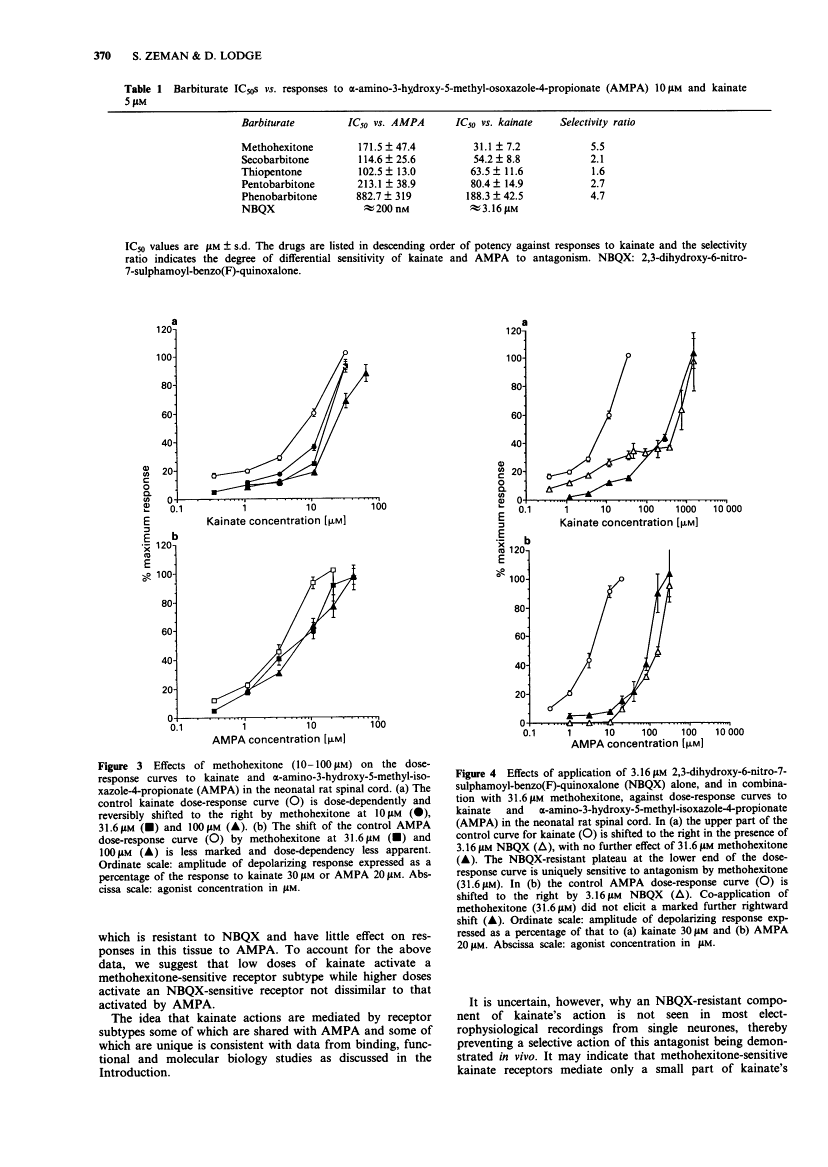
1. A grease-gap technique was used to record depolarizing responses to alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionate (AMPA), kainate and N-methyl-D-aspartate (NMDA) in the hemisected spinal cord of the neonatal rat. The pharmacology of non-NMDA subtypes of glutamate receptor was investigated with the novel quinoxalinedione, 2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo (F)-quinoxaline (NBQX) and with a series of barbiturates. 2. NBQX antagonized AMPA- and kainate-, but not NMDA- induced depolarizations. The near parallel shifts of the major part of the dose-response curves for AMPA and kainate by NBQX gave pA2 values (+/- s.e.) of 6.7 +/- 0.2 and 6.8 +/- 0.2 respectively, consistent with a common site of action for these two agonists. 3. Below the 50% level at which these pA2 values were calculated, however, an NBQX-resistant plateau was seen within the kainate, but not the AMPA, dose-response curve. 4. In decreasing order of potency, methohexitone, secobarbitone, thiopentone, pentobarbitone and phenobarbitone preferentially reduced kainate-, rather than AMPA- and NMDA-, induced depolarizations. Methohexitone was also the most selective with IC50S against kainate, AMPA and NMDA of 31 +/- 7, 172 +/- 47 and greater than 200 microM respectively. 5. The NBQX-resistant plateau seen within the kainate dose-response curve was reduced by methohexitone. Kainate antagonism by methohexitone was not reduced by 50 microM picrotoxin. 6. We conclude that, while mixed agonist actions may hamper demonstration of antagonist selectivity, depolarizations induced by the non-NMDA ionotropic agonists, AMPA and kainate, are mediated in part via distinct receptors.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986 Feb;87(2):345–355. [Europe PMC free article] [Abstract] [Google Scholar]
- Birch PJ, Grossman CJ, Hayes AG. 6,7-Dinitro-quinoxaline-2,3-dion and 6-nitro,7-cyano-quinoxaline-2,3-dion antagonise responses to NMDA in the rat spinal cord via an action at the strychnine-insensitive glycine receptor. Eur J Pharmacol. 1988 Oct 26;156(1):177–180. [Abstract] [Google Scholar]
- Campochiaro P, Coyle JT. Ontogenetic development of kainate neurotoxicity: correlates with glutamatergic innervation. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2025–2029. [Europe PMC free article] [Abstract] [Google Scholar]
- Collins GG, Anson J, Surtees L. Presynaptic kainate and N-methyl-D-aspartate receptors regulate excitatory amino acid release in the olfactory cortex. Brain Res. 1983 Apr 11;265(1):157–159. [Abstract] [Google Scholar]
- Cutler RW, Young J. Effect of barbiturates on release endogenous amino acids from rat cortex slices. Neurochem Res. 1979 Jun;4(3):319–329. [Abstract] [Google Scholar]
- Davies J, Watkins JC. Depressant actions of gamma-D-glutamylaminomethyl sulfonate (GAMS) on amino acid-induced and synaptic excitation in the cat spinal cord. Brain Res. 1985 Feb 18;327(1-2):113–120. [Abstract] [Google Scholar]
- Dingledine R. New wave of non-NMDA excitatory amino acid receptors. Trends Pharmacol Sci. 1991 Oct;12(10):360–362. [Abstract] [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991 Jun 27;351(6329):745–748. [Abstract] [Google Scholar]
- Evans RH. The effects of amino acids and antagonists on the isolated hemisected spinal cord of the immature rat. Br J Pharmacol. 1978 Feb;62(2):171–176. [Europe PMC free article] [Abstract] [Google Scholar]
- Fletcher EJ, Martin D, Aram JA, Lodge D, Honoré T. Quinoxalinediones selectively block quisqualate and kainate receptors and synaptic events in rat neocortex and hippocampus and frog spinal cord in vitro. Br J Pharmacol. 1988 Oct;95(2):585–597. [Europe PMC free article] [Abstract] [Google Scholar]
- Foster AC, Fagg GE. Acidic amino acid binding sites in mammalian neuronal membranes: their characteristics and relationship to synaptic receptors. Brain Res. 1984 May;319(2):103–164. [Abstract] [Google Scholar]
- Frandsen A, Quistorff B, Schousboe A. Phenobarbital protects cerebral cortex neurones against toxicity induced by kainate but not by other excitatory amino acids. Neurosci Lett. 1990 Mar 26;111(1-2):233–238. [Abstract] [Google Scholar]
- Gallo V, Giovannini C, Levi G. Modulation of non-N-methyl-D-aspartate receptors in cultured cerebellar granule cells. J Neurochem. 1990 May;54(5):1619–1625. [Abstract] [Google Scholar]
- Greenamyre JT, Young AB, Penney JB. Quantitative autoradiography of L-[3H]glutamate binding to rat brain. Neurosci Lett. 1983 Jun 16;37(2):155–160. [Abstract] [Google Scholar]
- Harrison NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985 Feb;84(2):381–391. [Europe PMC free article] [Abstract] [Google Scholar]
- Hollmann M, O'Shea-Greenfield A, Rogers SW, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989 Dec 7;342(6250):643–648. [Abstract] [Google Scholar]
- Honoré T, Davies SN, Drejer J, Fletcher EJ, Jacobsen P, Lodge D, Nielsen FE. Quinoxalinediones: potent competitive non-NMDA glutamate receptor antagonists. Science. 1988 Aug 5;241(4866):701–703. [Abstract] [Google Scholar]
- Iino M, Ozawa S, Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. [Abstract] [Google Scholar]
- Keinänen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990 Aug 3;249(4968):556–560. [Abstract] [Google Scholar]
- Kiskin NI, Krishtal OA, Tsyndrenko AYa Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett. 1986 Jan 30;63(3):225–230. [Abstract] [Google Scholar]
- Kiskin NI, Krishtal OA, Tsyndrenko A Ya. Cross-desensitization Reveals Pharmacological Specificity of Excitatory Amino Acid Receptors in Isolated Hippocampal Neurons. Eur J Neurosci. 1990;2(5):461–470. [Abstract] [Google Scholar]
- Krogsgaard-Larsen P, Honoré T, Hansen JJ, Curtis DR, Lodge D. New class of glutamate agonist structurally related to ibotenic acid. Nature. 1980 Mar 6;284(5751):64–66. [Abstract] [Google Scholar]
- Lodge D, Jones MG, Palmer AJ. Excitatory amino acids: new tools for old stories or pharmacological subtypes of glutamate receptors: electrophysiological studies. Can J Physiol Pharmacol. 1991 Jul;69(7):1123–1128. [Abstract] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. [Abstract] [Google Scholar]
- McLennan H, Liu J. The action of six antagonists of the excitatory amino acids on neurones of the rat spinal cord. Exp Brain Res. 1982;45(1-2):151–156. [Abstract] [Google Scholar]
- Monaghan DT, Bridges RJ, Cotman CW. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. [Abstract] [Google Scholar]
- Monaghan DT, Holets VR, Toy DW, Cotman CW. Anatomical distributions of four pharmacologically distinct 3H-L-glutamate binding sites. Nature. 1983 Nov 10;306(5939):176–179. [Abstract] [Google Scholar]
- Okazaki MM, Nadler JV. Protective effects of mossy fiber lesions against kainic acid-induced seizures and neuronal degeneration. Neuroscience. 1988 Sep;26(3):763–781. [Abstract] [Google Scholar]
- Olney JW. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science. 1969 May 9;164(3880):719–721. [Abstract] [Google Scholar]
- Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991 May;6(5):785–798. [Abstract] [Google Scholar]
- Pin JP, Van Vliet BJ, Bockaert J. Complex interaction between quisqualate and kainate receptors as revealed by measurement of GABA release from striatal neurons in primary culture. Eur J Pharmacol. 1989 Mar 7;172(1):81–91. [Abstract] [Google Scholar]
- Potashner SJ, Gerard D. Kainate-enhanced release of D-[3H]aspartate from cerebral cortex and striatum: reversal by baclofen and pentobarbital. J Neurochem. 1983 Jun;40(6):1548–1557. [Abstract] [Google Scholar]
- Sheardown MJ, Nielsen EO, Hansen AJ, Jacobsen P, Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990 Feb 2;247(4942):571–574. [Abstract] [Google Scholar]
- Stone TW. Sensitivity of hippocampal neurones to kainic acid, and antagonism by kynurenate. Br J Pharmacol. 1990 Dec;101(4):847–852. [Europe PMC free article] [Abstract] [Google Scholar]
- Stone WE, Javid MJ. Effects of anticonvulsants and glutamate antagonists on the convulsive action of kainic acid. Arch Int Pharmacodyn Ther. 1980 Jan;243(1):56–65. [Abstract] [Google Scholar]
- Teichberg VI, Eshhar N, Maoz I, Mano I, Ornstein D, Ortega A, Gregor P. Molecular characterization, ultrastructural localization and gene cloning of the chick cerebellar kainate receptor. Adv Exp Med Biol. 1990;268:73–78. [Abstract] [Google Scholar]
- Teichberg VI, Tal N, Goldberg O, Luini A. Barbiturates, alcohols and the CNS excitatory neurotransmission: specific effects on the kainate and quisqualate receptors. Brain Res. 1984 Jan 23;291(2):285–292. [Abstract] [Google Scholar]
- Turski L, Niemann W, Stephens DN. Differential effects of antiepileptic drugs and beta-carbolines on seizures induced by excitatory amino acids. Neuroscience. 1990;39(3):799–807. [Abstract] [Google Scholar]
- Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. [Abstract] [Google Scholar]
- Watkins JC, Pook PC, Sunter DC, Davies J, Honore T. Experiments with kainate and quisqualate agonists and antagonists in relation to the sub-classification of 'non-NMDA' receptors. Adv Exp Med Biol. 1990;268:49–55. [Abstract] [Google Scholar]
- Weakly JN. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. [Abstract] [Google Scholar]
- Wyllie DJ, Mathie A, Symonds CJ, Cull-Candy SG. Activation of glutamate receptors and glutamate uptake in identified macroglial cells in rat cerebellar cultures. J Physiol. 1991 Jan;432:235–258. [Abstract] [Google Scholar]
Associated Data
Articles from British Journal of Pharmacology are provided here courtesy of The British Pharmacological Society
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1476-5381.1992.tb14342.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1907489?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1476-5381.1992.tb14342.x
Article citations
A high-throughput model for investigating neuronal function and synaptic transmission in cultured neuronal networks.
Sci Rep, 7(1):14498, 03 Nov 2017
Cited by: 10 articles | PMID: 29101377 | PMCID: PMC5670206
Cell diversity and network dynamics in photosensitive human brain organoids.
Nature, 545(7652):48-53, 26 Apr 2017
Cited by: 602 articles | PMID: 28445462 | PMCID: PMC5659341
Distinct receptors underlie glutamatergic signalling in inspiratory rhythm-generating networks and motor output pathways in neonatal rat.
J Physiol, 586(9):2357-2370, 13 Mar 2008
Cited by: 9 articles | PMID: 18339693
The effects of AMPA receptor antagonists in models of stroke and neurodegeneration.
Eur J Pharmacol, 519(1-2):58-67, 01 Sep 2005
Cited by: 25 articles | PMID: 16112106
The role of AMPA and metabotropic glutamate receptors on morphine withdrawal in infant rats.
Int J Dev Neurosci, 22(5-6):379-395, 01 Aug 2004
Cited by: 13 articles | PMID: 15380837
Go to all (27) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparative patch clamp studies on the kinetics and selectivity of glutamate receptor antagonism by 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline (NBQX) and 1-(4-amino-phenyl)-4-methyl-7,8-methyl-endioxyl-5H-2,3-benzodiaze pine (GYKI 52466).
Neuropharmacology, 33(5):589-604, 01 May 1994
Cited by: 39 articles | PMID: 7523977
A comparison of the actions of agonists and antagonists at non-NMDA receptors of C fibres and motoneurones of the immature rat spinal cord in vitro.
Br J Pharmacol, 108(1):179-184, 01 Jan 1993
Cited by: 26 articles | PMID: 8094024 | PMCID: PMC1907711
Dicarboxyphenylglycines antagonize AMPA- but not kainate-induced depolarizations in neonatal rat motoneurones.
Eur J Pharmacol, 338(2):111-116, 01 Nov 1997
Cited by: 10 articles | PMID: 9455991
New developments in the molecular pharmacology of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate and kainate receptors.
Pharmacol Ther, 70(1):65-89, 01 Jan 1996
Cited by: 84 articles | PMID: 8804111
Review