Abstract
Free full text

Colony-stimulating factors activate human macrophages to inhibit intracellular growth of Histoplasma capsulatum yeasts.
Abstract
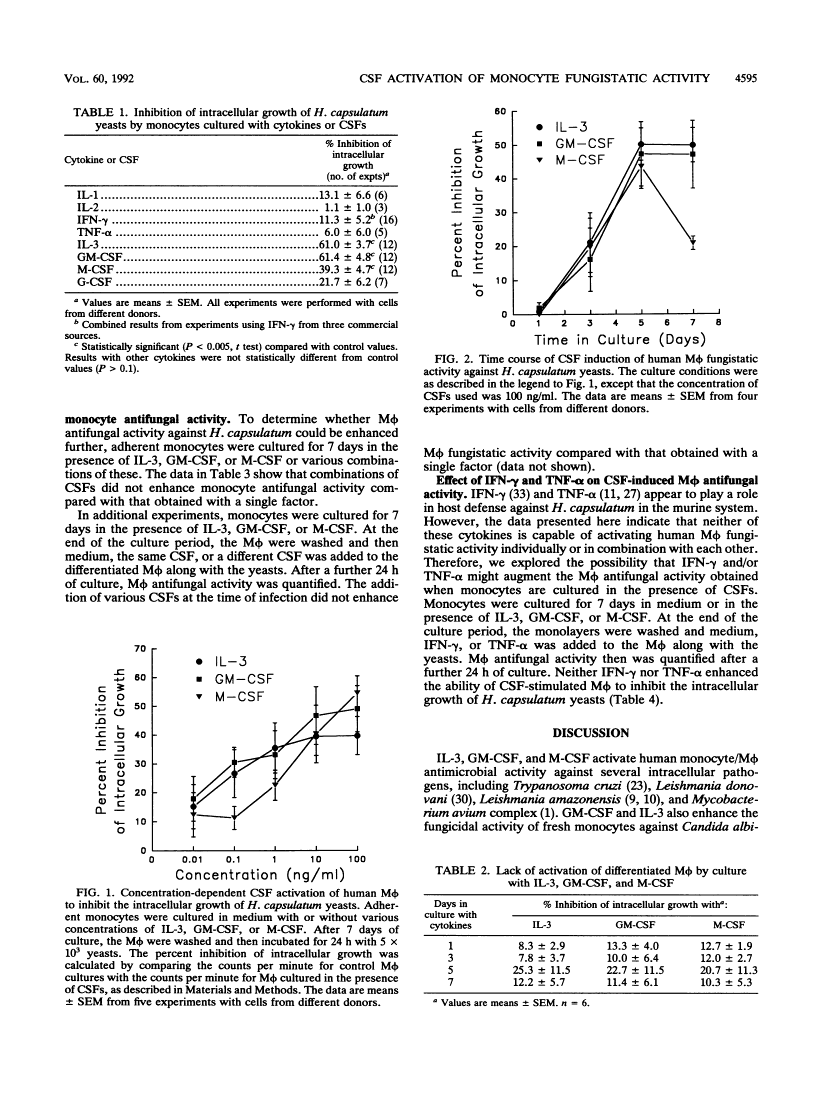
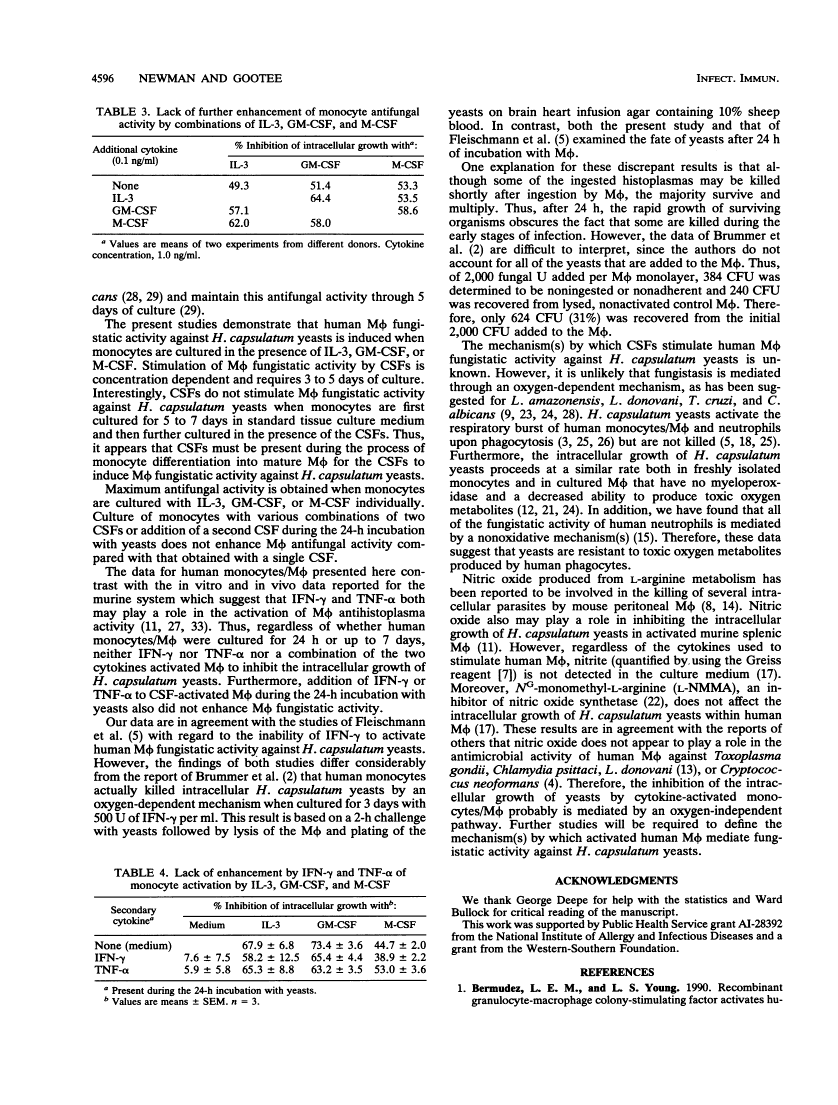
Recombinant cytokines and colony-stimulating factors (CSFs) were tested for their abilities to activate human monocytes/macrophages (M phi) to inhibit the intracellular growth of or kill Histoplasma capsulatum yeasts. None of the cytokines or CSFs or combinations of cytokines and CSFs activated M phi fungistatic activity when they were added to M phi monolayers concurrently with yeasts. In contrast, culture of monocytes for 7 days in the presence of interleukin 3, granulocyte-M phi CSF, or M phi CSF stimulated M phi fungistatic (but not fungicidal) activity against H. capsulatum yeasts in a concentration-dependent manner. Optimal activation of M phi by CSFs required 5 days of coculture, and the cultures had to be initiated with freshly isolated peripheral blood monocytes. Culture of monocytes with combinations of CSFs or addition of CSFs during the 24 h of coculture with the yeasts did not further enhance M phi fungistatic activity for H. capsulatum. Addition of gamma interferon or tumor necrosis factor alpha to CSF-activated M phi also did not enhance M phi fungistatic activity. These results suggest that interleukin 3, granulocyte-M phi CSF, and M phi CSF may play a role in the cell-mediated immune response to H. capsulatum by enhancing monocyte/M phi fungistatic activity.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez LE, Young LS. Recombinant granulocyte-macrophage colony-stimulating factor activates human macrophages to inhibit growth or kill Mycobacterium avium complex. J Leukoc Biol. 1990 Jul;48(1):67–73. [Abstract] [Google Scholar]
- Brummer E, Kurita N, Yoshida S, Nishimura K, Miyaji M. Killing of Histoplasma capsulatum by gamma-interferon-activated human monocyte-derived macrophages: evidence for a superoxide anion-dependent mechanism. J Med Microbiol. 1991 Jul;35(1):29–34. [Abstract] [Google Scholar]
- Bullock WE, Wright SD. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. [Europe PMC free article] [Abstract] [Google Scholar]
- Cameron ML, Granger DL, Weinberg JB, Kozumbo WJ, Koren HS. Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1313–1319. [Abstract] [Google Scholar]
- Fleischmann J, Wu-Hsieh B, Howard DH. The intracellular fate of Histoplasma capsulatum in human macrophages is unaffected by recombinant human interferon-gamma. J Infect Dis. 1990 Jan;161(1):143–145. [Abstract] [Google Scholar]
- Gmelig-Meyling F, Waldmann TA. Separation of human blood monocytes and lymphocytes on a continuous Percoll gradient. J Immunol Methods. 1980;33(1):1–9. [Abstract] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. [Abstract] [Google Scholar]
- Green SJ, Mellouk S, Hoffman SL, Meltzer MS, Nacy CA. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990 Aug;25(1-3):15–19. [Abstract] [Google Scholar]
- Ho JL, Reed SG, Sobel J, Arruda S, He SH, Wick EA, Grabstein KH. Interleukin-3 induces antimicrobial activity against Leishmania amazonensis and Trypanosoma cruzi and tumoricidal activity in human peripheral blood-derived macrophages. Infect Immun. 1992 May;60(5):1984–1993. [Europe PMC free article] [Abstract] [Google Scholar]
- Ho JL, Klempner MS. Diminished activity of protein kinase C in tetanus toxin-treated macrophages and in the spinal cord of mice manifesting generalized tetanus intoxication. J Infect Dis. 1988 May;157(5):925–933. [Abstract] [Google Scholar]
- Murray HW, Cartelli DM. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. [Europe PMC free article] [Abstract] [Google Scholar]
- Murray HW, Teitelbaum RF. L-arginine-dependent reactive nitrogen intermediates and the antimicrobial effect of activated human mononuclear phagocytes. J Infect Dis. 1992 Mar;165(3):513–517. [Abstract] [Google Scholar]
- Nathan CF, Hibbs JB., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. [Abstract] [Google Scholar]
- Newman SL, Bucher C, Rhodes J, Bullock WE. Phagocytosis of Histoplasma capsulatum yeasts and microconidia by human cultured macrophages and alveolar macrophages. Cellular cytoskeleton requirement for attachment and ingestion. J Clin Invest. 1990 Jan;85(1):223–230. [Europe PMC free article] [Abstract] [Google Scholar]
- Newman SL, Gootee L, Bucher C, Bullock WE. Inhibition of intracellular growth of Histoplasma capsulatum yeast cells by cytokine-activated human monocytes and macrophages. Infect Immun. 1991 Feb;59(2):737–741. [Europe PMC free article] [Abstract] [Google Scholar]
- Newman SL, Gootee L, Morris R, Bullock WE. Digestion of Histoplasma capsulatum yeasts by human macrophages. J Immunol. 1992 Jul 15;149(2):574–580. [Abstract] [Google Scholar]
- Newman SL, Musson RA, Henson PM. Development of functional complement receptors during in vitro maturation of human monocytes into macrophages. J Immunol. 1980 Nov;125(5):2236–2244. [Abstract] [Google Scholar]
- Pabst MJ, Hedegaard HB, Johnston RB., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [Abstract] [Google Scholar]
- Palmer RM, Rees DD, Ashton DS, Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. [Abstract] [Google Scholar]
- Reed SG, Nathan CF, Pihl DL, Rodricks P, Shanebeck K, Conlon PJ, Grabstein KH. Recombinant granulocyte/macrophage colony-stimulating factor activates macrophages to inhibit Trypanosoma cruzi and release hydrogen peroxide. Comparison with interferon gamma. J Exp Med. 1987 Dec 1;166(6):1734–1746. [Europe PMC free article] [Abstract] [Google Scholar]
- Sasada M, Kubo A, Nishimura T, Kakita T, Moriguchi T, Yamamoto K, Uchino H. Candidacidal activity of monocyte-derived human macrophages: relationship between Candida killing and oxygen radical generation by human macrophages. J Leukoc Biol. 1987 Apr;41(4):289–294. [Abstract] [Google Scholar]
- Schaffner A, Davis CE, Schaffner T, Markert M, Douglas H, Braude AI. In vitro susceptibility of fungi to killing by neutrophil granulocytes discriminates between primary pathogenicity and opportunism. J Clin Invest. 1986 Aug;78(2):511–524. [Europe PMC free article] [Abstract] [Google Scholar]
- Schnur RA, Newman SL. The respiratory burst response to Histoplasma capsulatum by human neutrophils. Evidence for intracellular trapping of superoxide anion. J Immunol. 1990 Jun 15;144(12):4765–4772. [Abstract] [Google Scholar]
- Smith JG, Magee DM, Williams DM, Graybill JR. Tumor necrosis factor-alpha plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990 Dec;162(6):1349–1353. [Abstract] [Google Scholar]
- Smith PD, Lamerson CL, Banks SM, Saini SS, Wahl LM, Calderone RA, Wahl SM. Granulocyte-macrophage colony-stimulating factor augments human monocyte fungicidal activity for Candida albicans. J Infect Dis. 1990 May;161(5):999–1005. [Abstract] [Google Scholar]
- Wang M, Friedman H, Djeu JY. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989 Jul 15;143(2):671–677. [Abstract] [Google Scholar]
- Weiser WY, Van Niel A, Clark SC, David JR, Remold HG. Recombinant human granulocyte/macrophage colony-stimulating factor activates intracellular killing of Leishmania donovani by human monocyte-derived macrophages. J Exp Med. 1987 Nov 1;166(5):1436–1446. [Europe PMC free article] [Abstract] [Google Scholar]
- Wolf JE, Abegg AL, Travis SJ, Kobayashi GS, Little JR. Effects of Histoplasma capsulatum on murine macrophage functions: inhibition of macrophage priming, oxidative burst, and antifungal activities. Infect Immun. 1989 Feb;57(2):513–519. [Europe PMC free article] [Abstract] [Google Scholar]
- Worsham PL, Goldman WE. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988 Jun;26(3):137–143. [Abstract] [Google Scholar]
- Wu-Hsieh BA, Howard DH. Inhibition of the intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987 Apr;55(4):1014–1016. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Infection and Immunity are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/iai.60.11.4593-4597.1992
Read article for free, from open access legal sources, via Unpaywall:
https://iai.asm.org/content/iai/60/11/4593.full.pdf
Free after 4 months at iai.asm.org
http://iai.asm.org/cgi/reprint/60/11/4593
Free to read at iai.asm.org
http://iai.asm.org/cgi/content/abstract/60/11/4593
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/iai.60.11.4593-4597.1992
Article citations
Utilization of a <i>Histoplasma capsulatum</i> zinc reporter reveals the complexities of fungal sensing of metal deprivation.
mSphere, 9(2):e0070423, 23 Jan 2024
Cited by: 0 articles | PMID: 38259064 | PMCID: PMC10900905
The HIF-1α/LC3-II Axis Impacts Fungal Immunity in Human Macrophages.
Infect Immun, 87(7):e00125-19, 20 Jun 2019
Cited by: 13 articles | PMID: 31036602 | PMCID: PMC6589057
Histoplasma Responses to Nutritional Immunity Imposed by Macrophage Activation.
J Fungi (Basel), 5(2):E45, 05 Jun 2019
Cited by: 12 articles | PMID: 31195617 | PMCID: PMC6616858
Review Free full text in Europe PMC
Sterol Regulatory Element Binding Protein (Srb1) Is Required for Hypoxic Adaptation and Virulence in the Dimorphic Fungus Histoplasma capsulatum.
PLoS One, 11(10):e0163849, 06 Oct 2016
Cited by: 10 articles | PMID: 27711233 | PMCID: PMC5053422
IL-4 Induces Metallothionein 3- and SLC30A4-Dependent Increase in Intracellular Zn(2+) that Promotes Pathogen Persistence in Macrophages.
Cell Rep, 16(12):3232-3246, 01 Sep 2016
Cited by: 25 articles | PMID: 27653687 | PMCID: PMC5603080
Go to all (48) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Activation of human macrophage fungistatic activity against Histoplasma capsulatum upon adherence to type 1 collagen matrices.
J Immunol, 158(4):1779-1786, 01 Feb 1997
Cited by: 24 articles | PMID: 9029116
Inhibition of intracellular growth of Histoplasma capsulatum yeast cells by cytokine-activated human monocytes and macrophages.
Infect Immun, 59(2):737-741, 01 Feb 1991
Cited by: 28 articles | PMID: 1898916 | PMCID: PMC257824
Comparison of the effects of IL-3, granulocyte-macrophage colony-stimulating factor, and macrophage colony-stimulating factor in supporting monocyte differentiation in culture. Analysis of macrophage antibody-dependent cellular cytotoxicity.
J Immunol, 145(2):607-615, 01 Jul 1990
Cited by: 105 articles | PMID: 2142182
Macrophages in host defense against Histoplasma capsulatum.
Trends Microbiol, 7(2):67-71, 01 Feb 1999
Cited by: 58 articles | PMID: 10081083
Review