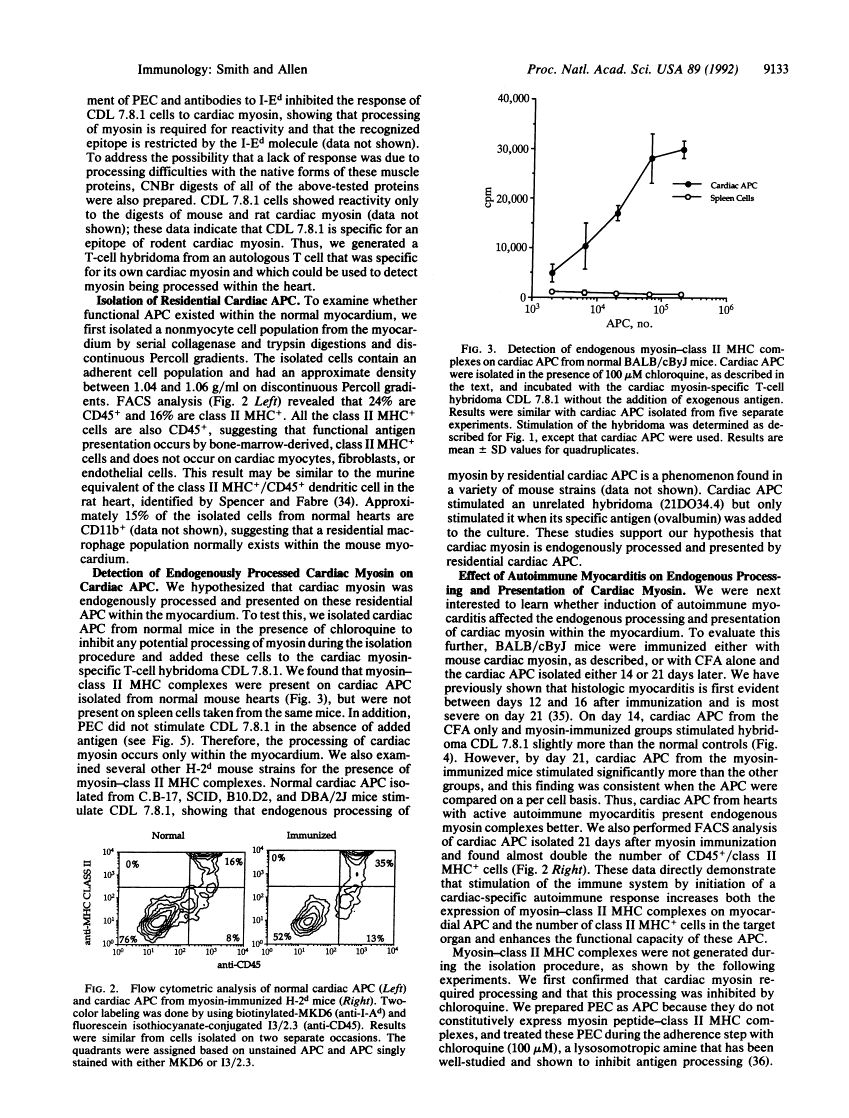
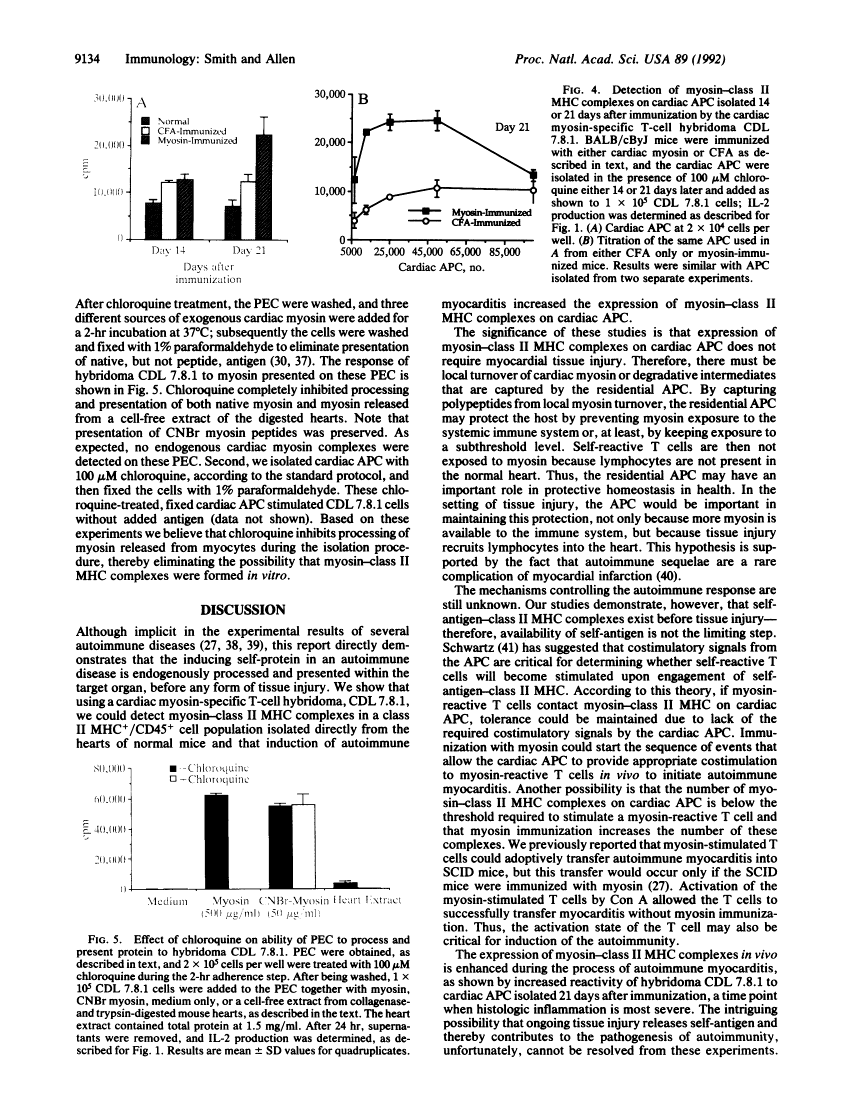
Abstract
Free full text

Expression of myosin-class II major histocompatibility complexes in the normal myocardium occurs before induction of autoimmune myocarditis.
Abstract
Determining how an autoimmune response is initiated is essential to understanding the mechanisms of autoimmunity. Self-reactive T cells, self-protein, and a failure of tolerance to that self-protein are all involved in the pathogenesis of autoimmune disease; yet it is not clear how self-reactive T cells find the target self-protein to initiate an autoimmune response. Although a variety of self-proteins have been shown to be presented on both class I and class II major histocompatibility complex (MHC) molecules, the relationship of these self-proteins to autoimmune disease has not been established. To explore this further, we generated a T-cell hybridoma that recognizes mouse cardiac myosin, the self-protein that induces murine autoimmune myocarditis. Using this hybridoma as a probe to detect myosin-class II MHC complexes, we isolated a class II MHC+/CD45+ residential antigen-presenting cell (APC) population directly from the hearts of normal mice and looked for evidence of endogenous processing of cardiac myosin by these APC. In this report we show that myosin-class II MHC complexes are found on residential APC in the normal mouse heart. Induction of autoimmune myocarditis increased the expression of myosin-class II MHC in the heart and enhanced their APC functions. This result is a direct demonstration that epitopes of a self-antigen involved in initiating an autoimmune disease are endogenously processed and presented within the target organ.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.2M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kappler JW, Staerz U, White J, Marrack PC. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. [Abstract] [Google Scholar]
- Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. [Abstract] [Google Scholar]
- Hengartner H, Odermatt B, Schneider R, Schreyer M, Wälle G, MacDonald HR, Zinkernagel RM. Deletion of self-reactive T cells before entry into the thymus medulla. Nature. 1988 Nov 24;336(6197):388–390. [Abstract] [Google Scholar]
- Bill J, Kanagawa O, Woodland DL, Palmer E. The MHC molecule I-E is necessary but not sufficient for the clonal deletion of V beta 11-bearing T cells. J Exp Med. 1989 Apr 1;169(4):1405–1419. [Europe PMC free article] [Abstract] [Google Scholar]
- Kisielow P, Blüthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. [Abstract] [Google Scholar]
- Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988 Nov 3;336(6194):73–76. [Abstract] [Google Scholar]
- Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. [Abstract] [Google Scholar]
- Burkly LC, Lo D, Flavell RA. Tolerance in transgenic mice expressing major histocompatibility molecules extrathymically on pancreatic cells. Science. 1990 Jun 15;248(4961):1364–1368. [Abstract] [Google Scholar]
- Murphy KM, Weaver CT, Elish M, Allen PM, Loh DY. Peripheral tolerance to allogeneic class II histocompatibility antigens expressed in transgenic mice: evidence against a clonal-deletion mechanism. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10034–10038. [Europe PMC free article] [Abstract] [Google Scholar]
- Schönrich G, Kalinke U, Momburg F, Malissen M, Schmitt-Verhulst AM, Malissen B, Hämmerling GJ, Arnold B. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell. 1991 Apr 19;65(2):293–304. [Abstract] [Google Scholar]
- Lorenz RG, Allen PM. Direct evidence for functional self-protein/Ia-molecule complexes in vivo. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5220–5223. [Europe PMC free article] [Abstract] [Google Scholar]
- Weiss S, Bogen B. B-lymphoma cells process and present their endogenous immunoglobulin to major histocompatibility complex-restricted T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(1):282–286. [Europe PMC free article] [Abstract] [Google Scholar]
- Stockinger B, Hausmann B. Induction of an immune response to a self antigen. Eur J Immunol. 1988 Feb;18(2):249–253. [Abstract] [Google Scholar]
- Winchester G, Sunshine GH, Nardi N, Mitchison NA. Antigen-presenting cells do not discriminate between self and nonself. Immunogenetics. 1984;19(6):487–491. [Abstract] [Google Scholar]
- Bikoff EK, Yu H, Eckhardt LA. T cell recognition of endogenous IgG2a expressed in B lymphoma cells. Eur J Immunol. 1988 Mar;18(3):341–348. [Abstract] [Google Scholar]
- Wekerle H, Schwab M, Linington C, Meyermann R. Antigen presentation in the peripheral nervous system: Schwann cells present endogenous myelin autoantigens to lymphocytes. Eur J Immunol. 1986 Dec;16(12):1551–1557. [Abstract] [Google Scholar]
- Falk K, Rötzschke O, Stevanović S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. [Abstract] [Google Scholar]
- Jardetzky TS, Lane WS, Robinson RA, Madden DR, Wiley DC. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. [Abstract] [Google Scholar]
- Rudensky AYu, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr On the complexity of self. Nature. 1991 Oct 17;353(6345):660–662. [Abstract] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. [Abstract] [Google Scholar]
- Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992 Mar 6;255(5049):1261–1263. [Abstract] [Google Scholar]
- Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. [Abstract] [Google Scholar]
- Singh VK, Yamaki K, Donoso LA, Shinohara T. Molecular mimicry. Yeast histone H3-induced experimental autoimmune uveitis. J Immunol. 1989 Mar 1;142(5):1512–1517. [Abstract] [Google Scholar]
- Bouvet JP, Couderc J, Bouthillier Y, Franc B, Decreusefond C, Mouton D. Collagen-induced arthritis in Biozzi mice. Joint involvement is not correlated with collagen II IgG2a autoantibodies nor restricted to only H-2q and H-2r. J Immunol. 1989 Sep 1;143(5):1537–1542. [Abstract] [Google Scholar]
- WITEBSKY E, ROSE NR, TERPLAN K, PAINE JR, EGAN RW. Chronic thyroiditis and autoimmunization. J Am Med Assoc. 1957 Jul 27;164(13):1439–1447. [Abstract] [Google Scholar]
- Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987 Dec 1;139(11):3630–3636. [Abstract] [Google Scholar]
- Smith SC, Allen PM. Myosin-induced acute myocarditis is a T cell-mediated disease. J Immunol. 1991 Oct 1;147(7):2141–2147. [Abstract] [Google Scholar]
- Shiverick KT, Thomas LL, Alpert NR. Purification of cardiac myosin. Application to hypertrophied myocardium. Biochim Biophys Acta. 1975 May 30;393(1):124–133. [Abstract] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. [Abstract] [Google Scholar]
- Allen PM, Unanue ER. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [Abstract] [Google Scholar]
- Lorenz RG, Blum JS, Allen PM. Constitutive competition by self proteins for antigen presentation can be overcome by receptor-enhanced uptake. J Immunol. 1990 Mar 1;144(5):1600–1606. [Abstract] [Google Scholar]
- Trowbridge IS. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. [Europe PMC free article] [Abstract] [Google Scholar]
- Spencer SC, Fabre JW. Characterization of the tissue macrophage and the interstitial dendritic cell as distinct leukocytes normally resident in the connective tissue of rat heart. J Exp Med. 1990 Jun 1;171(6):1841–1851. [Europe PMC free article] [Abstract] [Google Scholar]
- Smith SC, Allen PM. Neutralization of endogenous tumor necrosis factor ameliorates the severity of myosin-induced myocarditis. Circ Res. 1992 Apr;70(4):856–863. [Abstract] [Google Scholar]
- Ziegler HK, Unanue ER. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. [Europe PMC free article] [Abstract] [Google Scholar]
- Shimonkevitz R, Kappler J, Marrack P, Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. [Europe PMC free article] [Abstract] [Google Scholar]
- Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 317(6035):355–358. [Abstract] [Google Scholar]
- Haskins K, Portas M, Bergman B, Lafferty K, Bradley B. Pancreatic islet-specific T-cell clones from nonobese diabetic mice. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8000–8004. [Europe PMC free article] [Abstract] [Google Scholar]
- Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. [Abstract] [Google Scholar]
- Hart DN, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain. J Exp Med. 1981 Aug 1;154(2):347–361. [Europe PMC free article] [Abstract] [Google Scholar]
- Steiniger B, Klempnauer J, Wonigeit K. Phenotype and histological distribution of interstitial dendritic cells in the rat pancreas, liver, heart, and kidney. Transplantation. 1984 Aug;38(2):169–174. [Abstract] [Google Scholar]
- Larsen CP, Morris PJ, Austyn JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990 Jan 1;171(1):307–314. [Europe PMC free article] [Abstract] [Google Scholar]
- Herskowitz A, Ahmed-Ansari A, Neumann DA, Beschorner WE, Rose NR, Soule LM, Burek CL, Sell KW, Baughman KL. Induction of major histocompatibility complex antigens within the myocardium of patients with active myocarditis: a nonhistologic marker of myocarditis. J Am Coll Cardiol. 1990 Mar 1;15(3):624–632. [Abstract] [Google Scholar]
- Rose ML, Page C, Hengstenberg C, Yacoub MH. Identification of antigen presenting cells in normal and transplanted human heart: importance of endothelial cells. Hum Immunol. 1990 Jun;28(2):179–185. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.89.19.9131
Read article for free, from open access legal sources, via Unpaywall:
http://www.pnas.org/content/pnas/89/19/9131.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Transgenic Mice Expressing Functional TCRs Specific to Cardiac Myhc-α 334-352 on Both CD4 and CD8 T Cells Are Resistant to the Development of Myocarditis on C57BL/6 Genetic Background.
Cells, 12(19):2346, 25 Sep 2023
Cited by: 2 articles | PMID: 37830560 | PMCID: PMC10571761
Myocardial-Treg Crosstalk: How to Tame a Wolf.
Front Immunol, 13:914033, 25 May 2022
Cited by: 9 articles | PMID: 35693830 | PMCID: PMC9176752
Review Free full text in Europe PMC
Immune Cells and Immunotherapy for Cardiac Injury and Repair.
Circ Res, 128(11):1766-1779, 27 May 2021
Cited by: 86 articles | PMID: 34043424 | PMCID: PMC8171813
Review Free full text in Europe PMC
Role of Cardiac Macrophages on Cardiac Inflammation, Fibrosis and Tissue Repair.
Cells, 10(1):E51, 31 Dec 2020
Cited by: 136 articles | PMID: 33396359 | PMCID: PMC7824389
Review Free full text in Europe PMC
The role of B cells in heart failure and implications for future immunomodulatory treatment strategies.
ESC Heart Fail, 7(4):1387-1399, 13 Jun 2020
Cited by: 25 articles | PMID: 32533765 | PMCID: PMC7373901
Review Free full text in Europe PMC
Go to all (67) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Myocarditis-inducing epitope of myosin binds constitutively and stably to I-Ak on antigen-presenting cells in the heart.
J Exp Med, 182(5):1291-1300, 01 Nov 1995
Cited by: 83 articles | PMID: 7595200 | PMCID: PMC2192215
Identification and characterization of the antigen presenting cell in rat autoimmune myocarditis: evidence of bone marrow derivation and non-requirement for MHC class I compatibility with pathogenic T cells.
J Autoimmun, 15(3):369-379, 01 Nov 2000
Cited by: 5 articles | PMID: 11040077
Cardiac myosin-induced myocarditis: target recognition by autoreactive T cells requires prior activation of cardiac interstitial cells.
Lab Invest, 74(5):845-852, 01 May 1996
Cited by: 22 articles | PMID: 8642780
The role of T cells in myosin-induced autoimmune myocarditis.
Clin Immunol Immunopathol, 68(2):100-106, 01 Aug 1993
Cited by: 26 articles | PMID: 8358855
Review