Abstract
Objectives
To determine if atrophy rates were higher for entorhinal cortex (ERC) than for hippocampus in Alzheimer disease (AD), to determine the relationship between hippocampal atrophy rate and memory impairment, and to compare atrophy rates of ERC and hippocampus in differentiating between patients with AD and cognitively normal (CN) controls.Methods
Twenty patients with AD and 25 CN subjects had MRI scans and clinical evaluations twice approximately 1.9 years apart. ERC volumes were measured manually and hippocampal volumes were measured semiautomatically on volumetric T1-weighted MR images.Results
In AD, the atrophy rate of ERC (7.1 +/- 3.2%/year) was higher (p < 0.02) than that of hippocampus (5.9 +/- 2.4%/year). Furthermore, memory deficit in mild AD, measured with the Delayed List Verbal Recall test, correlated significantly with atrophy rates of both ERC (r = -0.61) and hippocampus (r = -0.59). Atrophy rates of ERC and hippocampus were comparable in differentiating between AD and CN. Using atrophy rates of ERC or hippocampus to detect a 20% treatment effect with 90% power (p < 0.05) would require about 100 completed patients per arm in a 2-year study.Conclusion
The finding in AD that the atrophy rate in the entorhinal cortex is higher than in the hippocampus is consistent with the view that AD pathology begins in the entorhinal cortex.Free full text

Higher atrophy rate of entorhinal cortex than hippocampus in AD
Abstract
Objectives
To determine if atrophy rates were higher for entorhinal cortex (ERC) than for hippocampus in Alzheimer disease (AD), to determine the relationship between hippocampal atrophy rate and memory impairment, and to compare atrophy rates of ERC and hippocampus in differentiating between patients with AD and cognitively normal (CN) controls.
Methods
Twenty patients with AD and 25 CN subjects had MRI scans and clinical evaluations twice approximately 1.9 years apart. ERC volumes were measured manually and hippocampal volumes were measured semiautomatically on volumetric T1-weighted MR images.
Results
In AD, the atrophy rate of ERC (7.1 ± 3.2%/year) was higher (p < 0.02) than that of hippocampus (5.9 ± 2.4%/year). Furthermore, memory deficit in mild AD, measured with the Delayed List Verbal Recall test, correlated significantly with atrophy rates of both ERC (r = −0.61) and hippocampus (r = −0.59). Atrophy rates of ERC and hippocampus were comparable in differentiating between AD and CN. Using atrophy rates of ERC or hippocampus to detect a 20% treatment effect with 90% power (p < 0.05) would require about 100 completed patients per arm in a 2-year study.
Conclusion
The finding in AD that the atrophy rate in the entorhinal cortex is higher than in the hippocampus is consistent with the view that AD pathology begins in the entorhinal cortex.
Both entorhinal cortex (ERC) and hippocampus are essential parts of the medial temporal lobe system that supports declarative (conscious) memory.1 Consistent with the observation that memory impairment is a prominent symptom of Alzheimer disease (AD), histopathologic studies found both structures involved early in AD pathology.2–4 Similarly, MRI studies on patients with AD consistently found volume losses in both ERC5–9 and hippocampus.10–14 Analyses of MRI data with high dimensional brain mapping methods, which make no assumptions about specific brain regions, also revealed substantial atrophy of regions in AD, encompassing the ERC and hippocampus.15–17 Furthermore, longitudinal MRI studies of AD found increased atrophy rates in the ERC18,19 and hippocampus.20–22 Taken together, these findings suggest that AD is associated with progressive atrophy of both ERC and hippocampus, providing potential surrogate markers for this disease.
It has been hypothesized that AD starts in the ERC before spreading to the hippocampus and eventually to the cortex.23 Furthermore, clinical studies of AD have shown that, as the disease progresses, the rate of cognitive decline increases,24,25 suggesting that the rate of neuron loss may also increase with time. Because AD has been proposed to start in the ERC and if the changes of rates of neurodegeneration over time are similar in both structures, it is therefore expected that the ERC would have a higher atrophy rate than the hippocampus. If so, measurement of ERC volume changes over time might be a more sensitive indicator of AD progression than measurement of hippocampal changes. Therefore, we tested the hypothesis that the atrophy rate was higher for ERC than for hippocampus in AD. Based on our previous finding of a correlation between increased atrophy rates of ERC and decreased memory functions,18 we tested if a similar relationship with memory decline was detected for atrophy rates of hippocampus.
ERC and hippocampus volumes obtained at a single time point have limited ability to distinguish patients with early AD from cognitively normal (CN) subjects, presumably because of the large variability in brain volumes between subjects.6–10 Previous cross-sectional MRI studies reported that volumes of ERC and hippocampus were comparable in discriminating between AD and CN.7,9 In addition, a longitudinal MR study showed that atrophy rates of ERC were better than volume measurements at a single time point in the discrimination between these two groups.18 However, whether atrophy rate of ERC is better than that of hippocampus to discriminate AD from CN has not been determined. Therefore, we compared atrophy rates of ERC and hippocampus to discriminate AD from CN. In addition, we computed sample sizes for hypothetical treatment trials of AD, based on observed atrophy rates of ERC and hippocampus.
Materials and methods
Twenty-two patients with AD and 27 CN subjects were selected from a larger database and matched for age, sex, and MRI scan intervals. The subjects with movement artifacts on T1-weighted MR images were excluded. MRI data of ERC were reported earlier for the majority of these subjects.18 Further, MRI data of two controls and six patients with AD were included in an earlier cross-sectional study for the validation of semiautomated hippocampal measurements.26 After visually inspecting results of hippocampal tracing using a semiautomated method,26 two patients with AD (one man/one woman) and two CN subjects (one man/one woman) were excluded from the analysis, because of obvious tracing errors that could not be corrected. Demographics of the remaining subjects are listed in table 1. Subject recruitment, clinical evaluation, and exclusion criteria were reported earlier.18 Patients with AD and CN subjects received a standard battery of cognitive and neuropsychological tests at the referral centers, including a Mini-Mental State Examination (MMSE)27 and a Delayed List Recall (DLR) test. DLR scores represent a percentage of correctly remembered words, normalized to the maximum number of words on each list. Clinical Dementia Rating scores were greater than or equal to 0.5 for patients with AD and zero for CN subjects at the baseline. None of the CN subjects declined to the status of mild cognitive impairment or AD during the MRI study period. All subjects or their guardians gave written informed consent before participating in the study, which was approved by the Committees of Human Research at the University of California, San Francisco and Davis.
Table 1
Subject demographics
| Characteristics | CN | AD |
|---|---|---|
| N (% female) | 25 (56) | 20 (60) |
| Age, y | 76.8 ± 7.8 | 75.3 ± 7.2 |
| MMSE score | 29.0 ± 1.0 | 21.0 ± 7.2* |
| DLR score, % | 90.2 ± 17.0 | 29.9 ± 24.5* |
| Scan interval, y | 2.0 ± 0.7 | 1.8 ± 0.7 |
Data at time of baseline MRI scan, represented as mean ± SD.
CN = cognitively normal controls; AD = Alzheimer disease; MMSE = Mini-Mental State Examination; DLR = Delayed List Recall (DLR), in percent of total number of words that needed to be remembered.
MRI data were obtained on a 1.5 T Siemens Vision System (Siemens Inc., Iselin, NJ) and volumetric T1-weighted magnetization-prepared rapid gradient echo and double spin echo images were acquired.18 One operator performed all ERC and hippocampal tracings of this study. ERC volumes were measured manually on T1-weighted images, as described earlier.18 Hippocampal volumes of first and second MRI scans of each subject were measured independently, using a semiautomated high dimensional brain-warping algorithm (Medtronic Surgical Navigation Technologies, Louisville, CO).26 An expert visually inspected semiautomated hippocampal tracing blinded to clinical information before data analysis began. Subjects whose tracings extended into nonhippocampal tissue were excluded. Because there were no obvious movement artifacts on T1-weighted images, incorrect tracing likely resulted from either erroneous spatial transformations to the brain template (because of extreme deformation of hippocampal shape) or from limitations related to a low contrast to noise ratio in the images. Atrophy rates were expressed as annual percentage change from baseline volume, according to (baseline volume − second volume)/(baseline volume * time interval) * 100. Volumes of ERC and hippocampus used in the discrimination between AD and CN were from the second MRI scans. Volumes were divided by total intracranial volume (TIV) to account for variable head sizes, as described previously in detail.9 TIV and white matter hyperintensities (WMH) were obtained using semiautomated tissue segmentation.28 Reliabilities of ERC and hippocampal measurements were determined by same-day test-retest MRI acquisitions on nine subjects to separate out between subject variability from within subject variability and noise and expressed as an intraclass correlation coefficient. The same operator who did all ERC and hippocampal measurements in this study performed the reliability test. Intraclass correlation coefficients were 0.99 for ERC and 0.94 for hippocampus.
Differences between AD and CN in atrophy rates of ERC and hippocampus were tested using multivariate analysis of variance, accounting for age, sex, and WMH volume when appropriate. To determine whether atrophy rates were higher for ERC than for hippocampus, two-tailed paired t-tests were performed separately for patients with AD and CN subjects. Relationships between measures were tested using Pearson product moment correlation coefficients. The ability of the different MRI measures to separate patients with AD from CN subjects was assessed using logistic regressions with group membership as the outcome variable and MRI measures as predictors. Furthermore, a receiver operator characteristics (ROC) analysis was used to evaluate the results by means of correct positive (sensitivity) and false negative (1 − specificity) classifications. Discriminatory powers between the measures were tested by comparing the areas under the ROC curves (AUC) using the Mann-Whitney method.29 Finally, sample size to detect a treatment effect on either ERC or hippocampal atrophy rates was based on two-tailed (p < 0.05) t-test using SamplePower (SPSS) software.
Results
The demographic data of the patients and control subjects who were included in the final analysis are listed in table 1. The groups were comparable in distributions of age (p = 0.5, t-test) and sex (χ2 = 1.7; p = 0.2). As expected, patients with AD had lower MMSE (p < 0.01, t-test) and DLR (p < 0.01, t-test) scores than CN subjects. In addition, WMH volumes were comparable between patients with AD and CN subjects. No correlation was found between follow-up periods and atrophy rates of ERC or hippocampus (both p > 0.1), suggesting no bias of atrophy rates due to different lengths of follow-up period.
Atrophy rates and baseline volumes of ERC and hippocampus are listed in table 2. Compared with CN subjects, patients with AD had greater atrophy rates of hippocampus (left F1,43 = 45.5, p < 0.001, right F1,43 = 38.4, p < 0.001). As shown before,18 patients with AD had greater atrophy rates of ERC (left F1,43 = 48.4, p < 0.001, right F1,43 = 42.8, p < 0.001) than CN subjects. On average, patients with AD had atrophy rates of 7.1 ± 3.2%/year for ERC and 5.9 ± 2.4%/year for hippocampus, while CN subjects had atrophy rates of 1.4 ± 2.0%/year for ERC and 0.8 ± 1.7%/year for hippocampus. Atrophy rates of both ERC and hippocampus in CN subjects were different from zero (p < 0.05, one sample t-test).
Table 2
Atrophy rate and follow-up volumes of entorhinal cortex (ERC) and hippocampus in patients with Alzheimer disease (AD) and cognitively normal controls (CN)
| Rates and volumes | AD | CN |
|---|---|---|
| ERC rate, %/y | ||
 Left Left | 7.5 ± 3.8 | 1.2 ± 2.1 |
 Right Right | 6.8 ± 3.1 | 1.6 ± 2.3 |
 Total Total | 7.1 ± 3.2* | 1.4 ± 2.0 |
| Hippocampal rate, %/y | ||
 Left Left | 6.3 ± 2.6 | 1.0 ± 2.6 |
 Right Right | 5.5 ± 2.8 | 0.6 ± 2.5 |
 Total Total | 5.9 ± 2.4* | 0.8 ± 1.7 |
| ERC volume, mm3 | ||
 Left Left | 709 ± 214 | 1,144 ± 384 |
 Right Right | 664 ± 305 | 1,119 ± 369 |
 Total Total | 1,274 ± 449 | 2,263 ± 684 |
| Hippocampal volume, mm3 | ||
 Left Left | 1,633 ± 302 | 2,250 ± 364 |
 Right Right | 1,716 ± 310 | 2,310 ± 332 |
 Total Total | 3,351 ± 585 | 4,561 ± 654 |
Data represented as mean ± SD. ERC volumes at baseline and rates were reported previously.17 p < 0.01 in all MRI measures between AD and CN.
Comparing ERC and hippocampal rates within each subject, patients with AD had greater atrophy rates of ERC than of hippocampus (p < 0.02, paired t-test). The coefficient of variance of atrophy rates of ERC (0.45), however, was higher than that of hippocampus (0.41) in AD. The relationship between atrophy rates of ERC and hippocampus within each subject is illustrated in figure 1, separately for AD and CN. Atrophy rates of ERC and hippocampus were strongly correlated in patients with AD (r = 0.76, p < 0.001), but not in CN subjects (r = 0.30, p < 0.15).
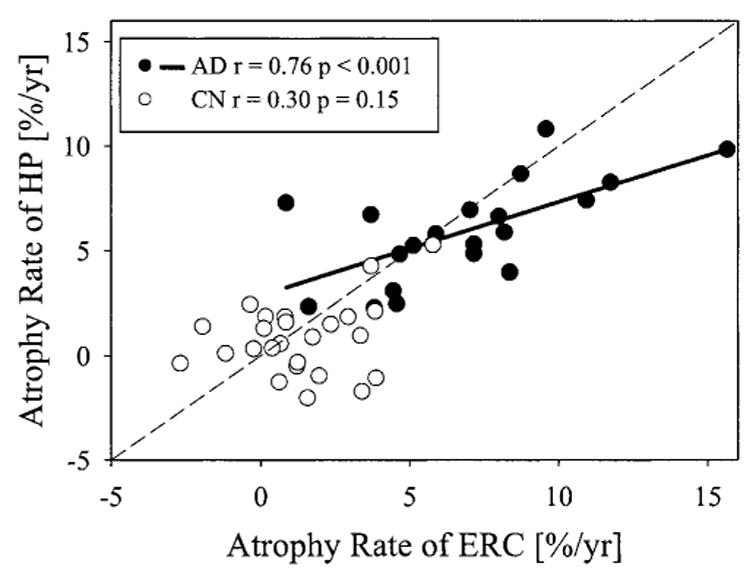
Relationship between atrophy rates of entorhinal cortex (ERC) and hippocampus (HP) in patients with Alzheimer disease (AD) and cognitively normal (CN) subjects. The dashed line is the line of identity. Subjects below the line of identity have higher atrophy rates in the ERC than in the hippocampus.
On average, hippocampi in AD were 27% smaller on the left (F1,43 = 19.7, p < 0.001) and 26% on the right (F1,43 = 25.1, p < 0.001) than in CN. As shown before,18 ERC in AD were 38% smaller on the left (F1,43 = 17.9, p < 0.001) and 40% on the right (F1,43 = 18.1, p < 0.001) than in CN. Side had no effect on the volume differences between the groups.
Next, we explored the relationship between memory deficits and atrophy rate measures. One patient with AD, who had problems with the DLR test because she was not a native English speaker, was excluded from this analysis. In AD, atrophy rates of ERC increased with decreasing DLR scores (r = −0.51, p < 0.05), as reported before.18 However, atrophy rates of hippocampus in these patients were not correlated with DLR scores (r = −0.20, p > 0.3). In contrast to DLR, MMSE scores were not significantly correlated with atrophy rates of ERC and hippocampus in AD. In CN subjects, no significant relations were found between DLR or MMSE scores and atrophy rates of ERC and hippocampus.
To further investigate the difference between ERC and hippocampal rates in their relationship to memory performance, especially with consideration of a potential floor effect of the memory test, we repeated the correlation analyses for patients with mild AD (11 out of 19, MMSE scores 24.6 ± 3.9) who scored better than zero on the DLR test. In this group of patients with mild AD, increased atrophy rates of hippocampus now correlated (r = −0.59, p < 0.05) with decreased DLR scores, in addition to a remaining correlation between atrophy rates of ERC and DLR scores (r = −0.61, p < 0.05), as depicted in figure 2. Correlations between atrophy rates of ERC or hippocampus and rates of DLR decline were not detected (both p > 0.05), independent of whether all patients with AD or only patients with DLR scores greater than zero (mildly demented) were included. MMSE scores, however, remained uncorrelated to atrophy rates of ERC (r = 0.11, p > 0.7) and hippocampus (r = 0.27, p > 0.4).
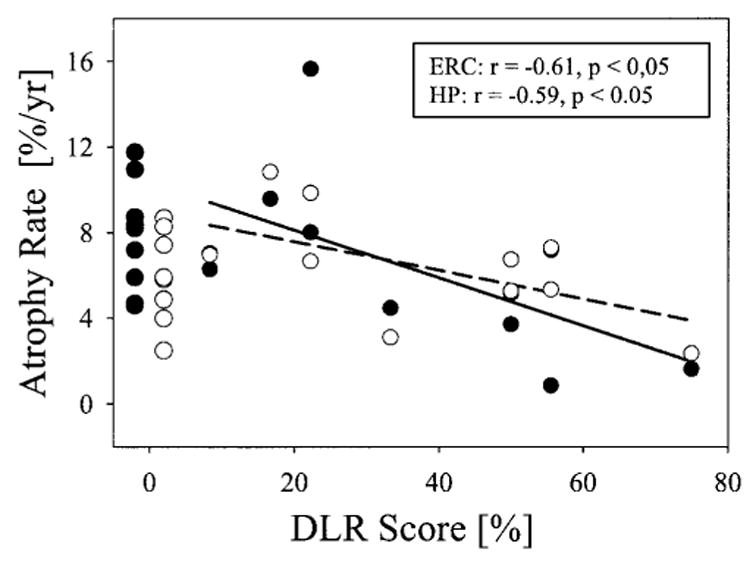
Atrophy rates of entorhinal cortex (ERC: ● and solid line) and hippocampus (HP: ● and dashed line) in patients with Alzheimer disease as a function of memory impairment, measured using the Delayed List Recall (DLR) test. DLR scores represent percentage of correctly remembered words, normalized to the maximum number of words on the list. To account for floor effects of DLR when patients were moderately to severely demented, only mildly impaired patients with DLR score greater than zero were included in regression analyses.
Next, we compared the statistical powers of atrophy rates and volume measurements of ERC and hippocampus at second MRI scans to differentiate between patients with AD and CN subjects. Results from a logistic regression analysis are listed in table 3 in terms of overall classification and sensitivity and specificity. In addition, ROC analyses were performed and the AUC from different MRI measures were compared. Using atrophy rates of ERC yielded an AUC of 0.96, which was larger (better differentiation) than (p < 0.01) an AUC of 0.88 using follow-up volumes of ERC. Similarly, using atrophy rates of hippocampus yielded an AUC of 0.97, which was larger (better differentiation) than (p < 0.01) the AUC of 0.91 using follow-up volumes of hippocampus. However, the difference between ROC curves using atrophy rates of ERC and hippocampus was not significant (p = 0.7). A combination of ERC and hippocampal atrophy rates did not further improve classification, as expected, because ERC and hippocampal atrophy rates were strongly correlated in AD.
Table 3
Classification between patients with Alzheimer disease and cognitively normal controls by logistic regression
| Classification | Volume* of ERC | Atrophy rate of ERC | Volume* of hippocampus | Atrophy rate of hippocampus |
|---|---|---|---|---|
| Overall correct classification, % | 82 | 91 | 82 | 87 |
| Sensitivity, % | 80 | 85 | 80 | 80 |
| Specificity, % | 84 | 96 | 84 | 92 |
ERC = entorhinal cortex.
Finally, we calculated the sample size that would be needed in a pharmacologic trial of AD (drug versus placebo) to detect a 20% treatment effect on ERC or hippocampal atrophy rates, assuming that a 100% treatment effect would completely inhibit atrophy. For 90% power and a 5% level of significance, 110 patients with AD per arm would be needed to detect a treatment effect with atrophy rates of ERC, whereas only 90 patients per arm would be needed using hippocampal atrophy rates. After accounting for a 10% failure rate in measuring hippocampus with automatic software, 100 patients per arm would be needed using atrophy rates of hippocampus. Even though ERC showed higher atrophy rates than hippocampus in AD, hippocampal rates had greater statistical power to detect treatment effects because the coefficient of variation of hippocampal rates was less than that of ERC rates. If it is assumed that 100% effective treatment of AD would slow the rate of atrophy to the rate of normal aging, rather than to zero rate of atrophy, the number of patients would need to be increased from 110 to 170 for ERC and from 100 to 133 for hippocampal measurements.
Discussion
The findings of this study were as follows: (1) atrophy rates of ERC were higher than of hippocampus in AD; (2) increased atrophy rates of both ERC and hippocampus were correlated with increased memory deficits in mild AD; (3) atrophy rates of ERC and hippocampus yielded comparable levels of differentiation between AD and CN; (4) hippocampus had greater statistical power to determine treatment effects than ERC in a hypothetical AD treatment trial.
The first finding was that patients with AD had higher atrophy rates of ERC than of hippocampus. Several MRI studies of AD, following patients longitudinally, reported increased atrophy rates for ERC,18,19 hippocampus,20–22 corpus callosum,30 and global brain.31–33 Furthermore, a pattern of regional atrophy rates was also found outside the limbic system in AD, as shown recently in a study using high-dimensional brain warping techniques.34 Together, these findings suggest that accelerated loss of brain tissue is a characteristic feature of AD, distinguishing this disorder from normal aging. By comparing atrophy rates within the subjects, we found that the atrophy rate of ERC was significantly higher than that of hippocampus in AD. Previous clinical studies demonstrated that the rates of cognitive decline accelerated with time in AD,24,25 suggesting accelerated neurodegeneration in AD. Similarly, one might expect that atrophy rates measured with MRI also accelerate. Assuming that degenerative processes proceed at similar rates in the ERC and hippocampus, one would therefore expect to find higher atrophy rates in the structure where neurodegeneration began earlier. Our results substantiate this hypothesis, which is consistent with the view of an earlier involvement of AD pathology in the ERC than the hippocampus.23 Another explanation for higher atrophy rates in the ERC than the hippocampus is that neurodegeneration proceeds at different rates, yet starting at the same time. Therefore, the current results are consistent with, but do not prove, that AD pathology begins in the ERC. More follow-up studies on subjects that have information about how many years they were into disease progression when the scans were done will be necessary to unambiguously determine if the ERC atrophies before the hippocampus. This study extended a previous study19 that found higher atrophy rates of ERC than hippocampus in familial AD to sporadic patients with AD who tend to be generally old. Because age was accounted for in our analysis, the difference between ERC and hippocampal rates cannot be explained by an accumulative effect of normal aging.
The second finding was that atrophy rates of both ERC and hippocampus in patients with mild AD (positive DLR scores) were positively correlated with memory deficits at baseline. This finding suggests that atrophy rates of ERC and hippocampus may be useful in monitoring AD progression. However, one has to be aware that patients with AD with DLR scores of zero were excluded to avoid complications of the DLR floor effect, and two tests had to be combined. In addition, we cannot explain the difference between ERC and hippocampal rates in correlations with DLR scores when all patients are included. Substantially more patients will be needed to contrast atrophy rates of ERC and hippocampus with memory decline. In contrast to DLR scores, there was no correlation between MMSE scores and atrophy rates of ERC and hippocampus. This is consistent with the view that MMSE score is an index of global cognitive functions and would not be expected to correlate highly with memory functions linked to ERC and hippocampus.
The third finding was that rate measurements of ERC or hippocampal atrophy were comparable in their ability to differentiate between patients with AD and CN subjects. However, measurements of atrophy rates of both ERC and hippocampus were substantially better than single time point volume measurements in differentiation between AD and CN. This may be explained by a large variability of brain substructure volumes between individuals, which should be reduced by calculating volume change over time due to self-correction. Limitations with cross-sectional measurements to differentiate between patients with AD and CN subjects have been reported before.6–10 Despite better discrimination with rate measurements, classification between patients with AD and CN subjects was not perfect because some of the CN subjects might have had prodromal AD, which may increase the rates of atrophy in these subjects, and some of the patients with a clinical diagnosis of AD (without autopsy confirmation) might have had other types of dementia, which may decrease rates of atrophy. It is also possible that there is substantial variability of rates of ERC or hippocampal atrophy in AD, which might overlap with normal controls. More neuropathologic studies are needed for these possibilities. Despite a higher atrophy rate of ERC than of hippocampus in AD, the discriminatory powers between patients with AD and CN subjects with both measures were comparable. This might be due to the greater variability of atrophy rates of ERC than that of hippocampus in AD, which offsets the advantages of higher atrophy rates of ERC.
The fourth finding was that in a hypothetical AD treatment trial, the hippocampus had greater statistical power in determining treatment effects than the ERC, presumably because variability of atrophy rates of ERC was higher than that of hippocampus. One possible explanation for a higher variability of atrophy rates for ERC over hippocampus is that ERC was measured by manual marking, in contrast to the semiautomated measurement of hippocampus. However, our test-retest studies of both methods showed similar reproducibility. Therefore, methodologic differences do not fully account for the higher variability of atrophy rates of ERC, and this increased variability of atrophy rates of ERC is likely due to higher biologic variability.
There were several limitations in this study. First, all subjects were followed up for only approximately 1.9 years and their diagnosis was not confirmed by neuropathology. Therefore, some of the CN subjects may have prodromal AD and some patients with AD may have other neuropathologies, which might skew the discriminatory powers of atrophy rates. Second, the relationships between DLR or MMSE scores and atrophy rate of ERC and hippocampus were tested using linear models. However, neuropsychological test scores of cognitive decline may not be linear throughout the course of AD.24,25 In addition, the decline of ERC and hippocampus was assumed to be linear, which may not be the case over interscan intervals of on average 1.9 years. Therefore, to the extent that volume decline increased with time, we may have overestimated atrophy rates. More patients with AD are needed to determine the patterns of the relationships between cognitive decline and atrophy rates of ERC and hippocampus in the whole range of AD. Third, different methods (manual versus semiautomated) were used to measure ERC and hippocampus in the same subjects. Although this could potentially introduce a systematic error, complicating comparisons between these two structures, this is unlikely to alter the results of this study because the validation of semiautomated hippocampal measurements showed a strong correlation with manual measurements (r = 0.92).26 Therefore, if manual tracing had been used for both ERC and hippocampus, a similar outcome would be expected.
Acknowledgments
The authors thank Diana Sacrey and Meera Krishnan for help with image processing.
References
Full text links
Read article at publisher's site: https://doi.org/10.1212/01.wnl.0000106462.72282.90
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc1820859?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1212/01.wnl.0000106462.72282.90
Article citations
Unveiling mitochondria as central components driving cognitive decline in alzheimer's disease through cross-transcriptomic analysis of hippocampus and entorhinal cortex microarray datasets.
Heliyon, 10(20):e39378, 15 Oct 2024
Cited by: 0 articles | PMID: 39498000 | PMCID: PMC11534180
Comparison of Quantitative Hippocampal Volumes and Structured Scoring Scales in Predicting Alzheimer Disease Diagnosis.
AJNR Am J Neuroradiol, 44(12):1411-1417, 11 Dec 2023
Cited by: 0 articles | PMID: 38050003
Functional MRI-specific alterations in frontoparietal network in mild cognitive impairment: an ALE meta-analysis.
Front Aging Neurosci, 15:1165908, 28 Jun 2023
Cited by: 1 article | PMID: 37448688 | PMCID: PMC10336325
Review Free full text in Europe PMC
Heart failure and cognitive impairment: A narrative review of neuroimaging mechanism from the perspective of brain MRI.
Front Neurosci, 17:1148400, 27 Mar 2023
Cited by: 2 articles | PMID: 37051150 | PMCID: PMC10083289
Review Free full text in Europe PMC
Study on the Role of an Erythrocyte Membrane-Coated Nanotheranostic System in Targeted Immune Regulation of Alzheimer's Disease.
Adv Sci (Weinh), 10(18):e2301361, 19 Apr 2023
Cited by: 6 articles | PMID: 37075744 | PMCID: PMC10288270
Go to all (122) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Atrophy rates of entorhinal cortex in AD and normal aging.
Neurology, 60(3):481-486, 01 Feb 2003
Cited by: 115 articles | PMID: 12578931 | PMCID: PMC1851672
Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease.
J Neurol Neurosurg Psychiatry, 71(4):441-447, 01 Oct 2001
Cited by: 379 articles | PMID: 11561025 | PMCID: PMC1763497
Effects of subcortical ischemic vascular dementia and AD on entorhinal cortex and hippocampus.
Neurology, 58(11):1635-1641, 01 Jun 2002
Cited by: 78 articles | PMID: 12058091 | PMCID: PMC1820858
Neuroimaging of hippocampal atrophy in early recognition of Alzheimer's disease--a critical appraisal after two decades of research.
Psychiatry Res Neuroimaging, 247:71-78, 01 Jan 2016
Cited by: 41 articles | PMID: 26774855
Review
Funding
Funders who supported this work.
NIA NIH HHS (4)
Grant ID: R01 AG010897
Grant ID: R01 AG10897
Grant ID: P01 AG012435
Grant ID: P01 AG12435





