Abstract
Free full text

Pax5 induces V-to-DJ rearrangements and locus contraction of the immunoglobulin heavy-chain gene
Abstract
The subnuclear location and chromatin state of the immunoglobulin heavy-chain (IgH) locus have been implicated in the control of V(D)J recombination. VH-to-DJH rearrangement of distal, but not proximal VH genes, furthermore, depends on the B-lineage commitment factor Pax5 (BSAP). Here we demonstrate that ectopic Pax5 expression from the Ikaros promoter induces proximal rather than distal VH–DJH rearrangements in IkPax5/+ thymocytes, thus recapitulating the loss-of-function phenotype of Pax5–/– pro-B cells. The phenotypic similarities of both cell types include (1) chromatin accessibility of distal VH genes in the absence of VH–DJH rearrangements, (2) expression of the B-cell-specific regulator EBF, (3) central location of IgH alleles within the nucleus, and (4) physical separation of distal VH genes from proximal segments in an extended IgH locus. Reconstitution of Pax5 expression in Pax5–/– pro-B cells induced large-scale contraction and distal VH–DJH rearrangements of the IgH locus. Hence, VH–DJH recombination is regulated in two steps during early B-lymphopoiesis. The IgH locus is first repositioned from its default location at the nuclear periphery toward the center of the nucleus, which facilitates proximal VH–DJH recombination. Pax5 subsequently activates locus contraction and distal VH–DJH rearrangements in collaboration with an unknown factor that is present in pro-B cells, but absent in thymocytes.
V(D)J recombination is of fundamental importance for the generation of diverse antigen receptor repertoires, as this process assembles the variable regions of immunoglobulin (Ig) and T-cell receptor (TCR) genes from discontinuous variable (V), diversity (D), and joining (J) gene segments during B- and T-cell development (Tonegawa 1983; Hesslein and Schatz 2001). All of these gene segments are flanked by conserved recombination signal sequences (RSSs) that constitute recognition sites for the V(D)J recombinase proteins RAG1 and RAG2 (Hesslein and Schatz 2001; Bassing et al. 2002). Upon binding and synapsis of two compatible RSS sites, the RAG1/2 complex introduces double-strand DNA breaks between the RSSs and flanking gene segments. Subsequently, the RAG proteins and repair factors of the nonhomologous end-joining machinery complete the recombination reaction by processing and religating the DNA ends (Hesslein and Schatz 2001; Bassing et al. 2002).
V(D)J recombination takes place only in lymphocytes, where it is tightly controlled in a lineage- and stage-specific manner. Within the B-lymphoid lineage, the immunoglobulin heavy-chain (IgH) locus is rearranged in pro-B cells prior to recombination of the Igκ and Igλ light-chain genes in pre-B cells, whereas the TCRβ and TCRα genes are rearranged in pro-T and pre-T cells, respectively (Hesslein and Schatz 2001; Bassing et al. 2002). As the RAG1 and RAG2 genes are expressed in all lymphoid progenitors (Igarashi et al. 2002) and immature T cells and B cells, V(D)J recombination is primarily regulated by limiting the accessibility of RSS sites within chromatin (Yancopoulos and Alt 1985; Stanhope-Baker et al. 1996; Krangel 2003). As a consequence, only the sites of particular gene segments are available for RAG1/2-mediated synapsis and DNA cleavage in different cell types and developmental stages.
V(D)J recombination of the IgH gene occurs in a defined temporal order with DH–JH rearrangements preceding VH–DJH recombination. The earliest lymphocyte progenitor (ELP; Igarashi et al. 2002) and later common lymphoid progenitor (CLP; Allman et al. 2003) already initiate DH–JH rearrangements, which are completed during development to the early pro-B-cell stage (fraction B; Li et al. 1993). As the ELP and CLP also give rise to T, NK, and dendritic cells (Kondo et al. 1997; Traver et al. 2000; Igarashi et al. 2002), it may not be surprising that these cell types carry DH–JH-rearranged IgH alleles at low frequency (Kurosawa et al. 1981; Born et al. 1988; Corcoran et al. 2003). Importantly, VH–DJH rearrangements could never be observed in thymocytes and dendritic cells (Kurosawa et al. 1981; Corcoran et al. 2003), as the second IgH rearrangement step takes place only in committed pro-B cells (fractions B and C; Li et al. 1993). Successful VH–DJH recombination leads to expression of the Igμ protein as part of the pre-B-cell receptor (pre-BCR), which acts as an important checkpoint to control the transition from the pro-B- to the pre-B-cell stage (Burrows et al. 2002).
The IgH locus with its 150–200 VH genes spans a large chromosomal region of ~3 Mb pairs (Chevillard et al. 2002), which is likely to be an impediment for efficient synapse formation and V(D)J recombination of distantly separated IgH gene segments. A recent fluorescent in situ hybridization (FISH) analysis revealed that V(D)J recombination correlates with changes in the subnuclear location and chromatin state of the IgH locus (Kosak et al. 2002). Both IgH alleles are present in an extended chromatin configuration at the nuclear periphery of non-B-lymphoid cells, whereas they are relocated to central positions of the nucleus and undergo large-scale contraction in committed pro-B cells (Kosak et al. 2002). Subnuclear compartmentalization was thus proposed as a novel mechanism for regulating IgH transcription and recombination during B-cell development (Kosak et al. 2002), particularly because the nuclear periphery in higher eukaryotes may function as a repressive compartment for transcriptional silencing (Baxter et al. 2002) in analogy to yeast (Hediger and Gasser 2002).
Differentiation of the CLP to committed pro-B cells critically depends on the three transcription factors E2A, EBF, and Pax5 (BSAP; Schebesta et al. 2002a). All three factors are also essential for V(D)J recombination of the IgH gene during early B-cell development. The absence of E2A or EBF arrests B-cell development at the stage of B220+ CD43+ progenitor cells (fraction A) that contain the IgH locus still in germ-line configuration (Bain et al. 1994; Lin and Grosschedl 1995). Both transcription factors appear to control the initial DH–JH rearrangement step by activating the expression of RAG1 and RAG2 (Bain et al. 1994; Lin and Grosschedl 1995; O'Riordan and Grosschedl 1999) and by promoting the accessibility of the DH–JH region to the V(D)J recombinase (Romanow et al. 2000). In the absence of Pax5, B-lymphopoiesis proceeds in the bone marrow to early pro-B cells (fraction B; Urbánek et al. 1994; Nutt et al. 1997), which still retain a broad lympho-myeloid developmental potential characteristic of uncommitted progenitors (Nutt et al. 1999; Rolink et al. 1999). Restoration of Pax5 expression in Pax5–/– pro-B cells suppresses this multilineage potential and rescues development to mature B cells, thus identifying Pax5 as the critical B-lineage commitment factor that restricts the developmental options of lymphoid progenitors to the B-cell pathway (Nutt et al. 1999). In addition, Pax5 controls the second, B-cell-restricted step of VH–DJH recombination, as rearrangements of the distal (5′) VHJ558 genes are ~50-fold reduced in Pax5–/– pro-B cells, although DH–JH recombination occurs at normal frequency (Nutt et al. 1997). Despite this defect, the distal VH genes are present in accessible chromatin, as indicated by their germ-line transcription and histone H3 acetylation in Pax5–/– pro-B cells (Hesslein et al. 2003). Interestingly, the VH–DJH recombination efficiency of Pax5–/– pro-B cells progressively increases with decreasing distance of the VH gene to the proximal DH–JH region (Hesslein et al. 2003). Hence, Pax5 is essential for the rearrangement of distal but not proximal VH genes.
Pax5 is expressed within the hematopoietic system exclusively from the pro-B- to the mature B-cell stage, consistent with its role as B-lineage commitment factor (Adams et al. 1992). We have recently complemented our Pax5 loss-of-function analyses with a gain-of-function approach by expressing a human Pax5 minigene under the control of the endogenous Ikaros (Ik) locus in all blood cell types (Souabni et al. 2002). Pan-hematopoietic expression of the IkPax5 allele efficiently promotes B-cell development at the expense of T-lymphopoiesis. Whereas ectopic Pax5 expression completely blocks T-cell development in chimeric mice reconstituted with wild-type and IkPax5/+ bone marrow, thymocyte differentiation is reduced and abnormal under noncompetitive conditions in IkPax5/+ mice (Souabni et al. 2002). At the molecular level, Pax5 down-regulates transcription of the T-cell specification gene Notch1 and activates expression of the B-lymphoid gene CD19 in thymocytes (Souabni et al. 2002).
By analyzing the rearrangement status of the IgH locus in IkPax5/+ thymocytes, we now demonstrate that ectopic Pax5 expression is sufficient to increase DH–JH recombination and to initiate VH–DJH rearrangements in immature cells of the T-lymphoid lineage. Unexpectedly, the VH–DJH recombination phenotype of Pax5-expressing thymocytes resembles that of Pax5-deficient pro-B cells. The distal VHJ558 and VH3609 genes are rearranged with very low efficiency in contrast to the proximal VHQ52 and VH7183 genes, although germ-line transcripts of the distal VHJ558 genes could be readily detected in IkPax5/+ pro-T cells as in Pax5–/– pro-B cells. Importantly, the expression of the B-cell-specific transcription factor EBF was induced to the same level in IkPax5/+ pro-T cells as in Pax5–/– pro-B cells. FISH analysis of three-dimensionally preserved nuclei further extended the phenotypic similarity between IkPax5/+ pro-T and Pax5–/– pro-B cells. The IgH alleles were centrally located in the nuclei of both cell types and were present in an extended chromatin state that physically separates the distal from the proximal VH genes. In contrast, the IgH loci were localized at the nuclear periphery in wild-type pro-T cells. In Pax5-expressing pro-B cells, the IgH loci were found in central nuclear positions and underwent large-scale contraction, as published (Kosak et al. 2002). Moreover, Pax5 induced IgH locus contraction and distal VH–DJH rearrangements in retrovirally reconstituted Pax5–/– pro-B cells. Based on these data, we propose a two-step model for the transcriptional activation of VH–DJH recombination in early B-cell development. The IgH locus is first relocated, possibly under the control of EBF, from the periphery to the center of the nucleus, thus facilitating VH–DJH recombination within the proximal domain of the IgH locus. Subsequently, Pax5 activates large-scale contraction and distal VH–DJH rearrangements of the IgH locus in collaboration with an unknown factor that is present in pro-B cells, but absent in thymocytes.
Results
Pax5 induces DH–JH and VH–DJH rearrangements atthe IgH locus in IkPax5/+ thymocytes
To investigate whether ectopic Pax5 expression is able to induce IgH rearrangements in T cells, we isolated CD4– CD8– double-negative (DN) pro-T cells and CD4+ CD8+ double-positive (DP) pre-T cells from the thymus of 2-week-old IkPax5/+ and control Ikneo/+ mice (Souabni et al. 2002) by FACS sorting after depletion of B220+ B-lymphocytes. As positive control, we sorted c-Kit+ B220+ pro-B cells from the bone marrow of wild-type and Pax5–/– mice (Urbánek et al. 1994). DNA was isolated from the different cell types and normalized by PCR amplification of a DNA fragment from the IgH Cμ region prior to quantitative PCR analysis of DH–JH and VH–DJH rearrangements (Fig. 1B). No VH–DJH rearrangements and only a low level of DH–JH rearrangements were detected in control Ikneo/+ thymocytes (Fig. 1B), as previously described for wild-type T cells (Kurosawa et al. 1981; Born et al. 1988). The frequency of DH–JH recombination was increased fivefold in IkPax5/+ thymocytes, reaching half the level observed in wild-type and Pax5–/– pro-B cells (Fig. 1B,C). Hence, ectopic Pax5 expression promotes further DH–JH recombination in thymocytes. More importantly, IkPax5/+ thymocytes carried VH–DJH rearrangements (Fig. 1B), which are normally restricted to the B-lymphoid lineage (Kurosawa et al. 1981). The proximal VH7183 and VHQ52 genes (Fig. 1A) were rearranged in IkPax5/+ pre-T cells as efficiently as in wild-type pro-B cells, whereas a slightly lower level of proximal VH7183 and VHQ52 gene rearrangements was detected in IkPax5/+ pro-T cells (Fig. 1B). Unexpectedly however, the recombination frequency of the more distal VHGam3.8, VH3609, and VHJ558 genes (Fig. 1A) was reduced 30- to 100-fold in IkPax5/+ pro-T and pre-T cells in contrast to wild-type pro-B cells (Fig. 1B). A similar position-dependent decrease of VH gene recombination was observed in Pax5–/– pro-B cells (Fig. 1B), in agreement with recently published data (Hesslein et al. 2003). Hence, ectopic expression of Pax5 leads to the same VH–DJH recombination phenotype in thymocytes as does the loss of Pax5 in pro-B cells. In summary, these data indicate that Pax5 is sufficient to induce V(D)J recombination within the proximal IgH domain in thymocytes. However, efficient rearrangement of the distal VH genes appears to require the cooperation of Pax5 with an un-identified second factor that is absent in Pax5-overexpressing T cells.

Pax5 induces DH–JH and VH–DJH rearrangements of the IgH locus in thymocytes. (A) Schematic diagram of the VH gene cluster of the IgH locus. Only the VH gene families analyzed are shown together with their transcriptional direction (arrow) and distal or proximal position within the VH gene cluster. (B) PCR detection of DH–JH and different VH–DJH rearrangements in IkPax5/+ and Ikneo/+ thymocytes (pro-T [DN] and pre-T [DP] cells) as well as in bone marrow Pax5+/+ and Pax5–/– pro-B cells, which were directly sorted from 2-week-old mice. Three-fold serial DNA dilutions were analyzed by PCR. Input DNA was normalized by amplification of a PCR fragment from the IgH Cμ regions, and DNA of the stromal ST2 cells was used as negative control. Numbers to the left indicate rearrangements to the JH1, JH2, and JH3 segments. (C) Quantitation of DH–JH recombination in sorted pro-B and pre-T cells. DH–JH rearrangements were analyzed by PCR in three independent preparations of sorted cells of the indicated genotypes. The average recombination frequency with its standard deviation is shown as relative percentage of the rearrangements detected in wild-type (wt) pro-B cells.
Surface Igμ expression in the absence of Igκ rearrangements in IkPax5/+ thymocytes
Sequence analysis of the VHDJH2 PCR fragments revealed that V(D)J recombination in IkPax5/+ pre-T cells involved different members of the VH7183 and VHQ52 gene families, although with a strong bias for more proximally located genes within each family (data not shown). Moreover, 13% of the sequenced PCR fragments contained a functional in-frame VH–DJH rearrangement (data not shown). This finding was confirmed by flow cytometric analysis demonstrating that functionally rearranged Igμ chains were expressed not only in the cytoplasm, but also on the cell surface of Thy1.2+ IkPax5/+ thymocytes at the expected frequency (Fig. 2A). However, no Vκ–Jκ rearrangements could be detected in IkPax5/+ pre-T cells, demonstrating that Pax5 is unable to induce rearrangements at the Igκ light-chain locus (Fig. 2B). As a consequence, the productively rearranged Igμ protein is likely to be expressed as part of the pre-BCR on the cell surface of IkPax5/+ thymocytes, which is consistent with expression data shown below. Most IkPax5/+ thymocytes expressed TCRβ on the cell surface (Fig. 2A), in agreement with the observation that Dβ2–Jβ2 and Vβ5.1–DJβ2 rearrangements were present at similar frequency in IkPax5/+ and control Ikneo/+ pre-T cells (Fig. 2B). These data further demonstrate that the IkPax5/+ thymocytes are of T-lymphoid origin despite ectopic expression of the B-lineage commitment factor Pax5 (Souabni et al. 2002).
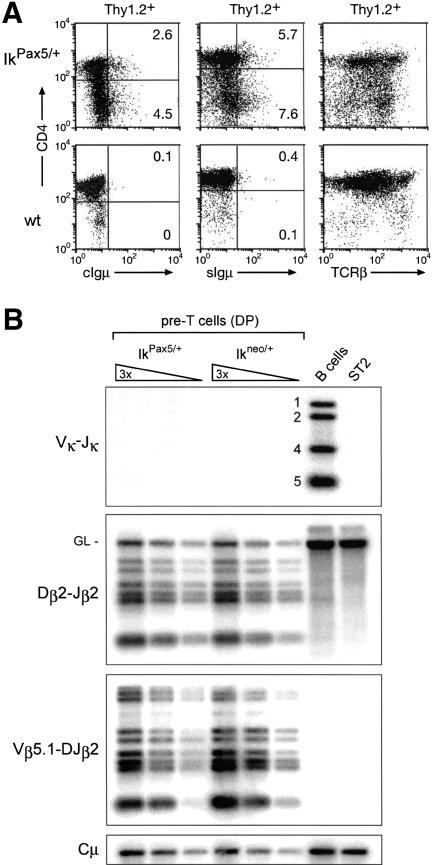
Surface Igμ expression in the absence of Igκ rearrangements in IkPax5/+ thymocytes. (A) Flow cytometric analysis. Cytoplasmic (c) and cell surface (s) expression of functionally rearranged Igμ protein and cell surface TCRβ expression were analyzed in wild-type (wt) and IkPax5/+ thymocytes (gated as Thy1.2+ cells). The percentage of Igμ+ cells is indicated. (B) Normal TCRβ recombination and absence of VK–JK rearrangements in IkPax5/+ thymocytes. The same sorted IkPax5/+ and Ikneo/+ pre-T cells, which were used for the experiment shown in Figure 1, were analyzed by PCR for VK–JK rearrangements at the Igκ locus and for Dβ2–Jβ2 and Vβ5.1–DJβ2 rearrangements at the TCRβ locus. Splenic B220+ B cells and stromal ST2 cells served as positive and negative controls, respectively. The numbers next to the B-cell lane indicate rearrangements to the JK1, JK2, JK4, and JK5 segments. GL denotes the position of the germ-line PCR product.
Conditional Pax5 activation induces VH–DJH rearrangements within the T-lymphoid lineage
The expression of RAG1 and RAG2 is initiated in the earliest lymphocyte progenitor (ELP), resulting in subsequent DH–JH recombination (Igarashi et al. 2002). Hence, the V(D)J rearrangement machinery is already active in lymphoid progenitors of the bone marrow prior to B- and T-lineage commitment. On the other hand, expression of the IkPax5 allele is initiated in the hematopoietic stem cell and is maintained in the progenitors and differentiating cells of all major hematopoietic lineages (Souabni et al. 2002). It is therefore conceivable that the Pax5-expressing lymphoid progenitors of IkPax5/+ mice may undergo VH–DJH rearrangements before migration to the thymus and initiation of T-cell development. Alternatively, the VH–DJH rearrangements present in IkPax5/+ thymocytes may originate within the T-lymphoid lineage. To distinguish between these two possibilities, we took advantage of the conditional Ikneo allele, which contains a floxed neomycin stop cassette upstream of the Pax5 minigene in the Ikaros locus (Souabni et al. 2002). Pax5 expression from this allele is only activated upon Cre recombinase-mediated deletion of the neomycin gene (Souabni et al. 2002). To this end, we crossed the Ikneo/+ mouse with a transgenic mouse, which expressed the Cre recombinase under the control of the proximal lck promoter (lck-cre) during pro-T-cell development (Lee et al. 2001; Wolfer et al. 2002). The lck-cre transgene was shown to initiate Cre-mediated inactivation of a floxed Notch1 allele in CD44+ CD25+ (DN2) pro-T cells and to complete gene deletion in CD44– CD25+ (DN3) pro-T cells (Wolfer et al. 2002). However, the same lckcre transgene deleted the floxed neomycin gene of the Ikneo allele with lower efficiency, as the activated IkPax5 allele was detected in only ~60% of pre-T cells (Fig. 3). Nevertheless, these thymocytes of Ikneo/+ lck-cre mice were characterized by an increase in DH–JH rearrangements and the presence of VH–DJH-rearranged IgH alleles (Fig. 3). The VH–DJH rearrangement frequency was highest for the proximal VH7183 genes and lowest for the distal VHJ558 gene family (Fig. 3), thus recapitulating the V(D)J recombination phenotype of IkPax5/+ thymocytes (Fig. 1B). The rearrangement frequency was, however, lower in Ikneo/+ lck-cre pre-T cells, possibly because of the inefficient activation of the Ikneo allele during T-cell development. Together these data unequivocally demonstrate that Pax5 is able to induce VH–DJH rearrangements within the T-lymphoid lineage.
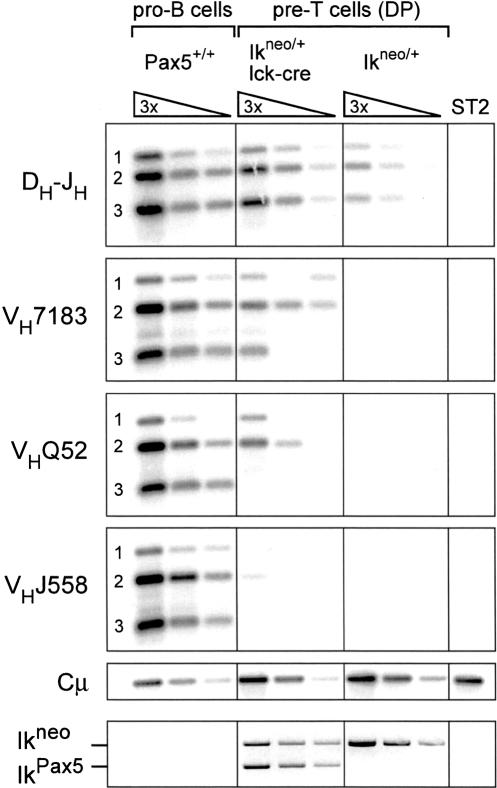
VH–DJH rearrangements upon conditional Pax5 activation in thymocytes. Pre-T cells were isolated by FACS sorting as DP thymocytes from Ikneo/+ mice carrying the lck-cre transgene followed by PCR quantification of DH–JH and VH–DJH rearrangements as described in Figure 1B. Ikneo/+ pre-T cells and stromal ST2 cells served as negative controls and Pax5+/+ pro-B cells as positive control for the detection of VH–DJH rearrangements. Cre-mediated conversion of the Ikneo to the IkPax5 allele was determined by PCR (bottom row).
Pax5 activates germ-line VH transcription and multiple B-lymphoid genes in pro-T cells
To study the extent of B-cell-specific gene activation by Pax5 in thymocytes, we purified pro-T cells from IkPax5/+ mice by depleting Lin+ cells (including B220+ B cells), followed by sorting for Thy1.2+ Lin– DN thymocytes. Flow cytometric reanalysis demonstrated that the sorted Thy1.2+ Lin– pro-T cells were purified to homogeneity (Fig. 4A). Moreover, RT–PCR analysis failed to detect rearranged Igκ and Igλ1 mRNAs in these sorted cells (Fig. 4B). We conclude, therefore, that the sorted IkPax5/+ pro-T cells were free of contaminating B cells. In addition, we sorted wild-type pro-T and pro-B cells as well as Pax5–/– pro-B cells. cDNA was prepared from all four cell types and normalized for equal expression of the control HPRT gene prior to semiquantitative RT–PCR analysis of B-cell-specific transcripts (Fig. 4C).
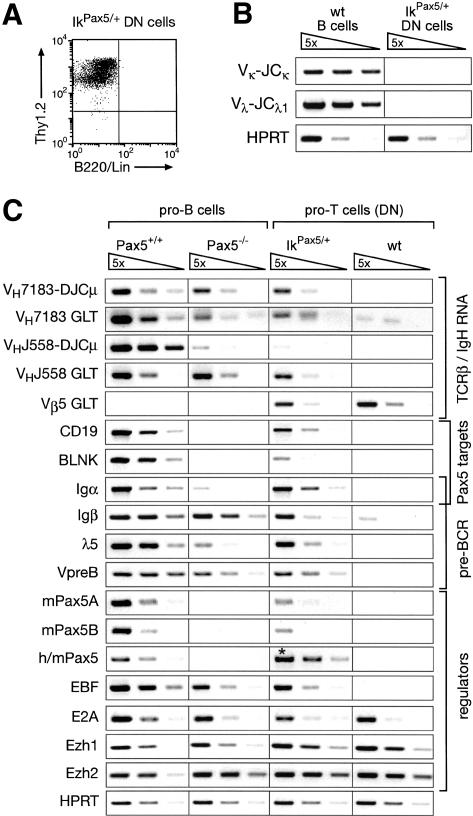
Activation of germ-line VH transcription and B-cell-specific gene expression in IkPax5/+ pro-T cells. (A) Flow cytometric reanalysis of sorted IkPax5/+ DN thymocytes. DN pro-T cells of 2-week-old mice were sorted as Thy1.2+ Lin– cells after depletion of Lin+ cells, which were stained with antibodies recognizing B220, CD4, CD8, DX5, CD11c, Mac-1, Gr-1, and Ter119. (B) Absence of B cells in the sorted IkPax5/+ pro-T cell population. Rearranged Igκ (VK–JCK) and Igλ1 (Vλ–JCλ1) mRNAs could not be detected by RT–PCR in purified IkPax5/+ pro-T cells in contrast to IgM+ IgD+ B cells isolated from wild-type (wt) spleen. The hypoxanthine phosphoribosyltransferase (HPRT) gene was equally expressed in both cell types. (C) B-cell-specific gene expression in IkPax5/+ pro-T cells. Transcripts of the indicated genes were analyzed by semiquantitative RT–PCR of five-fold serial dilutions of cDNA that was prepared from ex vivo sorted pro-B cells and pro-T cells of the indicated genotypes. The cDNA input was normalized according to the expression of the control HPRT gene. VHJ558-DJCμ and VH7183–DJCμ refer to rearranged Igμ mRNAs and GLT to the corresponding germline transcripts. Transgenic human (h) and endogenous mouse (m) Pax5 transcripts were amplified with conserved primers, giving rise to the same PCR fragment (denoted by an asterisk) for both transcripts.
The accessibility of a particular VH gene in active chromatin can be monitored by expression of its germline transcript (GLT), whereas the abundance of spliced Igμ (VH–DJCμ) mRNA is a direct measure of the VH–DJH recombination frequency in progenitor cells (Yancopoulos and Alt 1985). The germ-line transcripts of the proximal VH7183 gene family were expressed at a fivefold lower level in Pax5–/– pro-B cells and IkPax5/+ pro-T cells compared with wild-type pro-B cells. The rearranged mRNAs of the VH7183 genes were, however, present at similar abundance in all three cell types (Fig. 4C), consistent with efficient VH7183–DJH recombination in these cells (Fig. 1B; Hesslein et al. 2003). In contrast, the rearranged mRNA of the distal VHJ558 gene family was reduced ~100-fold in IkPax5/+ pro-T cells and Pax5–/– pro-B cells (Fig. 4C), which undergo VHJ558–DJH rearrangements with a similarly low efficiency compared with wild-type pro-B cells (Fig. 1B; Nutt et al. 1997). Germ-line VHJ558 transcripts were, however, present at a similar abundance in IkPax5/+ pro-T cells relative to wild-type and Pax5–/– pro-B cells (Fig. 4C). Germ-line transcripts of the TCR Vβ5 gene could not be detected in pro-B cells, but were expressed in IkPax5/+ pro-T cells, further confirming the T-lymphoid origin of these cells (Fig. 4C). These data indicate, therefore, that ectopic expression of Pax5 establishes an accessible chromatin state at both the proximal and distal VH genes in IkPax5/+ pro-T cells, although only the proximal VH genes undergo efficient VH–DJH recombination.
Ectopic Pax5 expression activated the Pax5 target genes CD19 (Nutt et al. 1998), BLNK (Schebesta et al. 2002b), and Igα (mb-1; Nutt et al. 1998) in IkPax5/+ pro-T cells (Fig. 4C). More surprisingly, these pro-T cells also expressed the genes Igβ (B29), λ5, and VpreB (Fig. 4C), which are known to be cooperatively regulated by the transcription factors EBF and E2A (Sigvardsson et al. 1997, 2002; O'Riordan and Grosschedl 1999). Consistent with this finding, Pax5 induced expression of the B-cell-specific EBF gene in IkPax5/+ pro-T cells to a level that is normally seen in wild-type and Pax5–/– pro-B cells. In contrast, the expression of E2A was similar and independent of Pax5 in pro-T cells as in pro-B cells (Fig. 4C). None of the B-cell-specific transcripts analyzed could be detected in wild-type pro-T cells except for low-level expression of the Igβ gene (Wang et al. 1998). Moreover, the efficient expression of all pre-BCR components (λ5, VpreB, Igα, and Igβ) strongly suggests that the rearranged Igμ protein is transported as part of the pre-BCR to the cell surface of IkPax5/+ thymocytes (Fig. 2A).
The Pax5 gene gives rise to two distinct mRNAs by alternative promoter usage and splicing of the different 5′-exons onto common coding sequences (Busslinger et al. 1996). The presence of the mouse Pax5A and Pax5B transcripts in IkPax5/+ pro-T cells demonstrated that the ectopically expressed human Pax5 protein is able to induce transcription of the endogenous Pax5 gene in thymocytes (Fig. 4C). This Pax5 activation is likely to be an indirect effect of EBF expression, which is known to regulate the Pax5 gene in pro-B cells (O'Riordan and Grosschedl 1999). PCR conditions, which detect human and mouse Pax5 mRNAs with equal efficiency, revealed that the human Pax5 minigene of the IkPax5 allele is expressed at a fivefold higher level in pro-T cells compared with the endogenous Pax5 gene in wild-type pro-B cells (Fig. 4C). In conclusion, moderate overexpression of Pax5 activates its own gene as well as many other B-cell-specific genes in IkPax5/+ pro-T cells.
Relocation of the IgH locus from the nuclear periphery to central positions in IkPax5/+ thymocytes
A recently published FISH analysis indicated that the subnuclear location and chromatin state of the IgH locus may regulate V(D)J recombination in non-B versus pro-B cells (Kosak et al. 2002). In particular, the 3-Mb-long IgH locus with its 150–200 VH genes was shown to be present in an extended chromatin state at the nuclear periphery of thymocytes (Kosak et al. 2002). To further investigate the discrepancy between distal and proximal VH gene recombination in IkPax5/+ thymocytes, we next used three-color 3D FISH analysis (Skok et al. 2001) to localize different IgH gene segments in three-dimensionally (3D) preserved nuclei by confocal laser scanning microscopy (Fig. 5). The locations of the different VH and Cγ1 gene probes used are shown in Figure 5A together with the minimal number of base pairs separating these probes in the IgH locus.
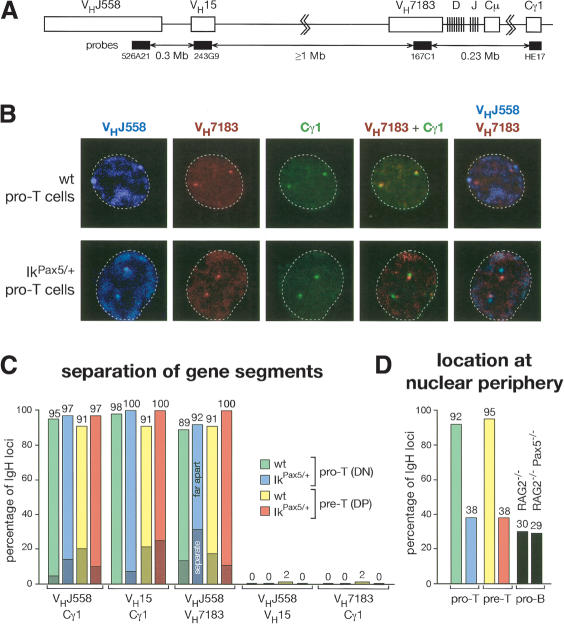
Subnuclear compartmentalization and extended chromatin state of VH genes in thymocyte nuclei. (A) Schematic diagram indicating the positions of the DNA probes in the IgH locus (not drawn to scale). The minimal number of megabase pairs (Mb) separating the probes was estimated according to the mouse genome database (Ensembl release of July 1, 2003). (B) Three-color 3D DNA-FISH analysis of the IgH locus. Confocal laser scanning microscopy identified the subnuclear locations of different gene segments of the two IgH alleles, which are shown on representative single optical sections through the nucleus of sorted wild-type (wt) and IkPax5/+ pro-T (DN) cells. The different probes with their colors are indicated. The VHJ558 BAC (526A21) was directly labeled with Cy5-dUTP (blue). The VH7183 (167C1) BACs as well as VH15 (243G9) BAC (not shown in panel B) were labeled with digoxygenin-dUTP and detected with rhodamine- and Texas red-coupled anti-digoxygenin antibodies (red). The Cγ1 probe (HE17) was labeled with biotin-dUTP and detected with FITC-avidin and biotinylated FITC-coupled anti-avidin antibodies (green). The IRES-GFP sequences, which were flanked by frt sites in the IkPax5 allele (Souabni et al. 2002), were specifically deleted for the three-color FISH analysis by mating IkPax5/+ mice with the transgenic ACTB:FLPe deleter line (Rodriguez et al. 2000). The contour of the cell is indicated by a broken line. (C) Statistical analysis of the distance between proximal and distal VH genes in thymocyte nuclei of the indicated genotypes. Signals of the different IgH gene probes, which were separated in the nucleus by 0.3–0.5 μm(separate; dark color) or 0.5–1.5 μm(far apart; light color), were scored as percentage of all signals analyzed. The actual numbers and sample sizes are shown in Supplementary Table 1A. (D) Statistical analysis of the peripheral location of distal VH genes in thymocytes and pro-B cells of the indicated genotypes.
As illustrated by the confocal images of Figure 5B, the distal VHJ558 and VH15 genes were colocalized at the nuclear periphery in 92%–95% of all wild-type pro-T and pre-T cells (Fig. 5D), in agreement with published data (Kosak et al. 2002). The signals of the distal VH genes were separated in only 2% of all wild-type thymocytes (Fig. 5C). In marked contrast, the distal VH genes were separated from the proximal VH7183 and Cγ1 genes by a distance of 0.3–1.5 μmin 89%–98% of the wild-type nuclei (Fig. 5C). In addition, the proximal IgH domain was positioned away from the nuclear periphery toward the center in 96% of wild-type pro-T and pre-T cells (Fig. 5B; data not shown). Hence, the IgH locus is anchored via the distal VH genes at the nuclear periphery and is oriented in its extended chromatin state toward the center, which may facilitate access of the V(D)J recombinase to the proximal IgH domain, thus accounting for the DH–JH rearrangements observed in wild-type thymocytes.
Ectopic Pax5 expression resulted in subnuclear repositioning of the IgH locus in pro-T and pre-T cells, as the majority (62%) of IgH loci were located at central positions in the nuclei of IkPax5/+ thymocytes (Fig. 5B,D). A similar percentage of centrally located IgH alleles was observed in RAG2–/– and Pax5–/– RAG2–/– pro-B cells (Figs. (Figs.5D,5D, ,6A),6A), indicating that the relocation of the IgH locus is as efficient in IkPax5/+ thymocytes as in pro-B cells. Importantly, the IgH locus remained in an extended chromatin state even in its more central location, as the distal VHJ558 and VH15 genes were still separated from the proximal VH7183 and Cγ1 genes in 92%–100% of all IkPax5/+ pro-T and pre-T cells (Fig. 5C). The physical separation of distal VH genes from the rearranged DJH segment is therefore a likely cause for the low VH–DJH recombination efficiency of distal VH genes in IkPax5/+ thymocytes.
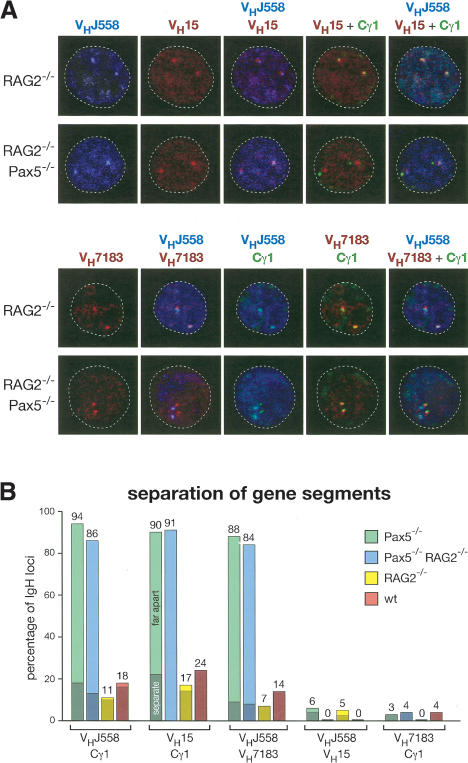
Absence of IgH locus contraction in the nucleus of Pax5–/– pro-B cells. (A) Three-color 3D DNA-FISH analysis of the IgH locus. In vitro cultured RAG2–/– and Pax5–/– RAG2–/– pro-B cells were analyzed by FISH and confocal microscopy as described in the legend for Figure 5B. (B) Statistical analysis of the distance between proximal and distal VH genes in pro-B cells of the indicated genotypes. A distance of 0.3–0.5 μm(separate) or 0.5–1.5 μm(far apart) between the signals is indicated by dark or light color, respectively. See Supplementary Table 1B for further information.
The IgH locus is present in an extended chromatin state in Pax5–/– pro-B cells
As the Pax5–/– pro-B cells and IkPax5/+ thymocytes exhibit a similar VH–DJH recombination phenotype, we next studied the nuclear location and chromatin state of IgH loci in wild-type and Pax5–/– pro-B cells. The IgH alleles were centrally located in ~70% of the pro-B cell nuclei regardless of the presence or absence of Pax5 (Figs. (Figs.5D,5D, ,6A).6A). Interestingly, the distal VHJ558 and VH15 genes were separated from the proximal VH7183 and Cγ1 genes in 84%–94% of all Pax5–/– pro-B cells (Fig. 6A,B), similar to the situation observed in IkPax5/+ pro-T cells (Fig. 5B,C). In contrast, the distal and proximal IgH gene segments were colocalized in 76%–86% of wild-type pro-B cells, whereas they were separated by only a short distance (0.3–0.5 μm) in the remaining 14%–24% of pro-B cells (Fig. 6B). These data therefore point to an essential role for Pax5 in regulating large-scale contraction of the IgH locus. To rule out the possibility that this Pax5 function depends on V(D)J recombination, we analyzed Pax5+/+ and Pax5–/– pro-B cells on a RAG2 mutant background, which prevents immunoglobulin gene rearrangements (Shinkai et al. 1992). The IgH locus was still in an extended state in Pax5–/– RAG2–/– pro-B cells and in a contracted state in Pax5+/+ RAG2–/– pro-B cells (Fig. 6A,B), indicating that Pax5-mediated contraction of the IgH locus can precede V(D)J recombination in pro-B cells.
Pax5 induces IgH locus contraction and distal VH–DJH rearrangements in pro-B cells
To directly demonstrate an involvement of Pax5 in IgH locus contraction and distal VH–DJH recombination, we used retroviral infection to restore Pax5 expression in in vitro cultured Pax5–/– pro-B cells. The retrovirus M-Pax5-iCD2 as well as the parental virus MiCD2 (Heavey et al. 2003) express a human CD2 indicator protein, which facilitated FACS sorting of the infected cells prior to PCR quantification of IgH rearrangements (Fig. 7A). DH–JH and proximal VH7183–DJH rearrangements were present at similar levels in Pax5–/– pro-B cells regardless of whether these cells were infected with the parental or Pax5-expressing virus (Fig. 7A). In contrast, retroviral Pax5 expression led to a 10-fold increase of distal VHJ558–DJH recombination above the level seen in in vitro cultured Pax5–/– pro-B cells (Fig. 7A). We next infected double-mutant Pax5–/– RAG2–/– pro-B cells to study the effect of Pax5 on IgH locus contraction in the absence of V(D)J recombination. As expected, the distal VHJ558 and VH15 genes were separated from the proximal VH7183 and Cγ1 genes in 94%–96% of the Pax5–/– RAG2–/– pro-B cells infected with the parental MiCD2 virus (Fig. 7B,C). In contrast, both distal and proximal gene segments were colocalized and thus present in a contracted state in 69%–75% of the Pax5-reconstituted Pax5–/– RAG2–/– pro-B cells, which compares favorably with the corresponding frequency (83%–93%) observed in Pax5+/+ RAG2–/– pro-B cells (Fig. 7B,C). We conclude therefore that Pax5 facilitates distal VH–DJH rearrangements by inducing IgH locus contraction in pro-B cells.

Distal VH gene rearrangements and IgH locus contraction upon reconstitution of Pax5 expression in Pax5–/– pro-B cells. (A) Induction of distal VH gene rearrangements by retroviral Pax5 expression in Pax5–/– pro-B cells. In vitro cultured Pax5–/– pro-B cells were infected with the M-Pax5-iCD2 or parental MiCD2 retrovirus followed by PCR analysis of DH–JH and VH–DJH rearrangements. Note that the starting Pax5–/– pro-B cell culture contained only two prominent DH–JH rearrangements (DH–JH2 and DH–JH3). (B) IgH locus contraction after retroviral reconstitution of Pax5 expression in Pax5–/– RAG2–/– pro-B cells. Double-mutant pro-B cells were infected with the M-Pax5-iCD2 or control MiCD2 virus prior to three-color 3D FISH analysis as described in the legend for Figure 5B. (C) Statistical analysis of the distance between proximal and distal VH genes in pro-B cells of the indicated genotypes. For further information, see Supplementary Table 1B. (D) Two-step model for IgH locus activation in early lymphopoiesis. A schematic diagram of the nucleus illustrates the location and contraction of proximal and distal VH genes in lymphoid progenitors, wild-type (wt), and IkPax5/+ pro-T cells as well as in Pax5–/–, retrovirally reconstituted Pax5–/– and wild-type pro-B cells. In a first step, the IgH locus is repositioned, possibly under the control of EBF, from the periphery to the center of the nucleus, resulting in proximal VH–DJH rearrangements. Pax5 subsequently activates large-scale contraction of the IgH locus, leading to distal VH–DJH rearrangements. Gray shading highlights the perinuclear compartment.
Discussion
Previous loss-of-function analyses identified an essential role for Pax5 in controlling VH–DJH recombination of distal but not proximal VH genes in pro-B cells (Nutt et al. 1997; Hesslein et al. 2003). Here we have reported the unexpected finding that ectopic expression of Pax5 promotes efficient rearrangement of proximal rather than distal VH genes in pro-T cells. The distal VHJ558 genes are, however, present in accessible chromatin in IkPax5/+ pro-T cells similar to Pax5–/– pro-B cells. Hence, the loss of Pax5 in pro-B cells results in the same VH–DJH recombination phenotype as expression of Pax5 in pro-T cells. This phenotypic similarity was further extended by FISH analyses demonstrating that the IgH loci in both cell types are located at central positions of the nucleus and are present in an extended chromatin state (Fig. 7D). As a consequence, the distal VH genes are separated from the proximal IgH domain by a large distance, which is likely to prevent efficient synapse formation between distal and proximal gene segments, thus resulting in a dramatic reduction of distal VH–DJH recombination in both cell types (Fig. 7D). The seemingly conflicting data of the Pax5 gain- and loss-of-function analyses may be explained by the existence of an unknown factor X that is expressed in B cells but not in T cells and that cooperates with Pax5 in the control of locus contraction and distal VH–DJH rearrangements (Fig. 7D). According to this hypothesis, the absence of factor X in IkPax5/+ pro-T cells or the loss of Pax5 in Pax5–/– pro-B cells results in equally inefficient recombination of distal VH genes. Importantly, the restoration of Pax5 expression in Pax5–/– pro-B cells unequivocally demonstrated that Pax5 promotes distal VH–DJH recombination by inducing large-scale contraction of the IgH locus, which leads to juxta-position of distal VH genes next to the proximal DHJH-rearranged gene segment.
The IgH locus contains 150–200 VH genes, which are positioned over a chromosomal region of ~3 Mb (Chevillard et al. 2002). The large size of the IgH locus is likely to constitute a mechanistic constraint for V(D)J recombination, as synapse formation between the distal VH genes and proximal DHJH-rearranged gene segment may be ineffective, resulting in a lower efficiency of VH–DJH recombination for distal relative to proximal VH genes. Indeed, VH–DJH recombination exhibits a marked preference for the utilization of the most proximal VH genes both in fetal and adult B-lymphopoiesis (Yancopoulos et al. 1984; Malynn et al. 1990). Even within the proximal VH7183 family, the more proximally located VH genes are preferentially used for V(D)J recombination (Williams et al. 2001). This position-dependent bias in VH gene rearrangements is observed already in wild-type pro-B cells, but is dramatically increased in the absence of Pax5, as Pax5–/– pro-B cells essentially fail to undergo distal VH–DJH recombination (Nutt et al. 1997). The recently published FISH analysis of Kosak et al. (2002) provided the first evidence that V(D)J recombination may be controlled by the subnuclear location and contraction state of the IgH locus. Our detailed V(D)J recombination and 3D FISH analyses have now considerably extended these published data by implicating the two B-cell-specific transcription factors EBF and Pax5 in controlling two separate steps of IgH locus activation. As illustrated by the two-step model in Figure 7D, the IgH locus is anchored via the distal VH genes at the nuclear periphery and is oriented in its extended chromatin state toward the center of the nucleus in all non-B cells such as pro-T cells. In this default configuration of the IgH locus, the distal VH genes are likely to be silenced, as the nuclear periphery may function as a repressive compartment in higher eukaryotes in analogy to yeast (Baxter et al. 2002; Hediger and Gasser 2002). The more centrally located, proximal IgH domain may, however, be accessible for the V(D)J recombinase, which could account for the low level of DH–JH rearrangements observed in thymocytes and dendritic cells (Kurosawa et al. 1981; Corcoran et al. 2003). The first step of IgH locus activation consists of relocation of the IgH locus from the nuclear periphery to a central position within the nucleus (Fig. 7D). In the absence of locus contraction, this subnuclear repositioning in concert with chromatin changes leads to proximal VH–DJH rearrangements as observed in IkPax5/+ pro-T cells and Pax5–/– pro-B cells. Interestingly, the subnuclear relocation of the IgH locus correlates with expression of the B-cell-specific transcription factor EBF in both cell types. EBF is known to function upstream of Pax5 in early B-lymphopoiesis, as it is normally expressed in a Pax5-independent manner in Pax5–/– pro-B cells (Nutt et al. 1997). Unexpectedly, ectopic expression of Pax5 activates EBF in IkPax5/+ pro-T cells, thus identifying a novel cross-regulatory interaction between these two transcription factors. Based on the correlation between EBF expression and IgH relocation, we hypothesize that EBF may be involved in controlling the first step of IgH locus activation. In a second step, Pax5 together with factor X induces IgH locus contraction and distal VH–DJH rearrangements in wild-type pro-B cells (Fig. 7D).
Another important regulatory step in V(D)J recombination controls the accessibility of the IgH locus (Yancopoulos and Alt 1985), which is determined by the local chromatin structure of the different gene segments (Stanhope-Baker et al. 1996; Maes et al. 2001). Histone acetylation, which is a characteristic feature of open chromatin, plays an important role in determining the chromatin accessibility of Ig and TCR loci (Chowdhury and Sen 2001; Krangel 2003). Analysis of the histone acetylation state revealed a stepwise activation of discrete chromatin domains in the IgH locus (Chowdhury and Sen 2001). A 120-kb genomic region encompassing the DH, JH, and Cμ gene segments is first hyperacetylated prior to V(D)J recombination. DH–JH rearrangements subsequently induce histone acetylation and rearrangements of the proximal VH genes (Chowdhury and Sen 2001). Finally, the distal 2-Mb domain containing the majority of VH genes is activated by IL-7 signaling (Chowdhury and Sen 2001) consistent with the observation that VH–DJH recombination of the distal, but not proximal VH genes is severely impaired in B-cell progenitors of IL-7Rα–/– mice (Corcoran et al. 1998). Despite the similar recombination phenotype of IL-7Rα–/– pro-B cells and IkPax5/+ thymocytes, we do not regard IL-7 signaling to be the missing component in T cells that normally cooperates with Pax5 in the control of distal VH–DJH rearrangements for the following reasons. First, IL-7 signaling is active during early T-cell development, where it plays an essential role in regulating cell proliferation and survival (Fry and Mackall 2002). Second, the expression of germ-line VHJ558 transcripts in IkPax5/+ pro-T cells indicates that the distal IgH domain is present in an accessible chromatin state (Fig. 4C), which is likely established under the influence of IL-7 signaling (Chowdhury and Sen 2001). Finally, the incubation of sorted IkPax5/+ pro-T cells in IL-7 medium for 2 d failed to promote distal VH–DJH rearrangements (M. Fuxa and M. Busslinger, unpubl.). Based on these considerations, we hypothesize that the recombination of distal VH genes is controlled by two independent pathways. IL-7 signaling is essential for the establishment of an accessible chromatin state in the distal IgH domain, whereas a second Pax5-dependent pathway is responsible for contraction of the activated VH locus, thus leading to distal VH–DJH rearrangements.
It is important to note that the Pax5-dependent contraction of the IgH locus occurs under conditions that do not affect the accessible chromatin state of the VH genes. Hence, locus contraction cannot be caused by chromatin condensation of the entire IgH locus. Interestingly, histone acetylation and thus chromatin accessibility are narrowly confined to individual VH gene segments, their promoters and RSS sites (Johnson et al. 2003). It is, therefore, conceivable that the intergenic regions between VH genes contain regulatory elements that control locus contraction. An interesting paradigmfor such regulatory sequences are the Polycomb response elements (PREs), which have the potential to formclusters with each other through the binding of Polycomb group (PcG) proteins (Pirrotta 1998; Francis and Kingston 2001; Orlando 2003). In apparent conflict with a possible role in locus contraction, the PcG proteins are generally thought to function in gene repression by maintaining the transcriptionally silent state (Pirrotta 1998; Francis and Kingston 2001; Orlando 2003). Interestingly however, the histone methyltransferase Ezh2 is not only a member of the PcG protein family, but is also an essential regulator of distal VH–DJH rearrangements (Su et al. 2003). Ezh2 and its related gene Ezh1 are similarly expressed at early stages of B- and T-cell development, as demonstrated by RT–PCR analysis of pro-B cells and immature thymocytes (Fig. 4C). Hence, Ezh1 and Ezh2 are unlikely candidates for factor X, which could, however, be another PcG protein that is expressed in pro-B cells, but not in thymocytes. We are presently testing the hypothesis that Pax5 cooperates with the PcG systemin controlling IgH locus contraction and distal VH–DJH rearrangements. It is, however, also conceivable that ectopic Pax5 expression in IkPax5/+ thymocytes fails to properly activate a Pax5 target gene, which codes for a chromatin regulator mediating IgH locus contraction.
Materials and methods
Mice
IkPax5/+ and Ikneo/+ (Souabni et al. 2002), Pax5–/– (Nutt et al. 1997), RAG2–/– (Shinkai et al. 1992), and lck-cre mice (Lee et al. 2001) were maintained and genotyped as described.
FACS sorting and analysis
The following phycoerythrin (PE)- or allophycocyanin (APC)-coupled antibodies were used for flow cytometry: anti-B220 (RA3-6B2), CD4 (L3T4), CD8 (53-6.7), CD11c (HL3), CD19 (1D3), DX5 (DX5), Gr-1 (RB6-8C5), c-Kit (2B8), IgM (M41.42), Mac-1 (M1/70), TCRβ (H57-597), Ter119 (TER-119), and Thy1.2 (30-H12) antibodies. Unspecific antibody binding was suppressed by preincubation of cells with CD16/CD32 Fc-block solution (PharMingen). Intracellular staining of cytoplasmic Igμ protein was performed as described (Thévenin et al. 1998). Pro-B cells were sorted as B220+ c-Kit+ cells after enrichment of c-Kit+ bone marrow cells by magnetic cell sorting (MACS). DP thymocytes were sorted as CD4+ CD8+ cells after depletion of B220+ cells. DN thymocytes were sorted as Thy1.2+ Lin– cells following depletion of Lin+ cells (stained with PE-anti-B220, CD4, CD8, DX5, CD11c, Mac-1, Gr-1, and Ter119 antibodies) with anti-PE MACS beads (Miltenyi Biotec).
V(D)J recombination analysis
Sorted cells from 2-week-old mice were digested with proteinase K, and DNA was isolated by phenol extraction and ethanol precipitation. PCR analyses of immunoglobulin genes were performed with published primers (Supplementary Table 2) as described (Schlissel et al. 1991; Angelin-Duclos and Calame 1998). TCRβ rearrangements were analyzed as described (Wolfer et al. 2002). PCR cycle numbers were adjusted to be in the linear range, based on the analysis of serially diluted DNA. PCR products were separated on agarose gels, transferred to a porablot NYamp membrane and analyzed by Southern blotting using published oligonucleotide probes (Supplementary Table 2; Schlissel et al. 1991).
RT–PCR analysis
RNA was prepared from sorted cells, using the Trizol Reagent (GIBCO-BRL). Reverse transcription (with randomhexamers) and semiquantitative PCR were performed as described (Horcher et al. 2001), using the primers shown in Supplementary Table 3. PCR products were separated on agarose gels and visualized by ethidiumbromide.
3D DNA-FISH and confocal analysis
Three-color 3D FISH experiments were carried out with sorted pro-T and pre-T cells as well as with in vitro cultured pro-B cells as previously described in detail (Skok et al. 2001). The fixation conditions used were designed to preserve nuclear integrity (Skok et al. 2001). Cells were analyzed by confocal microscopy on a Leica SP2 AOBS (Acousto Optical BeamSplitter) system. Optical Z-sections were collected at 0.3-μmsteps through individual nuclei. Only cells containing signals of both IgH loci were evaluated. DNA probes were prepared from the BACs 526A21 (VHJ558), 243G9 (VH10), and 167C1 (VH7183; Kosak et al. 2002), as well as from the plasmid pγ1/HE17 containing a 17-kb genomic insert of the Cγ1 region (Skok et al. 2001). The distance between the signals of the different IgH gene probes in the nucleus was measured on individual confocal images. A distance of 0.3–0.5 μmwas evaluated as “separate,” whereas “far apart” referred to a distance of 0.5–1.5 μm(Supplementary Table 1; Figs. Figs.5,5, ,6,6, ,77).
Retroviral infection of pro-B cells
A human Pax5 cDNA was inserted upstream of the IRES-hCD2t gene of the retroviral vector MiCD2, which results in expression of a C-terminally truncated (t) human (h) CD2 indicator protein (Heavey et al. 2003). The M-Pax5-iCD2 virus was generated and used for infection of Pax5–/– and Pax5–/– RAG2–/– pro-B cells as described (Heavey et al. 2003). Pax5 expression was verified by Western blot analysis of infected pro-B cells.
Acknowledgments
We thank C. Wilson for providing the lck-cre transgenic mouse, D. Hesslein for advice on rearrangement PCR assays, and G. Stengl for FACS sorting. This research was supported by Boehringer Ingelheim, the Austrian GEN-AU initiative (financed by BMBWK), and a Wellcome Trust University Award (J.S.).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Notes
Supplemental material is available at http://www.genesdev.org.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.291504.
References
- Adams B., Dörfler, P., Aguzzi, A., Kozmik, Z., Urbánek, P., Maurer-Fogy, I., and Busslinger, M. 1992. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes & Dev. 6: 1589–1607. [Abstract] [Google Scholar]
- Allman D., Sambandam, A., Kim, S., Miller, J.P., Pagan, A., Well, D., Meraz, A., and Bhandoola, A. 2003. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4: 168–174. [Abstract] [Google Scholar]
- Angelin-Duclos C. and Calame, K. 1998. Evidence that immunoglobulin VH-DJ recombination does not require germ line transcription of the recombining variable gene segment. Mol. Cell. Biol. 18: 6253–6264. [Europe PMC free article] [Abstract] [Google Scholar]
- Bain G., Maandag, E.C.R., Izon, D.J., Amsen, D., Kruisbeek, A.M., Weintraub, B.C., Krop, I., Schlissel, M.S., Feeney, A.J., van Roon, M., et al. 1994. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell 79: 885–892. [Abstract] [Google Scholar]
- Bassing C.H., Swat, W., and Alt, F.W. 2002. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109 Suppl.: S45–S55. [Abstract] [Google Scholar]
- Baxter J., Merkenschlager, M., and Fisher, A.G. 2002. Nuclear organisation and gene expression. Curr. Opin. Cell Biol. 14: 372–376. [Abstract] [Google Scholar]
- Born W., White, J., Kappler, J., and Marrack, P. 1988. Rearrangement of IgH genes in normal thymocyte development. J. Immunol. 140: 3228–3232. [Abstract] [Google Scholar]
- Burrows P.D., Stephan, R.P., Wang, Y.H., Lassoued, K., Zhang, Z., and Cooper, M.D. 2002. The transient expression of pre-B cell receptors governs B cell development. Semin. Immunol. 14: 343–349. [Abstract] [Google Scholar]
- Busslinger M., Klix, N., Pfeffer, P., Graninger, P.G., and Kozmik, Z. 1996. Deregulation of PAX-5 by translocation of the Eμ enhancer of the IgH locus adjacent to two alternative PAX-5 promoters in a diffuse large-cell lymphoma. Proc. Natl. Acad. Sci. 93: 6129–6134. [Europe PMC free article] [Abstract] [Google Scholar]
- Chevillard C., Ozaki, J., Herring, C.D., and Riblet, R. 2002. A three-megabase yeast artificial chromosome contig spanning the C57BL mouse Igh locus. J. Immunol. 168: 5659–5666. [Abstract] [Google Scholar]
- Chowdhury D. and Sen, R. 2001. Stepwise activation of the immunoglobulin μ heavy chain gene locus. EMBO J. 20: 6394–6403. [Europe PMC free article] [Abstract] [Google Scholar]
- Corcoran A.E., Riddell, A., Krooshoop, D., and Venkitaraman, A.R. 1998. Impaired immunoglobulin gene rearrangement in mice lacking the IL-7 receptor. Nature 391: 904–907. [Abstract] [Google Scholar]
- Corcoran L., Ferrero, I., Vremec, D., Lucas, K., Waithman, J., O'Keeffe, M., Wu, L., Wilson, A., and Shortman, K. 2003. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170: 4926–4932. [Abstract] [Google Scholar]
- Francis N.J. and Kingston, R.E. 2001. Mechanisms of transcriptional memory. Nat. Rev. Mol. Cell Biol. 2: 409–421. [Abstract] [Google Scholar]
- Fry T.J. and Mackall, C.L. 2002. Interleukin-7: From bench to clinic. Blood 99: 3892–3904. [Abstract] [Google Scholar]
- Heavey B., Charalambous, C., Cobaleda, C., and Busslinger, M. 2003. Myeloid lineage switch of Pax5 mutant but not wild-type B cell progenitors by C/EBPα and GATA factors. EMBO J. 22: 3887–3897. [Europe PMC free article] [Abstract] [Google Scholar]
- Hediger F. and Gasser, S.M. 2002. Nuclear organization and silencing: Putting things in their place. Nat. Cell Biol. 4: E53–E55. [Abstract] [Google Scholar]
- Hesslein D.G. and Schatz, D.G. 2001. Factors and forces controlling V(D)J recombination. Adv. Immunol. 78: 169–232. [Abstract] [Google Scholar]
- Hesslein D.G.T., Pflugh, D.L., Chowdhury, D., Bothwell, A.L.M., Sen, R., and Schatz, D.G. 2003. Pax5 is required for recombination of transcribed, acetylated, 5′ IgH V gene segments. Genes & Dev. 17: 37–42. [Europe PMC free article] [Abstract] [Google Scholar]
- Horcher M., Souabni, A., and Busslinger, M. 2001. Pax5/BSAP maintains the identity of B cells in late B lymphopoiesis. Immunity 14: 779–790. [Abstract] [Google Scholar]
- Igarashi H., Gregory, S.C., Yokota, T., Sakaguchi, N., and Kincade, P.W. 2002. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 17: 117–130. [Abstract] [Google Scholar]
- Johnson K., Angelin-Duclos, C., Park, S., and Calame, K.L. 2003. Changes in histone acetylation are associated with differences in accessibility of VH gene segments to V–DJ recombination during B-cell ontogeny and development. Mol. Cell. Biol. 23: 2438–2450. [Europe PMC free article] [Abstract] [Google Scholar]
- Kondo M., Weissman, I.L., and Akashi, K. 1997. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91: 661–672. [Abstract] [Google Scholar]
- Kosak S.T., Skok, J.A., Medina, K.L., Riblet, R., Le Beau, M.M., Fisher, A.G., and Singh, H. 2002. Subnuclear compartmentalization of immunoglobulin loci during lymphocyte development. Science 296: 158–162. [Abstract] [Google Scholar]
- Krangel M.S. 2003. Gene segment selection in V(D)J recombination: Accessibility and beyond. Nat. Immunol. 4: 624–630. [Abstract] [Google Scholar]
- Kurosawa Y., von Boehmer, H., Haas, W., Sakano, H., Trauneker, A., and Tonegawa, S. 1981. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature 290: 565–570. [Abstract] [Google Scholar]
- Lee P.P., Fitzpatrick, D.R., Beard, C., Jessup, H.K., Lehar, S., Makar, K.W., Perez-Melgosa, M., Sweetser, M.T., Schlissel, M.S., Nguyen, S., et al. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15: 763–774. [Abstract] [Google Scholar]
- Li Y.-S., Hayakawa, K., and Hardy, R.R. 1993. The regulated expression of B lineage-associated genes during B cell differentiation in bone marrow and fetal liver. J. Exp. Med. 178: 951–960. [Europe PMC free article] [Abstract] [Google Scholar]
- Lin H. and Grosschedl, R. 1995. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature 376: 263–267. [Abstract] [Google Scholar]
- Maes J., O'Neill, L.P., Cavelier, P., Turner, B.M., Rougeon, F., and Goodhardt, M. 2001. Chromatin remodeling at the Ig loci prior to V(D)J recombination. J. Immunol. 167: 866–874. [Abstract] [Google Scholar]
- Malynn B.A., Yancopoulos, G.D., Barth, J.E., Bona, C.A., and Alt, F.W. 1990. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J. Exp. Med. 171: 843–859. [Europe PMC free article] [Abstract] [Google Scholar]
- Nutt S.L., Urbánek, P., Rolink, A., and Busslinger, M. 1997. Essential functions of Pax5 (BSAP) in pro-B cell development: Difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes & Dev. 11: 476–491. [Abstract] [Google Scholar]
- Nutt S.L., Morrison, A.M., Dörfler, P., Rolink, A., and Busslinger, M. 1998. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J. 17: 2319–2333. [Europe PMC free article] [Abstract] [Google Scholar]
- Nutt S.L., Heavey, B., Rolink, A.G., and Busslinger, M. 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 401: 556–562. [Abstract] [Google Scholar]
- O'Riordan M. and Grosschedl, R. 1999. Coordinate regulation of B cell differentiation by the transcription factors EBF and E2A. Immunity 11: 21–31. [Abstract] [Google Scholar]
- Orlando V. 2003. Polycomb, epigenomes, and control of cell identity. Cell 112: 599–606. [Abstract] [Google Scholar]
- Pirrotta V. 1998. Polycombing the genome: PcG, trxG, and chromatin silencing. Cell 93: 333–336. [Abstract] [Google Scholar]
- Rodriguez C.I., Buchholz, F., Galloway, J., Sequerra, R., Kasper, J., Ayala, R., Stewart, A.F., and Dymecki, S.M. 2000. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25: 139–140. [Abstract] [Google Scholar]
- Rolink A.G., Nutt, S.L., Melchers, F., and Busslinger, M. 1999. Long-termin vivo reconstitution of T-cell development by Pax5-deficient B-cell progenitors. Nature 401: 603–606. [Abstract] [Google Scholar]
- Romanow W.J., Langerak, A.W., Goebel, P., Wolvers-Tettero, I.L.M., van Dongen, J.J.M., Feeney, A.J., and Murre, C. 2000. E2A and EBF act in synergy with the V(D)J recombinase to generate a diverse immunoglobulin repertoire in nonlymphoid cells. Mol. Cell 5: 343–353. [Abstract] [Google Scholar]
- Schebesta M., Heavey, B., and Busslinger, M. 2002a. Transcriptional control of B cell development. Curr. Opin. Immunol. 14: 216–223. [Abstract] [Google Scholar]
- Schebesta M., Pfeffer, P.L., and Busslinger, M. 2002b. Control of pre-BCR signaling by Pax5-dependent activation of the BLNK gene. Immunity 17: 473–485. [Abstract] [Google Scholar]
- Schlissel M.S., Corcoran, L.M., and Baltimore, D. 1991. Virus-transformed pre-B cells show ordered activation but not inactivation of immunoglobulin gene rearrangement and transcription. J. Exp. Med. 173: 711–720. [Europe PMC free article] [Abstract] [Google Scholar]
- Shinkai Y., Rathbun, G., Lam, K.-P., Oltz, E.M., Stewart, V., Mendelsohn, M., Charron, J., Datta, M., Young, F., Stall, A.M., et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68: 855–867. [Abstract] [Google Scholar]
- Sigvardsson M., O'Riordan, M., and Grosschedl, R. 1997. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity 7: 25–36. [Abstract] [Google Scholar]
- Sigvardsson M., Clark, D.R., Fitzsimmons, D., Doyle, M., Akerblad, P., Breslin, T., Bilke, S., Li, R., Yeamans, C., Zhang, G., and Hagman, J. 2002. Early B-cell factor, E2A, and Pax-5 cooperate to activate the early B cell-specific mb-1 promoter. Mol. Cell. Biol. 22: 8539–8551. [Europe PMC free article] [Abstract] [Google Scholar]
- Skok J.A., Brown, K.E., Azuara, V., Caparros, M.L., Baxter, J., Takacs, K., Dillon, N., Gray, D., Perry, R.P., Merkenschlager, M., et al. 2001. Nonequivalent nuclear location of immunoglobulin alleles in B lymphocytes. Nat. Immunol. 2: 848–854. [Abstract] [Google Scholar]
- Souabni A., Cobaleda, C., Schebesta, M., and Busslinger, M. 2002. Pax5 promotes B lymphopoiesis and blocks T cell development by repressing Notch1. Immunity 17: 781–793. [Abstract] [Google Scholar]
- Stanhope-Baker P., Hudson, K.M., Shaffer, A.L., Constantinescu, A., and Schlissel, M.S. 1996. Cell type-specific chromatin structure determines the targeting of V(D)J recombinase activity in vitro. Cell 85: 887–897. [Abstract] [Google Scholar]
- Su I.H., Basavaraj, A., Krutchinsky, A.N., Hobert, O., Ullrich, A., Chait, B.T., and Tarakhovsky, A. 2003. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat. Immunol. 4: 124–131. [Abstract] [Google Scholar]
- Thévenin C., Nutt, S.L., and Busslinger, M. 1998. Early function of Pax5 (BSAP) prior to the pre-B cell receptor stage of B lymphopoiesis. J. Exp. Med. 188: 735–744. [Europe PMC free article] [Abstract] [Google Scholar]
- Tonegawa S. 1983. Somatic generation of antibody diversity. Nature 302: 575–581. [Abstract] [Google Scholar]
- Traver D., Akashi, K., Manz, M., Merad, M., Miyamoto, T., Engleman, E.G., and Weissman, I.L. 2000. Development of CD8α-positive dendritic cells from a common myeloid progenitor. Science 290: 2152–2154. [Abstract] [Google Scholar]
- Urbánek P., Wang, Z.-Q., Fetka, I., Wagner, E.F., and Busslinger, M. 1994. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79: 901–912. [Abstract] [Google Scholar]
- Wang H., Diamond, R.A., and Rothenberg, E.V. 1998. Crosslineage expression of Ig-β (B29) in thymocytes: Positive and negative gene regulation to establish T cell identity. Proc. Natl. Acad. Sci. 95: 6831–6836. [Europe PMC free article] [Abstract] [Google Scholar]
- Williams G.S., Martinez, A., Montalbano, A., Tang, A., Mauhar, A., Ogwaro, K.M., Merz, D., Chevillard, C., Riblet, R., and Feeney, A.J. 2001. Unequal VH gene rearrangement frequency within the large VH7183 gene family is not due to recombination signal sequence variation, and mapping of the genes shows a bias of rearrangement based on chromosomal location. J. Immunol. 167: 257–263. [Abstract] [Google Scholar]
- Wolfer A., Wilson, A., Nemir, M., MacDonald, H.R., and Radtke, F. 2002. Inactivation of Notch1 impairs VDJβ rearrangement and allows pre-TCR-independent survival of early αβ lineage thymocytes. Immunity 16: 869–879. [Abstract] [Google Scholar]
- Yancopoulos G.D. and Alt, F.W. 1985. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell 40: 271–281. [Abstract] [Google Scholar]
- Yancopoulos G.D., Desiderio, S.V., Paskind, M., Kearney, J.F., Baltimore, D., and Alt, F.W. 1984. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature 311: 727–733. [Abstract] [Google Scholar]
Articles from Genes & Development are provided here courtesy of Cold Spring Harbor Laboratory Press
Full text links
Read article at publisher's site: https://doi.org/10.1101/gad.291504
Read article for free, from open access legal sources, via Unpaywall:
http://genesdev.cshlp.org/content/18/4/411.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102633365
Article citations
A SIRT7-dependent acetylation switch regulates early B cell differentiation and lineage commitment through Pax5.
Nat Immunol, 18 Oct 2024
Cited by: 0 articles | PMID: 39424985
HTS and scRNA-seq revealed that the location and RSS quality of the mammalian TRBV and TRBJ genes impact biased rearrangement.
BMC Genomics, 25(1):1010, 29 Oct 2024
Cited by: 0 articles | PMID: 39472808 | PMCID: PMC11520388
UPF1 plays critical roles in early B cell development.
Nat Commun, 15(1):5765, 09 Jul 2024
Cited by: 0 articles | PMID: 38982067 | PMCID: PMC11233602
Proximally biased V(D)J recombination in the clonal evolution of IGH alleles in KMT2A::AFF1 BCP-ALL of all age classes.
Hemasphere, 8(4):e71, 22 Apr 2024
Cited by: 0 articles | PMID: 38650597 | PMCID: PMC11033919
EBF1, PAX5, and MYC: regulation on B cell development and association with hematologic neoplasms.
Front Immunol, 15:1320689, 22 Jan 2024
Cited by: 0 articles | PMID: 38318177 | PMCID: PMC10839018
Review Free full text in Europe PMC
Go to all (287) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pax5 activates immunoglobulin heavy chain V to DJ rearrangement in transgenic thymocytes.
J Exp Med, 199(6):825-830, 08 Mar 2004
Cited by: 24 articles | PMID: 15007090 | PMCID: PMC2212727
Epigenetic modifications of the VH region after DJH recombination in Pro-B cells.
Immunology, 152(2):218-231, 23 Jun 2017
Cited by: 2 articles | PMID: 28502113
Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus.
Genes Dev, 11(4):476-491, 01 Feb 1997
Cited by: 257 articles | PMID: 9042861
Spatial Regulation of V-(D)J Recombination at Antigen Receptor Loci.
Adv Immunol, 128:93-121, 13 Aug 2015
Cited by: 28 articles | PMID: 26477366
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (1)
Grant ID: R01 GM086852
Wellcome Trust (1)
Nuclear organisations of immunoglobulin genes during the developmentmaturation and activation of B lymphocytes.
Dr Jane Skok, University College London
Grant ID: 067387




