Abstract
Free full text

Language: English |
Implication of the hypothalamic–pituitary–adrenal axis in the physiopathology of depression
Abstract
Major alterations of the hypothalamic–pituitary–adrenocortical (HPA) system that can be reversed by successful antidepressant therapy are often seen in depressed patients. Persuasive evidence points to the involvement of a dysfunctional glucocorticoid receptor (GR) system in these changes. Support for this also comes from studies of transgenic mice that express an antisense RNA, complementary to the GR mRNA, and have numerous neuroendocrine characteristics of human depression as well as altered behaviour. Many of these neuroendocrine and behavioural characteristics of the transgenic mice can be reversed by antidepressants. A possible explanation for this is that the antidepressant-induced increase in GRs renders the HPA axis more sensitive to glucocorticoid feedback. This new insight into antidepressant drug action suggests a novel approach to the development of antidepressant drugs.
Abstract
On constate souvent chez les patients déprimés des altérations majeures de l'axe hypothalamo- hypophyso-surrénalien (HPA) qu'il est possible d'inverser par une thérapie fructueuse aux antidépresseurs. Des données probantes convaincantes incriminent dans ces changements un système dysfonctionnel de récepteurs des glucocorticoïdes (RG). Des études portant sur des souris transgéniques qui expriment un ARN antisens, complémentaire de l'ARNm des RG, qui présentent de nombreuses caractéristiques neuroendocriniennes de la dépression humaine, ainsi que des altérations du comportement, appuient cette affirmation. Les antidépresseurs permettent d'inverser un grand nombre de ces caractéristiques neuroendocriniennes et comportementales des souris transgéniques. Le phénomène pourrait s'expliquer par l'augmentation, provoquée par les antidépresseurs, des RG qui sensibilise l'axe HPA à la rétroaction des glucocorticoïdes. Cette nouvelle connaissance de l'action des antidépresseurs indique une nouvelle façon d'aborder la mise au point de ces médicaments.
Introduction
The main driving force for hypothalamic–pituitary–adrenocortical (HPA) activation is hypothalamic corticotropin-releasing hormone (CRH) acting in synergy with vasopressin, which is produced in either the same or distinct neurons of the paraventricular nucleus, to enhance release of pituitary pro-opiomelanocortin (POMC)-derived peptides (corticotropin [ACTH] and endorphins). Adrenal glucocorticoid hormone secretion (corticosterone in rodents, cortisol in humans) is stimulated mainly by ACTH, although adrenocortical sensitivity to ACTH may be modified by sympathetic innervation of the adrenal gland.1 Different regulatory forces are superimposed on this system to coordinate adrenal secretions during periods of inactivity and stress. The first of these is a circadian rhythm of basal activity derived from the suprachiasmatic nucleus.2 Stress-induced responses of the HPA system involve afferent inputs from numerous other brain regions including noradrenergic innervation from the brainstem A1 and A2 cell groups and the pontine locus coeruleus,3 the amygdala,4,5 cerebral cortex and hippocampus.6 In general, the septum and hippocampus have inhibitory actions on HPA activity, whereas the effect of the amygdala is largely permissive. Another regulatory force on HPA system activity is provided by inhibitory feedback actions of adrenal steroids exerted through corticosteroid receptors located in different brain areas.
Glucocorticoid hormones terminate the stress response by negative feedback action at the level of the pituitary, hypothalamus and limbic brain areas, including the hippocampus, amygdala and septum. This action is mediated by 2 types of corticosteroid receptor that have been identified:7 the type I or mineralocorticoid receptor (MR) and the type II or glucocorticoid receptor (GR). The MR is mainly expressed either alone or together with the GR in hippocampal neurons, whereas the GR is more ubiquitously distributed in the brain,8 particularly in neurons. This dual system may be necessary to cope with corticosteroid concentrations ranging from 0.5 nmol/L to 50 nmol/L during the diurnal cycle and up to 100 nmol/L or more in response to stress.9 An adequate physiologic response to a wide hormone concentration range can be achieved by these 2 receptors, because the MR mediates the effects of, and possibly controls, low basal circadian levels of circulating glucocorticoids, and the GR appears to mediate the effects of high-stress levels of glucocorticoids and to be responsible for the negative feedback effects of glucocorticoids on the HPA system.7,9,10,11,12,13
HPA disturbances in affective disorders
Hypersecretion of ACTH and glucocorticoids at baseline and several neuroendocrine function tests convincingly indicate profound alterations of the HPA system in patients with severe depression. Features of affective disorders, secondary to Cushing's syndrome, are also frequently indistinguishable from those of patients with primary psychiatric illness.14 This role of the HPA system in both stress, a major problem of modern-day (and probably past) lifestyles, and its deregulation in mood disorders, which may, at least once during their lifetime, disrupt the lives of up to 15% of the population, underlies the importance of an understanding of the integrated central nervous system mechanisms responsible for HPA system regulation. In addition, it is now becoming apparent that many of the beneficial effects of antidepressants could be exerted through their actions on the HPA system. It is thus imperative to understand how normal HPA system regulation is disturbed in affective disorders and how antidepressants modulate the HPA system.
In certain depressed patients, cortisol secretion may be increased and may show an abnormal 24-hour secretory pattern and, unless exogenous CRH is administered, this pattern may be resistant to suppression by exogenous steroids.15,16,17 Somewhat surprisingly, very similar changes are also seen in the manic phase of bipolar illness.18 Lack of cortisol suppression by dexamethasone in depressed patients could indicate a primary hyperactivity of the adrenal glands rather than a central defect, but recent studies using CRH have eliminated this possibility.19,20,21,22 A key role of elevated CRH secretion in the development of depression is indicated by both clinical23 and preclinical24,25 studies. In patients with depression, elevated concentrations of CRH in the cerebrospinal fluid,26 increased numbers of CRH-containing cells in the paraventricular nucleus,27 decreased CRH binding in the frontal cortex28 and a blunted ACTH response to an intravenously administered test dose of CRH21,29 have been seen. These results are thought to reflect the desensitization of CRH receptors at corticotrophic cells and/or a restricted secretory response of ACTH to CRH caused by elevated basal cortisol levels. The latter mechanism is probably the most important, underscored by a normalized net ACTH output in patients with depression pretreated with metyrapone.30,31 Despite a blunted ACTH response to CRH, the associated cortisol response is unchanged, because ongoing HPA overactivity gradually produces adrenocortical hyperplasia rendering the gland hypersensitive to ACTH. Other non-ACTH mechanisms such as neural sympathetic factors or humoral factors from the immune system may also contribute to the dissociation between ACTH and cortisol in depression.
Genetics of affective disorders
Twin, adoption and family studies point to the inescapable conclusion of a genetic factor in affective disorders. Between 65% and 70% of monozygotic twins are concordant for the illness, whereas only 1%–15% of dizygotic twins are concordant.32,33
Is disturbed glucocorticoid feedback a trait of affective disorders?
Although a genetic predisposition may be necessary for the development of affective disorders, stressful life events often trigger their onset. To avoid detrimental consequences, the cascade of effects that constitute the adaptive response to this stress must be terminated effectively. The mechanisms for this appear to be defective in patients with depression.34,35,36,37
Elimination of primary adrenal gland hyperactivity19,20,21,22 now points to a key role for elevated CRH secretion23,24,25 in the mechanism leading to the failure of dexamethasone to suppress cortisol in depressed patients.
Glucocorticoids terminate the stress response by negative feedback actions mediated by corticosteroid receptor(s), and it is thus apparent that an understanding of their role in both stress and affective disorders is essential. This is underlined by the findings of Sapolsky et al,38 who demonstrated that elevated glucocorticoid levels lead to loss of the GR-containing cells in the hippocampus that mediate the glucocorticoid-induced suppression of CRH neurons in the paraventricular nucleus. Dexamethasone resistance in monkeys was also shown to be associated with a selective decrease of hippocampal GR.39 Loss of hippocampal GR in aged rats40,41 may also be pertinent to the increased corticosterone levels of these animals and can be compared to the higher cortisol levels of older depressed patients compared with younger ones.42
Diminished corticosteroid receptor concentrations caused by a malfunctioning of systems involved in the regulation of corticosteroid receptor gene expression could be a causative factor in the defective feedback action of cortisol seen in patients with severe depression and could thus explain their altered HPA system. Circumstantial findings of decreased GR concentrations in the lymphocytes of patients with depression underline this possibility.43 What is not known is whether a primary disturbance occurs at the level of corticosteroid receptors in limbic brain (MR and GR in the hippocampus, GR in the hypothalamus), thus modifying the fine tuning of ACTH secretion via CRH, vasopressin and POMC regulation, or whether other factors that drive expression of CRH and vasopressin and their release into hypophyseal portal blood result in an excess of ACTH and cortisol that secondarily decreases corticosteroid receptor capacity and function.
Transgenic mouse model of the neuroendocrine changes associated with affective disorders
Introduction of genes into the germ line of mice offers the possibility of generating animal models for human genetic disease. Because the GR is essential for feedback inhibition by glucocorticoids and because in depression adrenal steroids are unable to shut down HPA activity, a malfunction of the GR system may be present in depression, a view supported by the absence of any of the classic physical stigmata associated with Cushing's disease. If, in fact, a defective GR system (this does not indicate a defective receptor, only its regulation) is one characteristic of depressive disorders, an animal model of this disease could be produced, and Pepin et al44 made a transgenic mouse with decreased GR in the brain. We hoped that this transgenic model would shed light on whether the apparent lack of sensitivity to corticosteroids seen in the majority of patients with depression could result in neuroendocrine changes that are causally linked to pathogenesis as well as to the therapeutic effectiveness of antidepressant drugs. To make this transgenic mouse, we inverted an 1815-bp fragment of the 3' noncoding region of the type II GR cDNA downstream from a 2.3-kb EcoR1/Hind III human neurofilament gene promoter element so that it would generate an antisense RNA. When we used this same strategy and inserted the antisense RNA-generating transgene into the mouse genome, we succeeded in producing an animal with defective glucocorticoid feedback.44,45 The transgenic mice have HPA system changes characteristic of those seen in depression, including increased HPA activity (shown by increased plasma ACTH and corticosterone levels), a resistance to suppression of adrenocortical secretions by dexamethasone,45,46,47 feeding disturbances48 and cognitive deficits.49 We have shown that many of the behavioural changes in our transgenic mice are reversed after antidepressant treatment.50,51,52 Similar behavioural changes have also been noted in a more recently developed mouse with brain-specific loss of GR function.53 The HPA system of these mice is activated in the early morning, at a time when, in rodents, it is normally quiescent, and this sort of phase shift is also a characteristic of depressed patients.54 Findings in these transgenic mice thus support the hypothesis that a dysfunctional GR system could be involved in the production of the neuroendocrine components of affective disorders. Genetic studies do not implicate the GR itself to be a cause of affective disorders. It remains possible, however, that a brain-specific regulator of corticosteroid gene expression could be a plausible factor. These mice are, thus, appropriate for use as a model for studying the neuroendocrine characteristics of affective disorders and could help in evaluating the mechanism of action of antidepressant drugs.
Although a primary decrease in functional corticosteroid receptors can produce HPA system activation,45,47,55 the increased glucocorticoid levels could subsequently lead to secondary activation (or inhibition) of glucocorticoid-sensitive gene expression, and this could also lead to neurotransmitter imbalance.
Normalization of HPA activity by antidepressants
Depressed patients with symptoms of HPA malfunction respond to antidepressants with temporally associated changes in both mood and hormones. This challenged us to question whether antidepressants could elevate mood in patients with depression through their long-term effects on HPA system regulation.56,57
Two major classes of antidepressant drugs exist: monoamine oxidase inhibitors and monoamine reuptake inhibitors. This latter class is the most extensively used group of drugs to treat unipolar affective disorders. It is a structurally heterogeneous group of drugs that act by blocking the reuptake transporters of either, or both, norepinephrine and serotonin (5-HT).58 Normalization of the hyperactive HPA system occurs during successful antidepressant pharmacotherapy of depressive illness.34,59,60 One possible mechanism for this action of antidepressants could be an increase in the cellular corticosteroid receptor concentration rendering the HPA system more susceptible to feedback inhibition by cortisol. Work from my laboratory first showed that different types of antidepressant could in fact alter GR mRNA levels.61,62 Subsequent work has indicated that the capacity of brain tissues to bind corticosteroids63,64 and corticosteroid receptor immunoreactivity in the brain65 are also increased by antidepressants. Although it was known that antidepressants produce changes in monoaminergic neurotransmitter systems, these changes could not be correlated with therapeutic efficiencies or measures of hypothalamic–pituitary–adrenal function,66 suggesting that as-yet-unknown mechanisms of action may be operative. Although some actions of antidepressants on the GR may be exerted through postsynaptic or presynaptic influences on monoamine concentrations, an additional mechanism of action appears completely unrelated to this. Thus, we found increased GR mRNA levels irrespective of the preferential inhibitory action of antidepressants on the reuptake of different classes of monoamine neurotransmitters. Furthermore, we showed increased GR gene transcription in antidepressant-treated mouse fibroblast cells67 that do not possess monoamine reuptake mechanisms. We hypothesized that one action of antidepressants may be exerted at the genomic level (but not necessarily directly) to stimulate the transcription of the GR gene and that this action may not be limited to neurons. We investigated this in mouse fibroblast Ltk– cells and in the mouse neuroblastoma Neuro 2A cell line transfected with a plasmid DNA vector consisting of the glucocorticoid-responsive mouse mammary tumour virus (MMTV) promoter–enhancer element fused to a reporter gene (CAT). This allowed measurement of antidepressant-induced changes in glucocorticoid response because, at constant glucocorticoid concentrations, a linear relation between MMTV–CAT activity and GR concentrations existed.68 With this chimeric construct, we observed increased glucocorticoid-stimulated CAT activity when the cells were treated with the antidepressant desipramine. A different chimeric gene construct consisting of a 2.7-kb fragment of the GR gene promoter region fused to the CAT gene (pHGR2.7CAT) allowed more direct measurement of antidepressant action. Increased CAT activity was also seen when cells transfected with pHGR2.7CAT were treated with desipramine.69 Finally, GR mRNA concentrations and glucocorticoid-binding activity of neuroblastoma and fibroblast cells increased after treatment of cells with antidepressant.67
The time course of hippocampal and hypothalamic MR and GR concentration changes in rats treated with a tricyclic antidepressant,63 or with moclobemide,64 a reversible inhibitor of monoamine oxidase A, showed that both the MR and the GR are elevated between 2 and 5 weeks after the start of treatment. This suggests that antidepressant-induced changes in brain corticosteroid receptor capacity may underlie the observed simultaneous decrease in basal circulating ACTH and corticosterone levels and decreased adrenal size.63,64 Furthermore, when challenged by a stressor, antidepressant-treated rats showed a decreased ACTH and corticosterone response, possibly induced through enhanced effectiveness of negative corticosteroid feedback because of re-established GR and MR capacity.63,64 Some of these effects may be mediated through CRH because in rats that were treated daily with imipramine,69 or in amitriptyline-treated transgenic mice, the CRH mRNA levels in the paraventricular nucleus were decreased by 26%–37%.70,71
The fact that antidepressant therapy can normalize cortisol levels and restore the suppressibility of cortisol by dexamethasone34,59 suggests that the negative feedback action of cortisol at the limbic–hypothalamic level (possibly acting on CRH and arginine vasopressin secretion) is less effective in depressed patients and is restored to full efficiency by antidepressant therapy. One possible explanation for this is that neurons involved in the central control of CRH production have reduced GR concentrations and are therefore less able to curtail stress-evoked cortisol levels, leading to a deficient negative feedback effect on the secretion of CRH and arginine vasopressin. The time course of antidepressant actions on corticosteroid receptors coincides with their long-term actions on HPA system activity and follows closely that of clinical improvement of depression.34,59,60,61,62,70 The tissue specificity of antidepressant action on corticosteroid receptors is only partly known but, as indicated by actions in nonneuronal cell lines, may be more widespread than the brain areas involved in neuroendocrine regulation. If this action is also found in lymphocytes, a predictive test for antidepressant action could be envisaged.
Rationale and new hypothesis for antidepressant action
As described earlier, antidepressants clearly increase mRNA levels of the MR and the GR and hormone-binding activities.63,64,65,72 In transfected cells, GR gene promoter activity is stimulated by antidepressants,67 and several different antidepressants can modulate this GR in vivo.71 On the basis of these findings, we hypothesize that a primary action of antidepressants could be the stimulation of corticosteroid receptor gene expression with a resultant decrease in HPA system activity, including reduced expression of CRH, which has been implicated in the pathogenesis of depression. Antidepressants could thus increase the capacity of neurons involved in the regulation of the HPA system to respond to feedback inhibition by glucocorticoids, and they may have a common mechanism of action at the level of the corticosteroid receptor genes. Secondary effects, resulting from a reduction in glucocorticoid concentrations, would be exerted on the expression of genes susceptible to glucocorticoid control such as enzymes involved in neurotransmitter biosynthesis.73 The necessity of HPA system normalization for mood improvement remains speculative, although the fact that patients with hypercortisolemia (e.g., Cushing's syndrome) or on glucocorticoid therapy often have depressive symptoms, in addition to the poor treatment response in depressed patients with persistent HPA alteration, supports this hypothesis. If additional evidence could be accumulated that neuroendocrine alterations are causally involved in affective disorders, as some preliminary data suggest,17,74 this would provide a lead for the development of more effective drugs. Nevertheless, this work opens up a completely new insight into antidepressant drug action and suggests a line of approach to the development of new drugs by focusing on this action.
Antidepressants can also influence the HPA system by actions other than those mediated via corticosteroid receptors. Antidepressant action on monoamines, their receptors and transporters, and the second messenger systems involved, is a vast field and one that is being extensively studied by many different groups. The effects of various stressors on ACTH secretion and corticosterone concentrations are well documented, and many investigators have explored the role of monoaminergic neurotransmitters in these processes. Antidepressants do not, however, appear to have effects on the mRNA of serotonergic receptors, biosynthetic enzymes or transporters,75,76 suggesting that their action on the HPA system is exerted by other mechanisms. The role of brain 5-HT in the regulation of adrenocortical activity has been extensively investigated, but no consensus of opinion exists other than that it represents an essential regulatory component of the pituitary–adrenocortical response to stress. We have measured 5-HT concentrations and turnover in response to immobilization stress and found no modification in numerous hypothalamic and amygdaloid nuclei.77 Lesion of the central nucleus of the amygdala, however, increased 5-HT turnover in most hypothalamic nuclei and this was reversed by stress, suggesting an inverse relation between serotonergic activity and ACTH secretion.77 Increased HPA activity does indeed appear to coincide with changes in the 5-HT system, including increased 5-HT turnover,78 increased postsynaptic 5-HT2 receptors79 and decreased 5-HT1A receptors.80,81,82,83 These same changes are noted in depression,84 and tryptophan depletion leads to mood lowering.85 Perhaps the most important aspect of the 5-HT system is in relation to the negative feedback action of glucocorticoids.86 The retroinhibitory action of corticosterone is exerted via receptor sites localized in neurons of the hypothalamus, hippocampus, septum, amygdala and reticular formation. Innervation of these areas is provided by noradrenergic efferents from the locus coeruleus and the serotonergic raphe neurons. Although adrenalectomy or corticosteroid administration do not alter norepinephrine metabolism, corticosterone elevates 5-HT synthesis and concentrations and alters the dynamics of 5-HT release and uptake by synaptosomes.87 Lesion of the raphe nuclei by 5,7-dihydroxytryptamine decreased the GR and MR capacity of the hippocampus88,89 by a mechanism distinct from the downregulation that can be caused by high glucocorticoid concentrations,90 but lesion of the locus coeruleus by 6-hydroxydopamine had no effect on GR.91 The possibility remains that norepinephrine may exert actions on the HPA axis, but this is not well demonstrated by lesion studies. It is interesting to note that the antidepressant mirtazapine that acts as antagonist to presynaptic α2 receptors significantly reduces the hypercortisolemia associated with depression.92 Although the availability of new selective noradrenergic reuptake inhibitors with antidepressant profiles such as reboxetine could allow clarification of the role of norepinephrine, the results must be treated with caution because of the simultaneous effects on other monoaminergic systems.93 Lesion of cholinergic inputs to the hippocampus has the opposite effect on corticosteroid receptors,94 but this is probably not a component of antidepressant action considering their low reactivity with the cholinergic system. The action of antidepressants on the HPA axis, other than that exerted directly on corticosteroid receptor genes, is thus most likely to be exerted via the serotonergic and noradrenergic systems, and antidepressants could exert action on the HPA system through increased serotonergic/noradrenergic postsynaptic activation, leading to stimulation of corticosteroid receptor gene expression and increased sensitivity of the HPA system to retroinhibiton by corticosteroids. There would thus be a dual system for activation of corticosteroid receptor genes: directly, via unknown mechanisms, and via 5-HT (Fig. 1).
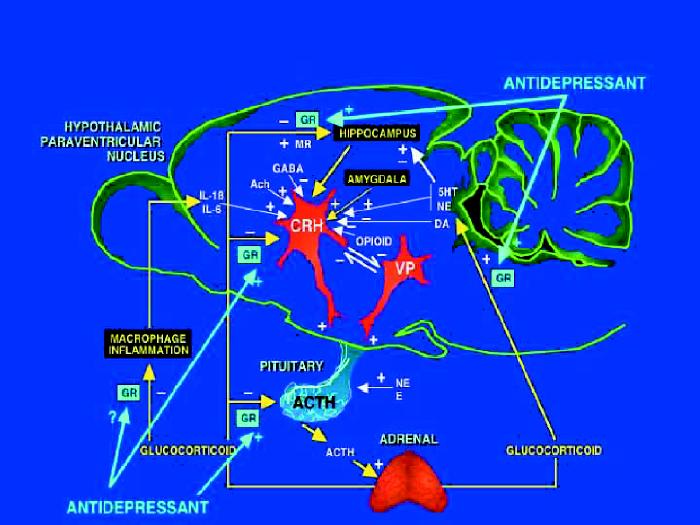
Fig. 1: Schematic representation of the hypothalamic–pituitary–adrenocortical (HPA) axis. Stimulatory (+) and inhibitory (–) actions of neural inputs to brain regions involved in HPA system regulation and the sites of corticosteroid regulation are shown. The sites at which antidepressants have stimulatory actions on the glucocorticoid receptor (GR), mineralocorticoid receptor (MR), or both, are indicated. IL = interleukin, GABA = γ-aminobutyric acid, Ach = acetylcholine, CRH = corticotropin-releasing hormone, VP = vasopressin, ACTH = corticotropin, NE = norepinephrine, E = epinephrine, 5HT = serotonin, DA = dopamine.
Footnotes
Competing interests: None declared.
Correspondence to: Dr. Nicholas Barden, Neuroscience Research Department, Centre Hospitalier de l'Université Laval Research Centre, 2705 boul. Laurier, RC-9800, Sainte-Foy QC G1V 4G2; fax 418 654-2753; ac.lavalu.luhcrc@nedrab
Submitted July 4, 2003; Revised Nov. 26, 2003; Accepted Dec. 1, 2003
References
Articles from Journal of Psychiatry & Neuroscience : JPN are provided here courtesy of Canadian Medical Association
Citations & impact
Impact metrics
Citations of article over time
Article citations
Tactile stimulation of young WAG/Rij rats prevents development of depression but not absence epilepsy.
Front Behav Neurosci, 18:1433431, 27 Jun 2024
Cited by: 0 articles | PMID: 38993266 | PMCID: PMC11236540
Potential role of gut microbiota in major depressive disorder: A review.
Heliyon, 10(12):e33157, 15 Jun 2024
Cited by: 0 articles | PMID: 39027446 | PMCID: PMC11254604
Review Free full text in Europe PMC
A theory of the neural mechanisms underlying negative cognitive bias in major depression.
Front Psychiatry, 15:1348474, 12 Mar 2024
Cited by: 1 article | PMID: 38532986 | PMCID: PMC10963437
Constipation preceding depression: a population-based cohort study.
EClinicalMedicine, 67:102371, 16 Jan 2024
Cited by: 4 articles | PMID: 38264501 | PMCID: PMC10803902
Bridging the Mind and Gut: Uncovering the Intricacies of Neurotransmitters, Neuropeptides, and their Influence on Neuropsychiatric Disorders.
Cent Nerv Syst Agents Med Chem, 24(1):2-21, 01 Jan 2024
Cited by: 2 articles | PMID: 38265387
Review
Go to all (195) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Involvement and role of antidepressant drugs of the hypothalamic-pituitary-adrenal axis and glucocorticoid receptor function.
Neuro Endocrinol Lett, 30(1):11-16, 01 Mar 2009
Cited by: 16 articles | PMID: 19300389
Review
Regulation of corticosteroid receptor gene expression in depression and antidepressant action.
J Psychiatry Neurosci, 24(1):25-39, 01 Jan 1999
Cited by: 32 articles | PMID: 9987205 | PMCID: PMC1188974
The effects of antidepressants on the hypothalamic-pituitary-adrenal axis.
Drug News Perspect, 19(10):603-608, 01 Dec 2006
Cited by: 64 articles | PMID: 17299602
Review
[The hypothalamic pituitary adrenal axis, glucocorticoid receptor function and relevance to depression].
Braz J Psychiatry, 26(3):189-201, 01 Sep 2004
Cited by: 72 articles | PMID: 15645065
Review



