Abstract
Free full text

Streptococcus pneumoniae and community-acquired pneumonia: A cause for concern
Abstract
Community-acquired pneumonia (CAP) is the sixth most common cause of death in the United States and the leading cause of death from infectious diseases. It is associated with significant morbidity and mortality, and poses a major economic burden to the healthcare system. Streptococcus pneumoniae is the leading cause of CAP. Other common bacterial causes include Haemophilus influenzae as well as atypical bacteria (Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella species). Increasing resistance to a variety of antimicrobial agents has been documented in S pneumoniae and is common in H influenzae as well. Successful empiric therapy is paramount to the management of CAP to avoid treatment failure and subsequent associated costs. Given that resistance is increasing among respiratory pathogens, and S pneumoniae is the most common etiologic agent identified in CAP, strategies for antimicrobial therapy should be based on the likely causative pathogen, the presence of risk factors for infection with resistant bacteria, and local resistance patterns.
Community-acquired pneumonia (CAP) is a common infection that is potentially life threatening, especially in older adults and those with comorbid disease.1 CAP is the sixth most common cause of death in the United States, and the leading cause of death from infectious diseases.2 In the United States, there are approximately 10 million physician visits annually for 3 to 4 million cases of CAP; nearly 500,000 adults are hospitalized and 50,000 die each year.3, 4 The prognosis of CAP ranges from rapid recovery without functional impairment to serious complications and death. Mortality from CAP is approximately 14% in hospitalized (nonsevere) patients but <1% in outpatients.5, 6 In addition, hospitalization determines the intensity of laboratory evaluation and antimicrobial therapy and affects overall cost. The costs associated with the management of CAP in the inpatient setting are significantly burdensome and may be 20 times higher than those associated with outpatient management.7
The overall economic burden of CAP is significant. It has been estimated that the total cost of treating patients aged ≥65 years is $4.8 billion each year (more than half of the total cost can be attributed to this age group) and $3.6 billion for patients <65 years.8 Most of the direct expense (>$8 billion per year) can be attributed to inpatient costs, including hospitalization, length of stay, room and board, and physician services. Medicare claims data from 1995 demonstrate that outpatient costs totaled $119 million (patients ≥65 years) and $266 million (patients <65 years).
Birnbaum and colleagues9 analyzed healthcare data for employees of a national Fortune 100 company to determine the employer's expenses for employees with pneumonia. The direct and indirect costs were 5 times higher for patients with pneumonia compared with those who did not have pneumonia ($11,544 vs. $2,368, respectively). Additionally, 59% of the costs could be attributed to the 10% of patients who were hospitalized for pneumonia. Because of the large economic burden resulting from inpatient treatment, therapies that are conducive to outpatient treatment may likely produce significant savings.
Several studies have shown that resistant pneumococcal infection in patients who require hospitalization is associated with increased length of stay,10, 11 mortality,10, 12, 13 and cost of care.10, 14 A case-control study in Iceland from 1988 to 199410 demonstrated increased costs from pneumonia caused by penicillin-resistant Streptococcus pneumoniae (PRSP) based on prolonged hospitalizations and use of more expensive antimicrobial agents. Results from a more recent study from 10 New York hospitals conducted between 1998 and 2000 demonstrated that PRSP is associated with a longer length of stay (9.7 days vs. 7.9 days) and greater total direct inpatient costs ($6,262 vs. $4,011).14 Clearly, there are economic consequences associated with the intensive management of patients who are infected with strains of S pneumoniae that are resistant to commonly used antimicrobials.
Observations of antimicrobial resistance and the increase in the number of likely causative pathogens have led to challenges in the management of CAP, and clinicians should consider these factors when selecting empiric therapy. Because the differences in clinical signs and symptoms for bacterial and viral etiologies are not clear, clinicians may have difficulty distinguishing between bacterial and viral causes of infection and determining whether antimicrobial therapy is warranted. Obtaining uncontaminated specimens from the suspected infection site to determine whether the etiology is bacterial is difficult and rarely done. Even when specimens are obtained, microbiological results are inconclusive approximately 50% of the time.15, 16, 17
The purpose of this article is to update clinicians on the etiology of CAP, with an emphasis on the most commonly identified pathogen, S pneumoniae, identify areas of increasing concern in the treatment of this infection, and provide practical considerations for the appropriate use of antimicrobial therapy in light of increasing resistance.
Pathogenesis of community-acquired pneumonia
There are several routes of pathogen acquisition involved in the pathophysiology of CAP. Aspiration of oropharyngeal contents is the most common route of acquisition but is often considered to be a subclinical aspiration.18 This should not be confused with aspiration pneumonia, which has an anaerobic etiology. The pathophysiology of 90% of pneumonias involves organisms that descend from the oropharynx into the lower respiratory tract. Other routes of pathogen acquisition include inhalation and spread along mucous membranes (viruses), hematogenous spread (Staphylococcus), and contiguous spread.18
Most people aspirate while sleeping, and some oropharyngeal secretions enter the lower respiratory tract, but because of a variety of defense mechanisms that exist in the airways (predominantly anatomical barriers [e.g., ciliary action]), most aspirated material has no clinical consequence. So the question arises: When does aspiration result in clinical pneumonia? Alterations in anatomical barriers, such as impaired ciliary action and affected inflammatory mucus production of the upper airways, can occur after viral infection, predisposing the lower airways to pneumonia. Also, impairment in the normal immune system, either in humoral or cell-mediated immunity, or phagocytic function, could result in organisms becoming pathogenic in the lower respiratory tract. Pneumonia usually occurs when there is a breakdown in the normal host defenses, when the organisms are extremely virulent, or when a large inoculum is introduced. In patients who experience recurrent episodes of pneumonia, certain immune system defects (i.e., human immunodeficiency virus [HIV] or immunoglobulin G deficiency) should be considered. Pneumococcal pneumonia and most other bacterial pneumonias are bacterial infections that occur secondary to viral infections in patients with damaged host defenses. Therefore, viral infection is a significant factor leading to many secondary bacterial infections.18, 19
Microbiology and etiology
Many studies have examined the etiology of CAP. Virtually all studies in a compilation of 15 trials showed that S pneumoniae was the most common pathogen (20%–60% of cases), followed by Haemophilus influenzae (3%–10%) (Table 1). 20 The atypical pathogens (Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella pneumophila) were variably implicated depending on the study, and viruses have become more appreciated as a common cause of pneumonia in adult patients. Other less common considerations (e.g., tuberculosis, Pneumocystis carinii, Q fever, fungi, and severe acute respiratory syndrome–associated coronavirus) exist, and clinicians should also consider these new and emerging pathogens as potential causes of pneumonia.
Table 1
Etiologic Agents in Community-Acquired Pneumonia*
| Etiologic Agents | Cases (%) |
|---|---|
| Bacteria | |
| Streptococcus pneumoniae | 20–60 |
| Haemophilus influenzae | 3–10 |
| Moraxella catarrhalis | 1–2 |
| Staphylococcus aureus | 3–5 |
| Other gram-negative species | 3–10 |
| Atypicals | |
| Mycoplasma spp | 1–6 |
| Chlamydia spp | 4–6 |
| Legionella spp | 2–8 |
| Viruses | 2–15 |
| Aspiration pneumonia | 6–10 |
| No diagnosis | 30–60 |
Adapted with permission from N Engl J Med.20
The majority of studies that have evaluated the etiology of CAP have consisted of patients in the hospital, where laboratory processes are more likely to be available for diagnosis. However, half of these cases still did not have defined etiologies. When patients were stratified by disease severity (ambulatory, hospitalized [nonsevere], and intensive care unit [ICU] [severe]), S pneumoniae was the most common cause of CAP among patients in all settings (Table 2). 1
Table 2
Etiology of Community-Acquired Pneumonia by Disease Severity (Descending Order of Incidence)
| Ambulatory Patients | Hospitalized (Non-ICU) | ICU (Severe) |
|---|---|---|
| • Streptococcus pneumoniae | • S pneumoniae | • S pneumoniae |
| • Mycoplasma pneumoniae | • M pneumoniae | • Legionella spp |
| • Haemophilus influenzae | • C pneumoniae | • H influenzae |
| • Chlamydia pneumoniae | • H influenzae | • Other gram-negative bacilli |
| • Respiratory viruses | • Legionella spp | • Staphylococcus aureus |
| • Aspiration | ||
| • Respiratory viruses |
ICU = intensive care unit.
Adapted from Lancet.1
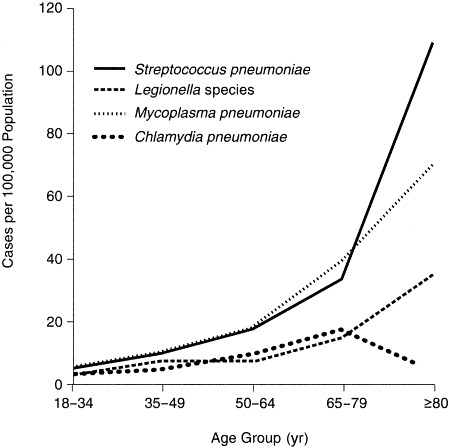
A study of 2,776 adult patients hospitalized with CAP in 2 counties in Ohio compared the incidence of etiologic agents based on patient age.4 As shown in Figure 1, the incidence of CAP requiring hospitalization caused by S pneumoniae, M pneumoniae, or L pneumophila increases with age. A study from Spain17 demonstrated that in patients without a defined etiology, the use of transthoracic needle aspiration changed the rank order of bacteria that cause CAP compared with the use of routine culture and serologic means (Table 3). Specifically, S pneumoniae became more frequently identified and prevalent than M pneumoniae, and more cases of H influenzae were identified. The use of more aggressive techniques of pathogen identification (skin and needle aspirates and molecular biological techniques [polymerase chain reaction]) improved diagnostic accuracy, and needle aspiration led to a positive microbial diagnosis in 83% of patients compared with 50% causality when conventional testing was used. However, it is unlikely that the use of transthoracic needle aspiration will become routine practice.
Table 3
Etiology of Community-Acquired Pneumonia Determined by Conventional Testing and Needle Aspirates of 109 Patients
| Conventional Testing (N = 54) | Plus Needle Aspirate (N = 90) | ||
|---|---|---|---|
| Etiologic Agent | % | Etiologic Agent | % |
| Mycoplasma pneumoniae | 35 | Streptococcus pneumoniae | 30 |
| Chlamydia pneumoniae | 17 | M pneumoniae | 22 |
| S pneumoniae | 17 | C pneumoniae | 13 |
| Influenza | 9 | Pneumocystis carinii | 8 |
| Chlamydia psittaci | 7 | Haemophilus influenzae | 7 |
| Mycoplasma tuberculosis | 6 | Influenza | 6 |
| P carinii | 6 | M tuberculosis | 4 |
| Coxiella burnetii | 4 | C psittaci | 4 |
| Defined etiology | 50 | Defined etiology | 83 |
Reprinted with permission from Am J Med.17
Two recent studies evaluated the etiology of mild (ambulatory) CAP. The most common pathogens identified were S pneumoniae, M pneumoniae, Chlamydia spp, and viruses (mostly influenza).15, 21 Mycoplasma was most common for patients <50 years and without significant comorbid conditions or abnormality of vital signs, whereas S pneumoniae was the most common pathogen for older patients or those with significant underlying disease.21
Risk factors for CAP
Several populations are at risk for developing CAP, including people who are very young (<6 years) or elderly (>65 years), those who smoke, or those with comorbid illness (e.g., chronic obstructive pulmonary disease [COPD], diabetes mellitus, renal insufficiency, chronic liver disease, heart failure, coronary artery disease, chronic neurologic disease, or malignancy).7 In many cases, these comorbidities may affect the host defense mechanisms previously mentioned. Thus, any person with ≥1 of these risk factors should be considered for microbiologic diagnosis and/or empiric therapy with a broad-spectrum antimicrobial to minimize the chance of treatment failure.
Patients with COPD are particularly at risk for developing pneumonia. The clinical manifestations of an acute exacerbation of COPD from pneumonia in such patients can be similar. In 1 study of patients admitted to the hospital for acute exacerbations, 10% to 16% were found to have pneumonia.22 Of note, although H influenzae is the most common cause of bacterial exacerbation of COPD, the most common pathogen identified as the cause of the pneumonia was S pneumoniae. Those patients with pneumonia tended to be more hypoxemic, have higher fever and more abrupt onset, and have a higher admittance rate to the ICU. These patients often required mechanical ventilation and had higher mortality and longer hospital stays.22, 23, 24
Clinical presentation of pneumonia
Because bacteriologic data may not be available for some time, if at all, a diagnosis of pneumonia is typically made based on clinical signs and symptoms. Patients may experience changes in body temperature (hypothermia or fever), rigors, sweats, cough (with or without production of sputum), alterations in color of respiratory secretions, chest discomfort, or dyspnea. Nonspecific symptoms including fatigue, myalgia, abdominal pain, anorexia, and headache also may be present.25 In addition to the assessment of physical findings through routine examination, a chest x-ray is essential to differentiate pneumonia from other causes of symptoms and for assessing disease severity, and is strongly recommended in recent CAP guidelines.19, 25
Pneumococcal pneumonia
Pneumococcal pneumonia comprises two thirds of all cases of bacterial pneumonia and is the most common cause of morbidity in patients with CAP.7 Because pneumococcal pneumonia is the most common form of CAP, empiric antimicrobial therapy must provide coverage of S pneumoniae, considering penicillin-resistant strains. As with other causes of pneumonia, patients who may have a predisposition to pneumococcal infection include those who are young, old, Native American, smokers, or have sickle-cell disease, Hodgkin disease, myeloma, HIV, asplenia, or alcoholism. Those who have abnormalities of immunoglobulin production or response to capsular polysaccharide may also be at greater risk and may be predisposed to this form of pneumonia. In addition to the aforementioned risk factors, patients who are particularly at risk for infection with PRSP include those who have received recent antimicrobial therapy (in the past 3 months), attend daycare, or are immunodeficient, institutionalized, or have been recently hospitalized.26
For severe cases or for those not responding to empiric treatment, the use of a protected specimen brush or transthoracic needle aspiration may be considered in an effort to identify the causative pathogen or pathogens. For the most part, it is not reliable practice to differentiate patients with different etiologic agents based on clinical manifestations alone, which is an important consideration when selecting empiric therapy.
Predictors of mortality
In patients who develop pneumonia, there are certain parameters that predict more severe consequences, particularly mortality. There are several factors that may increase mortality from CAP (Table 4). The 1 finding that has been associated with decreased mortality is pleuritic chest pain, which may have alerted patients to the presence of a problem and prompted them to seek treatment early.6
Table 4
Predictors of Increased Mortality in Community-Acquired Pneumonia
| • Male sex |
| • Tachypnea |
| • Hypothermia |
| • Diabetes mellitus |
| • Neoplastic disease |
| • Neurologic disease |
| • Leukopenia |
| • Bacteremia |
| • Multilobar infiltrates |
Adapted from JAMA.6
Mortality also has been associated with CAP etiology. In a meta-analysis of 127 reports of CAP published in the English language between 1966 and 1995, mortality rates were highest with gram-negative bacilli (Pseudomonas aeruginosa, 61.1%; Klebsiella species, 35.7%; Escherichia coli, 35.3%) and Staphylococcus aureus (31.8%). However, the prevalence of these bacteria is much lower than that of S pneumoniae, which accounted for two thirds of >7,000 cases in which an etiologic diagnosis was made and also accounted for the majority of CAP cases resulting in death (Table 5). 6 Low mortality rates were reported with Coxiella burnetii (0.5%) and M pneumoniae (1.4%), whereas S pneumoniae had a mortality rate of 12.3%, which approached the average mortality of 13.7%. The number of deaths from pneumococcus far surpass the number for other pathogens. Not only is S pneumoniae the most common cause of pneumonia, it is also the most common cause of death from pneumonia.
Table 5
Meta-Analysis Results of 127 Study Cohorts for Causes and Mortality of Community-Acquired Pneumonia by Infectious Agent
| Etiologic Agent (No. of Studies) | Patients (N) | Mortality (%) | Deaths (N)* |
|---|---|---|---|
| Streptococcus pneumoniae (59) | 4,432 | 12 | 545 |
| Haemophilus influenzae (27) | 833 | 7.4 | 62 |
| Mycoplasma pneumoniae (22) | 507 | 1.4 | 7 |
| Mixed bacteria (10) | 301 | 23.6 | 71 |
| Legionella† spp (20) | 272 | 14.7 | 40 |
| Viruses‡ (32) | 197 | 4.1 | 8 |
| Coxiella burnetii (7) | 182 | 0.5 | 1 |
| Staphylococcus aureus (25) | 157 | 31.8 | 50 |
| Klebsiella spp (12) | 56 | 35.7 | 20 |
| Chlamydia pneumoniae (2) | 41 | 9.8 | 4 |
| Chlamydia psittaci (8) | 32 | 0 | 0 |
| Pseudomonas aeruginosa (6) | 18 | 61.1 | 11 |
| Escherichia coli (6) | 17 | 35.3 | 6 |
| Proteus spp (3) | 12 | 8.3 | 1 |
| Streptococcus spp (types A and D) (3) | 6 | 16.7 | 1 |
| Unknown (27) | 11,229 | 12.8 | 1,437 |
Adapted with permission from JAMA.6
Pneumococcal resistance
Historically, clinicians prescribed β-lactams or other antimicrobials for empiric treatment of CAP with little concern about the susceptibility of the suspected pathogen to the chosen antimicrobial. However, during the past decade there has been an increase in antimicrobial resistance of the most common bacterial pathogens of CAP, especially S pneumoniae.27 In the United States, 29.6% of H influenzae isolates and >90% of Moraxella catarrhalis isolates currently produce β-lactamase, resulting in resistance to penicillins and many cephalosporins.28 More disturbing, however, is the increasing rate of resistance and level of resistance among S pneumoniae. The first reports of clinical resistance of S pneumoniae to penicillin appeared in the 1960s, nearly 20 years after the introduction of penicillin G.29 In the 1970s, pneumococcal resistance to penicillin and other antimicrobials was documented in South Africa, and in the 1980s, pneumococcal resistance was reported in many European, African, and Asian countries.29 In the United States, few strains of PRSP were recovered in a surveillance program conducted by the Centers for Disease Control and Prevention (CDC) in the 1980s.30 However, a sharp increase in the prevalence of PRSP occurred in the United States in the early 1990s (Figure 2) (G. V. Doern, personal communication, December 2002). The results of antimicrobial surveillance studies demonstrate that the prevalence of penicillin-nonsusceptible S pneumoniae (PNSP) in the United States was approximately 18% in 1990 to 1991 and almost 25% by 1994 to 1995 (G. V. Doern, personal communication, December 2002). In a study conducted in the winter of 1999 to 2000, 34.2% of S pneumoniae isolates (N = 1,531) were not susceptible to penicillin.31 Similar trends of increasing S pneumoniae resistance to penicillin have been observed worldwide.28, 29, 32, 33, 34, 35, 36, 37, 38
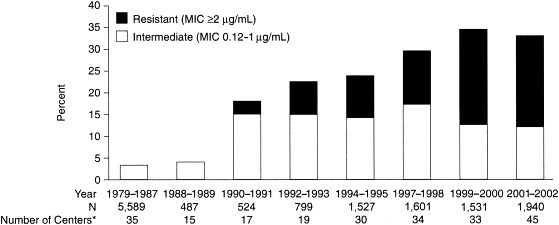
Trends in penicillin resistance among Streptococcus pneumoniae in the United States. *Number of centers contributing isolates. MIC = minimum inhibitory concentration. (Courtesy of G. V. Doern, personal communication, December 2002.)
Of particular concern when selecting an appropriate antimicrobial treatment for CAP are the increasing prevalences of high-level resistance and multidrug resistance that have been documented with S pneumoniae. High-level penicillin resistance (i.e., penicillin minimum inhibitory concentration [MIC] ≥2.0 μg/mL) among S pneumoniae has increased to a greater degree during the past 10 years than has intermediate resistance (MIC between 0.12 and 1.0 μg/mL) (Figure 2) (G. V. Doern, personal communication, December 2002). In addition to penicillin resistance, isolates of S pneumoniae have demonstrated increasing resistance to other classes of antimicrobials, including macrolides, tetracyclines, trimethoprim-sulfamethoxazole (TMP-SMX), and fluoroquinolones.28 In the United States, the prevalence of resistance to newer macrolides (e.g., azithromycin, clarithromycin) among S pneumoniae isolates is similar to that of erythromycin (approximately 24%).28 The observations regarding macrolide/azalide resistance have been coupled with recent reports of clinical failures with azithromycin and clarithromycin in pneumococcal pneumonia and bacteremia.39, 40, 41, 42
Resistance to fluoroquinolone antimicrobials also has increased in recent years. Between 1995 and 1997, resistance to ofloxacin increased from 2.6% to 3.8% in isolates obtained from the Active Bacterial Core Surveillance Program of the CDC.43 Furthermore, data from the 1998 to 2000 Alexander Project demonstrate that 6.7% of S pneumoniae isolates in the United States are resistant to ofloxacin.28 The prevalence of pneumococcal isolates in Canada with reduced susceptibility to fluoroquinolones increased from 0% in 1993 to 1.7% in 1997 to 1998.44 In a recent Canadian study of S pneumoniae respiratory isolates in elderly patients (≥65 years), Tang and colleagues45 reported resistance rates of 4.3% to levofloxacin. Similarly, resistance to fluoroquinolones in Hong Kong increased during a 3-year period from <0.5% for ofloxacin to 5.5% for levofloxacin.46 Fluoroquinolone resistance has manifested clinically as recent reports of levofloxacin treatment failure in patients with levofloxacin-resistant S pneumoniae infection.47, 48, 49, 50, 51, 52, 53
Cross-resistance has further complicated selection of antimicrobial therapy. Multidrug resistance and cross-resistance to other antimicrobials are common with S pneumoniae. Among 2,432 outpatient S pneumoniae respiratory isolates evaluated in a recent surveillance program in the United States, 62.9% were susceptible to penicillin, 12% were intermediately resistant, and 25% were highly resistant.28 Of S pneumoniae isolates that were intermediately or fully resistant to penicillin, 49.8% and 72.4%, respectively, also were resistant to macrolides/ azalides. Another study evaluated patterns of resistance from isolates from 1,703 patients with pneumococcal pneumonia.54 Of the isolates that were susceptible to penicillin, 3% and 6% were resistant to erythromycin and TMP-SMX, respectively. Among penicillin-resistant isolates, 40%, 60%, and 90% were resistant to cefotaxime, erythromycin, and TMP-SMX, respectively.
Drivers of resistance
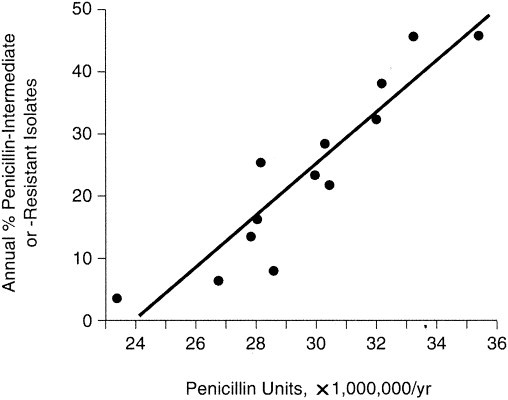
Factors that may drive resistance include antimicrobial consumption, particularly antimicrobial use in the past 3 months, inappropriate use of antimicrobials (e.g., suboptimal dosages), clonal spread of multidrug-resistant strains, and presence of comorbidities. Antimicrobial prescribing habits affect resistance patterns in that antimicrobial overuse contributes to resistance.44, 55, 56, 57 A study from Spain demonstrated that an increase in the use of penicillin was associated with an increase in penicillin-nonsusceptible isolates (Figure 3).55 Hyde and colleagues56 demonstrated that the use of macrolides in children was associated with an increase in macrolide resistance. Similarly, Chen and colleagues44 showed that an increase in the use of ciprofloxacin in Canada correlated with emergence of ciprofloxacin resistance among pneumococci. Results from Hong Kong demonstrate that patients previously exposed to fluoroquinolones were 10.6 times more likely to acquire levofloxacin-resistant isolates of S pneumoniae.46
Recent and inappropriate use of antimicrobials also are independent risk factors for development of resistance.57, 58 In a study by Guillemot and colleagues,58 β-lactam use within the previous 30 days (odds ratio [OR], 3.0; confidence interval [CI], 1.1–8.3), doses lower than clinically recommended (OR, 5.9; CI, 2.1–16.7), and treatment for >5 days (OR, 3.5; CI, 1.3–9.8) were identified as risk factors for penicillin-resistant pneumococcal nasopharyngeal carriage. A recent study by Yu and colleagues59 evaluated the clinical relevance of bacteremic pneumococcal pneumonia and the underlying factors associated with the development of resistance. The 2 factors that were shown to be independently associated with resistance on multivariate analysis were underlying disease (i.e., heart, liver, renal, or lung disease, or diabetes) (OR, 2.1; P <0.0001) and prior antimicrobial therapy (OR, 1.9; P <0.0091).
When strains of bacteria develop resistance, the resistance can spread, especially in the presence of extensive use of antimicrobials. A total of 90% of all drug-resistant S pneumoniae (DRSP) strains in the United States are caused by 5 different serotypes, and the dominant factor of emergence of PRSP in the United States has been the result of human-to-human spread of a few clonal groups.60
Clinical relevance of penicillin-resistant S pneumoniae
The clinical relevance of DRSP in meningitis is well understood. We know that adequate antimicrobial concentrations in the cerebrospinal fluid (CSF) must be attained to kill bacteria at that site, and that high concentrations can overcome low levels of resistance. Penicillin breakpoints have been defined based on CSF concentrations in which an MIC of ≤0.06 μg/mL is considered susceptible, an MIC of 0.12 to 1.0 μg/mL is intermediate, and an MIC of ≥2 μg/mL is resistant.61 In CAP, the clinical relevance of resistance has been less well understood until recently. Data on mortality rates from penicillin-susceptible versus β-lactam–resistant S pneumoniae are conflicting. Some studies controlling for potential confounding factors (e.g., age, underlying disease, severity of illness) have not shown a difference in mortality between patients with penicillin-susceptible pneumococci and those with PRSP.59 However, it is difficult to study the adverse impact of drug resistance on clinical outcomes for patients with pneumococcal pneumonia if patients are receiving antimicrobial therapy to which isolates are susceptible.62 Furthermore, many studies include S pneumoniae isolates with intermediate susceptibility to penicillin, which may not be as clinically relevant with regard to patient outcomes in CAP as infection with highly resistant (MIC ≥4 μg/mL) isolates.
Numerous studies conducted before 2000 were unable to consistently show a correlation between mortality and resistance, or demonstrate that penicillin resistance is clinically relevant (i.e., associated with clinical failures). However, most of those studies included isolates with intermediate susceptibility in the resistant group; in other words, there was a resistant group, which was a nonsusceptible group, and a susceptible group. In fact, before 2000, two thirds of the resistant groups included isolates that had intermediate susceptibility.
In a prospective, 10-year study in Spain,63 mortality was not correlated with resistance even though resistance to penicillin, cephalosporins, and erythromycin increased during the study period. In contrast, several studies have shown a significant association between mortality and high-level penicillin resistance (MIC ≥4 μg/mL) in S pneumoniae.12, 13, 62 In a study from the CDC, investigators found that after hospital day 4, the risk of death was 7 times greater in patients infected with high-level PRSP (MIC ≥4.0 μg/mL [OR, 7.1; 95% CI, 1.7–30.0]) than in patients infected with intermediate isolates (MIC 0.012–1.0 μg/mL [OR, 1.0; 95% CI, 0.3–3.0]).12 However, treatment and severity of disease were not recorded. Similarly, another study demonstrated that penicillin resistance (MIC ≥2.0 μg/mL) was an independent predictor of mortality in patients with pneumococcal bacteremia.13 In a trial of 192 patients, medical outcomes (in-hospital mortality, medical complication rates, and time to clinical stability) in patients with bacteremic pneumococcal pneumonia caused by penicillin-susceptible strains of S pneumoniae were compared with those in patients infected with PNSP.62 Compared with patients infected with penicillin-susceptible strains, patients infected with PNSP (MIC ≥0.12 μg/mL) had a greater risk of in-hospital death from pneumonia (relative risk [RR], 2.1; 95% CI, 1–4.3) (Figure 4). In addition, the risk of suppurative complications of infection was increased in patients infected with PNSP (RR, 4.5; 95% CI, 1.0–19.3). However, after adjustment for baseline differences in severity of illness, only the risk of suppurative complications of infection remained statistically significant (adjusted RR, 4.8; 95% CI, 1.2–18.8). A national, prospective, observational study of 844 patients reported similar results with 15% of isolates that were intermediately resistant and only 10% resistant to penicillin. This represents a different ratio of intermediate to resistant isolates (15% intermediate susceptibility [MIC 0.12–1 μg/mL] to 9.6% resistant [MIC ≥2 μg/mL])59 than exists in the United States, where there is more high-level resistance than intermediate resistance (12% intermediate susceptibility, 25% resistant).28 In this study, an association was identified between age, severity of illness, and comorbidity, but not with whether the isolates were PRSP. Similar results were found in the follow-up, case-control study of patients with bacteremic pneumococcal pneumonia,64 which addressed the limitations of the trial by Feikin and colleagues12 and controlled for risk factors, severity, and treatment. The findings from this multivariate analysis showed no contribution of antimicrobial resistance to mortality or requirement for ICU, but determined that more important predictors of outcome included severity of illness and whether there was a “do not resuscitate” order on the patient's chart.
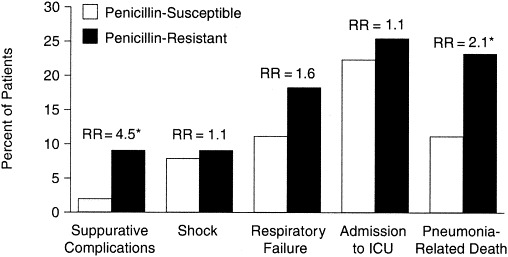
Outcomes for 192 hospitalized patients with pneumococcal pneumonia. *Statistically significant, unadjusted for other risk factors (when adjusted for other risk factors, the only outcome with significant differences was suppurative complications). ICU = intensive care unit; RR = unadjusted relative risk, Cochran-Mantel-Haenszel statistics. (Adapted from Clin Infect Dis.62)
Treatment considerations for antimicrobial-resistant pneumococcal community-acquired pneumonia
In light of increasing antimicrobial resistance among S pneumoniae and other common respiratory pathogens, physicians must consider local epidemiologic resistance patterns, patient risk factors for infection with drug-resistant pathogens, and the likely infecting pathogen when selecting appropriate therapy. Strategies for therapy of CAP must take into account the fact that S pneumoniae is the most common etiologic agent in the ambulatory, hospital (non-ICU), and severe (ICU) setting. In general, empiric therapy for all cases of CAP should cover the key respiratory pathogens and the atypical pathogens.
Several guidelines for the treatment of CAP have been developed to aid physicians in selection of appropriate therapy. The guidelines from the Infectious Diseases Society of America (IDSA) emphasize pathogen-directed treatment when there is a strongly suspected or determined etiology.7, 19 Empiric antimicrobial therapy should consider severity of illness, likely etiologic agent, resistance patterns among S pneumoniae (the most commonly identified pathogen), and comorbidities.19 It is important to stratify patients for appropriate use of antimicrobials (Table 6). For patients without significant comorbidities or recent use of antimicrobials, a macrolide or doxycycline is preferred. However, for patients at greater risk for DRSP, a respiratory fluoroquinolone or combination therapy with a β-lactam plus a macrolide (for “atypical” coverage) is recommended. The preferred β-lactam listed is high-dose amoxicillin (either as amoxicillin 3 g/day or amoxicillin-clavulanate 2 g b.i.d.). Similarly, the guidelines from the American Thoracic Society25 suggest empiric therapy based on the likely infecting pathogens. Combination therapy with oral β-lactams, such as cefpodoxime, cefuroxime, high-dose amoxicillin, or amoxicillin-clavulanate, plus a macrolide or doxycycline for “atypical” coverage, or monotherapy with a respiratory fluoroquinolone are indicated in outpatients with cardiopulmonary disease (congestive heart failure or COPD) and/or other modifying factors that place them at risk for infection with DRSP or gram-negative pathogens.
Table 6
Empiric Antimicrobial Therapy Recommendations for Outpatient Community-Acquired Pneumonia in Immunocompetent Adults from the 2003 Updated Guidelines of the Infectious Diseases Society of America
| Patient Variable | Preferred Treatment Options |
|---|---|
| Previously healthy | |
| No recent antibiotic therapy | A macrolide* or doxycycline |
| Recent antibiotic therapy† | A respiratory fluoroquinolone‡ alone or an advanced macrolide§ plus either high-dose amoxicillin or high-dose amoxicillin-clavulanate or high-dose amoxicillin-clavulanate |
| Comorbidities (COPD, diabetes mellitus, renal failure, congestive heart failure, or malignancy) | |
| No recent antibiotic therapy | An advanced macrolide§ or a respiratory fluoroquinolone |
| Recent antibiotic therapy | A respiratory fluoroquinolone alone or an advanced macrolide plus a β-lactam¶ |
COPD = chronic obstructive pulmonary disease.
Adapted from Clin Infect Dis.19
 Dosage for amoxicillin, 1 g orally t.i.d.; for amoxicillin-clavulanate, 2 g b.i.d.
Dosage for amoxicillin, 1 g orally t.i.d.; for amoxicillin-clavulanate, 2 g b.i.d.Another set of recommendations for CAP developed by the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group of the CDC emphasize treatment of the suspected etiology, S pneumoniae, and suggest consideration of those strains that are resistant to penicillin.65 This group recommends an oral β-lactam with good pneumococcal coverage (i.e., cefuroxime axetil, amoxicillin, or amoxicillin-clavulanate) for outpatient treatment of CAP (with the acknowledgment that these agents are not effective for “atypical” pathogens). Alternative outpatient antimicrobial treatments include a macrolide or doxycycline. The fluoroquinolones are not listed by the CDC group as first-line agents because of concern about emerging resistance.
A new, pharmacokinetically enhanced extended-release formulation of the β-lactam/β-lactamase inhibitor combination amoxicillin-clavulanate (2,000/125 mg b.i.d.) may be an appropriate therapy for outpatients with CAP, particularly those at risk for infection with S pneumoniae isolates that are resistant to penicillin.66 Extended-release amoxicillin-clavulanate provides a time above the MIC (T>MIC) of 38%, a value that is unattainable with older formulations for isolates with penicillin MICs of ≥4 μg/mL67 and perhaps up to 8 μg/mL. In addition, the clavulanate component, because of its ability to inhibit β-lactamase enzymes, provides coverage of β-lactamase–producing H influenzae and M catarrhalis, 2 possible causes of CAP. This formulation has been shown to be effective against S pneumoniae isolates with MICs of 4 μg/mL, with promising results for isolates with MICs up to 8 μg/mL. Data from 5,531 patients in 9 clinical trials (3 in acute bacterial sinusitis, 4 in CAP, 2 in acute exacerbation of chronic bronchitis) of extended-release amoxicillin-clavulanate evaluating the success rate of S pneumoniae eradication showed that relative to penicillin MICs, successful clinical outcomes were noted in 95.2% of patients when the pathogen isolated had an amoxicillin MIC of 4 μg/mL, and good clinical response was seen with higher MICs (1 of 1 eradication for MIC 8 μg/mL and MIC 16 μg/mL). Extended-release amoxicillin-clavulanate was 97.7% effective against erythromycin-resistant isolates (42 of 43 isolates tested).68
Conclusion
Because >75% of patients with CAP are treated as outpatients, clinicians must be cognizant of etiologic agents of CAP, local antimicrobial sensitivity patterns, and pharmacologic options for empiric therapy. The treatment of CAP has become increasingly complicated over time despite the development of newer, broader-spectrum antimicrobial agents. S pneumoniae is the most common cause of CAP, and empiric therapies should target this pathogen, considering drug-resistant isolates of this species. Even as the list of potential bacteriologic etiologies grows, treatment of CAP continues to be empiric because of the difficulties in obtaining uncontaminated specimens and differentiating between colonizing bacteria and those causing infection, and because serologic and culture results are not rapid and often fail to identify the causative pathogen. High rates of bacterial resistance to antimicrobials, particularly among S pneumoniae, limit the available therapeutic options at a time when more options are needed.
Distinguishing between nonbacterial and bacterial etiologies of respiratory tract infections (e.g., mild CAP, viral bronchitis) is a major challenge for physicians. Clinical distinguishing criteria are not clear, but some markers and recommendations are available to guide physicians in their decision making.69 The judicious prescribing of antimicrobial therapy is warranted not only because it is good clinical practice, but also because it is necessary to curtail current patterns of increasing bacterial resistance to commonly used antimicrobials. The choice of antimicrobial for a suspected infection of bacterial etiology should be made based on likely pathogens for a particular patient and the likelihood that the organism is resistant. Given that S pneumoniae is the most common cause of CAP, and penicillin resistance is increasing in this pathogen, extended-release amoxicillin-clavulanate is an appropriate antimicrobial in patients with CAP infected with known or suspected PRSP. In addition, patient education is expected to be of benefit in minimizing the use of antimicrobial therapy for infections of suspected viral etiology. As new specimen-sampling techniques and microbiologic detection methods become available, a shift from empiric therapy toward pathogen-directed therapy may result in improved outcomes and curb antimicrobial resistance.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.amjmed.2004.07.007
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc7147208?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.amjmed.2004.07.007
Article citations
Protective Effects of 23-Valent Pneumococcal Polysaccharide Vaccination Against Mortality: The VENUS Study.
Open Forum Infect Dis, 11(9):ofae530, 11 Sep 2024
Cited by: 0 articles | PMID: 39329110 | PMCID: PMC11425580
Isolation and Antimicrobial Resistance Patterns of Bacterial Pathogens from Community-Acquired Pneumonia at Adama Hospital Medical College, Adama, Ethiopia.
J Trop Med, 2024:8710163, 11 Jul 2024
Cited by: 0 articles | PMID: 39026529 | PMCID: PMC11257760
Which is a real valuable screening tool for lung cancer and measure thoracic diseases, chest radiography or low-dose computed tomography?: A review on the current status of Japan and other countries.
Medicine (Baltimore), 103(19):e38161, 01 May 2024
Cited by: 0 articles | PMID: 38728453 | PMCID: PMC11081589
Review Free full text in Europe PMC
Prevalence of Penicillin Resistance Among Streptococcus pneumoniae Isolates in a General Hospital in Southwest Saudi Arabia: A Five-Year Retrospective Study.
Cureus, 16(3):e55326, 01 Mar 2024
Cited by: 0 articles | PMID: 38559551 | PMCID: PMC10981866
Meningitis in critically ill patients admitted to intensive care unit for severe community-acquired pneumococcal pneumonia.
Ann Intensive Care, 13(1):129, 18 Dec 2023
Cited by: 0 articles | PMID: 38108904 | PMCID: PMC10728423
Go to all (57) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Antibacterial activity and PK/PD of ceftriaxone against penicillin-resistant Streptococcus pneumoniae and beta-lactamase-negative ampicillin-resistant Haemophilus influenzae isolates from patients with community-acquired pneumonia.
J Infect Chemother, 13(5):296-301, 30 Oct 2007
Cited by: 7 articles | PMID: 17982717
Garenoxacin activity against isolates form patients hospitalized with community-acquired pneumonia and multidrug-resistant Streptococcus pneumoniae.
Diagn Microbiol Infect Dis, 58(1):1-7, 03 Apr 2007
Cited by: 5 articles | PMID: 17408904
Pneumococcal community-acquired pneumonia in 148 hospitalized adult patients.
Eur J Clin Microbiol Infect Dis, 16(12):863-870, 01 Dec 1997
Cited by: 8 articles | PMID: 9495665
Evolving trends in Streptococcus pneumoniae resistance: implications for therapy of community-acquired bacterial pneumonia.
Int J Antimicrob Agents, 36(3):197-204, 16 Jun 2010
Cited by: 59 articles | PMID: 20558045
Review