Abstract
Free full text

A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor
Abstract
Background: The neurochemical and biological effects of antidepressant medications have become better defined over the last decade. When the anti-depressant bupropion was introduced in the United States in 1989, the specific pharmacologic basis of its clinical effects was uncertain. Research conducted over the past decade has significantly advanced the understanding of the neuropharmacology of bupropion and has demonstrated a novel mechanism of antidepressant activity. This article discusses the mechanism of action of bupropion and relates the drug's neuropharmacologic effects to its clinical efficacy and tolerability profiles.
Data Sources: Data were obtained via the MEDLINE database in an English-language search spanning the period 1965 to May 2002 and using the search terms bupropion, bupropion SR, and antidepressants, as well as from the manufacturer's bupropion databases.
Conclusions: The preclinical and clinical data show that bupropion acts via dual inhibition of norepinephrine and dopamine reuptake and is devoid of clinically significant serotonergic effects or direct effects on postsynaptic receptors. Dual norepinephrine and dopamine reuptake inhibition is associated with a unique clinical profile. Bupropion has demonstrated efficacy comparable to that of other antidepressants. However, because bupropion is a selective norepinephrine and dopamine reuptake inhibitor with no serotonergic activity, common antidepressant-associated side effects, such as sexual dysfunction, weight gain, and sedation, are not associated with bupropion therapy.
When introduced in the United States in 1989, bupropion was categorized as an “atypical” antidepressant because its neurotransmitter effects were undefined but known to differ from those of classical antidepressants (tricyclic antidepressants [TCAs] and monoamine oxidase inhibitors [MAOIs]) and selective serotonin reuptake inhibitors (SSRIs). Though the efficacy of bupropion is comparable to that of other antidepressants, including the SSRIs and TCAs,1–6 bupropion does not affect serotonin or postsynaptic receptors and therefore is an antidepressant with unique pharmacologic properties.7 This article discusses the pharmacology of bupropion, a compound currently available in 3 distinct but bioequivalent formulations8 (Wellbutrin, Wellbutrin SR [sustained-release], and Wellbutrin XL [extended release]) (Table 1), and relates the drug's neurotransmitter effects to clinical efficacy and tolerability. By understanding the neuropharmacologic basis of the clinical effects of antidepressants, health care providers can select among pharmacotherapies to better tailor treatments to the needs of their individual patients.
Table 1.
Pharmacokinetic Parameters of Bupropion Formulations at Steady State From Bupropion Bioequivalence Analysesa
NEUROBIOLOGY OF DEPRESSION
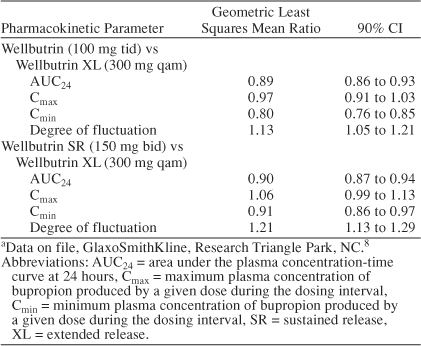
For nearly 4 decades, the monoamine hypothesis of depression has predominated.9 According to the monoamine hypothesis, depression is a neurochemical disorder arising from hypofunctioning of brain mono-amine systems including the serotonergic, noradrenergic, and/or dopaminergic pathways. This hypothesis arose from observations that the administration of classical antidepressants increased monoaminergic function, whereas monoamine depleters such as reserpine precipitated depressive symptoms in susceptible individuals.10,11 A large body of evidence from animal models and clinical studies in depressed patients also supported the monoamine hypothesis. For example, depressed patients were found to have subnormal cerebrospinal fluid levels of serotonin and norepinephrine metabolites as well as blunted neuroendocrine responses to monoamine agonists12–14; moreover, all currently available antidepressants acutely enhance some aspect of monoaminergic function (Table 2).11,15–18
Table 2.
Monoaminergic Effects of Common Antidepressantsa
In current conceptualizations of the neurobiology of depression, monoaminergic dysregulation is viewed more as an associated factor than as a primary cause. Depression and responses to antidepressants are thought to be mediated by yet to be fully defined final common physiologic pathway(s), the functions of which are modulated by the monoamines. Activity of specific monoaminergic pathways in this context are viewed as “upstream” events that influence “downstream” events, such as changes in gene expression and protein synthesis, which ultimately cause depression and modulate responses to antidepressants.14,16,19 Several observations support an “upstream” rather than primary role of monoamines in depression. First, whereas monoamine-enhancing effects of antidepressants are observed at the synaptic level within hours of the initial dose, the onset of clinical efficacy does not occur until days or weeks after initiation of antidepressant therapy,20 an observation consistent with the possibility that events downstream of and dependent upon monoamine activation are involved in the etiology of depression. Second, though all antidepressants marketed to date enhance monoaminergic neurotransmission, they have widely varying potencies for monoaminergic effects. For example, antidepressants differ by more than 1000-fold in potency at inhibiting monoamine reuptake, yet their efficacies are comparable and seemingly unrelated to potency.21 Third, although all antidepressants enhance monoaminergic neurotransmission, they do so via disparate mechanisms, consistent with the possibility that multiple monoamines influence final common pathways relevant to depression. Finally, more recent evidence suggests that antidepressants increase levels of brain-derived neurotrophic factor, a protein that has been found to promote cellular health.22 Antidepressants may thus play a neuroprotective role, a possibility supported by observations that hippocampal neurogenesis may be required for the behavior effects of antidepressants in mice23 and that progressive loss of hippocampal volume occurs during chronically untreated depression in humans.24,25
NEUROPHARMACOLOGY AND MECHANISM OF ACTION OF BUPROPION
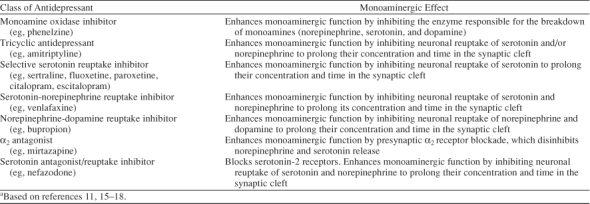
Animal research has demonstrated that bupropion enhances monoaminergic neurotransmission differently from other antidepressants.7 In rat and mouse studies, bupropion and its metabolites (hydroxybupropion, threo-hydrobupropion, and erythrohydrobupropion) did not alter serotonergic neurotransmission either presynaptically (by affecting serotonin release or reuptake) or postsynaptically (by binding to serotonin receptors).7,26 Rather, bupropion and its primary metabolite, hydroxybupropion, decreased the reuptake of dopamine and norepinephrine into rat and mouse synaptosomes (sacs formed by presynaptic neuronal membranes that mimic presynaptic neuronal terminal activity). In addition, the acute administration of bupropion reduced firing of dopamine and norepinephrine neurons in the brain stems of rats in a dose-dependent manner,7,26 an effect consistent with an increase in synaptic levels of dopamine and norepinephrine that in turn inhibits neuronal firing via an autoreceptor-mediated negative feedback mechanism. Furthermore, microdialysis studies that measured neurotransmitter levels in the nucleus accumbens of freely moving mice found extracellular dopamine and norepinephrine concentrations increased in response to bupropion administration in the Porsolt animal model of depression,27,28 and another microdialysis study29 has shown increased dopamine and norepinephrine concentrations in the rat prefrontal cortex in response to bupropion administration. Lastly, administration of dopamine- or norepinephrine-blocking drugs reduced the antidepressant effects of bupropion and its metabolite hydroxybupropion in animal models of depression.30 These preclinical data indicate that the mechanism of action of bupropion most likely involves its dual-reuptake inhibition of dopamine and norepinephrine (Figure 1).
Norepinephrine-Dopamine Reuptake Inhibitor (NDRI) Molecule Blocking Both Norepinephrine and Dopamine Reuptake Pumpsa
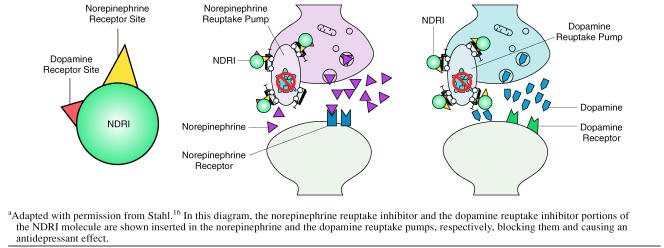
Clinical research and studies of human dopamine, nor-epinephrine, and serotonin transporters extend the pre-clinical findings. Therapeutic doses of bupropion given to depressed patients (N = 11) showed reduced whole-body turnover of norepinephrine without altering plasma nor-epinephrine levels, a finding that indicates significant central noradrenergic activity.31 In addition, 3 studies32–34 have investigated human dopamine transporter occupancy by bupropion and its metabolites. In a study32 conducted in healthy volunteers (N = 6) using positron emission tomography (PET), bupropion and its metabolites effectively bound to striatal dopamine transporters under steady-state conditions with therapeutic oral dosing of bupropion SR (150 mg b.i.d.). The mean dopamine transporter occupancy was 26.0% (SD = 8.3) at 3 hours after the last dose of bupropion SR, and this level was maintained through the last PET assessment at 24 hours after dosing (25.2% occupancy, SD = 9.7) (Figures 2 and and3).3). This degree of dopamine transporter occupancy was corroborated in a study of depressed patients33 (N = 7) using single photon emission computed tomography (SPECT), which found a mean bupropion dopamine transporter occupancy of 25.4% (SD = 20.9) at steady state following therapeutic dosing of bupropion SR (150 mg b.i.d.). In contrast, Meyer and colleagues34 reported dopamine transporter occupancy in depressed patients (N = 8) of only 14% following treatment with bupropion. However, interpretation of these data is difficult given that the report lacks an index of the variability in the data, the time course of dopamine effects, and evidence that patients were at steady state.
In Vivo Binding of 11C-βCIT-FE, a Selective Dopamine Transporter-Binding Radioligand, at Baseline and 3, 12, and 24 Hours After Cessation of Steady-State Dosing With Bupropion SRa
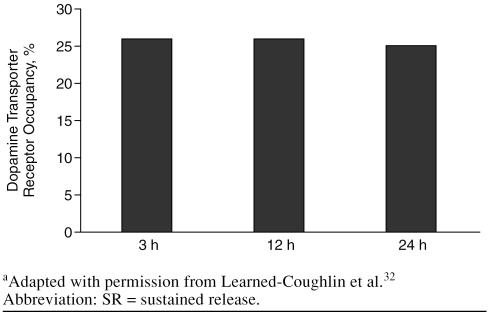
Mean Dopamine Transporter Receptor Occupancy of Bupropion 3, 12, and 24 Hours After Cessation of Steady-State Dosing With Bupropion SRa
The effects of bupropion and its metabolites on mono-amine reuptake have been further characterized in vitro using cells expressing human transporters for dopamine, norepinephrine, and serotonin.8 Bupropion with its metabolites inhibited reuptake at human transporters for both dopamine and norepinephrine, with slightly greater functional potency at the dopamine transporter than at the nor-epinephrine transporter. Inhibition of serotonin reuptake via the serotonin transporter was negligible even at the highest concentration tested. Combined relative potencies for bupropion and its metabolites at human dopamine and norepinephrine transporters are presented in Figure 4. When interpreting these data, it is important to note both the relatively high (~10:1) brain-to-plasma ratio for bupropion and its metabolites as well as the plasma pharma-cokinetic profile of parent drug and metabolites. Brain concentrations of bupropion and its major metabolites remain above the 50% inhibitory concentrations (IC50) for brain dopamine and norepinephrine transporters throughout the typical 12-hour dosing interval of bupropion SR. These data confirm that bupropion is a dual norepinephrine and dopamine reuptake inhibitor (NDRI) in humans at clinically relevant doses.31 Results of other studies15,26 have shown that bupropion and its metabolites do not have appreciable affinity for postsynaptic receptors including histamine, α- or β-adrenergic, serotonin, dopamine, or acetylcholine receptors. The lack of affinity for these postsynaptic receptors differentiates bupropion from the TCAs and some of the other new-generation antidepressants that have relatively high affinities for histamine, acetylcholine, and/or α- adrenergic receptors.20
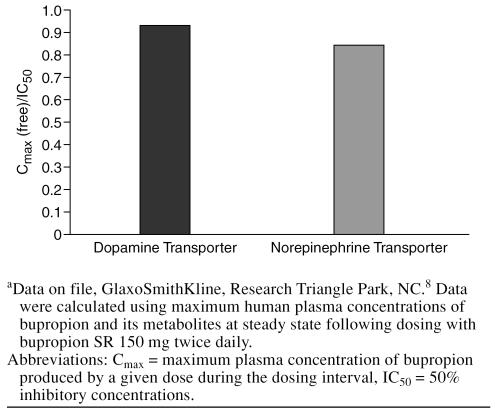
Combined Relative In Vitro Potency (Cmax/IC50) for Bupropion and Metabolites at Human Dopamine and Norepinephrine Transportersa
Considered in aggregate, these data demonstrate that bupropion inhibits the reuptake of norepinephrine and dopamine in humans without affecting release or transport of other neurotransmitters and without binding to other neurotransmitter receptors. This pharmacologic profile is unique to bupropion, which is currently the only available NDRI shown to increase dopamine neurotransmission in both the nucleus accumbens and the prefrontal cortex.
NEUROPHARMACOLOGY OF BUPROPION
The specific neurotransmitter(s) affected by antidepressants and the potency of these neurotransmitter effects do not necessarily predict antidepressant efficacy. Regardless of pharmacologic profiles, the effectiveness of antidepressant medications is generally comparable among and within classes, as was found in the evidence report of the Agency for Healthcare Policy and Research35 and is reflected in the positions of the American Psychiatric Association,36,37 reviewers for the Cochrane Library,38 and clinical experts publishing independently of these organizations.39,40 Though bupropion is distinguished from other antidepressants by its pharmacology, multiple head-to-head trials1–6 comparing bupropion with SSRIs and TCAs have demonstrated comparable antidepressant efficacy, and a pooled analysis41 of all bupropion comparative trials with SSRIs demonstrated identical remission rates (47%). Moreover, bupropion has demonstrated comparable efficacy when administered in conjunction with the SSRI sertraline in treating depression (and anxious symptoms of depression) even among patients with high levels of anxiety at baseline.42,43
The distinctive neuropharmacologic properties of bupropion do, however, have clinical implications with regard to clinical application and therapeutic spectrum in individual patients. For example, in addition to its use as a first-line antidepressant, bupropion is frequently used to augment the efficacy44–49 and mitigate side effects50–59 of serotonergic antidepressants. Bupropion is also effective for other disorders characterized by dysfunctional noradrenergic and/or dopaminergic neurotransmission. By inhibiting dopamine reuptake, bupropion confers anti-craving and antiwithdrawal effects that make it an effective smoking-cessation aid.60 Smoking-cessation clinical trial results with bupropion show that short- and long-term abstinence rates approximately double when compared with placebo or the nicotine patch.60 Bupropion has also demonstrated efficacy in the treatment of attention-deficit/hyperactivity disorder (ADHD),61–63 which is thought to involve both noradrenergic and dopaminergic dysregulation, and it is the only antidepressant to have demonstrated efficacy in reducing the risk of seasonal depressive relapse when taken prophylactically for seasonal affective disorder (SAD)64; noradrenergic and dopaminergic abnormalities have been implicated in the pathogenesis of both ADHD61–63 and SAD.65–67 Further data suggesting that bupropion is less likely than TCAs to cause a switch into mania in bipolar depression have made bupropion a preferred treatment option for bipolar depression.36,68–70 It has been hypothesized that bupropion's relatively low risk of inducing mania may be related to its absence of serotonergic properties or effects on postsynaptic β-receptors.71,72 In contrast, although other antidepressants such as the SSRIs, dual serotonin and norepinephrine reuptake inhibitors (SNRIs), TCAs, and MAOIs are frequently used to treat a wide variety of anxiety disorders, bupropion has not been well studied for the treatment of anxiety disorders.
Clinical Tolerability
Unlike therapeutic effects, which may not be observed for several weeks, most side effects occur within hours to days of initiation of an antidepressant.73 This observation suggests that acute tolerability of antidepressants, unlike antidepressant efficacy, is directly related to acute synaptic effects on monoaminergic and other systems.
Clinical data demonstrate that specific neurotransmitter effects are associated with distinct side effect profiles (Table 3).17,18,20,74,75 Antidepressant-induced side effects are attributed to drug activity at central or peripheral synapses where agents either bind to neurotransmitter receptors and influence cellular function or alter concentrations of endogenous neurotransmitters that then bind to neurotransmitter receptors. Because the acute pharmacologic effects of bupropion are unique among currently marketed antidepressants, bupropion also demonstrates a distinct tolerability profile. Across 3 randomized, placebo-controlled studies (987 patients treated with bupropion SR [100–400 mg/day] and 385 placebo-treated patients), adverse events occurring significantly more frequently with bupropion than placebo were dry mouth (16% vs. 7%), nausea (12.5% vs. 7.5%), and insomnia (10.5% vs. 6.5%), respectively.76 These side effects have also been reported with other antidepressants. However, bupropion's tolerability profile differs from those of other antidepressants in that some adverse events do not occur significantly more frequently with bupropion than placebo, including sexual dysfunction, weight gain, and sedation—side effects that occur often with other antidepressants.
Table 3.
Biochemical Pharmacologic Mechanisms and Their Possible Side Effecta

The association of SSRIs, TCAs, MAOIs, and SNRIs with sexual dysfunction is well established.77,78 In a study reported in 2002,79 37% of 6297 patients consulting 1101 U.S. primary care clinics reported sexual problems associated with antidepressant use. Sexual dysfunction as measured by the Changes in Sexual Functioning Questionnaire was 4 to 6 times more likely to occur with anti-depressants affecting serotonergic function compared with bupropion, which was associated with the lowest risk of sexual dysfunction. Comparator studies of bupropion and SSRIs corroborate these findings.2,3,80–82 In addition, bupropion has been successfully substituted for other antidepressants that cause sexual dysfunction83,84 and has been effective as an antidote for sexual dysfunction caused by other antidepressants in numerous uncontrolled studies50,54–56,59 and in 2 of 3 placebo-controlled clinical trials.51,52,57 Adjunctive bupropion treatment to reverse a variety of antidepressant-induced sexual side effects was more successful when administered as regular daily doses rather than occasional as-needed use.50 In the trial in which bupropion was not effective as an antidote,57 it is possible that an inadequate dose of bupropion was used and/or that the sexual functioning rating scale used (the Arizona Sexual Experience Scale) lacked adequate sensitivity to detect antidepressant-associated sexual dysfunction.
In addition to sexual dysfunction, weight gain may occur frequently with some classes of antidepressants.85–89 With respect to SSRIs, evidence suggests weight gain may occur during long-term treatment (possibly via a serotonergic mechanism such as down-regulation of 5-HT2C receptors, although antihistaminergic effects may also contribute).90,91 In contrast, bupropion has not been associated with weight gain. Depression trials suggest that bupropion is weight-neutral in patients at or below ideal body weight at baseline but is associated with modest weight loss, proportional to initial body mass index.76,92–94 In addition, bupropion has demonstrated efficacy as an adjunct for weight loss in nondepressed, obese individuals.95,96 The mechanism of the weight-reducing effect of bupropion has not been determined, although it is noteworthy that both dopaminergic and noradrenergic brain pathways have critical roles in the regulation of appetite, satiety, and feeding behavior.97,98
Bupropion, unlike many other antidepressants, is not associated with sedation. The incidence of sedation in controlled clinical trials of bupropion did not differ between bupropion SR and placebo.76 In addition, in a pooled analysis41 of all studies comparing bupropion with SSRIs, bupropion was associated with significantly lower rates of sedation than were the SSRIs.
An often-debated issue is the incidence of seizure associated with antidepressant therapy. Most antidepressant clinical trials report that the seizure incidence ranges from 0.1% to 0.3% for the newer-generation antidepressants 99–103 and up to 1.1% for the TCAs.104–106 The spontaneous seizure rate reported in the general population is approximately 0.1%.107,108 For bupropion, the incidence of seizure reported in the product information for the older, immediate-release formulation (Wellbutrin) is 0.4% at doses up to 450 mg/day,109 and for Wellbutrin SR and Zyban (also a sustained-release formulation), 0.1% for doses up to 300 mg/day.109 In addition, a recently conducted review8 by the manufacturer of its clinical trials database for the sustained-release formulation of bupropion (N = 15,213) showed an overall seizure incidence of 0.07% at doses up to 400 mg/day. The mechanisms by which antidepressants may lower the seizure threshold are largely unknown.
Considered together, these data show that dual inhibition of norepinephrine and dopamine reuptake with bupropion results in a side effect profile distinct from that of antidepressants with other mechanisms of action. Although many antidepressants are associated with side effects such as sexual dysfunction, weight gain, and sedation, bupropion's side effect profile differs and consists primarily of dry mouth, nausea, and insomnia.
CONCLUSIONS
Preclinical and clinical data demonstrate that bupropion acts via dual inhibition of norepinephrine and dopamine reuptake, which constitutes a novel mechanism of antidepressant action. As such, bupropion is associated with a unique clinical profile with efficacy comparable to that of other antidepressants. Devoid of clinically significant serotonergic effects or direct effects on postsynaptic receptors, bupropion—the only currently available NDRI—is as effective as other antidepressants but does not cause common antidepressant-associated side effects such as sexual dysfunction, weight gain, and sedation. These data support the use of bupropion as a first-line antidepressant as well as its possible utility as augmentation therapy.
Drug names: amitriptyline (Elavil and others), bupropion (Wellbutrin, Zyban, and others), citalopram (Celexa), escitalopram (Lexapro), fluoxetine (Prozac and others), mirtazapine (Remeron), paroxetine (Paxil and others), phenelzine (Nardil), reserpine (Serpalan and others), sertraline (Zoloft), venlafaxine (Effexor).
Footnotes
Dr. Stahl has been a consultant for, received honoraria from, or conducted clinical research supported by Abbott, Asahi Kasei, AstraZeneca, Bristol-Myers Squibb, Cephalon, Cypress Bioscience, Eli Lilly, GlaxoSmithKline, Organon, Otsuka, Pfizer, Pierre Fabre, and Wyeth. Dr. Pradko has been a consultant for and has served on the speakers or advisory board of GlaxoSmithKline. Drs. Haight, Modell, Rockett, and Learned-Coughlin are employees of GlaxoSmithKline.
REFERENCES
- Kavoussi RJ, Segraves RT, and Hughes AR. et al. Double-blind comparison of bupropion sustained-release and sertraline in depressed outpatients. J Clin Psychiatry. 1997 58:532–537. [Abstract] [Google Scholar]
- Croft H, Settle E, and Houser T. et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther. 1999 21:643–658. [Abstract] [Google Scholar]
- Coleman CC, Cunningham LA, and Foster VJ. et al. Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of bupropion sustained-release and sertraline treatment. Ann Clin Psychiatry. 1999 11:205–215. [Abstract] [Google Scholar]
- Weihs KL, Settle EC, and Batey SR. et al. Bupropion sustained-release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiatry. 2000 61:196–202. [Abstract] [Google Scholar]
- Weisler RH, Johnston JA, and Lineberry CG. et al. Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychopharmacol. 1994 14:170–179. [Abstract] [Google Scholar]
- Chouinard G. Bupropion and amitriptyline in the treatment of depressed patients. J Clin Psychiatry. 1983;44:121–129. [Abstract] [Google Scholar]
- Ferris RM, Cooper BR. Mechanism of antidepressant activity of bupropion. J Clin Psychiatry Monograph. 1993;11(1):2–14. [Abstract] [Google Scholar]
- GlaxoSmithKline. data on file, Research Triangle Park, NC. 2002. [Google Scholar]
- Hirschfeld RMA. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry. 2000;61(suppl 6):4–6. [Abstract] [Google Scholar]
- Shore PA, Silver SL, Brodie BB. Interaction of reserpine, serotonin, and lysergic acid diethylamide in brain. Science. 1955;122:284–285. [Abstract] [Google Scholar]
- Stahl SM. Basic psychopharmacology of antidepressants, pt 1: antidepressants have seven distinct mechanisms of action. J Clin Psychiatry. 1998;59(suppl 4):5–14. [Abstract] [Google Scholar]
- Bonhomme N, Esposito E. Involvement of serotonin and dopamine in the mechanism of action of novel antidepressant drugs: a review. J Clin Psychopharmacol. 1998;18:447–454. [Abstract] [Google Scholar]
- Rush AJ, Giles DE, and Schlesser MA. et al. The dexamethasone suppression test in patients with mood disorders. J Clin Psychiatry. 1996 57:470–484. [Abstract] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nature Medicine. 2001;7:541–547. [Abstract] [Google Scholar]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and anxiety disorders. In: Hardman JG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill. 2001 447–483. [Google Scholar]
- Stahl SM. Essential Psychopharmacology. 2nd ed. New York, NY: Cambridge University Press. 2000 [Google Scholar]
- Remeron [package, insert]. West Orange, NJ: Organon. 2002. [Google Scholar]
- Serzone [package, insert]. Princeton, NJ: Bristol-Myers Squibb Company. 2002. [Google Scholar]
- Krystal JH, D'Souza DC, and Sanacora G. et al. Current perspectives on the pathophysiology of schizophrenia, depression, and anxiety disorders. Med Clin North Am. 2001 85:559–577. [Abstract] [Google Scholar]
- Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 suppl 2):1S–9S. [Abstract] [Google Scholar]
- Tatsumi M, Groshan K, and Blakely RD. et al. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997 340:249–258. [Abstract] [Google Scholar]
- Holden C. ed. Random samples: don't go off the Prozac. Science. 2003 301:760–761. [Google Scholar]
- Santarelli L, Saxe M, and Gross C. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003 301:805–809. [Abstract] [Google Scholar]
- Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. [Abstract] [Google Scholar]
- Gelenberg AJ. ed. How do antidepressants work? Biological Therapies in Psychiatry. 2003 26:44. [Google Scholar]
- Ascher JA, Cole JO, and Colin J-N. et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995 56:395–401. [Abstract] [Google Scholar]
- Nomikos GC, Damsma G, and Wenkstern D. et al. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989 2:273–279. [Abstract] [Google Scholar]
- Nomikos GC, Damsma G, and Wenkstern D. et al. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology. 1992 7:7–14. [Abstract] [Google Scholar]
- Li SX, Perry KW, Wong DT. Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of the rat. Neuropharmacology. 2002;42:181–190. [Abstract] [Google Scholar]
- Cooper BR, Hester TJ, Maxwell RA. Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther. 1980;215:127–134. [Abstract] [Google Scholar]
- Golden RN, Rudorfer MV, and Sherer MA. et al. Bupropion in depression, 1: biochemical effects and clinical response. Arch Gen Psychiatry. 1988 45:139–143. [Abstract] [Google Scholar]
- Learned-Coughlin SM, Bergström M, and Savitcheva I. et al. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry. 2003 54:800–805. [Abstract] [Google Scholar]
- Szabó Z, Àrgyelán M, and Kanyó B. et al. The effect of bupropion on the activity of dopamine transporter in depression: preliminary results [abstract]. Eur Neuropsychopharmacol. 2003 13(suppl 4):S210. [Google Scholar]
- Meyer JH, Goulding VS, and Wilson AA. et al. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl). 2002 163:102–105. [Abstract] [Google Scholar]
- Mulrow CD, Williams JW Jr, and Madjukar T. et al. Treatment of depression: newer pharmacotherapies. Rockville, Md: Agency for Health Care Policy and Research, US Dept of Health and Human Services. 1999 AHCPR publication 99-E014. [Abstract] [Google Scholar]
- American Psychiatric Association. Practice Guideline of Patients With Major Depressive Disorder [Revision] Am J Psychiatry. 2000;157(suppl 4):1–45. [Abstract] [Google Scholar]
- American Psychiatric Association. Major Depressive Disorder: A Patient and Family Guide, 2001. Available at: www.psych.org. Accessed June 7, 2001. [Google Scholar]
- Geddes JR, Freemantle N, and Mason J. et al. SSRIs versus other anti-depressants for depressive disorder. Cochrane Database Syst Rev 2000(2):CD001851. [Abstract] [Google Scholar]
- Steffens DC, Krishnan KR, Helms MJ. Are SSRIs better than TCAs? comparison of SSRIs and TCAs: a meta-analysis. Depress Anxiety. 1997;6:10–18. [Abstract] [Google Scholar]
- Montgomery SA, Kasper S. Comparison of compliance between serotonin reuptake inhibitors and tricycles antidepressants: a meta-analysis. Int Clin Psychopharmacol. 1995;9(suppl 4):33–40. [Abstract] [Google Scholar]
- Thase ME, Haight BR, and Richard NE. et al. Remission rates following therapy with bupropion or SSRIs. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- Rush AJ, Batey S, and Donahue R. et al. Does pretreatment anxiety predict response to either bupropion SR or sertraline? J Affect Disord. 2001 64:81–87. [Abstract] [Google Scholar]
- Trivedi MH, Rush AJ, and Carmody TJ. et al. Do bupropion SR and sertraline differ in their effects on anxiety in depressed patients? J Clin Psychiatry. 2001 62:776–781. [Abstract] [Google Scholar]
- Bodkin JA, Lasser RA, and Wines JD. et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant immunotherapy. J Clin Psychiatry. 1997 58:137–145. [Abstract] [Google Scholar]
- Boyer WF, Feighner JP. The combined use of fluoxetine and bupropion [poster]. Presented at the 146th annual meeting of the American Psychiatric Association. 22–27May1993 San Francisco, Calif. [Google Scholar]
- DeBattista C, Solvason HB, and Poirier J. et al. A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol. 2003 23:27–30. [Abstract] [Google Scholar]
- Ramasubbu R. Treatment of resistant depression by adding noradrenergic agents to lithium augmentation of SSRIs. Ann Pharmacother. 2002;36:634–640. [Abstract] [Google Scholar]
- Spier SA. Use of bupropion with SRIs and venlafaxine. Depress Anxiety. 1998;7:73–75. [Abstract] [Google Scholar]
- Yeghiya M, Danielyan A, and Khachatur G. et al. Augmentation of SSRIs with bupropion in treatment resistant depression in adolescents [abstract]. Presented at the 156th annual meeting of the American Psychiatric Association. 17–22May2003 San Francisco, Calif. [Google Scholar]
- Ashton AK, Rosen RC. Bupropion as an antidote for serotonin reuptake inhibitor–induced sexual dysfunction. J Clin Psychiatry. 1998;59:112–115. [Abstract] [Google Scholar]
- Clayton AH, Warnock J, and Kornstein SG. et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. J Clin Psychiatry. 2004 65:62–67. [Abstract] [Google Scholar]
- DeBattista C, Solvason HB, and Fleming S. et al. A placebo-controlled, double-blind study of bupropion SR in the treatment of SSRI-induced sexual dysfunction [poster]. Presented at the 154th annual meeting of the American Psychiatric Association. 5–10May2001 New Orleans, La. [Abstract] [Google Scholar]
- Dording CM, Peterson TJ, and Mischoulon D. et al. The management of SSRI-induced side effects: a survey of psychiatrists. In: New Research Abstracts of the 153rd Annual Meeting of the American Psychiatric Association. 13–18May2000 Chicago, Ill. Abstract NR42:67. [Abstract] [Google Scholar]
- Gitlin MJ, Suri R, and Alshuler L. et al. Bupropion sustained release as a treatment of SSRI-induced sexual side effects. J Sex Marital Ther. 2002 28:131–138. [Abstract] [Google Scholar]
- Kennedy SH, McCann SM, and Masellis M. et al. Combining bupropion SR with venlafaxine, paroxetine, and fluoxetine: a preliminary report on pharmacokinetic, therapeutic, and sexual dysfunction effects. J Clin Psychiatry. 2002 63:181–186. [Abstract] [Google Scholar]
- Labatte LA, Grimes JB, and Hines A. et al. Bupropion treatment of serotonin reuptake antidepressant-associated sexual dysfunction. Ann Clin Psychiatry. 1997 9:241–245. [Abstract] [Google Scholar]
- Masand PS, Ashton A, and Gupta S. et al. Sustained-release bupropion for SSRI-induced sexual dysfunction: a randomized double-blind placebo-controlled, parallel group study. Am J Psychiatry. 2001 158:805–807. [Abstract] [Google Scholar]
- Perlis RH, Fava M, and Nierenberg AA. et al. Strategies for treatment of SSRI-associated sexual dysfunction: a survey of an academic psychopharmacology practice. Harv Rev Psychiatry. 2002 10:109–114. [Abstract] [Google Scholar]
- Solvason HB, DeBattista C, and Kendrick E. et al. Bupropion SR in the treatment of SSRI-induced sexual dysfunction [poster]. Presented at the 40th annual meeting of the New Clinical Drug Evaluation Unit Program (NCDEU). May 30–June 2, 2000 Boca Raton, Fla. [Google Scholar]
- Johnston JA, Schmidt G, and Ascher J. et al. Pharmacokinetic optimization of bupropion SR for smoking cessation. Drugs. 2002 62(suppl 2):11–24. [Abstract] [Google Scholar]
- Barrickman LL, Perry PJ, and Allen AJ. et al. Bupropion versus methylphenidate in the treatment of attention-deficit hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 1995 34:649–657. [Abstract] [Google Scholar]
- Hudziak JJ, Wilens TE, and Rosenthal NE. et al. The efficacy of extended-release bupropion in adult ADHD [poster]. Presented at the 42nd annual meeting of the American College of Neuropsychopharmacology. 7–11December2003 San Juan, Puerto Rico. [Google Scholar]
- Conners CK, Casat CD, and Gualtieri CT. et al. Bupropion hydrochloride in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996 34:1314–1321. [Abstract] [Google Scholar]
- Rosenthal NE, Modell JG, and Harriett A. et al. Wellbutrin XL for the prevention of seasonal depressive episodes [poster]. Presented at the 42nd annual meeting of the American College of Neuropsychopharmacology. 7–11December2003 San Juan, Puerto Rico. [Google Scholar]
- Depue RA, Arbisi P, and Krauss S. et al. Seasonal independence of low prolactin concentrations and high spontaneous eye blink rates in unipolar and bipolar II seasonal affective disorder. Arch Gen Psychiatry. 1990 47:356–364. [Abstract] [Google Scholar]
- Neumeister A, Turner EH, and Matthews JR. et al. Effects of tryptophan depletion vs catecholamine depletion in patients with seasonal affective disorder in remission with light therapy. Arch Gen Psychiatry. 1998 55:524–530. [Abstract] [Google Scholar]
- Partonen T. Dopamine and circadian rhythms in seasonal affective disorder. Med Hypotheses. 1996;47:191–192. [Abstract] [Google Scholar]
- Sachs GS, Lafer B, and Stoll AL. et al. A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry. 1994 55:391–393. [Abstract] [Google Scholar]
- Sachs GS, Printz DJ, and Kahn DA. et al. The Expert Consensus Guideline Series: Medication Treatment of Bipolar Disorder 2000. Postgrad Med. 2000 Special Report. 1–102. [Abstract] [Google Scholar]
- Haykal RF, Akiskal HS. Bupropion as a promising approach to rapid cycling bipolar II patients. J Clin Psychiatry. 1990;51:450–455. [Abstract] [Google Scholar]
- Ferris RM, Cooper BR, Maxwell RA. Studies of bupropion's mechanism of antidepressant activity. J Clin Psychiatry. 1983;44:74–78. [Abstract] [Google Scholar]
- Wilens TE, Prince JB, and Spencer T. et al. An open trial of bupropion for the treatment of adults with attention-deficit/hyperactivity disorder and bipolar disorder. Biol Psychiatry. 2003 54:9–16. [Abstract] [Google Scholar]
- Richelson E. Pharmacology of antidepressants: characteristics of the ideal drug. Mayo Clin Proc. 1994;69:1069–1081. [Abstract] [Google Scholar]
- Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry. 1998;59(suppl 18):23–29. [Abstract] [Google Scholar]
- Horst WD, Preskorn SH. Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affect Disord. 1998;51:237–254. [Abstract] [Google Scholar]
- Settle EC, Stahl SM, and Batey SR. et al. Safety profile of sustained-release bupropion in depression: results of three clinical trials. Clin Ther. 1999 3:454–463. [Abstract] [Google Scholar]
- Clayton AH, West SG. The effects of antidepressants on human sexuality. Primary Psychiatry. 2003;10:62–70. [Google Scholar]
- Rothschild AJ. Sexual side effects of antidepressants. J Clin Psychiatry. 2000;61(suppl 11):28–36. [Abstract] [Google Scholar]
- Clayton AH, Pradko JF, and Croft HA. et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002 63:357–366. [Abstract] [Google Scholar]
- Coleman CC, King BR, and Bolden-Watson C. et al. A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained-release and fluoxetine. Clin Ther. 2001 23:1040–1058. [Abstract] [Google Scholar]
- Segraves RT, Kavoussi R, and Hughes AR. et al. Evaluation of sexual functioning in depressed outpatients: a double-blind comparison of sustained-release bupropion and sertraline treatment. J Clin Psychopharmacol. 2000 20:122–128. [Abstract] [Google Scholar]
- Modell JG, Katholi CR, and Modell JD. et al. Comparative sexual side effects of bupropion, fluoxetine, paroxetine, and sertraline. Clin Pharmacol Ther. 1997 61:476–487. [Abstract] [Google Scholar]
- Clayton AH, McGarvey EL, and Abouesh AI. et al. Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J Clin Psychiatry. 2001 62:185–190. [Abstract] [Google Scholar]
- Walker PW, Cole JO, Gardner EA. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry. 1993;54:459–463. [Abstract] [Google Scholar]
- Ansseau M, van Frenckell R, and Mertens C. et al. Controlled comparison of two doses of milnacipran and amitriptyline in major depressive inpatients. Psychopharmacology (Berl). 1989 98:163–168. [Abstract] [Google Scholar]
- Berken GH, Weinstein DO, Stern WC. Weight gain: a side effect of tricyclic antidepressants. J Affect Disord. 1984;7:133–138. [Abstract] [Google Scholar]
- Fernstrom MH, Krowinski RL, Kupfer DJ. Chronic imipramine treatment and weight gain. Psychiatry Res. 1986;17:269–273. [Abstract] [Google Scholar]
- Fernstrom MH, Kupfer DJ. Antidepressant-induced weight gain: a comparison study of four medications. Psychiatry Res. 1988;26:265–271. [Abstract] [Google Scholar]
- Garland EJ, Remick RA, Zis AP. Weight gain with antidepressants and lithium. J Clin Psychopharmacol. 1988;8:323–330. [Abstract] [Google Scholar]
- Stocchi F, Nordera G, and Jokinen RH. et al, for the Paroxetine Generalized Anxiety Disorder Study Team. Efficacy and tolerability of paroxetine for the long-term treatment of generalized anxiety disorder. J Clin Psychiatry. 2003 64:250–258. [Abstract] [Google Scholar]
- Michelson D, Amsterdam JD, and Quitkin FM. et al. Changes in weight during a 1-year trial of fluoxetine. Am J Psychiatry. 1999 156:1170–1176. [Abstract] [Google Scholar]
- Croft H, Houser TL, and Jamerson BD. et al. Effect on body weight of bupropion sustained-release in patients with major depression treated for 52 weeks. Clin Ther. 2002 24:662–672. [Abstract] [Google Scholar]
- Harto-Truax N, Stern WC, and Miller LL. et al. Effects of bupropion on body weight. J Clin Psychiatry. 1983 44:183–186. [Abstract] [Google Scholar]
- Westlund R, Haight BR, and Harriett A. et al. Effect on body weight of bupropion in patients with major depression [poster]. Presented at the 16th Annual US Psychiatric & Mental Health Congress. 6–9November2003 Orlando, Fla. [Google Scholar]
- Anderson JW, Greenway FL, and Fojioka K. et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002 10:633–641. [Abstract] [Google Scholar]
- Jain AK, Kaplan RA, and Gadde KM. et al. Bupropion SR for weight loss in obese patients with depressive symptoms: results of a double-blind, placebo-controlled study. Obes Res. 2002 10:1049–1056. [Abstract] [Google Scholar]
- Wellman PJ, Davies BR, and Morien A. et al. Modulation of feeding by hypothalamic paraventricular nucleus alpha 1- and alpha 2-adrenergic receptor. Life Sci. 1993 53:669–679. [Abstract] [Google Scholar]
- Terry P, Gilbert DB, Cooper SJ. Dopamine receptor subtype agonists and feeding behavior. Obes Res. 1995;3(suppl 4):515S–523S. [Abstract] [Google Scholar]
- Celexa [package, insert]. St Louis, Mo: Forest Laboratories. 2002. [Google Scholar]
- Prozac [package, insert]. Indianapolis, Ind: Eli Lilly and Company. 2002. [Google Scholar]
- Paxil [package, insert]. Research Triangle Park, NC: GlaxoSmithKline. 2002. [Google Scholar]
- Zoloft [package, insert]. New York, NY: Pfizer Inc. 2002. [Google Scholar]
- Effexor [package, insert]. Philadelphia, Pa: Wyeth. 2002. [Google Scholar]
- Jick H, Dinan B, and Hunter JR. et al. Tricyclic antidepressants and convulsions. J Clin Psychopharmacol. 1983 3:182–185. [Abstract] [Google Scholar]
- Lowry MR, Dunner FJ. Seizures during tricyclic therapy. Am J Psychiatry. 1980;137:1461–1462. [Abstract] [Google Scholar]
- Rosenstein DL, Nelson JC, Jacobs SC. Seizures associated with antidepressants: a review. J Clin Psychiatry. 1993;54:289–299. [Abstract] [Google Scholar]
- Pisani F, Oteri G, and Costa C. et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002 25:91–110. [Abstract] [Google Scholar]
- Pisani F, Spina E, Oteri G. Antidepressant drugs and seizure susceptibility: from in vitro data to clinical practice. Epilepsia. 1999;40(suppl 10):S48–S56. [Abstract] [Google Scholar]
- Wellbutrin [package, insert]. Research Triangle Park, NC: GlaxoSmithKline. 2002. [Google Scholar]
Articles from Primary Care Companion to The Journal of Clinical Psychiatry are provided here courtesy of Physicians Postgraduate Press, Inc.
Full text links
Read article at publisher's site: https://doi.org/10.4088/pcc.v06n0403
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc514842?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.4088/pcc.v06n0403
Article citations
Recent advances in the synthesis of antidepressant derivatives: pharmacologic insights for mood disorders.
3 Biotech, 14(11):260, 05 Oct 2024
Cited by: 0 articles | PMID: 39376479
Review
Role of vesicular monoamine transporter-2 for treating attention deficit hyperactivity disorder: a review.
Psychopharmacology (Berl), 241(11):2191-2203, 20 Sep 2024
Cited by: 0 articles | PMID: 39302436
Review
Glutamatergic Modulators for Major Depression from Theory to Clinical Use.
CNS Drugs, 38(11):869-890, 16 Aug 2024
Cited by: 0 articles | PMID: 39150594 | PMCID: PMC11486832
Review Free full text in Europe PMC
Interactions of antidepressants with concomitant medications-safety of complex therapies in multimorbidities.
Pharmacol Rep, 76(4):714-739, 16 Jul 2024
Cited by: 0 articles | PMID: 39012418 | PMCID: PMC11294384
Review Free full text in Europe PMC
Bupropion-disguised chest pain presenting in a middle-aged male: a case report and review of literature.
Ann Med Surg (Lond), 86(7):4213-4216, 28 May 2024
Cited by: 0 articles | PMID: 38989170
Go to all (245) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Other Antidepressants.
Handb Exp Pharmacol, 250:325-355, 01 Jan 2019
Cited by: 19 articles | PMID: 30194544
15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL.
Prim Care Companion J Clin Psychiatry, 7(3):106-113, 01 Jan 2005
Cited by: 141 articles | PMID: 16027765 | PMCID: PMC1163271
The efficacy and tolerability of bupropion in the treatment of major depressive disorder.
Clin Drug Investig, 31 Suppl 1:5-17, 01 Oct 2011
Cited by: 17 articles | PMID: 22015858
Review
Bupropion: a review of its use in the management of major depressive disorder.
Drugs, 68(5):653-689, 01 Jan 2008
Cited by: 59 articles | PMID: 18370448
Review





