Abstract
Free full text

Microglial Phagocytosis of Fibrillar β-Amyloid through a β1 Integrin-Dependent Mechanism
Abstract
Microglia are the principle immune effector and phagocytic cells in the CNS. These cells are associated with fibrillar β-amyloid (fAβ)-containing plaques found in the brains of Alzheimer's disease (AD) patients. The plaque-associated microglia undergo a phenotypic conversion into an activated phenotype and are responsible for the development of a focal inflammatory response that exacerbates and accelerates the disease process. Paradoxically, despite the presence of abundant activated microglia in the brain of AD patients, these cells fail to mount a phagocytic response to Aβ deposits but can efficiently phagocytose Aβ fibrils and plaques in vitro.
We report that exposure of microglia to fAβ in vitro induces phagocytosis through mechanisms distinct from those used by the classical phagocytic receptors, the Ig receptors (FcRγI and FcγRIII) or complement receptors. Microglia interact with fAβ through a recently characterized Aβ cell surface receptor complex comprising the B-class scavenger receptor CD36, α6β1 integrin, and CD47 (integrin-associated protein). Antagonists specific for each component of the receptor complex blocks fAβ-stimulated phagocytosis. These data demonstrated that engagement of this ensemble of receptors is required for induction of phagocytosis. The phagocytic response stimulated by this receptor complex is driven principally by a β1 integrin-linked process that is morphologically and mechanistically distinct from the classical type I and type II phagocytic mechanisms. These data provide evidence for phagocytic uptake of fAβ through a receptor-mediated, nonclassical phagocytic mechanism.
Introduction
Microglia are the principal immune effector cell in the brain and are competent phagocytes (Streit et al., 2004). Phagocytosis is stimulated by specific epitopes on phagocytic targets and requires activation of downstream signaling cascades that lead to the rearrangement of the actin cytoskeleton and engulfment of the particle (Aderem and Underhill, 1999). The two most prominent microglia phagocytic receptors are the Ig receptors (FcRγI and FcγRIII/FcR) and the complement receptor 3 (CR3). Engagement of the FcR by immune IgG initiates type I phagocytosis (Kaplan, 1977; Caron and Hall, 1998), characterized by extension of pseudopodia and envelopment of the phagocytic target. This mechanism relies on the action of tyrosine kinases and the small G-proteins Rac and Cdc42. In contrast, type II phagocytosis results from ligation of CR3, a β2 integrin (also known as Mac-1 orαmβ2), by complement-opsonized particles (Kaplan, 1977). This phagocytic mechanism is dependent on activation of RhoA (Caron and Hall, 1998) and requires microtubules (Newman et al., 1991). Engagement of CR3 leads to a “passive” phagocytic response, with the phagocytic target sinking into the cell membrane.
It has been appreciated recently that the enteropathogenic bacteria Yersinia uses a novel mechanism for stimulating its phagocytic uptake by macrophages. The Yersinia cell surface protein invasin binds to the β1 subunit of various integrin pairs (Isberg et al., 2000) and stimulates phagocytosis. Although the signaling mechanism for this phagocytic pathway has not been completely elucidated, Rac1 activation is a critical step, driving actin polymerization and phagosome formation (McGee et al., 2003).
The brains of Alzheimer's disease (AD) patients are characterized by extensive deposition of fibrillar forms of β-amyloid (fAβ), which activate nearby microglial cells. Microglia interact with fAβ through a recently identified cell surface receptor complex (Bamberger et al., 2003). The functional components of this receptor complex include the α6β1 integrin, CD47, and the B-class scavenger receptor CD36. The binding of fAβ to this ensemble of receptors results in the activation of tyrosine kinase-based signaling cascades, leading to an acquisition of a proinflammatory phenotype (McDonald et al., 1997; Combs et al., 1999). The activation of microglia elicits both cytokine and chemokine production, resulting in the recruitment of microglia to Aβ-containing plaques (Rogers and Lue, 2001). However, microglia fail to efficiently remove amyloid deposits from the AD brain or its murine models. Indeed, serial three-dimensional reconstruction of Aβ plaques in an animal model of AD revealed that the Aβ deposits were enveloped by microglial processes but were not internalized by microglia (Stalder et al., 2001). Paradoxically, microglial cells have a well documented capacity to mount a phagocytic response to fAβ (Ard et al., 1996; Paresce et al., 1996; Bard et al., 2000) or to isolated senile plaques in vitro (DeWitt et al., 1998). Significantly, in animal models of AD, the administration of antibodies to Aβ, either directly to the brain (Lombardo et al., 2003) or after either active (Schenk et al., 1999) or passive (Bard et al., 2000; Wilcock et al., 2003) immunization, results in phagocytic removal of Aβ deposits. These data argue that in vivo microglia can mount a robust phagocytic response if stimulated by antibody-opsonized Aβ through engagement of microglial FcR. Golde and colleagues have provided data that argue alternative mechanisms are also involved in removal of Aβ from the brain (Das et al., 2003).
The aim of this study was to investigate the molecular mechanisms subserving microglia phagocytosis of fAβ in vitro to gain insight into how this response is regulated in the brain. We report that engagement of the microglial Aβ cell surface receptor complex by fAβ peptides stimulates phagocytosis through a novel β1 integrin-linked process that is mechanistically distinct from classical phagocytic mechanisms.
Materials and Methods
Materials. Zymosan was purchased from Sigma (St. Louis, MO) and was opsonized with complement by incubating with mouse or rabbit serum for 30 min at 37°C, followed by 10 washes with 0.85% NaCl before resuspension in PBS. Immune IgG was prepared from goat anti-rabbit IgG from Sigma and rabbit anti-mouse IgG from Cappel (Aurora, OH) in a 5:1 ratio. The anti-α-actinin antibody, fucoidan, and nocodazole were also purchased from Sigma. Aβ25-35, Aβ1-42, Aβ42-1, and amylin peptides were purchased from American Peptide Company (Sunnyvale, CA). Aβ peptides corresponding to amino acids 25-35 and 1-42 were dissolved in sterile water and incubated for 5 d at 37°C to allow for fibrilization. We cannot rule out that the Aβ1-42 preparation may contain some oligomers. 4N1K and RHD-containing peptides were purchased from Bachem (Torrence, CA). Glutathione S-transferase (GST)-CD36 peptide was a gift from Dr. S. Frieda Pearce (Cornell University, Ithaca, NY). The anti-β1 antibody was purchased from Immunotech (Marseille, France). The Mac-1 antibody was purchased from Chemicon (Temecula, CA). The anti-CD18 antibody was purchased from BD Biosciences (Lexington, KY). Invasin was a gift from Dr. Ralph Isberg (Tufts University, Medford, MA). SP-DiOC18, Nile red fluorospheres (1 μm microspheres), AlexaFluor 488 phalloidin, and AlexaFluor 488 goat anti-mouse IgM were purchased from Molecular Probes (Eugene, OR). Cy3 was purchased from Amersham Biosciences (Piscataway, NJ). Piceatannol, LY294002, and 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo [3,4d]pyrimadine (PP2) were purchased from Alexis Biochemicals (San Diego, CA). Blebbistatin was purchased from Calbiochem (La Jolla, CA).
Tissue culture. The immortalized murine microglia cell line BV-2 was grown and maintained in DMEM containing 2% fetal bovine serum) and gentamycin in 5% CO2. Primary microglia were derived from postnatal day 1-2 mouse brains (C57BL/6J) as described previously (McDonald et al., 1997).
Phagocytosis assay. BV-2 cells were collected, and 1 × 106 cells were plated in 35 mm plates overnight. The medium was removed and replaced with serum-free DMEM. After 3 hr, the cells were treated for 30 min in the absence or presence of antagonists to receptor subunits: 4N1K, GST-CD36, fucoidan, the RHD-containing peptide, anti-β1 antibody, invasin, Mac-1 antibody, and anti-CD18 antibody, or in the presence or absence of signaling inhibitors (nocodazole, piceatannol, PP2, LY294002, or blebbistatin). The cells were then stimulated by the addition of 60 μm (63.6 μg/ml) fAβ25-35 peptide, 5 μm (22.6 μg/ml) fAβ1-42 peptide, 1 mg/ml immune IgG, 1 mg/ml opsonized zymosan, or 10 μm (39 μg/ml) amylin, unless otherwise stated, for 30 min. Fluorescent microspheres were then added to the cells for 30 min after having been washed in PBS containing 1 mg/ml BSA. The fluorescent microspheres were used as a marker of fluid phase phagocytosis. Cells were then fixed with 2% paraformaldehyde, and three random fields of cells (>100 cells) were counted on an inverted microscope.
To determine phagocytic efficiency, the number of microspheres per cell were counted. The microspheres per cell were assigned points that increased linearly such that a cell received 1 point for ingesting one microsphere, 2 points for two microspheres, and so on, up to a maximum of 6 points for more than five microspheres ingested per cell. The number of cells containing x microspheres was multiplied by the point value. Each of these values are then added together and divided by the total number of cells counted. The phagocytic efficiency is based on a weighted average of ingested microspheres per cell.
Preparation of Cy3-labeled Aβ1-42. Aβ1-42 was dissolved in sterile water and then diluted to a concentration of 1 μg/μl in 50 mm sodium borate, pH 9.3 (Paresce et al., 1996). Cy3 (200μg) was added to Aβ1-42 (250 μg) solution and rotated for 30 min at room temperature. The labeled fibrillar peptide was separated from unincorporated dye by centrifugation.
Phagocytosis of Cy3-labeled Aβ1-42. Coverslips were coated with poly-l-lysine before plating 150,000 BV-2 cells per coverslip resting in a 24-well plate overnight. The medium was changed to serum-free DMEM, and, 3 hr later, the cells were incubated in the presence or absence of unlabeled fAβ25-35 for 30 min. Cy3-labeled fAβ1-42 (Cy3Aβ) was then added. After 30 min, the cells were washed with cold PBS. The cells were then fixed with 4% paraformaldehyde and washed again with cold PBS. To label the internal and external membranes of the cells, 2 μm SP-DiOC18 was added to the cells for 10 min. The cells were again washed with PBS before being rinsed with water and mounted on a glass slide. The slides were then viewed, and images were obtained using a confocal microscope.
Phagocytosis of immobilized and free stimulating ligands. BV-2 cells were collected, and 1 × 106 cells were plated in 35 mm plates overnight. The medium was changed to serum-free DMEM, and, after 3 hr, the cells were treated with 60 μm (63.6 μg/ml) fAβ25-35 peptide, 1 mg/ml immune IgG, or 1 mg/ml opsonized zymosan, and fluorescent microspheres coated in PBS containing 1 mg/ml BSA were then added to the wells stimulated with free ligands. The immobilized samples comprised microspheres, which were coated with the respective stimulating ligand (Kopec and Carroll, 1998). To coat the microspheres, the same concentration of stimulating ligand was added to the microspheres and placed in a 37°C incubator for 30 min. After cells had been exposed to ligands and microspheres for 40 min, they were fixed with 2% paraformaldehyde, and three random fields of cells were counted.
Phalloidin staining. BV-2 cells were collected and then plated on coverslips (150,000 BV-2 cells per coverslip) resting in a 24-well plate overnight. The medium was changed to serum-free DMEM, and, 3 hr later, the cells were incubated in the presence or absence of the stimulating ligand for 30 min before the addition of microspheres for 30 min. The cells were rinsed with PBS before being fixed in 2% paraformaldehyde and washed again in PBS. The cells were then incubated at room temperature with 0.1% Triton X-100 buffer for 5 min and washed again in PBS. Finally, AlexaFluor 488 phalloidin (1.6 U diluted in PBS) was added to the coverslips and incubated at room temperature protected from light for 30 min. The coverslips were mounted on glass slides.
α-Actinin staining. The BV-2 cells were prepared for α-actinin staining as described above. After incubation with 0.1% Triton X-100, the cells were then incubated with blocking buffer composed of PBS containing 10% goat serum and 0.1% Triton X-100 buffer for 30 min. The cells were then incubated with anti-α-actinin antibody (1:500) in blocking buffer overnight at 4°C protected from light. The coverslips were then washed with PBS and incubated for 2 hr with blocking buffer containing AlexaFluor488 goat anti-mouse IgM at room temperature protected from light. The coverslips were washed four times with PBS before being dipped in water and mounted on glass slides.
Results
BV-2 cells exhibit ligand-stimulated phagocytosis through classical and nonclassical mechanisms
We have established a microglial phagocytosis assay that measures ligand-stimulated phagocytosis as monitored by the ingestion of fluorescent microspheres. BV-2 cells were used because they have been proven to mimic behaviors of primary microglia (Bocchini et al., 1992; Laurenzi et al., 2001) and exhibit a robust phagocytic response (Kopec and Carroll, 1998). We initially demonstrated that these cells could be stimulated to phagocytose during activation of the classical phagocytic receptors, FcR or CR3, after incubation with immune IgG or opsonized zymosan, respectively (Fig. 1C,D). These data demonstrate the functional linkage of these receptors to the phagocytic machinery of the cell.

BV-2 cells exhibit ligand-stimulated phagocytosis through classical and nonclassical mechanisms. Dose dependence of phagocytosis stimulated by fAβ25-35 (A), fAβ1-42 (B), immune IgG (C), mouse complement-opsonized zymosan (D) (dark speckles), and rabbit complement-opsonized zymosan (light speckles) in BV-2 cells is shown. BV-2 cells were incubated with the indicated ligand for 30 min before being exposed to microspheres for an additional 30 min. E, The number of microspheres taken up per BV-2 cell in untreated cells or treated with fAβ25-35 (60 μm; 63.6 μg/ml), immune IgG (1 mg/ml), and mouse complement-opsonized zymosan (mOZ) (1 mg/ml) are shown. BV-2 cells were incubated with the indicated ligand for 30 min before the addition of microspheres for 30 min. F, Phagocytic efficiency of cells stimulated with fAβ25-35 (60 μm; 63.6 μg/ml), immune IgG (1 mg/ml), and mouse complement-opsonized zymosan (mOZ) (1 mg/ml). G, BV-2 cells were treated with amylin (10 μm; 39 μg/ml), fAβ25-35 (60 μm; 63.6 μg/ml), invasin397 (Inv 397) (5 μg/ml), Aβ42-1 (5 μm; 22.6 μg/ml), the reverse β amyloid peptide, nonfibrillar (n-f) Aβ25-35 (60 μm; 63.6 μg/ml), and invasin 195 (Inv 195) (5 μg/ml) for 30 min before a 30 min incubation with microspheres. H, Antibodies directed against CR3 [Mac-1 (5 μg/ml) and CD18 (5 μg/ml)] were added to BV-2 cells before exposure to the stimulating ligand. These experiments are representative of three independent studies. *p < 0.01; **p < 0.001 compared with control.
Exposure of the microglia to fAβ peptides stimulated a phagocytic response of similar magnitude. We found that both fAβ1-42 and fAβ25-35 were able to elicit phagocytic responses by the microglial cells (Fig. 1A,B). We have documented previously that microglia respond to fibrillar forms (but not nonfibrillar forms) of Aβ1-40, Aβ1-42, and Aβ25-35 (Terzi et al., 1994) by stimulation of intracellular signaling cascades (McDonald et al., 1997; Combs et al., 1999; Bamberger et al., 2003). When the experiments were conducted at 4°C, stimulation of phagocytosis was not detected (data not shown). The reverse Aβ peptide, Aβ42-1, as well as nonfibrillar Aβ25-35, were unable to stimulate phagocytosis, demonstrating that only fibrillar Aβ peptides can elicit this response (Fig. 1G). Similarly, human amylin fibrils were also able to stimulate phagocytosis of microspheres (Fig. 1G). Stimulation of microglia resulted in both an increase in the fraction of phagocytic cells and the efficiency of uptake of microspheres (Fig. 1E,F). Phagocytic efficiency is defined as the weighted average of microspheres ingested per stimulus, as described in Materials and Methods.
The specificity of the phagocytic response was tested with antibodies directed against CR3. BV-2 cells were pretreated with antibodies directed against CR3 (anti-CD18 antibody and Mac-1 antibody) before addition of stimulating ligands. The antibodies inhibited only phagocytosis stimulated by opsonized zymosan without affecting fAβ and immune IgG-mediated phagocytosis (Fig. 1H). Addition of the anti-CR3 antibodies in the absence of phagocytic stimulating ligands did not elicit a phagocytic response. Thus, the CR3-mediated phagocytic response is elicited exclusively by opsonized zymosan, not fAβ or immune IgG.
The β1 integrin has been shown recently to be responsible for the phagocytic uptake of the bacterium Yersinia by macrophages (Dersch and Isberg, 2000; Isberg et al., 2000). The Yersinia cell surface protein invasin specifically and uniquely binds to the β1 integrin component of various integrin pairs. We tested the ability of two forms of invasin for their ability to stimulate phagocytosis in BV-2 cells. A truncated form of invasin, invasin195, binds to the β1 integrin but does not stimulate microglial phagocytosis (Fig. 1G). The full-length form of invasin, invasin397, binds to the β1 integrin and activates intracellular signaling cascades, which stimulates the phagocytic machinery (Wiedemann et al., 2001). Indeed, invasin397 elicited a phagocytic response during exposure to BV-2 microglial cells (Fig. 1G), demonstrating that this nonclassical mechanism is operational in these cells.
Based on our finding that the various ligands stimulated the phagocytic receptors on BV-2 cells, we next examined whether the ligand was a more efficient stimulus bound to the phagocytic target or free in solution. We assessed this by comparing phagocytosis in cells that were incubated with the stimulus added to the medium compared with cells that had been exposed to microspheres coated with the stimulating ligand. We established that cells stimulated with free or bound ligands both stimulated phagocytosis; however, ligands free in the surrounding media exhibited a modestly higher level of phagocytosis than cells that were exposed to ligands immobilized on the microsphere surface (Fig. 2). The results were similar for all of the stimulating ligands. The enhanced phagocytic response is likely attributable to engagement of a larger number of receptors by the free ligands compared with cells that contacted ligand only on the surface of the microspheres.
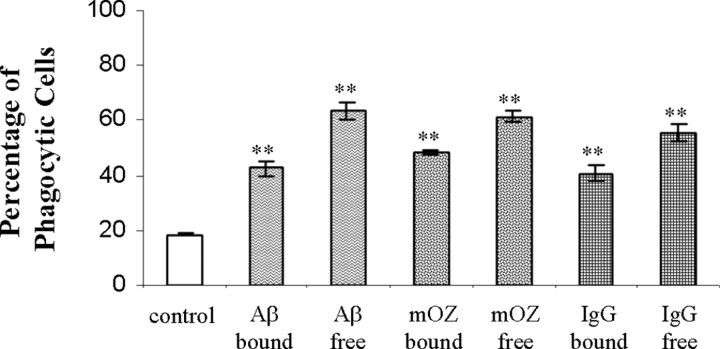
Free and immobilized ligands both stimulate phagocytosis. BV-2 cells were exposed to fAβ25-35, immune IgG, or mouse complement-opsonized zymosan (mOZ) either free in the media or immobilized on the surface of the microspheres for 40 min before being scored for ingestion of microspheres. The data shown are representative of three independent experiments. **p < 0.001 compared with control.
BV-2 cells phagocytose Aβ1-42 fibrils
To directly establish that the BV-2 microglia phagocytose fAβ, the cells were exposed to Cy3Aβ, allowing direct visualization of Aβ fibrils. Confocal images were taken of BV-2 cells after incubation with Cy3Aβ. To verify uptake by the phagocytes, Z-scan images through the microglia were obtained and showed that the Cy3Aβ has been internalized and is not above the cell or adherent to the cell surface (Fig. 3). The cells exhibited a more robust phagocytic response after a pretreatment with unlabeled fAβ25-35 for 30 min before the addition of Cy3Aβ. This result is attributable to higher receptor occupancy and enhanced stimulation of the phagocytic response after the priming of cells with the unlabeled fAβ peptide. This experiment demonstrated that fAβ stimulates phagocytic machinery of the BV-2 cells and confirms that the cells were capable of ingesting fAβ. The Cy3Aβ is trafficked into phagosomes after having been ingested. These findings are consistent with those of Ard et al. (1996) who observed in electron micrographs that anti-Aβ-immunopositive material was localized in membrane-bound intracellular vesicles.
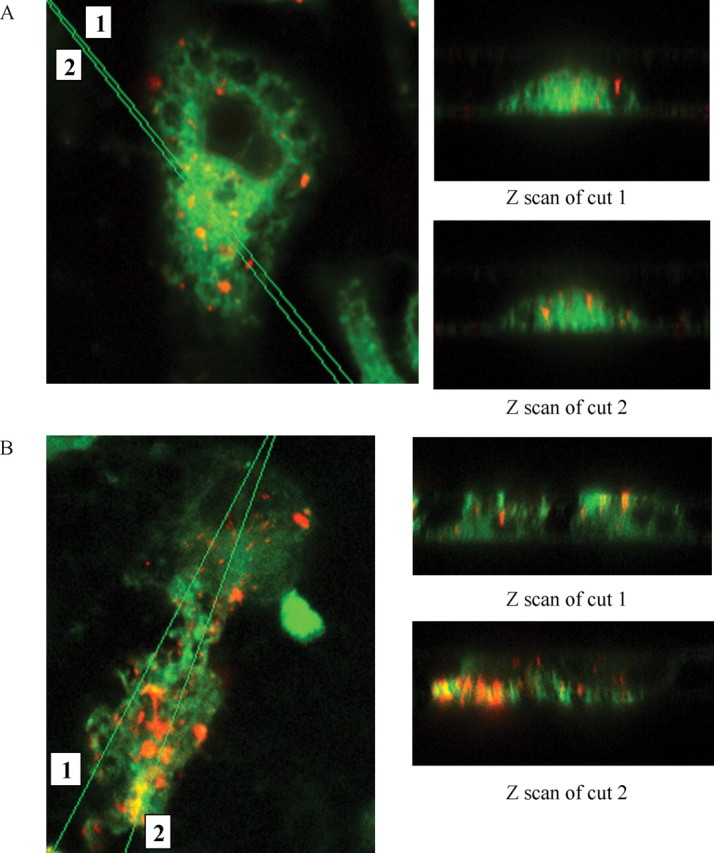
BV-2 cells phagocytose fibrillar Aβ1-42 labeled with Cy3. Confocal images (100×) of BV-2 cells ingesting Cy3-labeled fAβ1-42 are shown in the x-y plane, as well as Z scans of cuts through the cells. A, BV-2 cells were incubated with Cy3Aβ (40μg/ml) for 30 min before being fixed and stained with DiO (green) to label internal and external cell membranes. B, BV-2 cells were pretreated with unlabeled fAβ25-35 (60 μm) for 30 min before adding Cy3Aβ (40 μg/ml). The ingested Cy3Aβ (red) is trafficked to intracellular vesicles. This experiment is representative of three independent experiments.
Antagonists to the microglial Aβ cell surface receptor complex inhibit fAβ-mediated phagocytosis
Recently, we have identified a multicomponent microglial Aβ cell surface receptor complex (Bamberger et al., 2003). The individual subunits of this receptor complex bind fAβ and then act in concert to initiate intracellular signaling and proinflammatory activation of microglia and monocytes. To test whether the microglial Aβ cell surface receptor complex was mechanistically linked to the phagocytic machinery and the induction of phagocytosis, we evaluated the effects of a series of antagonists to elements of the receptor complex. The peptide antagonist 4N1K is derived from thrombospondin and binds to CD47, blocking the interaction of CD47 with other ligands (Chung et al., 1997). Incubation of cells with 4N1K before fAβ stimulation of phagocytosis inhibited ingestion of microspheres (Fig. 4A). The CD47 antagonist did not affect phagocytosis induced by opsonized zymosan or immune IgG. Fucoidan acts to inhibit both class A and B scavenger receptor interactions (Husemann et al., 2002). GSTCD36 comprises a peptide derived from the extracellular binding domain of CD36 coupled with a GST tag. This peptide specifically blocks the interaction between B-class scavenger receptor CD36 and other ligands (Pearce et al., 1995). Both of the scavenger receptor antagonists, GST-CD36 and fucoidan, inhibited fAβ-stimulated phagocytosis yet had no effect on the engulfment of microspheres stimulated by opsonized zymosan or immune IgG (Fig. 4A). To establish the participation of the α6β1 integrin in fAβ-stimulated phagocytosis, three antagonists directed toward the β1 integrin were used. A peptide containing an RHD epitope that binds β1 integrins (Ghiso et al., 1992) exhibited an inhibitory effect, as did the anti-β1 antibody (Fig. 4A,B). A truncated form of the highly specific β1 integrin ligand invasin195 acts as a functional antagonist of β1 integrin activation that is responsible for initiating the phagocytosis of Yersinia (Isberg et al., 2000; Wiedemann et al., 2001). Importantly, invasin195 blocked fAβ-stimulated phagocytosis (Fig. 4C). These data provide direct evidence for the requirement of fAβ interaction with the β1 integrin to stimulate phagocytosis. The above results demonstrate that each of the receptor complex components is essential and that they act in concert to initiate phagocytosis stimulated by fAβ.
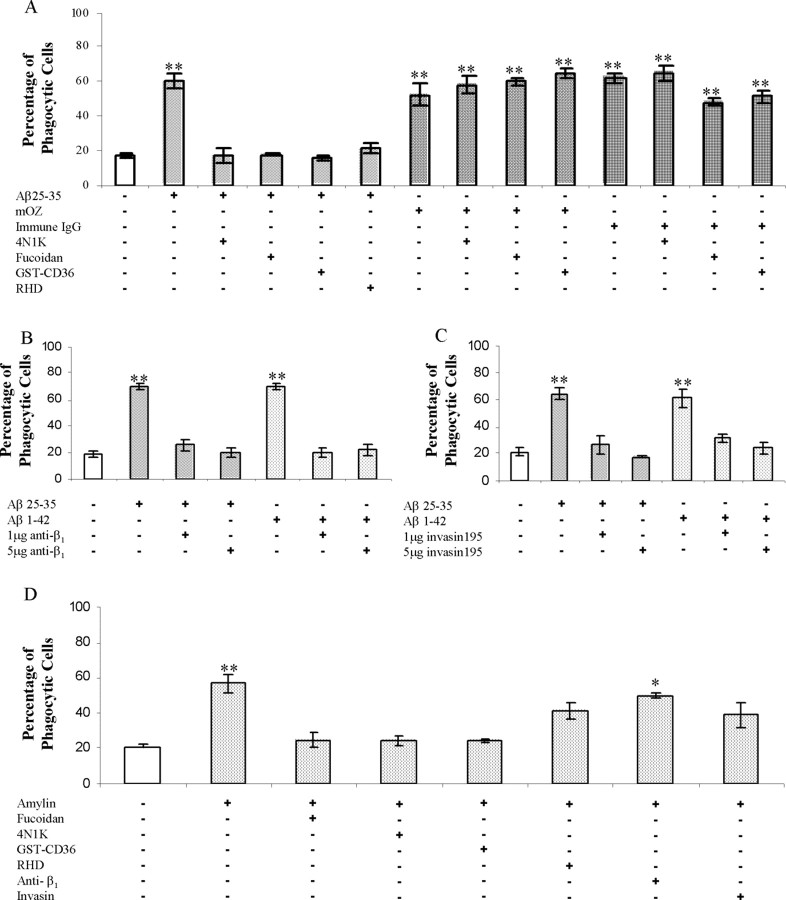
fAβ elicits phagocytosis through stimulation of the microglial Aβ cell surface receptor complex. A, Antagonists of the microglial Aβ cell surface receptor complex were tested for their ability to inhibit fAβ-mediated phagocytosis. Antagonists of CD47 (100 μm 4N1K), the β1 integrin (100 μg/ml RHD), and scavenger receptors (300 μg/ml fucoidan and 100 nm GST-CD36) were added to BV-2 cells 30 min before stimulation with the indicated ligand. mOZ, Mouse complement-opsonized zymosan. B, C, The anti-β1 antibody as well as invasin 195, a specificβ1 integrin ligand, were added to BV-2 cells 30 min before stimulation with fAβ. D, Antagonists were added to BV-2 for 30 min before amylin stimulation using the same concentrations as in A. These experiments are representative of three independent experiments. *p < 0.01; **p < 0.001 compared with control.
Microglia and macrophages recognize and respond to other fibrillar peptides. We evaluated the specificity of the microglial Aβ cell surface receptor complex by testing the ability of the fibrillar peptide amylin to induce phagocytosis. Amylin fibrils stimulated a phagocytic response by BV-2 microglia (Figs. (Figs.1G,1G, ,4D).4D). The specificity of the amylin phagocytic response was tested by the addition of antagonists to the Aβ receptor complex. Importantly, CD47 and scavenger receptor antagonists blocked amylin-stimulated phagocytosis (Fig. 4D). However, β1 integrin antagonists did not produce a strong inhibition of phagocytosis. These data suggest that amylin fibrils are recognized by microglia through an analogous but distinct receptor complex that includes CD47 and scavenger receptors but not the β1 integrin.
fAβ stimulates phagocytosis in primary microglia
Primary murine microglia were evaluated for their capacity to phagocytose microspheres. Microglia stimulated with fAβ25-35 or fAβ1-42 induced phagocytosis of microspheres (Fig. 5A). Antagonists directed against elements of the microglial Aβ cell surface receptor complex inhibited this stimulation of phagocytosis (Fig. 5B). These data establish that the microglial Aβ cell surface receptor complex is present and functions as a phagocytic receptor on primary microglia.

fAβ stimulates phagocytosis through the microglial Aβ cell surface receptor complex in primary murine microglia. A, Neonatal primary microglial cells were treated with fAβ25-35 (60 μm; 63.6 μg/ml) and fAβ1-42 (5 μm; 22.6 μg/ml) for 30 min before the addition of microspheres. B, Antagonists to the microglial Aβ cell surface receptor complex were tested in primary microglial cells as described in Figure 4. The data are representative of three independent experiments. *p < 0.01; **p < 0.001; #p < 0.05 compared with control.
Cytoskeletal morphology differentiates types of phagocytosis
We asked whether cells phagocytosing fAβ could be distinguished morphologically from cells treated with immune IgG or opsonized zymosan. Actin polymerization is the principle force driving phagocytosis (Aderem and Underhill, 1999). To compare actin dynamics and cell morphologies, cells were stained with phalloidin after treatment with the stimulating ligands. We found that fAβ and invasin397 treatment resulted in flattening of the cells with development of numerous projecting processes (Fig. 6A). The morphology of these cells was similar to that elicited by incubation with immune IgG. However, the fAβ- and invasin397-stimulated cells had finger-like projections that were not present on the immune IgG-stimulated cells. In contrast, opsonized zymosan-treated cells exhibited a rounded morphology and became flattened. Cells incubated with invasin195 exhibit a morphology similar to control cells. The differences in cell morphology seen with actin staining are probably reflective of the different Rho GTPases that are used in the various phagocytic mechanisms. Immune IgG-, fAβ-, and invasin397-treated cells exhibit similar morphologies, consistent with the involvement of Rac1 activation in the cytoskeleton reorganization elicited by each of these ligands.
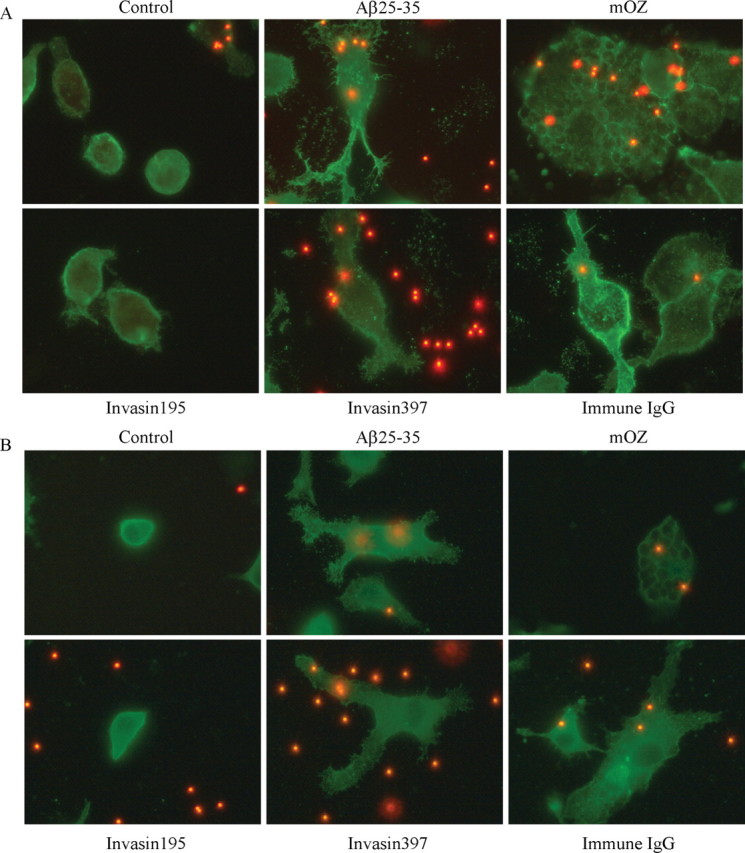
Cytoskeletal morphology differentiates types of phagocytosis. Phalloidin (A), which stains f-actin, andα-actinin (B) was used as cytoskeletal markers to compare cellular morphologies. BV-2 cells were treated with invasin 195 (1 μg/ml), Aβ25-35 (60 μm; 63.6 μg/ml), invasin397 (1 μg/ml), immune IgG (1 mg/ml), and mouse complement-opsonized zymosan (mOZ) (1 mg/ml) for 30 min before an additional 30 min incubation with microspheres. Images of cells were taken at 100×. These experiments are representative of three independent studies.
α-Actinin is another cytoskeletal protein that participates in phagocytosis (Allen and Aderem, 1996). Cells were stimulated with the individual ligands and stained for α-actinin. We observed a similar pattern of α-actinin organization in the fAβ-, invasin397-, and immune IgG-treated cells (Fig. 6B). These conditions resulted in some punctate staining; however, the α-actinin was generally dispersed through the cells and was concentrated along the cell periphery. The opsonized zymosan-treated cells exhibited a significantly different morphology with distinct intracellular phagosomes lined with α-actinin. The invasin195-stimulated cells appeared similar to the control cells.
Phagocytosis of fAβ uses a nonclassical pathway
To establish the cellular mechanisms through which fAβ is phagocytosed by microglia, we attempted to ascertain whether fAβ was taken up by classical phagocytic mechanisms by examination of functional requirements for cytoskeletal elements in the process. Microtubules have been shown to be essential for CR3-mediated, type II but not type I phagocytosis (Newman et al., 1991). We used nocodazole to inhibit microtubule dynamics and found that pretreatment with the drug inhibited opsonized zymosan-stimulated phagocytosis but had no effect on that stimulated by immune IgG, validating the differential role for microtubules in the classical phagocytic mechanisms (Fig. 7A). Significantly, nocodazole treatment before fAβ stimulation caused a reduction in phagocytosis; however, the drug did not abolish the phagocytic response such as seen with the CR3-stimulated type II phagocytosis. Myosin II has been shown to play a critical role in type II phagocytosis. Olazabal et al. (2002) reported that CR3 uses myosin II for both particle internalization and actin cup formation; however, FcR only uses myosin II for particle internalization (Olazabal et al., 2002). To establish a role for myosin II in fAβ-mediated phagocytosis, we treated cells with a highly specific myosin II inhibitor, blebbistatin (Straight et al., 2003). We observed the predicted inhibition of CR3-mediated phagocytosis but observed only a modest inhibition of phagocytosis elicited by fAβ treatment (Fig. 7B). These data demonstrate that fAβ-stimulated phagocytosis is only partially reliant on microtubules and myosin II, in contrast to type I, which does not require these elements, and type II, which requires both cytoskeletal networks to mount the phagocytic response.

Cytoskeletal perturbation and signaling inhibitors differentiate types of phagocytosis. A, Nocodazole (5 μg/ml) was added to BV-2 cells to inhibit microtubule dynamics 45 min before stimulation with fAβ25-35 (60 μm; 63.6 μg/ml), immune IgG (1 mg/ml), and mouse complement-opsonized zymosan (mOZ) (1 mg/ml). B, Blebbistatin (100 μm) was added to BV-2 cells 30 min before stimulation with the indicated ligand. C, Piceatannol (10 μm), a Syk inhibitor, PP2 (10 μm), a Src inhibitor, and LY294002 (10 μm), a PI3K inhibitor, were added to BV-2 cells 30 min before stimulation with the indicated ligand. The data are representative of five independent experiments. **p < 0.001 compared with control.
The tyrosine kinases Syk and Src have been shown to be important for type I phagocytosis (Greenberg et al., 1993). To determine whether fAβ-mediated phagocytosis used these signaling elements, inhibitors directed against Syk and Src were tested. Pretreatment of BV-2 cells with piceatannol, a Syk inhibitor, produced an inhibition of fAβ, as well as immune IgG-mediated, phagocytosis (Fig. 7C). Piceatannol did not, however, significantly reduce opsonized zymosan-stimulated phagocytosis. These data verify that Syk plays a role in immune IgG- as well as fAβ-mediated phagocytosis. These findings are consistent with our previous observation that Syk is activated after fAβ exposure of the cells and is essential for activation of downstream signaling cascades (McDonald et al., 1997; Combs et al., 1999). We found that inhibition of Src with PP2 and phosphatidylinositol 3-kinase (PI3K) with LY294002 resulted in general inhibition of phagocytosis elicited by all of the phagocytic stimuli. This result is consistent with the common and essential role for these enzymes in the phagocytic process (Greenberg, 1995, 1999; Aderem and Underhill, 1999).
Discussion
Our interest in microglial phagocytic mechanisms was stimulated by the observation that senile plaques in the AD brain are surrounded by phenotypically activated microglia, but these cells are ineffective in removing the insoluble deposits of Aβ from the intact brain. Microglia are competent phagocytes and are efficient in phagocytic uptake of amyloid aggregates (Ard et al., 1996; Paresce et al., 1996; Bard et al., 2000) and senile plaques themselves (DeWitt et al., 1998) when examined in vitro. This issue has taken on greater significance with the development of vaccination approaches for the treatment of AD (Schenk et al., 1999; Lombardo et al., 2003; Wilcock et al., 2003). There is compelling evidence that introduction of anti-Aβ antibodies into the brain (by either direct administration or leakage past the blood-brain barrier) results in a robust phagocytic response by plaque-associated microglia, resulting in the dissolution and removal of deposited Aβ in the brain (Schenk et al., 1999; Bard et al., 2000; Wilcock et al., 2003). Schenk et al. (1999) have argued that this result is attributable to the recognition of the Aβ-antibody complex by microglial FcR that are effectively linked to the phagocytic machinery. This conclusion was supported by ex vivo assays in which antibodies directed against Aβ prompted microglia cells to phagocytose plaques from brain sections in an FcR-dependent manner (Bard et al., 2000). The participation of FcR in Aβ phagocytosis is consistent with previous reports of enhanced phagocytosis of Aβ-IgG conjugates in vitro (Paresce et al., 1996; Brazil et al., 2000). The requirement of FcR for removal of Aβ deposits in vivo has been contested (Das et al., 2003), and there is clear evidence that alternative mechanisms are capable of removing Aβ deposits (Wyss-Coray et al., 2002; Wilcock et al., 2004).
The role of complement in Aβ deposition and removal in the brain has been controversial. The association of the complement component C1q with Aβ was reported to inhibit microglial phagocytosis (Webster et al., 2000) or enhance uptake if immune complexes were present (Webster et al., 2001). Wyss-Coray et al. (2002) have recently shed significant new light on this issue by demonstrating that there is a complement-dependent process that is responsible for removing Aβ deposits from the brain. Mice overexpressing a complement inhibitor exhibited a dramatic increase of plaque deposition. These findings suggest that there is a constitutive, but low-efficiency, complement-dependent process that suppresses Aβ deposition and facilitates its removal from the brain. However, these processes are not efficient enough to block the appearance of Aβ plaques.
The principal aim of the present study was to establish the cellular mechanisms by which fAβ is phagocytosed by microglia. Although published studies have provided evidence of fAβ stimulation of microglial phagocytosis of microspheres and fAβ itself in vitro (Ard et al., 1996; Kopec and Carroll, 1998; Kitamura et al., 2003), how this response was stimulated and linked to the phagocytic machinery was unexplored. We have demonstrated that fAβ must engage each element of the multicomponent receptor complex to stimulate the phagocytic response. The organization and composition of the fAβ receptor complex is similar to that used by macrophages to respond to other fibrillar peptides, such as thrombospondin and Bordetella pertussis filamentous hemaglutinin (Bamberger et al., 2003).
The critical involvement of scavenger receptors in Aβ-stimulated phagocytosis is consistent with a number of other studies that have shown that both A- and B-class scavenger receptors play an essential role in microglial activation and uptake of Aβ. Indeed, the scavenger receptor-dependent phagocytosis of fAβ is substantially more robust than that elicited by FcR stimulation. Both Webster et al. (2001) and Das et al. (2003) demonstrated Aβ phagocytosis in the presence of immune complexes in vitro; however, this result was only observed with the addition of fucoidan, which acted to block scavenger receptor binding. We and others have demonstrated the requirement for the B-class scavenger receptor CD36 participation in fAβ-stimulated microglia activation (Moore et al., 2002; Bamberger et al., 2003; El Khoury et al., 2003). The present study has established that CD36 is also essential for stimulation of fAβ phagocytosis by microglia. The microglial scavenger receptor A (SRA) has been shown previously to bind and participate in the internalization of fAβ (Paresce et al., 1996; Webster et al., 2000). However, it does not appear to play an essential role in these events. Microglia from SRA knock-out mice exhibit a modestly reduced ability to take up Aβ in vitro (Chung et al., 2001), and inactivation of the SRA gene in APP overexpressing mice has no effect on Aβ deposition (Huang et al., 1999). These findings are consistent with our observation that SRA participates in binding of Aβ fibrils to microglia but does not participate in stimulation of intracellular signaling (Bamberger et al., 2003).
The identification of a novel β1 integrin-dependent mechanism required for the phagocytosis of the bacteria Yersinia led us to question whether a similar mechanism might mediate Aβ-stimulated phagocytosis because the β1 integrin is a critical component of the microglial Aβ cell surface receptor complex (Bamberger et al., 2003). β1 integrin engagement has been shown to stimulate signaling pathways leading to Rac1 activation, catalyzing actin reorganization and phagosome formation (McGee et al., 2003). Immune IgG-, fAβ-, and invasin397-treated cells have similar morphologies and actin distribution, likely as the result of common signaling through Rac. Both FcR and the β1 integrin have a functional linkage to the tyrosine kinase Syk and its downstream effector Rac (Miller et al., 1999; Pradip et al., 2003). The data presented here suggest that we have uncovered a nonclassical type of phagocytosis for fAβ that is governed by β1 integrin signaling.
We have been able to differentiate between β1 integrin-mediated phagocytosis and classical phagocytic mechanisms on the basis of their requirement for microtubule integrity. fAβ-stimulated phagocytosis was partially inhibited by nocodazole pretreatment, yet cells were still capable of ingesting microspheres. This finding suggests that microtubules participate in fAβ-mediated phagocytosis, but they are not essential and clearly distinguish this mechanism from type II phagocytosis. These findings are consistent with that of Weltzien and Pachter (2000), who have shown that disruption of microtubules with colchicine inhibits phagocytosis without affecting the binding of fAβ to the macrophage cell surface. Significantly, disruption of microtubules with nocodazole decreased phagocytosis of Yersinia in HeLa cells (McGee et al., 2003).
In summary, these experiments describe a new phagocytic receptor complex that resides on the microglia cell surface and mediates the phagocytic response during exposure to fAβ. It remains unclear why the plaque-associated microglia in vivo are unable to effectively phagocytose the amyloid deposits despite their physical association with the plaques (Stalder et al., 2001). It is possible that other plaque constituents block the interaction of the microglia with the plaque, as has been suggested for C1q (Webster et al., 2000). We favor a model in which autocrine proinflammatory cytokine stimulation of the microglia acts to suppress activation of the phagocytic machinery and suggest that anti-inflammatory therapies may act to facilitate removal of fAβ and ameliorate amyloid pathology in the AD brain.
Footnotes
This work was supported by National Institutes of Health Grant AG20202 and by grants from the Blanchette Hooker Rockefeller Foundation and the Coins for Alzheimer's Research Trust Fund of Rotary International. We thank Drs. Ralph Isberg, Frieda Pearce, Ingo Autenrieth, Guntram Grassl, and Tom Egelhoff for providing us with reagents and Maryanne Pendergast for assistance with the confocal microscope.
Correspondence should be addressed to Gary Landreth, Alzheimer Research Laboratory, E504, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106. E-mail:ude.urwc@2leg.
Copyright © 2004 Society for Neuroscience 0270-6474/04/249838-09$15.00/0
References
- Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593-623. [Abstract] [Google Scholar]
- Allen LA, Aderem A (1996) Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med 184: 627-637. [Europe PMC free article] [Abstract] [Google Scholar]
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD (1996) Scavenging of Alzheimer's amyloid beta-protein by microglia in culture. J Neurosci Res 43: 190-202. [Abstract] [Google Scholar]
- Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE (2003) A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci 23: 2665-2674. [Europe PMC free article] [Abstract] [Google Scholar]
- Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T (2000) Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916-919. [Abstract] [Google Scholar]
- Bocchini V, Mazzolla R, Barluzzi R, Blasi E, Sick P, Kettenmann H (1992) An immortalized cell line expresses properties of activated microglial cells. J Neurosci Res 31: 616-621. [Abstract] [Google Scholar]
- Brazil MI, Chung H, Maxfield FR (2000) Effects of incorporation of immunoglobulin G and complement component C1q on uptake and degradation of Alzheimer's disease amyloid fibrils by microglia. J Biol Chem 275: 16941-16947. [Abstract] [Google Scholar]
- Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282: 1717-1721. [Abstract] [Google Scholar]
- Chung H, Brazil MI, Irizarry MC, Hyman BT, Maxfield FR (2001) Uptake of fibrillar beta-amyloid by microglia isolated from MSR-A (type I and type II) knockout mice. NeuroReport 12: 1151-1154. [Abstract] [Google Scholar]
- Chung J, Gao AG, Frazier WA (1997) Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3. J Biol Chem 272: 14740-14746. [Abstract] [Google Scholar]
- Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE (1999) Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of β-amyloid and prion proteins. J Neurosci 19: 928-939. [Abstract] [Google Scholar]
- Das P, Howard V, Loosbrock N, Dickson D, Murphy MP, Golde TE (2003) Amyloid-β immunization effectively reduces amyloid deposition in FcRγ -/- knock-out mice. J Neurosci 23: 8532-8538. [Europe PMC free article] [Abstract] [Google Scholar]
- Dersch P, Isberg RR (2000) An immunoglobulin superfamily-like domain unique to the Yersinia pseudotuberculosis invasin protein is required for stimulation of bacterial uptake via integrin receptors. Infect Immun 68: 2930-2938. [Europe PMC free article] [Abstract] [Google Scholar]
- DeWitt DA, Perry G, Cohen M, Doller C, Silver J (1998) Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Exp Neurol 149: 329-340. [Abstract] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD (2003) CD36 mediates the innate host response to beta-amyloid. J Exp Med 197: 1657-1666. [Europe PMC free article] [Abstract] [Google Scholar]
- Ghiso J, Rostagno A, Gardella JE, Liem L, Gorevic PD, Frangione B (1992) A 109-amino-acid C-terminal fragment of Alzheimer's-disease amyloid precursor protein contains a sequence, -RHDS-, that promotes cell adhesion. Biochem J 288: 1053-1059. [Europe PMC free article] [Abstract] [Google Scholar]
- Greenberg S (1995) Signal transduction of phagocytosis. Trends Cell Biol 5: 93-99. [Abstract] [Google Scholar]
- Greenberg S (1999) Modular components of phagocytosis. J Leukoc Biol 66: 712-717. [Abstract] [Google Scholar]
- Greenberg S, Chang P, Silverstein SC (1993) Tyrosine phosphorylation is required for Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med 177: 529-534. [Europe PMC free article] [Abstract] [Google Scholar]
- Huang F, Buttini M, Wyss-Coray T, McConlogue L, Kodama T, Pitas RE, Mucke L (1999) Elimination of the class A scavenger receptor does not affect amyloid plaque formation or neurodegeneration in transgenic mice expressing human amyloid protein precursors. Am J Pathol 155: 1741-1747. [Europe PMC free article] [Abstract] [Google Scholar]
- Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC (2002) Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia 40: 195-205. [Abstract] [Google Scholar]
- Isberg RR, Hamburger Z, Dersch P (2000) Signaling and invasin-promoted uptake via integrin receptors. Microbes Infect 2: 793-801. [Abstract] [Google Scholar]
- Kaplan G (1977) Differences in the mode of phagocytosis with Fc and C3 receptors in macrophages. Scand J Immunol 6: 797-807. [Abstract] [Google Scholar]
- Kitamura Y, Shibagaki K, Takata K, Tsuchiya D, Taniguchi T, Gebicke-Haerter PJ, Miki H, Takenawa T, Shimohama S (2003) Involvement of Wiskott-Aldrich syndrome protein family verprolin-homologous protein (WAVE) and Rac1 in the phagocytosis of amyloid-beta(1-42) in rat microglia. J Pharmacol Sci 92: 115-123. [Abstract] [Google Scholar]
- Kopec KK, Carroll RT (1998) Alzheimer's beta-amyloid peptide 1-42 induces a phagocytic response in murine microglia. J Neurochem 71: 2123-2131. [Abstract] [Google Scholar]
- Laurenzi MA, Arcuri C, Rossi R, Marconi P, Bocchini V (2001) Effects of microenvironment on morphology and function of the microglial cell line BV-2. Neurochem Res 26: 1209-1216. [Abstract] [Google Scholar]
- Lombardo JA, Stern EA, McLellan ME, Kajdasz ST, Hickey GA, Bacskai BJ, Hyman BT (2003) Amyloid-beta antibody treatment leads to rapid normalization of plaque-induced neuritic alterations. J Neurosci 23: 10879-10883. [Europe PMC free article] [Abstract] [Google Scholar]
- McDonald DR, Brunden KR, Landreth GE (1997) Amyloid fibrils activate tyrosine kinase-dependent signaling and superoxide production in microglia. J Neurosci 17: 2284-2294. [Europe PMC free article] [Abstract] [Google Scholar]
- McGee K, Holmfeldt P, Fallman M (2003) Microtubule-dependent regulation of Rho GTPases during internalisation of Yersinia pseudotuberculosis. FEBS Lett 533: 35-41. [Abstract] [Google Scholar]
- Miller LA, Hong JJ, Kinch MS, Harrison ML, Geahlen RL (1999) The engagement of beta1 integrins on promonocytic cells promotes phosphorylation of Syk and formation of a protein complex containing Lyn and beta1 integrin. Eur J Immunol 29: 1426-1434. [Abstract] [Google Scholar]
- Moore KJ, El Khoury J, Medeiros LA, Terada K, Geula C, Luster AD, Freeman MW (2002) A CD36-initiated signaling cascade mediates inflammatory effects of beta-amyloid. J Biol Chem 277: 47373-47379. [Abstract] [Google Scholar]
- Newman SL, Mikus LK, Tucci MA (1991) Differential requirements for cellular cytoskeleton in human macrophage complement receptor- and Fc receptor-mediated phagocytosis. J Immunol 146: 967-974. [Abstract] [Google Scholar]
- Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM (2002) Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol 12: 1413-1418. [Abstract] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR (1996) Microglial cells internalize aggregates of the Alzheimer's disease amyloid beta-protein via a scavenger receptor. Neuron 17: 553-565. [Abstract] [Google Scholar]
- Pearce S, Wu J, Silverstein RL (1995) Recombinant GST/CD36 fusion proteins define a thrombospondin binding domain. Evidence for a single calcium-dependent binding site on CD36. J Biol Chem 270: 2981-2986. [Abstract] [Google Scholar]
- Pradip D, Peng X, Durden DL (2003) Rac2 specificity in macrophage integrin signaling: potential role for Syk kinase. J Biol Chem 278: 41661-41669. [Abstract] [Google Scholar]
- Rogers J, Lue LF (2001) Microglial chemotaxis, activation, and phagocytosis of amyloid beta-peptide as linked phenomena in Alzheimer's disease. Neurochem Int 39: 333-340. [Abstract] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P (1999) Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173-177. [Abstract] [Google Scholar]
- Stalder M, Deller T, Staufenbiel M, Jucker M (2001) 3D-Reconstruction of microglia and amyloid in APP23 transgenic mice: no evidence of intracellular amyloid. Neurobiol Aging 22: 427-434. [Abstract] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ (2003) Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 299: 1743-1747. [Abstract] [Google Scholar]
- Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1: 14. [Europe PMC free article] [Abstract] [Google Scholar]
- Terzi E, Holzemann G, Seelig J (1994) Alzheimer beta-amyloid peptide 25-35: electrostatic interactions with phospholipid membranes. Biochemistry 33: 7434-7441. [Abstract] [Google Scholar]
- Webster SD, Yang AJ, Margol L, Garzon-Rodriguez W, Glabe CG, Tenner AJ (2000) Complement component C1q modulates the phagocytosis of Abeta by microglia. Exp Neurol 161: 127-138. [Abstract] [Google Scholar]
- Webster SD, Galvan MD, Ferran E, Garzon-Rodriguez W, Glabe CG, Tenner AJ (2001) Antibody-mediated phagocytosis of the amyloid beta-peptide in microglia is differentially modulated by C1q. J Immunol 166: 7496-7503. [Abstract] [Google Scholar]
- Weltzien RB, Pachter JS (2000) Visualization of beta-amyloid peptide (Abeta) phagocytosis by human mononuclear phagocytes: dependency on Abeta aggregate size. J Neurosci Res 59: 522-527. [Abstract] [Google Scholar]
- Wiedemann A, Linder S, Grassl G, Albert M, Autenrieth I, Aepfelbacher M (2001) Yersinia enterocolitica invasin triggers phagocytosis via beta1 integrins, CDC42Hs and WASp in macrophages. Cell Microbiol 3: 693-702. [Abstract] [Google Scholar]
- Wilcock D, Rojiani A, Rosenthal A, Levkowitz G, Subbarao S, Alamed J, Wilson D, Wilson N, Freeman M, Gordon M, Morgan D (2004) Passive amyloid immunotherapy clears amyloid and transiently activates microglia in a transgenic mouse model of amyloid deposition. J Neurosci 24: 6144-6151. [Europe PMC free article] [Abstract] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D (2003) Intracranially administered anti-Aβ antibodies reduce β-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci 23: 3745-3751. [Europe PMC free article] [Abstract] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E (2002) Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci USA 99: 10837-10842. [Europe PMC free article] [Abstract] [Google Scholar]
Articles from The Journal of Neuroscience are provided here courtesy of Society for Neuroscience
Full text links
Read article at publisher's site: https://doi.org/10.1523/jneurosci.2557-04.2004
Read article for free, from open access legal sources, via Unpaywall:
https://www.jneurosci.org/content/jneuro/24/44/9838.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1523/jneurosci.2557-04.2004
Article citations
The Role of CXCR3 in Nervous System-Related Diseases.
Mediators Inflamm, 2024:8347647, 11 Oct 2024
Cited by: 0 articles | PMID: 39429695 | PMCID: PMC11488998
Review Free full text in Europe PMC
Cognitive Effects of Simulated Galactic Cosmic Radiation Are Mediated by ApoE Status, Sex, and Environment in APP Knock-In Mice.
Int J Mol Sci, 25(17):9379, 29 Aug 2024
Cited by: 0 articles | PMID: 39273325 | PMCID: PMC11394682
Hybrid Amyloid Quantum Dot Nano-Bio Assemblies to Probe Neuroinflammatory Damage.
ACS Chem Neurosci, 15(17):3124-3135, 15 Aug 2024
Cited by: 0 articles | PMID: 39146244 | PMCID: PMC11378299
Enhanced phagocytosis associated with multinucleated microglia via Pyk2 inhibition in an acute β-amyloid infusion model.
J Neuroinflammation, 21(1):196, 06 Aug 2024
Cited by: 0 articles | PMID: 39107821 | PMCID: PMC11301859
Downregulation of Fidgetin-Like 2 Increases Microglial Function: The Relationship Between Microtubules, Morphology, and Activity.
Mol Neurobiol, 19 Aug 2024
Cited by: 0 articles | PMID: 39160390
Go to all (283) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Microglial phagocytosis induced by fibrillar β-amyloid is attenuated by oligomeric β-amyloid: implications for Alzheimer's disease.
Mol Neurodegener, 6:45, 30 Jun 2011
Cited by: 137 articles | PMID: 21718498 | PMCID: PMC3149591
CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation.
J Neurosci, 29(38):11982-11992, 01 Sep 2009
Cited by: 368 articles | PMID: 19776284 | PMCID: PMC2778845
Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines.
J Neurosci, 25(36):8240-8249, 01 Sep 2005
Cited by: 300 articles | PMID: 16148231 | PMCID: PMC6725530
Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo.
J Neurosci, 18(6):2161-2173, 01 Mar 1998
Cited by: 231 articles | PMID: 9482801 | PMCID: PMC6792921
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIA NIH HHS (2)
Grant ID: AG20202
Grant ID: R01 AG020202





