Abstract
Background
Blood viscosity is fundamentally important in clinical practice yet the apparent viscosity at very low shear rates is not well understood. Various conditions such as blunt trauma may lead to the appearance of zones inside the vessel where shear stress equals zero. The aim of this research was to determine the blood viscosity and quantitative aspects of rouleau formation from erythrocytes at yield velocity (and therefore shear stress) equal to zero. Various fundamental differential equations and aspects of multiphase medium theory have been used. The equations were solved by a method of approximation. Experiments were conducted in an aerodynamic tube.Results
The following were determined: (1) The dependence of the viscosity of a mixture on volume fraction during sedimentation of a group of particles (forming no aggregates), confirmed by published experimental data on the volume fractions of the second phase (f2) up to 0.6; (2) The dependence of the viscosity of the mixture on the volume fraction of erythrocytes during sedimentation of rouleaux when yield velocity is zero; (3) The increase in the viscosity of a mixture with an increasing erythrocyte concentration when yield velocity is zero; (4) The dependence of the quantity of rouleaux on shear stress (the higher the shear stress, the fewer the rouleaux) and on erythrocyte concentration (the more erythrocytes, the more rouleaux are formed).Conclusions
This work represents one of few attempts to estimate extreme values of viscosity at low shear rate. It may further our understanding of the mechanism of blunt trauma to the vessel wall and therefore of conditions such as traumatic acute myocardial infarction. Such estimates are also clinically significant, since abnormal values of blood viscosity have been observed in many pathological conditions such as traumatic crush syndrome, cancer, acute myocardial infarction and peripheral vascular disease.Free full text

Mathematical model of blunt injury to the vascular wall via formation of rouleaux and changes in local hemodynamic and rheological factors. Implications for the mechanism of traumatic myocardial infarction
Abstract
Background
Blood viscosity is fundamentally important in clinical practice yet the apparent viscosity at very low shear rates is not well understood. Various conditions such as blunt trauma may lead to the appearance of zones inside the vessel where shear stress equals zero. The aim of this research was to determine the blood viscosity and quantitative aspects of rouleau formation from erythrocytes at yield velocity (and therefore shear stress) equal to zero. Various fundamental differential equations and aspects of multiphase medium theory have been used. The equations were solved by a method of approximation. Experiments were conducted in an aerodynamic tube.
Results
The following were determined: (1) The dependence of the viscosity of a mixture on volume fraction during sedimentation of a group of particles (forming no aggregates), confirmed by published experimental data on the volume fractions of the second phase (f2) up to 0.6; (2) The dependence of the viscosity of the mixture on the volume fraction of erythrocytes during sedimentation of rouleaux when yield velocity is zero; (3) The increase in the viscosity of a mixture with an increasing erythrocyte concentration when yield velocity is zero; (4) The dependence of the quantity of rouleaux on shear stress (the higher the shear stress, the fewer the rouleaux) and on erythrocyte concentration (the more erythrocytes, the more rouleaux are formed).
Conclusions
This work represents one of few attempts to estimate extreme values of viscosity at low shear rate. It may further our understanding of the mechanism of blunt trauma to the vessel wall and therefore of conditions such as traumatic acute myocardial infarction. Such estimates are also clinically significant, since abnormal values of blood viscosity have been observed in many pathological conditions such as traumatic crush syndrome, cancer, acute myocardial infarction and peripheral vascular disease.
Introduction
Blood is a liquid-liquid suspension because erythrocytes exhibit fluid-like behavior under certain shear conditions [1]. The dependence of viscosity on shear rate is one of the most widely used rheological measurements [2]. Normal blood also thins when it is sheared, therefore its apparent viscosity is highly sensitive to shear rates below 100 s-1 [2,3].
The objective of this research was to determine blood viscosity at yield velocity (and therefore shear stress) equal to zero. Our previous studies have shown that conditions such as blunt trauma to large vessels may lead to boundary layer separation where du/dy = 0, i.e. to the appearance of zones where shear stress equals zero [4]. A further aim of this research was to evaluate quantitative aspects of rouleau formation from erythrocytes when the yield velocity is equal to zero.
Methods
Various calculations have been made for the viscosity of a mixture and the coefficient of constraint [5-7]. There is considerable variation in such calculations, resulting from different combinations of phases. This variation apparently reflects the non-Newtonian nature of concentrated viscous disperse mixtures and the insufficiency of the variables ρ and μ alone (where ρ is density and μ is viscosity) to determine the mechanical properties of such mixtures. In this regard, experiments over the range of operating parameters are needed for any mixture to determine pressure loss using different rheological models; in particular, the model of a viscous fluid with an effective viscosity coefficient. It must be noted that when f2 > 0.1 (where f2 is the volume fraction of the second phase), not only the shape and size of the erythrocytes but also the irregular arrangement of the particles and their collisions with each other and with the solid walls have substantial effects on the effective viscosity and other rheological characteristics of the mixture [8,9].
The problems mentioned above have led to studies of group sedimentation at f2 > 0.1 in the interpenetrating model of two- or multi-phase media [10]. These studies usually deal with either high- or low-concentration mixtures. Mechanisms of sedimentation in moderately concentrated mixtures, which are rather common, have not been fully investigated. Mathematical modeling of group sedimentation of particles (in our case, rouleaux) in two-phase interpenetrating media [11] should take into account not only the Stokes force [12] but also other forces that are given in [13]:
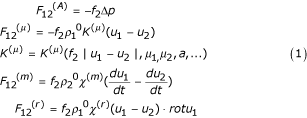
where F12(A) is a buoyancy force, p- pressure difference, χ(m)- coefficient of constraint, ρ- density of the first phase, K(μ) – coefficient of phase interaction, μ1 and μ2 – viscosities of the first and second phases, f2 – the volume fraction of the second phase. It is also important to calculate μ, the viscosity of the blood mixture, which depends on the volume fraction of particles. In this case it is possible to determine the force F12(μ). F12(μ) is a frictional force or Stokes force that results from viscous forces involved in the interaction between phases. F12(μ) is calculated using the difference between velocities (slippage) u1 - u2, the particle size a, the quantities and shapes of inclusions, and the physical properties of the phases (see equation 1). (The effects of the shape and multiplicity of particles, and of some other variables included in the expression for F12(μ), are accounted for in coefficients K(μ) in (1)).
Using all of the above, I shall determine blood viscosity as a variable dependent on a volume fraction of particles. This will allow me to determine blood viscosity at a yield velocity of zero, and the number of rouleaux as a variable dependent on erythrocyte concentration, shear stress and yield velocity.
Determination of viscosity of a mixture as a variable dependent on volume fraction of particles
Sedimentation of a single particle is based on the Stokes law, according to which a frictional force resulting from the motion of spherical particles with diameter d and velocity V in a medium of viscosity μ is expressed by the equation:
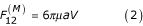
where a – radius of particles (inclusions) and V – velocity of particle precipitation.
In the general case of a multiphase medium, the frictional force or Stokes force F12(μ), which results from viscous forces involved in the interactions between phases, is calculated using the difference between velocities (slippage) u1 - u2, the particle size a, the quantity and shape of inclusions, and the physical properties of the phases. Multiphase models are based on the idea of interpenetrating media, where the system of particles is replaced by a mathematical continuum and particle size is considerably less than the distance over which flow conditions may change [11].
The force of gravity acting on a particle is calculated using the specific gravity of the particle; that is:
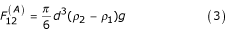
where ρ1;ρ2;g are respectively the density of the fluid, the density of the particle, and the acceleration due to gravity.
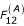 is a buoyancy force (Archimedes force);
is a buoyancy force (Archimedes force);
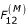 is a frictional force or Stokes force.
is a frictional force or Stokes force.
Force 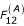 causes a particle to accelerate. In addition to gravity, the particle is affected by the frictional force, which acts in the opposite direction and has a value directly proportional to the velocity according to the Stokes law. This means that force
causes a particle to accelerate. In addition to gravity, the particle is affected by the frictional force, which acts in the opposite direction and has a value directly proportional to the velocity according to the Stokes law. This means that force 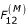 and gravity
and gravity 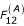 tend to cancel each other out. Therefore, the motion proceeds with a constant velocity V that can be determined from equations (2) and (3):
tend to cancel each other out. Therefore, the motion proceeds with a constant velocity V that can be determined from equations (2) and (3):
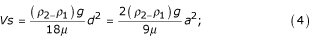
where Vs – velocity of precipitation of a single particle.
Sometimes investigators have to deal with the sedimentation of multiple particles in concentrated mixtures. Formulae for the velocity of sedimentation of particles, dependent on the concentration and velocity of a single particle in an infinite fluid, can be derived using statements from the interpenetrating model [13] and the Euler equation [14]. Assuming that a specific volume has two phases differing in specific gravity, the particles with the greater specific gravity will start moving down a channel, so that a process of mutual penetration occurs.
The flow of the fluid can be expressed by criterion equations:
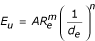
where Eu – Euler number, A – coefficient of proportionality, Re – Reynolds number; or:
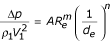
In the process of sedimentation when the concentration of inclusions is rather high and the particle size is small, flow is laminar; m = - 1 and n = 1 (where m and n are criterion coefficients).
Taking into account data from [13]:
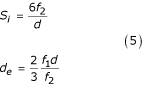
where Si – particle surface area; f1 – volume fraction of the first phase; f2 – volume fraction of the second phase
Dividing the continuity equation:
V1S = V1iS1
by S, I obtain:
V1 = f1V1i
where S is the area of the canal section.
Therefore:
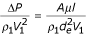
Using equations (5) and (2), I can transform the last equation into the Kozeny-Carman formula for restrained sedimentation in a laminar flow:
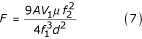
where A lies within the range 80–110.
Dividing equation (7) by the number of particles per unit of volume allows the resistance force applied by the fluid to a single particle to be derived as:
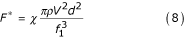
Where F* – resistance force created by the fluid and acting on a single particle, and χ – coefficient of resistance for precipitation of multiple particles.
The resistance force applied to a single particle during precipitation in a fluid is known to be [12,15]:
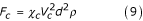
For particles suspended in a fluid:
F* = F12
therefore from (8) and (9) it follows that:
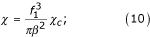
where β – the ratio of the velocity of sedimentation of the group of particles to the velocity of sedimentation of a single particle, and χc – the coefficient of resistance when precipitating a single particle in an infinite fluid.
From (10), when f1 → 1 it follows that:
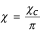
when the Reynolds numbers are small:
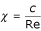
where c – constant.
Therefore, it can be assumed that:
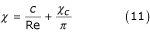
From equations (10) and (11) it follows that:
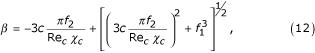
where:
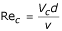
where ν – the coefficient of viscosity.
When the motion is laminar, according to the Stokes law:
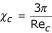
Substituting this expression in equation (12), it follows that:
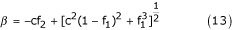
If one considers the sedimentation of a particle in a suspension with viscosity μm and density ρm, then the equilibrium equation [13] can be expressed as:
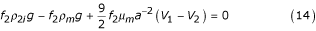
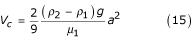
ρm = f1ρ1i + f2ρ2i
Using equations (14), (15) and (3) and the condition V1 = 0 it follows that:

Substituting the relative velocity equation (13) into equation (17), it follows that:
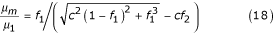
When f1 → 1 and c = 2.5, this reduces to the Einstein formula:
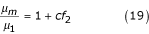
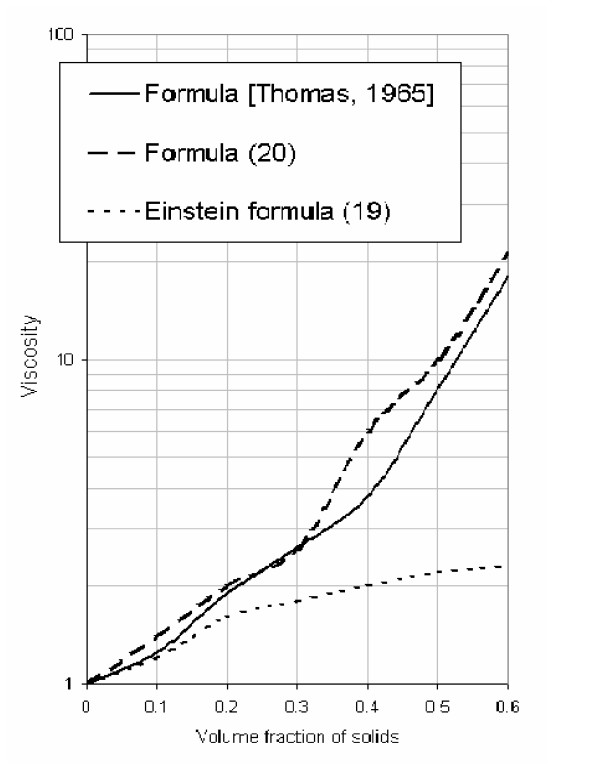
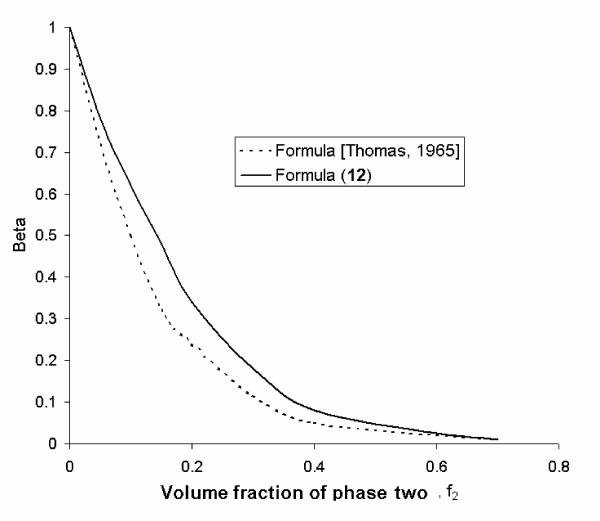
From the calculation given in Figure Figure1,1, it follows that equation (18) is consistent with the experimental data (up to f2 = 0.5 when c = 2.5) obtained by other investigators [6,7] regarding the velocity changes in suspensions for a wide range of fluids and particle sizes as well as particle compositions. Figure Figure22 shows the relationship between relative sedimentation velocity and particle concentration. The relationship between relative velocity, viscosity and volume fraction is also consistent with experimental data [6,7].
Determination of viscosity when yield velocity equals zero
The value of viscosity derived in equation (18) describes the sedimentation of solid particles, that is particles that do not form rouleaux. I shall now determine the viscosity of blood when the yield velocity is zero. It is known [16] that if whole blood (in which coagulation is prevented) is placed in a vertically-positioned capillary tube, erythrocytes will aggregate into rouleaux and then sediment. Therefore the viscosity μ1 must be determined in blood that has minimal numbers of rouleaux, and it is necessary to take into account the effect on rouleau sedimentation of erythrocytes that remain suspended. Such a condition occurs when the yield velocity is high (500 – 1000 s-1) and the number of rouleaux is minimal. This condition can be expressed by equations (18) or (19) when f1 → 1 and c = 2.5; that is rouleaux do not sediment in plasma but rather in a mixture of erythrocytes, plasma and a certain number of rouleaux.
Calculations made according to equations (18) or (19) when f1 → 1 and c = 2.5 yield the following results:
μ1 = 6.8 mNsm-2 when concentration of erythrocytes is 28.7%
μ1 = 8.8 mNsm-2 when concentration of erythrocytes is 48%
μ1 = 10 mNsm-2 when concentration of erythrocytes is 58.9%
These data are consistent with experimental data [16] when the yield velocity ranges from 500 to 1000 s-1. Thus, using the effect of the viscosity of the mixture from equations (18) and (19), I can calculate the viscosity of the blood at zero velocity by means of the following equation:
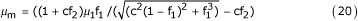
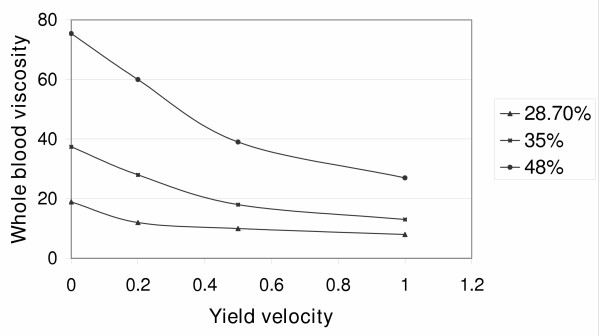
In this equation, when coefficient c = 2.5, there is a minimal number of rouleaux at μ1 = 3 to 4 mNsm-2 (the value of viscosity when the maximum yield velocity is more than 500 s-1). Figure Figure3,3, where the viscosity at zero yield velocity is plotted on the Y axis, shows that viscosity increases with increasing concentration. Thus an increase in erythrocyte concentration results in an increase of viscosity.
I shall now determine the shear stress at various concentrations and yield velocities. Table Table11 shows that an increase of shear stress causes a decrease of viscosity. Thus, an increase in the concentration of erythrocytes will result in an increase of viscosity and a decrease in shear stress. It can be assumed that a maximal number of rouleaux is formed when the yield velocity is zero, since there are no forces that disassemble them. Then I can determine the number of rouleaux at different values of viscosity and shear stress. Table Table22 shows these data and indicates that the main source of rouleaux is the erythrocytes themselves. The higher the erythrocyte concentration, the more rouleaux remain in the blood despite an increase in the forces that destroy them. It is also clear that an increase in shear stress results in a decrease of the number of rouleaux.
Table 1
Relationship between shear stress and viscosity
| Yield velocity (s-1) | The volume fraction of the second phase | Viscosity (mNsm-2) | Shear stress (N/m2) |
| 0.2 | 28.7 | 13 | 0.0026 |
| 35.9 | 30 | 0.006 | |
| 48 | 63 | 0.0126 | |
| 5 | 28.7 | 6 | 0.03 |
| 35.9 | 8 | 0.04 | |
| 48 | 15 | 0.075 | |
| 100 | 28.7 | 4 | 0.4 |
| 35.9 | 5 | 0.5 | |
| 48 | 6 | 0.6 | |
| 500 | 28.7 | 3 | 1.5 |
| 35.9 | 3 | 1.5 | |
| 48 | 4 | 2 | |
Table 2
The relationship between erythrocyte concentration and number of rouleaux
| Yield velocity (s-1) | Concentration % | Viscosity (mNsm-2) | Rouleaux concentration % | Concentration of destroyed rouleaux % | Shear stress (N/m2) |
| 0.2 | 28.7 | 15 | 65.2 | 34.8 | 0.0026 |
| 35.9 | 30 | 81 | 19 | 0.006 | |
| 48 | 63 | 83 | 17 | 0.0126 | |
| 5 | 28.7 | 6 | 26 | 74 | 0.03 |
| 35.9 | 8 | 21.3 | 78.7 | 0.04 | |
| 48 | 15 | 20 | 80 | 0.075 | |
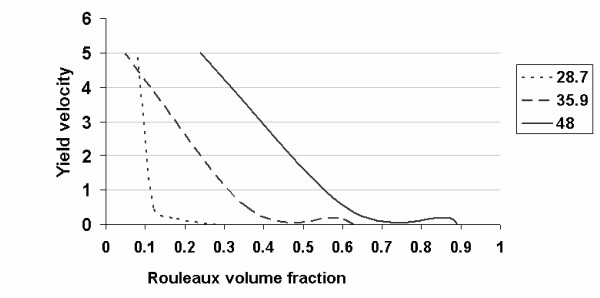
I can now determine the concentration of rouleaux, assuming that viscosity is determined by the numbers of erythrocytes only at a high yield velocity (since high yield velocities destroy rouleaux). Granted this assumption, the viscosity is determined according to the Einstein equation (18) and (19). Viscosity at decreasing yield velocity is determined by both erythrocytes and newly-formed rouleaux. Then, according to equation (20), I obtain the result presented in Figure Figure4:4: the number of rouleaux decreases sharply with increasing yield velocity. Therefore, the number of rouleaux depends on the concentration of erythrocytes.
The quantity of rouleaux depends on shear stress (the higher the shear stress, the lower the rouleaux content of the blood) and erythrocyte concentration (the more erythrocytes, the more rouleaux will be formed). I can now determine whether all rouleaux are interconnected and what kind of cohesive forces operate among them. It is known that at low yield velocities, a greater fraction of the erythrocytes form rouleaux [16]. These long columns of erythrocytes have a certain stiffness and might interweave to form a single structure [16]. It is hypothesized that cohesive forces may vary among rouleaux. This phenomenon makes the properties of blood resemble those of a solid body. When the yield velocity increases, the length of the rouleaux gradually decreases and ultimately only stand-alone erythrocytes are left.
To test this hypothesis, an experiment was conducted in which the breaking force and shear stress were those that naturally destroy rouleaux, but the cohesive forces were different. In an aerodynamic tube, a laminar boundary layer was created on a flat surface with the required shear stress on the surface of the wall [4]. On this surface, fine particles of equal diameter were placed (the cohesive force ranged from 0.0027 mN to 0.035 mN). From this information I could determine the destruction, i.e. the detachment and separation of particles from the surface. The results of the experiment are given in Table Table33.
Table 3
The relationship between shear stress, particle diameter and damage to the wall
| Shear stress (N/m2) | Diameter of particles (mm) | Damage (g/s) |
| 0.043 | 0.25–0.63 | 0.002 |
| 0.051 | 0.25–0.63 | 0.03 |
| 0.092 | 0.25–0.63 | 0.07 |
| 0.13 | 0.25–0.63 | 0.122 |
| 0.13 | 0.5–0.63 | 0.05 |
| 0.158 | 0.5–0.63 | 0.1 |
Table Table33 shows that destruction of rouleaux decreases with increasing particle diameter (which means increasing cohesive force). Conversely, the destruction of rouleaux increases with increasing shear stress. It can be supposed that an increase in shear stress destroys rouleaux that have a cohesive force lower than the breaking force. A further increase in shear stress will lead to the destruction of rouleaux with a greater cohesive force.
Summary of results
The following have been determined
1. The dependence of the viscosity of a mixture on volume fraction during sedimentation of a group of particles (forming no aggregates), confirmed by published experimental data [7] for volume fractions of the second phase (f2) up to 0.6.
2. The dependence of viscosity of a mixture on the volume fraction of erythrocytes during sedimentation of rouleaux when the yield velocity is zero.
3. Increase in the velocity of a mixture with an increasing concentration of erythrocytes when yield velocity is zero.
4. An increased erythrocyte concentration results in an increase of viscosity of the mixture, and an increase in shear stress results in a decrease of viscosity of the mixture.
5. The quantity of rouleaux depends on shear stress (the higher the shear stress, the fewer rouleaux in the blood) and erythrocyte concentration (the more erythrocytes, the more rouleaux are formed).
6. With an increase in shear stress, those rouleaux are destroyed whose cohesive force is weaker than the breaking force. A further increase in shear stress will start to destroy rouleaux that have a greater cohesive force.
Discussion
The role of the non-Newtonian viscosity of blood has remained a continuing challenge. Currently, the apparent viscosity at very low shear rates is considered as "effectively infinite immediately before the substance yields and begins to flow" [17]. Traditionally, Casson or Herschel-Bulkley models are used to measure both the yield stress of blood and shear thinning viscosity [18]. Human blood however does not comply with Casson's equation at a very low shear rate [13]. Other attempts to obtain finite viscosity values failed to take into account the hydrodynamic interactions between particles, or the complications related to aggregates [2]. Although an attempt to estimate blood viscosity at a very low shear rate has been made, no study has estimated the viscosity of blood when yield velocity equals zero.
The mathematical model created in this study used the most fundamental differential equations that have ever been derived to estimate blood viscosity. Depending on erythrocyte concentration, this model estimates the blood viscosity at zero yield stress. It takes into account the following factors: (1) Erythrocytes sediment as a group and not as single particles; (2) Erythrocytes interact with each other; (3) Erythrocytes sediment as a rouleaux; (4) Such rouleaux sediment within an erythrocyte-containing medium.
In general, abnormal values of blood viscosity can be observed in such pathologies as cancer [19,20], peripheral vascular disease [19,20] and acute myocardial infarction [19,20]. Blood hyperviscosity may impair the circulation and cause ischemia and local necrosis through decreased capillary perfusion [21]. Blood hyperviscosity due to abnormal red cell aggregation has been found in patients with diabetes, hyperlipidemia and cancer [22]. Estimation of blood viscosity is, however, particularly important in trauma patients. It is known that blunt trauma to vascular walls may lead to conditions for boundary layer separation [4]. Physically, this can be explained as follows [12]: flow retarded at the surface has low kinetic energy and cannot enter the high pressure zone, therefore it separates from the vessel wall and moves into the inner flow. It should be noted that under normal physiological conditions, the boundary layer does not separate [16]. Shear stress in the zone of boundary layer separation is equal to zero [4]. Therefore, in accordance with the above, trauma may create transient conditions for the formation of rouleaux or for the interlacing of existing rouleaux that have formed in the flowing blood [16], since there is no breaking force at zero shear and yield velocity. A certain number of rouleaux can then enter the arterial branching zone, where the shear velocity and shear stress on the internal wall are low [16], and these rouleaux might attach to the vessel wall, potentially causing atheromatosis. Such arterial branching zones could also be injured by blunt forces, which will also lead to boundary layer separation [4]. Therefore, rouleaux will be formed with low shear velocity and low shear stress on the internal wall [16], also creating conditions for atheromatosis.
Therefore, our understanding of the mechanism of blunt trauma to the vascular wall, which takes into account local hemodynamic and rheological factors, can be summarized in the following way. Trauma leads to the appearance of zones with high shear stress (as the result of injury to part of the vessel) and low or zero shear stress (within the zone of boundary layer separation) [4]. We have reported that high shear stress (exceeding the physiological value) may potentially damage the endothelium [4] and increase platelet aggregation [23,24], possibly leading to thrombus formation. On the other hand, trauma may lead to boundary layer separation, resulting in the appearance of a zone with zero shear stress and zero yield velocity [4]. This may result, according to current research, in an increase of blood viscosity through increased erythrocyte aggregation and rouleaux formation. Such hyperviscosity has been reported in patients with traumatic crush syndrome and also has been studied in animals exposed to traumatic crush [25]. As noted above, hyperviscosity may worsen the blood circulation and cause ischemia and local necrosis through deterioration in capillary perfusion [21].
This work also establishes a quantitative relationship between the extent of rouleaux formation and shear stress. According to current results, the number of rouleaux increases with decreasing shear stress, and this trend becomes more pronounced as the shear stress approaches zero. Rouleaux continue to form inside what I call the "hemodynamic shade". This "hemodynamic shade" creates a stagnant zone that can be characterized by a secondary flow and a boundary. Hemodynamic stress outside this zone, however, is still significant enough to destroy and entrain rouleaux. The "hemodynamic shade" zone can also be characterized by a significant deterioration of mass exchange due to the attachment of rouleaux to the vessel wall. This may decrease the permeability of the endothelium [16] and decrease the rate of removal of lipids and lipoproteins, which in turn can lead to the formation of lipid stripes directed along the blood flow and located in the "hemodynamic shade" of the original attached rouleaux. The escalating formation of rouleaux continues within the entire "hemodymanic shade" zone.
The model of traumatic damage to the vessel that takes into account local rheological and hemodynamic factors could be applied to many internal injuries involving an elastic vessel wall and a blunt traumatic mechanism. One example is traumatic myocardial infarction, which can result from blunt trauma to the coronary vessels. It should be noted that patients with blunt trauma may develop acute myocardial infarction; such patients may benefit from screening procedures such as electrocardiography, which might improve their chances of survival [8,26-49]. In a large cross-sectional observational study, abdominal, pelvic and blunt cardiac injuries were found to be significantly associated with acute myocardial infarction even after controlling for confounders such as mechanism and severity of injury, age, sex, race, source of payment, alcohol and cocaine use [50]. Intracoronary thrombosis has been suggested as one of the mechanisms of acute myocardial infarction in young people due to trauma, since other "atherosclerotic" mechanisms do not apply [38,42]. Nonetheless, the exact mechanism of traumatic myocardial infarction remains unclear. Current research suggests that blunt trauma may result in the appearance of a region of very low or zero shear stress, where hyperviscosity and increased rouleaux formation are likely to appear. Large quantities of rouleaux may be transported in the bloodstream toward the more distal parts of the coronary vessels, causing their occlusion. Caimi et al. [51], for instance, observed that blood viscosity at low shear rate is the only hemorheological factor that significantly increases the risk of acute myocardial infarction in young people. On the other hand, blunt trauma may result in traumatic compression of the vessel wall with high shear stress [4]. Increased shear stress itself may cause rupture of a coronary atherosclerotic plaque [52]. In addition, high shear stress may result in increased platelet aggregation [23,24], often leading to thrombus formation.
In summary, there is still a gap in our understanding of all quantitative aspects of the extreme values of viscosity at low and zero shear rates [3]. To the best of my knowledge, the work described in this paper represents one of the few attempts to estimate extreme values of viscosity at low shear rate. An understanding of the precise mechanisms that affect blood viscosity would be of clinical significance.
Acknowledgements
The author gratefully acknowledges the contribution of Prof. Paul Agutter for his valuable comments.
References
- Baskurt OK, Tugral E, Neu B, Meiselman HJ. Particle electrophoresis as a tool to understand the aggregation behavior of red blood cells. Electrophoresis. 2002;23:2103–9. 10.1002/1522-2683(200207)23:13<2103::AID-ELPS2103>3.0.CO;2-O. [Abstract] [CrossRef] [Google Scholar]
- Yeow YL, Wickramasinghe SR, Leong YK, Han B. Model-independent relationships between hematocrit, blood viscosity, and yield stress derived from Couette viscometry data. Biotechnol Prog. 2002;18:1068–75. 10.1021/bp025558k. [Abstract] [CrossRef] [Google Scholar]
- Quemada D. Blood rheology and its implication in flow of blood. Wien and New York, International Center for Mechanical Sciences: Springer-Verlag; 1983. [Google Scholar]
- Ismailov RM, Shevchuk NA, Schwerha J, Keller L, Khusanov H. Blunt trauma to large vessels: a mathematical study. Biomed Eng Online. 2004;3:14. 10.1186/1475-925X-3-14. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Faizullaev FD. Laminar motion of multiphase media in conduits. New York: Consultants Bureau; 1969. [Google Scholar]
- Herczinsky R, Piénkowska I. Towards a Statistical Theory of Suspension. Ann Rev Fluid Mech. 1980;12:237–69. 10.1146/annurev.fl.12.010180.001321. [CrossRef] [Google Scholar]
- Thomas DG. Transport Characteristics of Suspension: VIII. A Note on the Viscosity of Newtonian Suspensions of Uniform Spherical Particles. J Coll Sci. 1965;20:267–77. 10.1016/0095-8522(65)90016-4. [CrossRef] [Google Scholar]
- Losev ES. A physical model of gravitational erythrocyte sedimentation. Biofizika. 1992;37:1057–62. [Abstract] [Google Scholar]
- Gavalov SM. Mechanism of fractional erythrocyte sedimentation rate. Sovetskaya Meditsina. 1957;21:62–6. Russian. [Abstract] [Google Scholar]
- Batchelor GK. An introduction to fluid dynamics. Cambridge: Cambridge University Press; 1967. [Google Scholar]
- Rakhmatullin KA. Foundations of gas dynamics of mutually penetrable flows of compressible media. Prikladnaya Matematika Mekhanika. 1956;20:184–195. Russian. [Google Scholar]
- Schlichting H. Boundary layer theory. New York: McGraw-Hill Book Co; 1968. [Google Scholar]
- Nigmatullin RI. Basic mechanics of multiphase media. Moscow: Nauka; 1978. In Russian. [Google Scholar]
- Malinovskaya TA. Separation of suspension in industry of limited synthesis. Moscow: Nauka; 1971. [Google Scholar]
- Nigmatullin RI. Mechanics of heterogeneous media. Moscow: Nauka; 1978. [Google Scholar]
- Caro CG. The mechanics of the circulation. Oxford: Oxford University Press; 1978. [Google Scholar]
- Happel J, Brenner H. Low Reynolds Number Hydrodynamics. New York: McGraw-Hill; 1963. [Google Scholar]
- Zhang JB, Kuang ZB. Study on blood constitutive parameters in different blood constitutive equations. J Biomech. 2000;33:355–60. 10.1016/S0021-9290(99)00101-3. [Abstract] [CrossRef] [Google Scholar]
- Chmiel H, Anadere I, Walitza E. The determination of blood viscoelasticity in clinical hemorheology. Biorheology. 1990;27:883–94. [Abstract] [Google Scholar]
- Anadere I, Chmiel H, Hess H, Thurston GB. Clinical blood rheology. Biorheology. 1979;16:171–8. [Abstract] [Google Scholar]
- Kwaan HC, Bongu A. The hyperviscosity syndromes. Semin Thromb Hemost. 1999;25:199–208. [Abstract] [Google Scholar]
- Dintenfass L. Modifications of blood rheology during aging and age-related pathological conditions. Aging (Milano) 1989;1:99–125. [Abstract] [Google Scholar]
- Jen CJ, McIntire LV. Characteristics of shear-induced aggregation in whole blood. J Lab Clin Med. 1984;103:115–24. [Abstract] [Google Scholar]
- Wagner CT, Kroll MH, Chow TW, Hellums JD, Schafer AI. Epinephrine and shear stress synergistically induce platelet aggregation via a mechanism that partially bypasses VWF-GP IB interactions. Biorheology. 1996;33:209–29. 10.1016/0006-355X(96)00018-2. [Abstract] [CrossRef] [Google Scholar]
- Chernysheva GA, Plotnikov MB, Smol'yakova VI, Avdoshin AD, Saratikov AS, Sutormina TG. Relationship between rheological and hemodynamic changes in rats with crush syndrome. Bull Exp Biol Med. 2000;130:1048–50. 10.1023/A:1002827230305. [Abstract] [CrossRef] [Google Scholar]
- Rab SM. Traumatic myocardial infarction. Br J Clin Pract. 1969;23:172–3. [Abstract] [Google Scholar]
- Jones FL., Jr Transmural myocardial necrosis after nonpenetrating cardiac trauma. Am J Cardiol. 1970;26:419–22. 10.1016/0002-9149(70)90740-X. [Abstract] [CrossRef] [Google Scholar]
- Fu M, Wu CJ, Hsieh MJ. Coronary dissection and myocardial infarction following blunt chest trauma. J Formos Med Assoc. 1999;98:136–40. [Abstract] [Google Scholar]
- Fang BR, Li CT. Acute myocardial infarction following blunt chest trauma. Eur Heart J. 1994;15:705–7. [Abstract] [Google Scholar]
- Atalar E, Acil T, Aytemir K, Ozer N, Ovunc K, Aksoyek S, Kes S, Ozmen F. Acute anterior myocardial infarction following a mild nonpenetrating chest trauma--a case report. Angiology. 2001;52:279–82. [Abstract] [Google Scholar]
- Lee HY, Ju YM, Lee MH, Lee SJ, Chang WH, Imm CW. A case of post-traumatic coronary occlusion. Korean J Intern Med. 1991;6:33–7. [Abstract] [Google Scholar]
- Candell J, Valle V, Paya J, Cortadellas J, Esplugas E, Rius J. Post-traumatic coronary occlusion and early left ventricular aneurysm. Am Heart J. 1979;97:509–12. 10.1016/0002-8703(79)90400-9. [Abstract] [CrossRef] [Google Scholar]
- Pifarre R, Grieco J, Garibaldi A, Sullivan HJ, Montoya A, Bakhos M. Acute coronary artery occlusion secondary to blunt chest trauma. J Thorac Cardiovasc Surg. 1982;83:122–5. [Abstract] [Google Scholar]
- Watt AH, Stephens MR. Myocardial infarction after blunt chest trauma incurred during rugby football that later required cardiac transplantation. Br Heart J. 1986;55:408–10. [Europe PMC free article] [Abstract] [Google Scholar]
- Jokl E, Greenstein J. Fatal coronary thrombosis in a boy of ten years. Lancet. 1944;2:659. 10.1016/S0140-6736(00)46019-8. [CrossRef] [Google Scholar]
- Pringle SD, Davidson KG. Myocardial infarction caused by coronary artery damage from blunt chest injury. Br Heart J. 1987;57:375–6. [Europe PMC free article] [Abstract] [Google Scholar]
- Kahn JK, Buda AJ. Long-term follow-up of coronary artery occlusion secondary to blunt chest trauma. Am Heart J. 1987;113:207–10. 10.1016/0002-8703(87)90035-4. [Abstract] [CrossRef] [Google Scholar]
- Boland J, Limet R, Trotteur G, Legrand V, Kulbertus H. Left main coronary dissection after mild chest trauma. Favorable evolution with fibrinolytic and surgical therapies. Chest. 1988;93:213–4. [Abstract] [Google Scholar]
- Oliva PB, Hilgenberg A, McElroy D. Obstruction of the proximal right coronary artery with acute inferior infarction due to blunt chest trauma. Ann Intern Med. 1979;91:205–7. [Abstract] [Google Scholar]
- Haas JM, Peterson CR, Jones RC. Subintimal dissection of the cornary arteries. A complication of selective coronary arteriography and the transfemoral percutaneous approach. Circulation. 1968;38:678–83. [Abstract] [Google Scholar]
- Cheng TO, Adkins PC. Traumatic aneurysm of left anterior descending coronary artery with fistulous opening into left ventricle and left ventricular aneurysm after stab wound of chest. Report of case with successful surgical repair. Am J Cardiol. 1973;31:384–90. 10.1016/0002-9149(73)90273-7. [Abstract] [CrossRef] [Google Scholar]
- Vlay SC, Blumenthal DS, Shoback D, Fehir K, Bulkley BH. Delayed acute myocardial infarction after blunt chest trauma in a young woman. Am Heart J. 1980;100:907–16. 10.1016/0002-8703(80)90073-3. [Abstract] [CrossRef] [Google Scholar]
- Goulah RD, Rose MR, Strober M, Haft JI. Coronary dissection following chest trauma with systemic emboli. Chest. 1988;93:887–8. [Abstract] [Google Scholar]
- Kohli S, Saperia GM, Waksmonski CA, Pezzella S, Singh JB. Coronary artery dissection secondary to blunt chest trauma. Cathet Cardiovasc Diagn. 1988;15:179–83. [Abstract] [Google Scholar]
- de Feyter PJ, Roos JP. Traumatic myocardial infarction with subsequent normal coronary arteriogram. Eur J Cardiol. 1977;6:25–31. [Abstract] [Google Scholar]
- Espinosa R, Badui E, Castano R, Madrid R. Acute posteroinferior wall myocardial infarction secondary to football chest trauma. Chest. 1985;88:928–30. [Abstract] [Google Scholar]
- Lascault G, Komajda M, Drobinski G, Grosgogeat Y. Left coronary artery aneurysm and anteroseptal acute myocardial infarction following blunt chest trauma. Eur Heart J. 1986;7:538–40. [Abstract] [Google Scholar]
- Moosikasuwan JB, Thomas JM, Buchman TG. Myocardial infarction as a complication of injury. J Am Coll Surg. 2000;190:665–70. 10.1016/S1072-7515(00)00263-5. [Abstract] [CrossRef] [Google Scholar]
- Chun JH, Lee SC, Gwon HC, Lee SH, Hong KP, Seo JD, Lee WR. Left main coronary artery dissection after blunt chest trauma presented as acute anterior myocardial infarction: assessment by intravascular ultrasound: a case report. J Korean Med Sci. 1998;13:325–7. [Europe PMC free article] [Abstract] [Google Scholar]
- Ismailov RM, Ness RB, Weiss HB, Lawrence BA, Miller TR. Trauma associated with acute myocardial infarction in a multi-state hospitalized population. Int J Cardiol. [Abstract]
- Caimi G, Hoffmann E, Montana M, Canino B, Dispensa F, Catania A, Lo Presti R. Haemorheological pattern in young adults with acute myocardial infarction. Clin Hemorheol Microcirc. 2003;29:11–8. [Abstract] [Google Scholar]
- Gertz SD, Roberts WC. Hemodynamic shear force in rupture of coronary arterial atherosclerotic plaques. Am J Cardiol. 1990;66:1368–72. 10.1016/0002-9149(90)91170-B. [Abstract] [CrossRef] [Google Scholar]
Articles from Theoretical Biology & Medical Modelling are provided here courtesy of BMC
Full text links
Read article at publisher's site: https://doi.org/10.1186/1742-4682-2-13
Read article for free, from open access legal sources, via Unpaywall:
https://tbiomed.biomedcentral.com/counter/pdf/10.1186/1742-4682-2-13
Citations & impact
Impact metrics
Citations of article over time
Article citations
Myocardial Injury Caused by Severe Blow: Importance of Carefulness in Accurate Diagnosis.
Int Heart J, 59(4):845-847, 23 May 2018
Cited by: 0 articles | PMID: 29794383
Coronary Thrombosis without Dissection following Blunt Trauma.
Case Rep Cardiol, 2016:8671015, 23 Feb 2016
Cited by: 5 articles | PMID: 27006836 | PMCID: PMC4781932
Circle of Willis atherosclerosis, Alzheimer's disease and the Dean number.
World J Cardiol, 5(10):394-396, 01 Oct 2013
Cited by: 1 article | PMID: 24198911 | PMCID: PMC3817283
Tricuspid valve chordal rupture due to airbag injury and review of pathophysiological mechanisms.
Interact Cardiovasc Thorac Surg, 15(3):555-557, 07 Jun 2012
Cited by: 8 articles | PMID: 22678241 | PMCID: PMC3422951
Trauma associated with cardiac dysrhythmias: results from a large matched case-control study.
J Trauma, 62(5):1186-1191, 01 May 2007
Cited by: 9 articles | PMID: 17495723
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Arch vessel injury: geometrical considerations. Implications for the mechanism of traumatic myocardial infarction II.
World J Emerg Surg, 1:28, 08 Sep 2006
Cited by: 4 articles | PMID: 16961917 | PMCID: PMC1570452
New trends in clinical hemorheology: an introduction to the concept of the hemorheological profile.
Schweiz Med Wochenschr Suppl, 43:41-49, 01 Jan 1991
Cited by: 6 articles | PMID: 1843037
Review
Temperature-dependent threshold shear stress of red blood cell aggregation.
J Biomech, 43(3):546-550, 29 Oct 2009
Cited by: 18 articles | PMID: 19878949
Mathematical model describing erythrocyte sedimentation rate. Implications for blood viscosity changes in traumatic shock and crush syndrome.
Biomed Eng Online, 4:24, 04 Apr 2005
Cited by: 4 articles | PMID: 15807888 | PMCID: PMC1090599

 1
1